A Lipid Primer - the Diversity of Natural Lipids
Definitions
Although most scientists with experience in this field tend to have a firm understanding of what is meant by the term "lipid", there is no widely accepted definition. General textbooks usually describe lipids in woolly terms as a group of naturally occurring compounds, which have in common a ready solubility in such organic solvents as hydrocarbons, chloroform and alcohols. They include a diverse range of compounds, like fatty acids, oxylipins and their ester or amide derivatives (e.g., triacylglycerols, phospholipids, sphingolipids), hydrocarbons, carotenoids, terpenes, sterols, polyketides and bile acids. Many of these compounds may appear to have little otherwise to relate them, but this definition may be a useful guide for non-scientists in that it encompasses the common fats and oils encountered in everyday life.
 In fact, for scientists, a definition of this kind is
misleading as many of the substances that are now widely regarded as lipids may be almost as soluble in water as in organic solvents.
Although the international bodies that usually decide such matters have shirked the task,
a more specific definition of lipids than one based simply on solubility is necessary, and one based on biochemical mechanisms has been put
forward by LipidMaps (see our page on Nomenclature for a more detailed discussion), but it will only be meaningful
to those with an advanced knowledge of biochemistry.
While some scientists seem to consider that all organic compounds other than proteins, carbohydrates and nucleotides are lipids.
this would make my task as a chronicler of the subject much more difficult.
More seriously, there is a danger that the term 'lipid' then becomes a catch-all (or even a dustbin!) for all classes of organic molecules
that do not fit into other categories.
In fact, for scientists, a definition of this kind is
misleading as many of the substances that are now widely regarded as lipids may be almost as soluble in water as in organic solvents.
Although the international bodies that usually decide such matters have shirked the task,
a more specific definition of lipids than one based simply on solubility is necessary, and one based on biochemical mechanisms has been put
forward by LipidMaps (see our page on Nomenclature for a more detailed discussion), but it will only be meaningful
to those with an advanced knowledge of biochemistry.
While some scientists seem to consider that all organic compounds other than proteins, carbohydrates and nucleotides are lipids.
this would make my task as a chronicler of the subject much more difficult.
More seriously, there is a danger that the term 'lipid' then becomes a catch-all (or even a dustbin!) for all classes of organic molecules
that do not fit into other categories.
I would prefer to restrict the use of "lipid" to fatty acids or closely related compounds and their naturally occurring derivatives (esters or amides), what might be termed "main-stream lipids", together with isoprenoids such as sterols, carotenoids and most fat-soluble vitamins, which co-occur in membranes. Others will certainly disagree, but my own preferred definition avoids considerations of solubility, biosynthesis or function, and excludes such polar molecules as most polyketides. It is -
Lipids comprise a heterogeneous class of predominantly hydrophobic organic molecules of relatively low molecular weight (commonly <1000) that are defined by the presence either of linear alkyl chains, usually with even-numbers of carbon atoms and saturated or unsaturated with double bonds in characteristic positions, or of isoprene units in linear or cyclic structures. These can contain variable numbers of oxygenated substituents such as carboxylic acid, hydroxyl groups and/or other heteroatoms, such as nitrogen in amines/amides. Often, these are linked covalently to glycerol, carbohydrates, phosphate and other small polar entities, which can render the molecules more amphiphilic.
 The key building blocks
of main-stream lipids are fatty acids, which comprise a linear alkyl chain with mainly even numbers of carbon atoms
(commonly C14 to C22), which can be saturated or unsaturated, and with a terminal carboxyl group;
further substituent groups can include oxygen atoms and ring structures.
While fatty acids per se have important biological properties, they occur most often in esterified form as ester or amide components
of other lipids.
The key building blocks
of main-stream lipids are fatty acids, which comprise a linear alkyl chain with mainly even numbers of carbon atoms
(commonly C14 to C22), which can be saturated or unsaturated, and with a terminal carboxyl group;
further substituent groups can include oxygen atoms and ring structures.
While fatty acids per se have important biological properties, they occur most often in esterified form as ester or amide components
of other lipids.
The most common lipid classes in nature consist of fatty acids linked by an ester bond to the trihydric alcohol - glycerol, or to other alcohols such as cholesterol, or by amide bonds to sphingoid bases or on occasion to other amines. In addition, lipid classes may contain alkyl moieties other than fatty acids, phosphoric acid, organic bases, carbohydrates and many more components, which can be released by various hydrolytic procedures.
A further subdivision into two broad classes is convenient for analytical purposes. Simple lipids are defined as those that on hydrolysis yield at most two types of primary product per mole (e.g., triacylglycerols and cholesterol esters), while complex lipids yield three or more primary hydrolysis products per mole. Alternatively, the terms "neutral" and "polar" lipids, respectively, are used to define these groups, but they are less exact. The complex lipids are best considered in terms of the glycerophospholipids (or simply if less accurately as phospholipids), which contain a polar phosphorus moiety and a glycerol backbone, sphingolipids containing a sphingoid base, or glycolipids (both glycoglycerolipids and glycosphingolipids), which contain a polar carbohydrate moiety. The picture is further complicated by the existence of phosphoglycolipids and sphingophospholipids (e.g., sphingomyelin).
The lipidome is the comprehensive spectrum of lipids in a tissue, organelle or membrane, while the science of lipidomics has been defined as the analysis of lipids on the systems-level scale together with their interacting factors.
Fatty Acids
Fatty acids are often considered to be the defining components of lipids. The common fatty acids of plant tissues are C16 and C18 straight-chain compounds with zero to three double bonds of a cis (or Z) configuration. Such fatty acids are also abundant in animal tissues, together with other even numbered components with a somewhat wider range of chain-lengths and up to six cis double bonds separated by methylene groups (methylene-interrupted), but the compositions are dependent on those received from the diet as well as those synthesised within tissues. Bacteria tend to produce saturated (including branched chain) and monoenoic fatty acids. The systematic and trivial names of those fatty acids encountered most often, together with their shorthand designations, are listed in the table.
The common fatty acids of animal and plant origin |
||
| Systematic name | Trivial name | Shorthand |
| Saturated fatty acids | ||
| ethanoic | acetic | 2:0 |
| butanoic | butyric | 4:0 |
| hexanoic | caproic | 6:0 |
| octanoic | caprylic | 8:0 |
| decanoic | capric | 10:0 |
| dodecanoic | lauric | 12:0 |
| tetradecanoic | myristic | 14:0 |
| hexadecanoic | palmitic | 16:0 |
| octadecanoic | stearic | 18:0 |
| eicosanoic | arachidic | 20:0 |
| docosanoic | behenic | 22:0 |
| Monoenoic fatty acids | ||
| 9Z-hexadecenoic | palmitoleic | 16:1(n-7) |
| 9Z-octadecenoic | oleic | 18:1(n-9) |
| 11Z-octadecenoic | cis-vaccenic | 18:1(n-7) |
| 13Z-docosenoic | erucic | 22:1(n-9) |
| 15Z-tetracosenoic | nervonic | 24:1(n-9) |
| Polyunsaturated fatty acids* | ||
| 9,12-octadecadienoic | linoleic | 18:2(n-6) |
| 6,9,12-octadecatrienoic | γ-linolenic | 18:3(n-6) |
| 9,12,15-octadecatrienoic | α-linolenic | 18:3(n-3) |
| 5,8,11,14-eicosatetraenoic | arachidonic | 20:4(n-6) |
| 5,8,11,14,17-eicosapentaenoic | EPA | 20:5(n-3) |
| 4,7,10,13,16,19-docosahexaenoic | DHA | 22:6(n-3) |
| * all double bonds are of the cis configuration | ||
The most abundant saturated fatty acid in nature is hexadecanoic or palmitic acid. It can be designated a "16:0" fatty acid, the first numerals denoting the number of carbon atoms in the aliphatic chain and the second, after the colon, denoting the number of double bonds. All the even-numbered saturated fatty acids from C2 to C30 and beyond have been found in nature, but only the 14:0 to 18:0 homologues are likely to be encountered in appreciable concentrations in glycerolipids, other than in a restricted range of commercial fats and oils. Sphingolipids are a further exception in that they tend to contain longer-chain components.
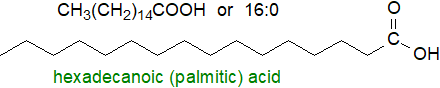
Oleic or cis-9- or 9Z-octadecenoic acid, the most abundant monoenoic fatty acid in nature, is designated as an "18:1" fatty acid, or more precisely as 9c‑18:1 or as 18:1(n-9), the latter to indicate that the last double bond is 9 carbon atoms from the terminal methyl group. The second form of the nomenclature is of value to biochemists and nutritionists in relation to most polyunsaturated fatty acids (two or more cis-double bonds). The more common cis-monoenoic acids fall into the same range of chain-lengths, i.e., 16:1(n‑7) and 18:1(n-9), though 20:1 and 22:1 are abundant in fish.
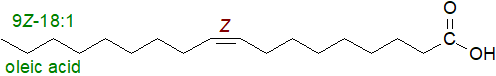
Fatty acids with double bonds of the trans (or E) configuration are found occasionally in natural lipids, or they are formed during industrial processing (refining or hydrogenation) and so enter the food chain, where their suitability for human nutrition is controversial. They tend to be minor components only of animal tissue lipids, other than of ruminants, where they are formed naturally by biohydrogenation of dietary fatty acids in the rumen.
The C18 polyunsaturated fatty acids, linoleic or 9Z,12Z-octadecadienoic acid (18:2(n-6)) and α-linolenic or 9Z,12Z,15Z-octadecatrienoic acid (18:3(n-3)), are major components of most plant lipids, including many of the vegetable oils of commerce. The cis-double bonds in these and related fatty acids are separated by one methylene group, i.e., they are 'methylene-interrupted'.
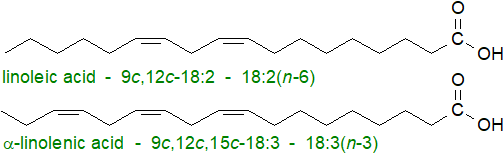
They are essential fatty acids in that they cannot be synthesised in animals but are required as dietary constituents for health and development. As linoleic acid is almost always present in foods, it tends to be relatively abundant in animal tissues; α-linolenic acid is most abundant in vegetable sources. In turn, these fatty acids are the biosynthetic precursors in animals of families of C20 and C22 polyunsaturated fatty acids with three to six cis-double bonds via sequential desaturation and chain-elongation steps (desaturases in animal tissues can only insert a double bond on the carboxyl side of an existing double bond). Those fatty acids derived from linoleic acid (omega-6 series), especially arachidonic acid (20:4(n-6)), are also necessary constituents of the membrane phospholipids in mammalian tissues as well as being the precursors of biologically active molecules such as the prostaglandins and other oxygenated metabolites (oxylipins). α-Linolenic acid is the precursor of the family of polyunsaturated fatty acids of the (n-3) or omega-3 series, such as eicosapentaenoic acid (20:5(n-3) or EPA) and docosahexaenoic acid (22:6(n-3) or DHA), which tend to be major components of phospholipids in all tissues, notably the eye and brain, as well as being substantial components of fish oils. In turn, these are utilized for synthesis of a separate range of oxylipins, the specialized pro-resolving mediators.
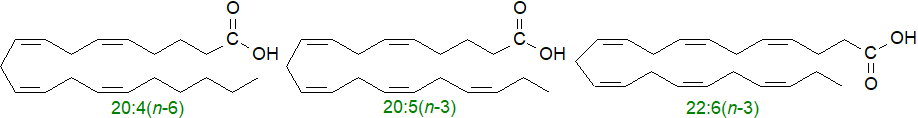
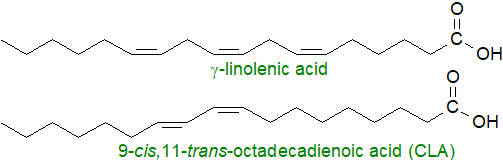
Other fatty acids may not be 'essential' in the above sense, but they can have vital functions in tissues. For example, palmitic acid is the biosynthetic precursor of sphingoid bases and thence of all sphingolipids (see below), and it is a necessary component of one class of proteolipids, i.e., protein-lipid complexes. On the other hand, in a dietary excess, it may be harmful. Oleic acid amounts to 70% of the fatty acids in olive oil and thence of the Mediterranean diet, which is generally considered to promote good health, and it can act as a mediator of tissue processes. Many other fatty acids of nutritional value do of course exist in nature, and there is some interest in γ‑linolenic acid (6,9,12‑octadecatrienoic acid, 18:3(n‑6), GLA), available from evening primrose oil, while conjugated linoleic acid (mainly 9Z,11E‑octadecadienoate) or 'CLA' is a natural constituent of dairy products and is claimed to be health promoting.
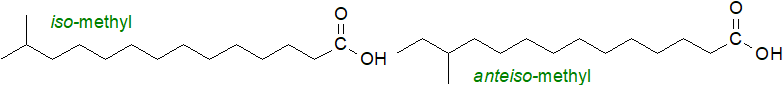
Branched chain fatty acids are synthesised by many microorganisms (most often with an iso- or an anteiso-methyl branch), and they are produced to a limited extent in higher organisms. They enter animal tissues mainly from the intestinal microbiome and via the diet, where ruminant fats are a common source. Phytanic acid or 3,7,11,15-tetramethylhexadecanoic acid is a metabolite of phytol (from chlorophyll) in animal tissues, although it is usually present at low levels only.
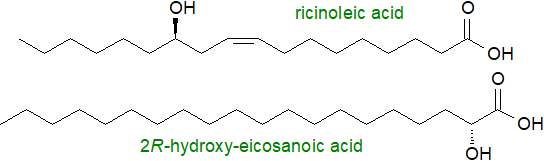
Fatty acids with many other substituent groups are present in some species of plants and microorganisms, and they may be encountered in animal tissues, which they can enter via the food chain. These substituents include acetylenic and allenic bonds, conjugated double bonds, ring structures and hydroxy-, epoxy-, keto and furan-groups. 2‑Hydroxy fatty acids are synthesised in animal and plant tissues, where they are often major constituents of the sphingolipids, while 12‑hydroxy-octadec-9Z-enoic or 'ricinoleic' acid is the main fatty acid constituent of castor oil.
Eicosanoids and Related Oxylipins
Oxylipins can be defined as a family of oxygenated natural products that are formed from fatty acids by pathways involving at least one step of mono- or dioxygen-dependent oxidation catalysed by various enzymes (or by autoxidation). All can have profound impacts upon tissue metabolism at minute concentrations. The term 'eicosanoid' is used to embrace those oxylipins derived from C20 fatty acids in animal tissues, including prostaglandins, thromboxanes and leukotrienes, and the key precursor fatty acids are 8Z,11Z,14Z-eicosatrienoic (dihomo-γ-linolenic or 20:3(n-6)), 5Z,8Z,11Z,14Z-eicosatetraenoic (arachidonic or 20:4(n-6)) and 5Z,8Z,11Z,14Z,17Z-eicosapentaenoic (20:5(n-3) or EPA) acids. Docosanoids (resolvins, protectins and maresins or 'specialized pro-resolving mediators') derived from docosahexaenoic acids (DHA) (and eicosapentaenoic (EPA)) and have properties that in the main tend to oppose or balance those of the eicosanoids.
In all of these, the activity depends on the precise positions and stereochemistry of ring structures, double bonds and hydroxyl groups in the alkyl chain, mainly when they are in an unesterified form, but a small proportion are present in tissues as components of complex lipids. Other oxylipins are produced from the same precursors by non-enzymic means, e.g., the isoprostanes. Together they constitute an epilipidome, a subset of the natural lipidome required to regulate complex metabolic pathways.
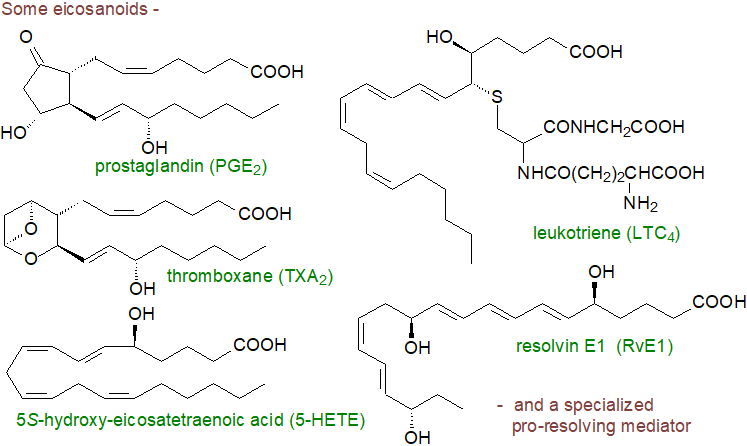
Those eicosanoids derived from arachidonic acid illustrated above have been most studied to date, although specialized pro-resolving mediators are increasingly the focus of research together with octadecanoids derived from linoleate. The prostaglandins and thromboxanes have cyclic structures generated by cyclooxygenase enzymes, and they often take part in the processes of inflammation. The hydroxy-eicosatetraenoic acids (HETE) are products of lipoxygenases and cytochrome P450 oxidases, and of these the 5‑lipoxygenase is notable in that it synthesises the first intermediate in the biosynthesis of leukotrienes. Although many of these are pro-inflammatory, the lipoxins derived from arachidonate and the specialized pro-resolving mediators formed from EPA and DHA are anti-inflammatory and promote the resolution of inflammation.
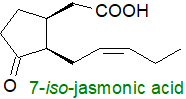 Plant products such as the jasmonates
and many other oxylipins are derived primarily from α‑linolenic (18:3(n-3)) acid,
and they are also generated by the action of lipoxygenases and other oxidative enzymes;
(+)-7-jasmonoyl-L-isoleucine rather than jasmonic acid per se is the main active form.
They are plant hormones, which are intimately associated with the growth and development of plants, and they are part of the signalling
response to physical damage by animals, insects and pathogens, and to other environmental stresses.
Despite their differing origins, there are obvious structural similarities between the jasmonates and prostanoids.
Plant products such as the jasmonates
and many other oxylipins are derived primarily from α‑linolenic (18:3(n-3)) acid,
and they are also generated by the action of lipoxygenases and other oxidative enzymes;
(+)-7-jasmonoyl-L-isoleucine rather than jasmonic acid per se is the main active form.
They are plant hormones, which are intimately associated with the growth and development of plants, and they are part of the signalling
response to physical damage by animals, insects and pathogens, and to other environmental stresses.
Despite their differing origins, there are obvious structural similarities between the jasmonates and prostanoids.
Simple Lipids
Triacylglycerols: Most of the fatty acids in living tissues are present in an esterified form (in excess in the free form they can be toxic). Nearly all the fats and oils of animal and plant origin of commercial value consist mainly of the simple lipid class triacylglycerols (termed "triglycerides" in the older literature), which contain a glycerol moiety with each hydroxyl group esterified to a fatty acid. The primary purpose of these lipid is to serve as a reservoir of fatty acids as a source of energy and of structural components for membranes. While both butter and corn oil consist largely of triacylglycerols, they differ substantially in their fatty acid compositions and thence their nutritional and physical properties. In nature, they are synthesised by enzyme systems, which determine that a centre of asymmetry is created about carbon-2 of the glycerol backbone, so they exist in enantiomeric forms, i.e., with different fatty acids in each of the three positions.
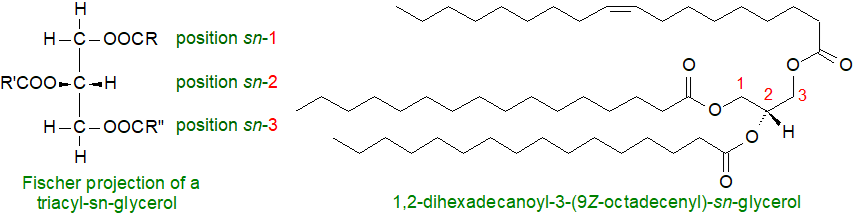
A stereospecific numbering system (sn) has been recommended to describe these forms. In a Fischer projection of a natural L-glycerol derivative, the secondary hydroxyl group is shown to the left of C-2; the carbon atom above this then becomes C-1 and that below is C-3. The prefix "sn" is placed before the stem name of the compound when the stereochemistry is defined. As an example, the single molecular species 1,2‑dihexadecanoyl-3-(9Z-octadecenoyl)-sn-glycerol is illustrated. From a practical standpoint, the composition of position sn-2 of dietary triacylglycerols is often considered most relevant to their subsequent metabolism.
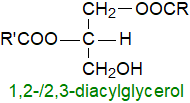 Diacylglycerols
(formerly termed "diglycerides") and monoacylglycerols ("monoglycerides") contain two moles and one mole of fatty
acids per mole of glycerol, respectively, and they exist in various isomeric forms; they are sometimes termed collectively "partial glycerides".
Although they are rarely present at greater than trace levels in fresh animal and plant tissues,
1,2-diacyl-sn-glycerols are intermediates in the biosynthesis of triacylglycerols and other glycerolipids,
and they are cellular messengers produced by hydrolysis of phosphatidylinositol and related lipids by a phospholipase C.
Some synthetic analogues have commercial applications.
Diacylglycerols
(formerly termed "diglycerides") and monoacylglycerols ("monoglycerides") contain two moles and one mole of fatty
acids per mole of glycerol, respectively, and they exist in various isomeric forms; they are sometimes termed collectively "partial glycerides".
Although they are rarely present at greater than trace levels in fresh animal and plant tissues,
1,2-diacyl-sn-glycerols are intermediates in the biosynthesis of triacylglycerols and other glycerolipids,
and they are cellular messengers produced by hydrolysis of phosphatidylinositol and related lipids by a phospholipase C.
Some synthetic analogues have commercial applications.
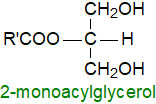 2-Monoacyl-sn-glycerols are formed as intermediates or end-products of the
enzymatic hydrolysis of triacylglycerols during digestion in the gut, and these and other positional isomers are powerful surfactants.
2‑Arachidonoylglycerol is an endocannabinoid, as is anandamide
or N‑arachidonoylethanolamine; they are so-named because they are similar pharmacologically to phytocannabinoids
from cannabis.
2-Monoacyl-sn-glycerols are formed as intermediates or end-products of the
enzymatic hydrolysis of triacylglycerols during digestion in the gut, and these and other positional isomers are powerful surfactants.
2‑Arachidonoylglycerol is an endocannabinoid, as is anandamide
or N‑arachidonoylethanolamine; they are so-named because they are similar pharmacologically to phytocannabinoids
from cannabis.
Acyl migration occurs rapidly in partial glycerides at room temperature, but especially on heating, in alcoholic solvents or in the presence of acid or base, so care is required for their isolation or analysis if the stereochemistry is to be retained. Synthetic 1/3‑monoacylglycerols are used in commerce as surfactants.
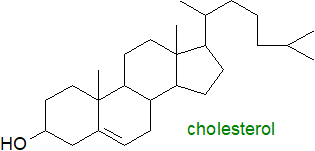 Cholesterol is by far the most common member of the
sterol lipid class in animal tissues; it has a tetracyclic ring system with a double bond in one of the rings
and one free hydroxyl group.
It is found both in the free state and in esterified form, i.e., as cholesterol esters.
Other related sterols are present in free and esterified form in animal tissues, but at trace levels only.
In spite of its bad reputation when in excess, cholesterol is required to maintain the fluidity of cellular membranes and as the
biosynthetic precursor of the bile acids, vitamin D and steroidal hormones.
Cholesterol is by far the most common member of the
sterol lipid class in animal tissues; it has a tetracyclic ring system with a double bond in one of the rings
and one free hydroxyl group.
It is found both in the free state and in esterified form, i.e., as cholesterol esters.
Other related sterols are present in free and esterified form in animal tissues, but at trace levels only.
In spite of its bad reputation when in excess, cholesterol is required to maintain the fluidity of cellular membranes and as the
biosynthetic precursor of the bile acids, vitamin D and steroidal hormones.
In plants, cholesterol is rarely detected in other than small amounts, but such phytosterols as sitosterol, stigmasterol, avenasterol, campesterol and brassicasterol, and their fatty acid esters are usually present, while ergosterol is characteristic of yeasts; they have similar functions in cells. Hopanoids are structurally related lipids produced by some bacteria.
Waxes: Wax esters are the most common and abundant components of waxes, and in their most common form, they consist of fatty acids esterified to long-chain alcohols with comparable chain-lengths; the latter tend to be saturated or have one double bond only. Such compounds are found in animal, plant and microbial tissues, often on exposed surfaces, where they act or in defence against predators, as waterproofing and for lubrication, although they can also be energy stores.

In some tissues, such as skin, avian preen glands or plant leaf surfaces, the wax components are much more complicated in their structures and compositions, and they can contain aliphatic diols, free alcohols, hydrocarbons (squalene, nonacosane, etc.), aldehydes and ketones.
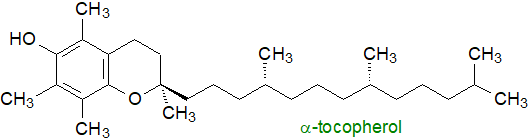
Tocopherols (collectively termed ‘vitamin E’) are substituted benzopyranols (methyl tocols) that occur in vegetable oils with different forms (α-, β-, γ- and δ-) recognized according to the number or position of methyl groups on the aromatic ring; tocotrienols have the same ring structures but with three double bonds in the aliphatic chain. α-Tocopherol (with the greatest vitamin E activity) illustrated is a natural antioxidant but is also utilized for metabolic purposes. Retinoids (vitamin A) are further lipid-soluble isoprenoids derived from dietary carotenoids, that are essential for vision as well as growth, development and reproduction. Vitamins D and K are likewise classified as fat-soluble or lipidic molecules.
Free (unesterified) fatty acids are minor constituents of living tissues, but they are required as precursors of other lipids, as an energy source and as cellular messengers.
Glycerophospholipids
Phospholipids are the definitive constituents of most natural membranes with each phospholipid class having its own ionic state that governs its location and how it operates within a bilayer. In addition to serving as structural components, they participate in innumerable processes in cells, sometimes as integral components of enzyme complexes. As amphiphilic molecules, phospholipids form micelles spontaneously in aqueous media.
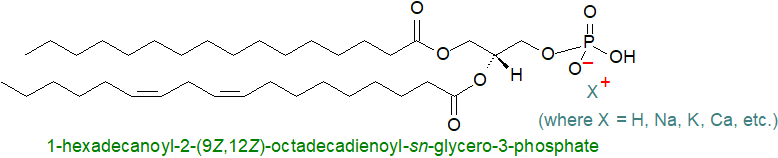
Phosphatidic acid or 1,2-diacyl-sn-glycerol-3-phosphate is found in trace amounts only in tissues under normal circumstances, but it has great metabolic importance as a biosynthetic precursor of most other glycerolipids. In all organisms but in plants especially, it is a signalling molecule. It is strongly acidic and is usually isolated as a mixed salt.
Lysophosphatidic acid with one mole of fatty acid per mole of lipid (in position sn-1) is a marker for ovarian cancer, and it is a cellular messenger that is a ligand for several receptors. Other lysophospholipids have their own functions in tissues.
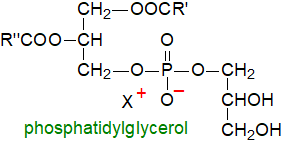 Phosphatidylglycerol
or 1,2-diacyl-sn-glycerol-3-phosphoryl-1'-sn-glycerol
tends to be a trace constituent of most tissues, but it is often the main component of some bacterial membranes.
Its physical properties are a factor in lung surfactant, and in plant chloroplasts, it has a role in photosynthesis.
In both animals and plants, it is the biosynthetic precursor of cardiolipin.
In some bacteria, the 3'-hydroxyl of the phosphatidylglycerol moiety is linked to an amino acid (lysine, ornithine or alanine)
to form an O‑aminoacylphosphatidylglycerol or complex 'lipoamino acid'.
Phosphatidylglycerol
or 1,2-diacyl-sn-glycerol-3-phosphoryl-1'-sn-glycerol
tends to be a trace constituent of most tissues, but it is often the main component of some bacterial membranes.
Its physical properties are a factor in lung surfactant, and in plant chloroplasts, it has a role in photosynthesis.
In both animals and plants, it is the biosynthetic precursor of cardiolipin.
In some bacteria, the 3'-hydroxyl of the phosphatidylglycerol moiety is linked to an amino acid (lysine, ornithine or alanine)
to form an O‑aminoacylphosphatidylglycerol or complex 'lipoamino acid'.
Cardiolipin (diphosphatidylglycerol or more precisely 1,3-bis(sn-3'-phosphatidyl)-sn-glycerol) is a unique phospholipid with a dimeric structure in essence, having four acyl groups and potentially carrying two negative charges (and is thus an acidic lipid). As a major constituent of mitochondrial lipids (heart muscle is a rich source), it is an integral component of the enzyme complexes concerned with oxidative phosphorylation and ATP production, it is essential for their efficient operation. In animal tissues, a high proportion is a single molecular species, the tetra-linoleoyl form.
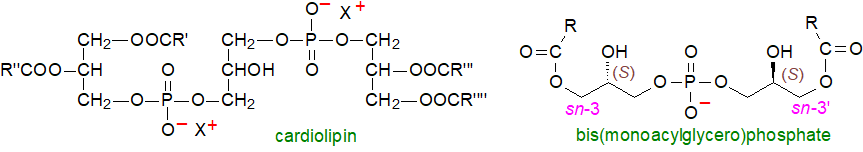
Bis(monoacylglycero)phosphate or lysobisphosphatidic acid is an interesting lipid in that its stereochemical configuration differs from that of all other animal glycero-phospholipids; the phosphodiester moiety is linked to positions sn-1 and sn-1' of glycerol, rather than to positions sn-3 and 3', while the fatty acids are esterified to the sn-2 positions. It is usually a minor component only of animal tissues, but it is enriched in the lysosomal membranes and is considered to be a marker for this organelle. Because of the unusual stereochemistry, it is resistant to most lipases in lysosomes.
Phosphatidylcholine or 1,2-diacyl-sn-glycerol-3-phosphorylcholine is zwitterionic and is usually the most abundant lipid in the membranes of animal tissues and often of plant membranes, but only rarely of bacteria. With the other choline-containing phospholipid, sphingomyelin, it is a structural component that constitutes much of the lipid in the external monolayer of the plasma membrane of animal cells. The trivial name "lecithin" is sometimes applied, but this term is now used more often for the mixed phospholipid by‑products of seed oil refining.
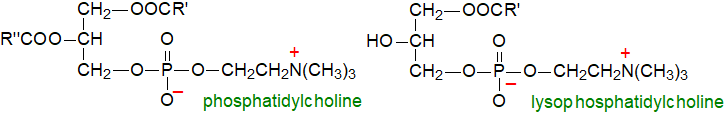
Lysophosphatidylcholine, which contains only one fatty acid moiety in each molecule usually in position sn-1, is sometimes present as a minor component of tissues, although it is relatively abundant bound to albumin in plasma. Like all lysophospholipids, it is a powerful surfactant and is more soluble in water than most other glycerolipids; in excess, it can be disruptive to tissues.
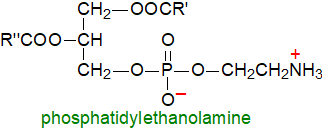 Phosphatidylethanolamine (once given the trivial name "cephalin") is
often the second most abundant phospholipid class in animal and plant tissues, and it can be the main lipid class in bacteria.
In some circumstances, the amine group can be methylated enzymically to
yield first phosphatidyl-N-monomethylethanolamine and then phosphatidyl-N,N-dimethylethanolamine,
but these intermediates rarely accumulate in significant amounts; the eventual product is phosphatidylcholine.
N‑Acylphosphatidylethanolamine is a minor component of some plant tissues (cereals are a rich source),
and it is occasionally found in animal tissues, where it is the precursor of some biologically active
amides.
Lysophosphatidylethanolamine contains only one mole of fatty acid per mole of lipid.
Phosphatidylethanolamine (once given the trivial name "cephalin") is
often the second most abundant phospholipid class in animal and plant tissues, and it can be the main lipid class in bacteria.
In some circumstances, the amine group can be methylated enzymically to
yield first phosphatidyl-N-monomethylethanolamine and then phosphatidyl-N,N-dimethylethanolamine,
but these intermediates rarely accumulate in significant amounts; the eventual product is phosphatidylcholine.
N‑Acylphosphatidylethanolamine is a minor component of some plant tissues (cereals are a rich source),
and it is occasionally found in animal tissues, where it is the precursor of some biologically active
amides.
Lysophosphatidylethanolamine contains only one mole of fatty acid per mole of lipid.
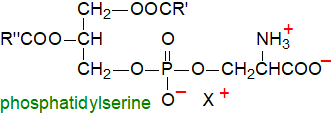 Phosphatidylserine is
a weakly acidic lipid that is present in most tissues of animals and plants and in microorganisms.
Under normal conditions, it is located entirely in the inner monolayer leaflet of the plasma membrane and other cellular membranes.
Phosphatidylserine is a cofactor for the stimulation of protein kinase C, and it is involved in many other processes,
including blood coagulation and bone formation.
As part of the mechanism of apoptosis (programmed cell death), it migrates to the outer leaflet of the plasma membrane
where it acts as an "eat-me" signal to macrophages.
N‑Acylphosphatidylserine has been detected in some animal tissues.
Phosphatidylserine is
a weakly acidic lipid that is present in most tissues of animals and plants and in microorganisms.
Under normal conditions, it is located entirely in the inner monolayer leaflet of the plasma membrane and other cellular membranes.
Phosphatidylserine is a cofactor for the stimulation of protein kinase C, and it is involved in many other processes,
including blood coagulation and bone formation.
As part of the mechanism of apoptosis (programmed cell death), it migrates to the outer leaflet of the plasma membrane
where it acts as an "eat-me" signal to macrophages.
N‑Acylphosphatidylserine has been detected in some animal tissues.
Phosphatidylinositol, containing the optically inactive form of inositol, myo‑inositol, is a common constituent of animal, plant and microbial lipids. In animal tissues especially, it may be accompanied by small amounts of phosphatidylinositol 4‑phosphate and phosphatidylinositol 4,5-bisphosphate (and other 'poly-phosphoinositides'), each of which is required for its own metabolic purpose, often in characteristic membranes. These compounds turn over rapidly in animal cells, and they are converted to metabolites such as diacylglycerols and inositol phosphates, which regulate many metabolic processes. Of these, diacylglycerols regulate a group of enzymes known as protein kinase C, which in turn control many vital aspects of cellular metabolism, including differentiation, proliferation, metabolism and apoptosis. In addition, phosphatidylinositol is the primary source of the arachidonic acid used for eicosanoid synthesis in animals, and it is an anchor that can link a variety of proteins to the external leaflet of the plasma membrane via a glycosyl bridge (glycosylphosphatidylinositol(GPI)-anchored proteins).
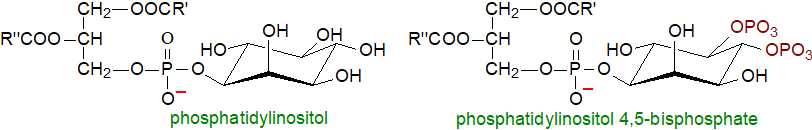
Ether lipids: Many glycerolipids, but mainly phospholipids of animal and microbial origin but not those of plants, contain aliphatic residues linked either by an ether bond or a vinyl ether bond to position sn-1 of L-glycerol. They can be abundant in the phospholipids of animals and microorganisms, but mainly in the phosphatidylethanolamine (PE) fraction. When a lipid contains a vinyl ether bond, the generic term "plasmalogen" is often used, and it has been recommended that the alkyl- and alk‑1‑enyl-forms of PE should be termed "plasmanylethanolamine" and "plasmenylethanolamine", respectively. The mechanisms for biosynthesis of plasmalogens in animals and bacteria are so different that they are believed to have evolved separately.
1-Alkyl-2,3-diacyl-sn-glycerols, ether analogues of triacylglycerols, tend to be present in trace amounts only in animal tissues, but they can be major constituents of certain fish oils such as those of sharks. Related compounds containing a 1‑alk‑1'‑enyl moiety ('neutral plasmalogens') are only occasionally detected.
On hydrolysis of glycerolipids containing an alkyl ether bond, 1-alkyl-sn-glycerols are formed and can be isolated for analysis. When plasmalogens are hydrolysed under basic conditions, 1-alkenyl-sn-glycerols are released, but aldehydes are produced from the latter on acidic hydrolysis. With both types, the aliphatic residues generally have a chain-length of 16 or 18, and they are saturated or may contain one further double bond, which is remote from the ether linkage.
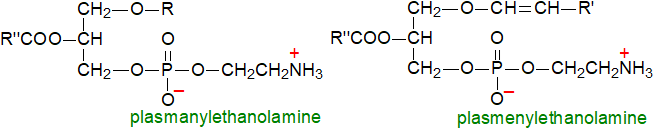
'Platelet-activating factor' or 1-alkyl-2-acetyl-sn-glycerophosphorylcholine is a distinctive ether-containing phospholipid from animals that has been studied intensively because it can exert profound effects at minute concentrations. It causes aggregation of blood platelets at concentrations as low as 10‑11M, and it induces a hypertensive response at very low levels; it is a mediator of inflammation and is a signalling molecule.
Glycoglycerolipids
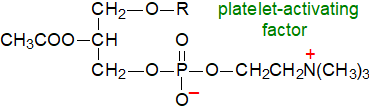
In the photosynthetic tissues of plants, a substantial proportion of the lipids consists of 1,2-diacyl-sn-glycerols joined by a glycosidic linkage at position sn‑3 to a carbohydrate moiety. The main components are the mono- and digalactosyldiacylglycerols, but related compounds have been found with up to four galactose units, or in which one or more of these is replaced by glucose moieties, while a 6‑O-acyl-monogalactosyldiacylglycerol is occasionally detected. Monogalactosyldiacylglycerols are essential to photosynthesis as integral components of the photosystem complexes. They have a comparable role in membranes to the glycerophospholipids for which they can sometimes substitute in part when phosphorus is limiting as a nutrient. Digalactosyldiacylglycerols are present in extra-plastid membranes under some conditions, including the plasma membrane where they are located on the inner leaflet.
A related unique plant glycolipid is sulfoquinovosyldiacylglycerol or the 'plant sulfolipid'. Rather than a relatively common sulfate moiety, it contains a sulfonic acid residue linked by a carbon-sulfur bond to the 6‑deoxyglucose moiety of a monoglycosyldiacylglycerol and is found exclusively in the membranes of chloroplasts where it is another component of the photosynthetic mechanism. It is a major reservoir of organic sulfur in the biosphere.
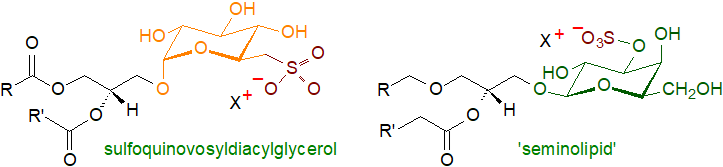
Monogalactosyldiacylglycerols are not solely plant lipids as they have been found in small amounts in brain and nervous tissue in some animals. A range of complex glyceroglycolipids have been characterized from intestinal tract and lung tissue that exist in both diacyl and alkylacyl forms. Such compounds are destroyed by some of the methods used in the isolation of glycosphingolipids, so they can be missed during analysis and may be more widespread than has been thought. A complex glycoglycero-sulfolipid, termed seminolipid, of which the main component is 1-O-hexadecyl-2-O-hexadecanoyl-3-O-(3'-sulfo-β-D-galactopyranosyl)-sn-glycerol, the principal glycolipid in testis and sperm. It differs from the comparable plant lipid in having a sulfate as opposed to a sulfonate linkage to the carbohydrate moiety as well as having an ether-linked alkyl group in position sn-1. A further range of highly complex glycoglycerolipids occur in bacteria and other micro-organisms, often with mannose as a carbohydrate moiety.
Sphingolipids
Sphingolipids are major component of animal and plant tissues but are only rarely encountered in bacteria. They consist of long-chain or sphingoid bases linked by an amide bond to a fatty acid and via the terminal hydroxyl group to complex carbohydrate or phosphorus-containing moieties. While they have some similar functions in membranes to the complex glycerolipids, they have their own characteristic metabolism.
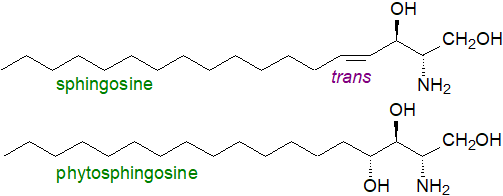 Long-chain bases
(sphingoids or sphingoid bases) are the distinguishing structural unit of sphingolipids.
They are long-chain (12 to 22 carbon atoms) aliphatic amines (secondary), which contain two or three adjacent hydroxyl groups and often a
trans-double bond in position 4.
The commonest or most abundant of these in animal tissues is
sphingosine ((2S,3R,4E)-2-amino-4-octadecen-1,3-diol).
More than a hundred different long-chain bases have been found in animals, plants and microorganisms, and many of these may occur in a
single tissue
but almost always as part of a complex lipid as opposed to in the free form.
The aliphatic chains can be saturated, monounsaturated and diunsaturated, with double bonds in various positions and
of either the cis or trans configuration; they may sometimes have methyl substituents.
In plants, phytosphingosine ((2S,3S,4R)-2-amino-octadecanetriol) with three hydroxyl groups
is the most common long-chain base.
Long-chain bases
(sphingoids or sphingoid bases) are the distinguishing structural unit of sphingolipids.
They are long-chain (12 to 22 carbon atoms) aliphatic amines (secondary), which contain two or three adjacent hydroxyl groups and often a
trans-double bond in position 4.
The commonest or most abundant of these in animal tissues is
sphingosine ((2S,3R,4E)-2-amino-4-octadecen-1,3-diol).
More than a hundred different long-chain bases have been found in animals, plants and microorganisms, and many of these may occur in a
single tissue
but almost always as part of a complex lipid as opposed to in the free form.
The aliphatic chains can be saturated, monounsaturated and diunsaturated, with double bonds in various positions and
of either the cis or trans configuration; they may sometimes have methyl substituents.
In plants, phytosphingosine ((2S,3S,4R)-2-amino-octadecanetriol) with three hydroxyl groups
is the most common long-chain base.
As a shorthand convenience, a nomenclature like that for fatty acids can be used, i.e., the chain-length and number of double bonds in the sphingoid base are denoted in the same manner with the prefix "d" or "t" to designate di- and trihydroxy bases, respectively. Thus, sphingosine is d18:1 and phytosphingosine is t18:0.
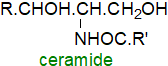 Ceramides contain fatty acids linked by an amide bond to the amine group
of a long-chain base.
In general, they are present at low levels only in tissues, but they are intermediates in the biosynthesis of the complex sphingolipids,
in the regulation of apoptosis, and in cell differentiation, transformation and proliferation, and they are signalling molecules in cells.
Unusual ceramides are located in the epidermis of the most studied species, pig and humans; the fatty acids with an amide link to the sphingoid
base are C30 and C32 in chain length with an ω‑hydroxyl group to which the essential fatty acid, linoleic acid,
specifically is esterified.
These are ultimately modified to form a covalent link to proteins in the epidermis where they form the
impermeable barrier to the external environment that prevents the loss of moisture through the skin.
Ceramides contain fatty acids linked by an amide bond to the amine group
of a long-chain base.
In general, they are present at low levels only in tissues, but they are intermediates in the biosynthesis of the complex sphingolipids,
in the regulation of apoptosis, and in cell differentiation, transformation and proliferation, and they are signalling molecules in cells.
Unusual ceramides are located in the epidermis of the most studied species, pig and humans; the fatty acids with an amide link to the sphingoid
base are C30 and C32 in chain length with an ω‑hydroxyl group to which the essential fatty acid, linoleic acid,
specifically is esterified.
These are ultimately modified to form a covalent link to proteins in the epidermis where they form the
impermeable barrier to the external environment that prevents the loss of moisture through the skin.
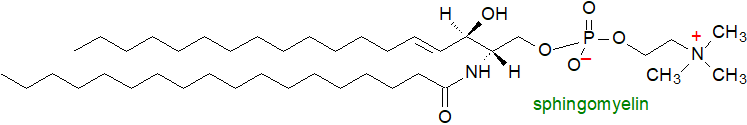
Sphingomyelin is a sphingophospholipid and consists of a ceramide unit linked at position 1 to phosphorylcholine; it is found as a major component of the complex lipids of all animal tissues but not of plants or micro-organisms. It resembles phosphatidylcholine in some of its physical properties and can apparently substitute in part for this in membranes, although it has its own role as a major constituent of the plasma membrane of cells, where it is concentrated together with sphingoglycolipids and cholesterol in tightly organized sub-domains termed 'rafts'. Sphingosine tends to be the most abundant long-chain base constituent, and it is usually accompanied by sphinganine and C20 homologues. Sphingomyelin is a precursor for many sphingolipid metabolites that take part in cellular signalling, including sphingosine-1-phosphate (see below), as part of the 'sphingomyelin cycle'. A correct balance between the various metabolites is necessary for good health. Niemann-Pick disease types A and B are rare lipid storage disorders that result from a deficiency in the enzyme responsible for the degradation of sphingomyelin.
A similar lipid ceramide phosphorylethanolamine is found in the lipids of insects and some freshwater invertebrates, while the phosphonolipid analogue, ceramide 2‑aminoethylphosphonic acid, has been detected in sea anemones and protozoa. Ceramide phosphorylinositol is present in some organisms, and like phosphatidylinositol, it can be an anchor unit for oligosaccharide-linked proteins in membranes (more...).
The most widespread glycosphingolipids are the monoglycosylceramides, and they consist of a basic ceramide unit linked by a glycosidic bond at carbon 1 of the long-chain base to glucose or galactose. They were first found in brain lipids, where the principal form is galactosylceramide ('cerebroside'), but they are now known to be ubiquitous constituents of animal tissues. Glucosylceramide is an abundant lipid in skin where it is part of the water permeability barrier, but it is also the biosynthetic precursor of lactosylceramide and thence of the complex oligoglycolipids and gangliosides in other organs. O-Acyl-glycosylceramides have been detected in small amounts in some tissues, as have cerebrosides with other monosaccharides such as xylose, mannose and fucose. In plants, glucosylceramide is present in membranes, where the main long-chain base is phytosphingosine.
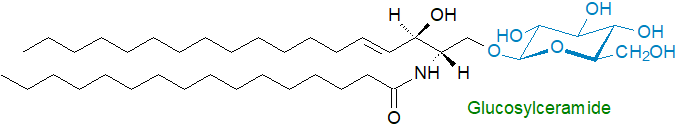
Di-, tri- and tetraglycosylceramides (oligoglycosylceramides) are present in most animal tissues at lower levels. The most common diglycosyl form is lactosylceramide, and it can be accompanied by related compounds containing further galactose or galactosamine residues. Tri- and tetraglycosylceramides with a terminal galactosamine residue are sometimes termed 'globosides', while glycolipids containing fucose are known as 'fucolipids'; that illustrated is the main glycosphingolipid in erythrocytes.
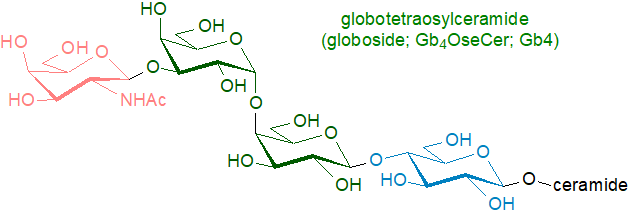
Lactosylceramide is the biosynthetic precursor of most of these oligoglycosphingolipids with further monosaccharide residues being added to the end of the carbohydrate chain (up to as many as twenty in some tissues). They are an element of the immune response system, and some are involved in the antigenicity of blood group determinants, while others bind to certain toxins or bacteria. As the complex glycosyl moiety is of primary relevance in this context, it has received most attention from investigators. However, certain of these lipids have been found to have distinctive long-chain base and fatty acid compositions, which enhance their activity.
Sulfate esters of galactosylceramide and lactosylceramide (sulfoglycosphingolipids - often referred to as "sulfatides" or "lipid sulfates"), with the sulfate group linked to position 3 of the galactosyl moiety, are major components of brain lipids, but they are found in trace amounts only in other tissues apart from the kidney where they are involved in ion transport.
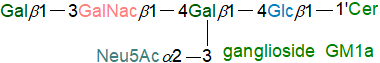 Gangliosides are highly complex oligoglycosylceramides, which contain one or more
sialic acid groups (N‑acetyl derivative of neuraminic acid (Neu5Ac)) with glucose, galactose and galactosamine as sugar moieties.
The polar and ionic nature of these lipids renders them soluble in water (contrary to some definitions of a lipid).
They were first found in the ganglion cells of the central nervous system, hence the name, but are now known to be present in most animal tissues.
Like the neutral oligoglycosylceramides, the carbohydrate and sialic acid residues are mainly responsible for the observed interactions
with cellular and extra-cellular components, although the long-chain base and fatty acid components can vary between tissues and species
and are presumably related in some way to their function.
Gangliosides have been shown to control growth and differentiation of cells, and they have roles in the immune defence system.
They act as receptors for many tissue metabolites and in this way may regulate cell signalling, and they bind to various bacterial
toxins, such as those from botulinum, tetanus and cholera.
Several unpleasant lipidoses have been identified involving storage of excessive amounts of gangliosides (and other oligoglycosylceramides)
in tissues, the best known of which is Tay-Sachs disease, and aberrant metabolism has been found in many cancers.
Gangliosides are highly complex oligoglycosylceramides, which contain one or more
sialic acid groups (N‑acetyl derivative of neuraminic acid (Neu5Ac)) with glucose, galactose and galactosamine as sugar moieties.
The polar and ionic nature of these lipids renders them soluble in water (contrary to some definitions of a lipid).
They were first found in the ganglion cells of the central nervous system, hence the name, but are now known to be present in most animal tissues.
Like the neutral oligoglycosylceramides, the carbohydrate and sialic acid residues are mainly responsible for the observed interactions
with cellular and extra-cellular components, although the long-chain base and fatty acid components can vary between tissues and species
and are presumably related in some way to their function.
Gangliosides have been shown to control growth and differentiation of cells, and they have roles in the immune defence system.
They act as receptors for many tissue metabolites and in this way may regulate cell signalling, and they bind to various bacterial
toxins, such as those from botulinum, tetanus and cholera.
Several unpleasant lipidoses have been identified involving storage of excessive amounts of gangliosides (and other oligoglycosylceramides)
in tissues, the best known of which is Tay-Sachs disease, and aberrant metabolism has been found in many cancers.
Plants do not contain gangliosides, but complex sphingolipids, phytoglycosphingolipids containing glucosamine, glucuronic acid and mannose linked to the ceramide via phosphorylinositol, are now known to be major components of the membranes in plant tissues and in fungi.
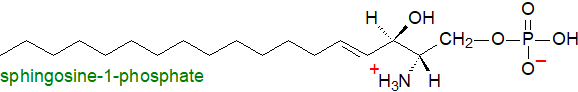
Sphingosine-1-phosphate is one of the simplest sphingolipids structurally (akin to lysophospholipids). It is present at low levels only in animal tissues, but it is a pivotal lipid in many cellular signalling pathways (balancing the actions of ceramide and ceramide-1-phosphate). For example, within cells, sphingosine-1-phosphate promotes cellular division (mitosis), while in the blood it affects platelet aggregation and thrombosis. It is an intermediate in the catabolism of sphingolipids.
Fatty acids of sphingolipids: Although structures of fatty acids are discussed in greater depth above, it is worth noting that the fatty acyl groups of sphingolipids are very different from those in the glycerolipids. They tend to consist of long-chain (C16 up to C26 but occasionally longer) odd- and even-numbered saturated or monoenoic fatty acids and related 2-D-hydroxy fatty acids, both in plant and animal tissues. Linoleic acid may be present at low levels in sphingolipids from animal tissues, but polyunsaturated compounds are rarely found (although their presence is often reported in error).
Other Lipids
Of course, many more lipids occur in nature than can be described in this document. I have not touched here on arsenolipids, archaeal lipids, betaine lipids, rhamnolipids, some of the fat-soluble vitamins, lipoamino acids, anandamide (endocannabinoids) and other simple amides, proteolipids, lipoproteins, and lipopolysaccharides, to name but a few, but there is information on these and many other lipids elsewhere on this website. New lipids with novel structures, biochemistry and pharmacology continue to be found, and no doubt many remain to be discovered.
Suggested Reading
Other than here, our host site LIPID MAPS® and Claude Leray's website www.cyberlipid.org are the best sources of information on lipid structures. I can recommend -
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Gunstone, F.D., Harwood, J.L. and Dijkstra, A.J. (Editors), The Lipid Handbook (3nd Edition) (CRC Press, Boca Raton) (2007) - see CRC Press.
- Gurr, M.I., Harwood, J.L., Frayn, K.N., Murphy, D.J. and Michell, R.H. Lipids: Biochemistry, Biotechnology and Health (6th Edition). (Wiley-Blackwell) (2016).
- Hannun, Y.A., Merrill, A.H. and Luberto, C. The bioactive sphingolipid playbook. A primer for the uninitiated as well as sphingolipidologists. J. Lipid Res., 66, 100813 (2025); DOI.
- Jouhet, J. and others. Plant and algal lipidomes: Analysis, composition, and their societal significance. Prog. Lipid Res., 96, 101290 (2024); DOI.
- Leray, C. Les Lipides dans le Monde Vivant. Introduction à la Lipidomique. (Lavoisier, France) (2010) - see Lavoisier.
- Penkov, S. and Fedorova, M. Membrane epilipidome-lipid modifications, their dynamics, and functional significance. Cold Spring Harbor Persp. Biol., 16, a041417 (2024); DOI.
- Ridgway, N.D. and McLeod, R.S. (Editors) Biochemistry of Lipids, Lipoproteins and Membranes (6th Edition). (Elsevier, Amsterdam) (2016) - see Science Direct - now in a 7th edition (2021).
- Tietel, Z. and others. An overview of food lipids toward food lipidomics. Comp. Rev. Food Sci. Food Safety, 22, 4302-4354 (2023); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: July 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
