Fatty Acids: Acetylenic and Allenic
 Aliphatic compounds
containing acetylenic (alkyne or triple) and allenic bonds, including alcohols, aldehydes, ketones, hydrocarbons and fatty acids,
are widespread in nature.
Innumerable different structures (more than 2,000) have been characterized, and such compounds have many different biological
functions, although they are usually present at rather low levels as secondary metabolites.
However, fatty acids with acetylenic bonds are sometimes found as major components in certain seed oils of higher plants,
and they are present in the lipids of mosses, fungi and algae.
They have also been detected in primitive marine animals, such as sponges, and in some insects and bacteria.
Aliphatic compounds
containing acetylenic (alkyne or triple) and allenic bonds, including alcohols, aldehydes, ketones, hydrocarbons and fatty acids,
are widespread in nature.
Innumerable different structures (more than 2,000) have been characterized, and such compounds have many different biological
functions, although they are usually present at rather low levels as secondary metabolites.
However, fatty acids with acetylenic bonds are sometimes found as major components in certain seed oils of higher plants,
and they are present in the lipids of mosses, fungi and algae.
They have also been detected in primitive marine animals, such as sponges, and in some insects and bacteria.
Discussion of the structures, occurrence and biochemistry of all of these natural lipids would be a daunting task, so a few only of the more noteworthy of those fatty acids with acetylenic bonds have been chosen for discussion below, although selected references to other acetylenic lipids are listed at the end of this document. While their primary functions are in the organisms in which they are produced, often as defence compounds, some natural acetylenic fatty acids have useful pharmaceutical properties, for example as antibacterial and anti-cancer agents, and synthetic analogues are under development for clinical testing. They are chemically unstable and highly reactive compounds, especially in the presence of oxygen.
1. Acetylenic Fatty Acids from Plants and Fungi
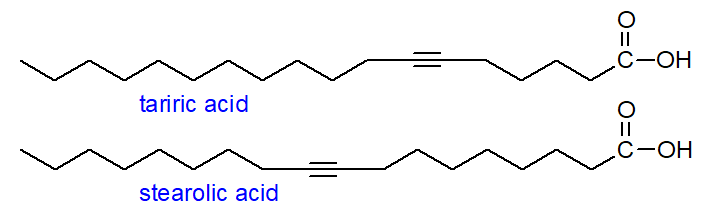
Monoynoic fatty acids: Tariric (octadec-6-ynoic) acid was identified as long ago as 1892 by Arnaud in seed oils of Picramnia species, where it can amount to as much as 95% of the total fatty acids. It was much later before stearolic (octadec-9-ynoic) acid was detected in a seed oil, although it was well known as a product of chemical synthesis; it is only encountered at low levels in seed oils of the Santalaceae and Olacaceae. These fatty acids have been detected together with an isomer with the acetylenic bond in position 12 in mosses and liverworts.

Non-conjugated enynoic fatty acids: Crepenynic acid or octadec-9Z-en,12-ynoic acid, sometimes abbreviated to 9Z,12a-18:2, was first found in 1964 as a major component of the seed oil of Crepis foetida and subsequently in other seed oils of this and some other plant families.

It is now known to be derived biosynthetically from linoleic acid (see below) and is itself the precursor of a substantial number of acetylenic metabolites required for diverse purposes in plants. Other isomers or related acids found in seed oils include octadec-6Z-en,9-ynoic (from Ongokea klaineana), hexadec-15-en,12-ynoic (‘scleropyric’ from Scleropyrum wallichianum), and octadec-9-yn,17-enoic (from Alvardoa amorphoides) acids; sterculynic acid (cis-9,10-methylene-9-octadecen-17-ynoic acid) is described in our web page on cyclic fatty acids. Crepenynic acid has been detected in mosses together with the isomers 6a,9Z-18:2 and 9a,12Z-18:2, while related fatty acids with up to three hydroxyl groups have been found in Basidomycetes (mushrooms). Although it was first detected in seed oils, crepenynic acid is now known to occur in other parts of higher plants such as the flowers and leaves of Afzelia cuanzensis, where it occurs with 14,15‑dehydrocrepenynic acid. In seed oils of two species of Atractylodes, it is located only in positions 1/3 of the triacylglycerols and not in position sn-2.
Mosses (Bryophytes), algae and fungi can contain polyunsaturated fatty acids similar to the conventional common range of (n‑3) and (n‑6) families, but in which the first double bond is instead acetylenic. These include 6a,9Z,12Z-18:3, 8a,11Z,14Z-20:3, 6a,9Z,12Z,15Z-18:4 and 5a,8Z,11Z,14Z-20:4, the last two of which are illustrated. These are precursors of a wide range of acetylenic oxylipins that are generated on wounding, and act in the defence against attack by fungi, bacteria and even herbivores, such as slugs.

Conjugated enynoic fatty acids (one acetylenic bond): Ximenynic or octadecen-9-yn,11E-enoic acid was first isolated and identified from seed oils of the Ximenia genus, where it occurs together with a series of homologues up to C26 in chain-length, presumably produced by chain-elongation. ‘Santalbic’ acid (from Santalum spicatum) is an earlier name from before the structure was determined independently. It is reported to have anti-inflammation, anticancer, antimicrobial and larvicidal properties in vitro and in vivo. The structurally related pyrulic (heptadecen-8-yn,10E-enoic) acid has been characterized from a seed oil (Pyrularia pubera, Santalaceae).

Dehydrocrepenynic acid or (9Z,14Z-octadecadien-12-ynoic acid) with an additional methylene-interrupted double bond is occasionally found with crepenynic acid in seed oils but more often in fungi, including some mushrooms. 10E,16-Heptadecadien-8-ynoic acid and a related C18 fatty acid (from Acanthosyris spinescens) also have an enyne system.
Fatty acids with an acetylenic bond in conjugation with two double bonds occur in seed oils, including 11E,13E-octadecadien-9-ynoic acid (from Ximenia americana), heisteric or 7Z,11E-octadecadien-9-ynoic acid from Heisteria silvanii, and 8E,10E-octadecadien-12-ynoic acid from Tanacetum (Chrysanthemum) corymbosum together with crepenynic acid.
Poly-acetylenic fatty acids: Octadeca-9,11-diynoic acid is the simplest of several di- and polyacetylenic fatty acids present in isano seed oil (Onguekoa gore) and other species from the Olacaceae. Others include isanic (17-octadecen-9,11-diynoic), bolekic (13Z‑octadecen-9,11-diynoic) and 13Z,17-octadecadien-9,11-diynoic acids. Similarly, several fatty acids with ene-diyne structures have been isolated from the Santalaceae, including exocarpic (13E-octadecen-9,11-diynoic) acid.

One of the first polyacetylenic fatty acids to be isolated was dehydromatricaria (2E-decen-4,6,8-triynoic) acid in roots of Solidago species (Compositae) as long ago as 1826, although it was very much later before it was properly characterized together with some related compounds (see dihydromatricaria acid below). Triynoic acids have been detected in the Santalaceae, while 17‑octadecen-9,11,13-triynoic (oropheic) acid has been found in leaves of Orophea enneandra (Annonaceae). The stems and leaves of Scurrula atropurpurea, a parasitic plant that attacks the tea plant, contain three C18 polyacetylenic fatty acids that have been used in traditional medicine for the treatment of cancer in Indonesia.

Complex polyacetylenic fatty acids occur in fungi, including a C9 triynoic fatty acid with a terminal triple bond from Basidiomycetes and three phomallenic acids with an allenyldiyne structure from a Phoma sp. (one of which is illustrated). Some fungi and algae contain complex acetylenic fatty acids with additional hydroxyl, keto and epoxyl groups or bromine atoms. The slime mould Lycogala epidendrum contains triacylglycerols to which acetylenic-epoxy acids, such as 8R,9R-epoxy-17-octadecen-4,6-diynoic acid are esterified ('lycogarides').
Allenic fatty acids: The first allenic fatty acid to be described from a higher plant was
8‑hydroxy-5,6-octadienoic acid, which is found in estolide linkage with 2,4-decadienoic acid in the seed oil of Sapium sebiferum,
the Chinese tallow tree.
Laballenic or 5R,6‑octadecadienoic acid is a major constituent of the seed oil of Leonotis nepetaefolia
(Labiateae).
It is noteworthy that the allenic group is responsible for the marked optical activity ([α]D = −47°)
of the fatty acid, a much greater effect than with other common substituents.
The first allenic fatty acid to be described from a higher plant was
8‑hydroxy-5,6-octadienoic acid, which is found in estolide linkage with 2,4-decadienoic acid in the seed oil of Sapium sebiferum,
the Chinese tallow tree.
Laballenic or 5R,6‑octadecadienoic acid is a major constituent of the seed oil of Leonotis nepetaefolia
(Labiateae).
It is noteworthy that the allenic group is responsible for the marked optical activity ([α]D = −47°)
of the fatty acid, a much greater effect than with other common substituents.
Subsequently, a C20 homologue of laballenic (i.e., phlomic) acid and lamenallenic acid (5,6,16-18:3) were isolated from other seed oils of the Labiateae. Fatty acids with allene and cumulene (-CH=C=C=CH-) groups are now known to be widespread if minor components in higher plants and fungi (as oxylipins), and even in primitive animals, cf., the phomallenic acids discussed above.
Biosynthesis of acetylenic fatty acids: While many aspects of the mechanism for crepenynic acid biosynthesis in seed oils remain to be ascertained, the primary precursor is linoleic acid as illustrated.
 |
| Figure 1. Biosynthesis of acetylenic fatty acids in higher plants. |
Linoleic acid (1) is converted by a Δ12 acetylenase, related to the FAD2 desaturases, which abstracts two hydrogens from position 12/13, to form crepenynic acid (2). To produce a conjugated ynene system in dehydrocrepenynic acid (3), a further double bond is inserted at position 14 by a Δ14 desaturase, and this can be followed by the action of a Δ14 acetylenase to form 9Z-octadecen-12,14-dynoic acid (4). The last can be further desaturated, or it can enter the complex secondary metabolic pathways that lead to the polyacetylenes. It has been suggested that as a triple bond in a substrate imparts rotational and rigidity constraints to a molecule as well as reducing the carbon-carbon bond length of part of the acyl chain, sequential introduction of adjacent substituent groups by the same or similar enzymes is facilitated, leading to the formation of the innumerable secondary metabolites with acetylenic bonds in conjugation.
The acetylenases for the biosynthesis of tariric and stearolic acids and for front-end acetylenation of polyunsaturated fatty acids in mosses and algae are structurally distinct from the comparable enzymes for crepenynic acid biosynthesis and metabolism in higher plants. The Δ6 fatty acyl acetylenase from the moss Ceratodon purpureus is a bifunctional enzyme that is also able to insert a Δ6 double bond.
 As
crepenynic acid is the main precursor of polyacetylenic compounds in plants of which nearly 2,000 have been characterized, its biosynthesis
and subsequent metabolism have received intensive study.
These metabolites are naturally occurring fungicides, antibacterials and pesticides, and some of them have pharmaceutical potential.
For example, falcarinol and other falcarins from carrots, which utilize a family of aberrant FADS2 enzymes to synthesise the acetylenic
fatty acid precursors, have anti-cancer, anti-inflammatory and neuroprotective properties.
As
crepenynic acid is the main precursor of polyacetylenic compounds in plants of which nearly 2,000 have been characterized, its biosynthesis
and subsequent metabolism have received intensive study.
These metabolites are naturally occurring fungicides, antibacterials and pesticides, and some of them have pharmaceutical potential.
For example, falcarinol and other falcarins from carrots, which utilize a family of aberrant FADS2 enzymes to synthesise the acetylenic
fatty acid precursors, have anti-cancer, anti-inflammatory and neuroprotective properties.
2. Acetylenic Fatty Acids from the Animal Kingdom
Many different fatty acids containing triple bonds have been isolated from sponges and corals, and these can be highly complex molecules with very long chains and one to four triple bonds, e.g., haliclonyne is a C47 oxo-octahydroxy-dientetraynoic acid from a marine sponge Haliclona sp. Others are known with bromine and thiophene substituents in addition to hydroxyl groups, and 18‑bromooctadeca-(9Z,17E)-diene-7,15-diynoic and 18‑bromooctadeca-(9E,17E)-diene-5,7,15-triynoic acids were isolated from the sponge Xestospongia testudinaria. Formulae of a few of the large number of such fatty acids found in sponges are illustrated, i.e., from the sponges Xestospongia testudinaria (1), Oceanapia sp. (2) and Stellata sp. (3).

 8Z-Dihydromatricaria acid is a defence compound that has long been known from plants of the family Asteraceae and from
fungi, but it is also synthesised in insects such as the soldier beetle (Chauliognathus lecontei).
It is of interest that the enzymes responsible for its biosynthesis differ between plants and beetles,
although both utilize the same precursors, first linoleate and eventually 9Z,16Z-octadecadiene-12,14-diynoic acid,
suggesting that the relevant genes developed independently.
In beetles of the family Lycidae, lycidic or 5E,7E-octadecadien-9-ynoic acid is formed as a defence against predators, while
the moth Thaumetopoea pityocampa makes 13Z-hexadecen-11-ynyl acetate as a sex pheromone,
with hexadec-11-ynoic acid as a biosynthetic intermediate.
8Z-Dihydromatricaria acid is a defence compound that has long been known from plants of the family Asteraceae and from
fungi, but it is also synthesised in insects such as the soldier beetle (Chauliognathus lecontei).
It is of interest that the enzymes responsible for its biosynthesis differ between plants and beetles,
although both utilize the same precursors, first linoleate and eventually 9Z,16Z-octadecadiene-12,14-diynoic acid,
suggesting that the relevant genes developed independently.
In beetles of the family Lycidae, lycidic or 5E,7E-octadecadien-9-ynoic acid is formed as a defence against predators, while
the moth Thaumetopoea pityocampa makes 13Z-hexadecen-11-ynyl acetate as a sex pheromone,
with hexadec-11-ynoic acid as a biosynthetic intermediate.
3. Acetylenic Fatty Acids from Bacteria
The bacterial family Actinomycetes produce mycomycin, one of the first polyacetylenic fatty acids to be characterized with two triple bonds and an allenic structure, i.e., tridecatetra-3,5,7,8-en-10,12-diynoic acid, although many similar compounds are now known. Others synthesise poly-ynoic fatty acids with a conjugated triple bond system starting with a terminal alkyne, and they use them as chemical weapons against predators and competitors. Pseudomonas protegens Cab57, a biocontrol agent against plant pathogens, produces four such fatty acids termed protegencins A to D with four triple bonds in conjugation that are active against the fungal plant pathogen Pythium ultimum. Related families of polyynoic fatty acids are present in other bacterial genera, e.g., the caryoynencins from Pseudomonas (now Trinickia) caryophylli. Many cyanobacteria species, and especially the genus Nostoc, synthesise lipopeptides which contain medium-chain fatty acids with terminal acetylenic groups.

A study of the biosynthesis of the protegencins has shown that polyyne biosynthesis is a thio-templated enzyme complex, containing a fatty acyl-AMP ligase, a designated acyl carrier protein and a thioesterase, with three desaturases working synergistically to introduce the four triple bonds.
4. Analysis
Fatty acids with more than one triple bond are highly susceptible to autoxidation so should be handled in an inert atmosphere if possible. GC-MS has been widely used for analysis (see our mass spectrometry pages), but there are also suitable LC-MS procedures.
Recommended Reading
- Badami, R.C. and Patil, K.B. Structure and occurrence of unusual fatty acids in minor seed oils. Prog. Lipid Res., 19, 119-153 (1981); DOI.
- Barnathan, G. Acides gras inhabituels des organismes marins: une illustration de la biodiversité moléculaire marine. Oléag. Corps Gras, Lipides, 17, 238-250 (2010); DOI.
 Casillas-Vargas, G.,
Ocasio-Malavé, C., Medina, S., Morales-Guzmán, C., Del Valle, R.G., Carballeira, N.M. and Sanabria-Ríos, D.J.
Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation
of antibacterial agents. Prog. Lipid Res., 82, 101093 (2021);
DOI.
Casillas-Vargas, G.,
Ocasio-Malavé, C., Medina, S., Morales-Guzmán, C., Del Valle, R.G., Carballeira, N.M. and Sanabria-Ríos, D.J.
Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation
of antibacterial agents. Prog. Lipid Res., 82, 101093 (2021);
DOI.- Christensen, L.P. Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules, 25, 2568 (2020); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Dembitsky, V.M. and Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res., 46, 328-375 (2007); DOI.
- Kim, H.J., Ishida, K. and Hertweck, C. Thiotemplated biosynthesis of bacterial polyyne fatty acids by a designated desaturase triad. Chembiochem, 23, e202200430 (2022); DOI.
- Kuklev, D.V., Domb, A.J. and Dembitsky, V.M. Bioactive acetylenic metabolites. Phytomedicine, 20, 1145-1159 (2013); DOI.
- Li, X., Lv, J.-M., Hu, D. and Abe, I. Biosynthesis of alkyne-containing natural products. RSC Chem. Biol., 2, 166-180 (2021); DOI.
- Minto, R.E. and Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res., 47, 233-306 (2008); DOI.
- Negri, R. Polyacetylenes from terrestrial plants and fungi: Recent phytochemical and biological advances. Fitoterapia, 106, 92-109 (2015); DOI.
- Santos, P., Busta, L., Yim, W.C., Cahoon, E.B. and Kosma, D.K. Structural diversity, biosynthesis, and function of plant falcarin-type polyacetylenic lipids. J. Exp. Botany, 73, 2889-2904 (2022); DOI.
- Zhou, Z.F., Menna, M., Cai, Y.S. and Guo, Y.W. Polyacetylenes of marine origin: chemistry and bioactivity. Chem. Rev., 115, 1543-1596 (2015); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: October 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
