Isoprostanes and Related Oxylipins
Products of Non-Enzymatic Oxidation
Isoprostanes are prostaglandin-like compounds produced in tissues primarily but not exclusively from esterified arachidonic acid by non-enzymatic reactions catalysed by the free radicals of reactive oxygen species (ROS) in vivo, and in contrast to prostaglandins per se, they do not require cyclooxygenases (COX-1 and COX-2) for their formation. As autoxidation reactions of this kind lack specificity, many different structural and stereoisomers are formed with in general a short half-life, yet some of them are potent lipid mediators in tissues, especially in the lungs and kidney, and they may have a role in normal physiology. They are invaluable markers for oxidative stress, and importantly they can be assayed by non-invasive means in urine. Although much of the early work has been concerned with metabolites of arachidonic acid, related oxylipins are formed from eicosapentaenoic and docosahexaenoic acids in animals, but rarely from linoleate. In plants, α‑linolenic acid is the precursor of phytoprostanes (discussed with the plant oxylipins), but isoprostanes derived from arachidonic and eicosapentaenoic acids have been detected in green algae.
Isoprostanes were first produced in the test tube as long ago as 1967, but it was 1990 before it was reported that they were formed in appreciable amounts in vivo and had functions in living tissues. Since then, the topic has expanded apace, although research may still be hampered by a lack of commercial standards.
In contrast to the conventional prostanoids, which are produced mainly in the form of the free acids, the isoprostanes are synthesised in an ester-bound state generally in position sn-2 of phospholipids in situ in membranes. For practical convenience they are illustrated here mainly as the unesterified (free) acids, and this may be the main form in which they affect tissue metabolism following release by enzymatic hydrolysis. In the context of non-enzymatic oxidation or autoxidation in general, many oxylipins are formed that do not contain cyclic structures, and our web pages on oxidized phospholipids, oxidized sterols and tocopherols, the last as antioxidants, contain more information on mechanistic aspects.
1. Structures and Occurrence
Basic structures: Isoprostanes resemble normal prostanoids in some ways, and the most abundant form is analogous to prostaglandin F2α, often with PGD2 and PGE2, but they differ in many aspects of their stereochemistry from those produced enzymatically. The side chains are mainly cis to the cyclopentane ring (in the same plane) as this is more stable thermodynamically, although trans isomers (spatially opposed as in normal prostanoids) do occur. Four regioisomers of the F-, D- and E‑series isoprostanes are possible, and each of these are potentially produced in eight diastereomeric forms, i.e., numerous isomers can exist of each series. Two nomenclature systems have been proposed, and one of them has been recommended by IUPAC (Taber, D.F. et al. (1997); DOI, now updated by Oger, C. et al. (2024) - DOI). To distinguish them from the normal prostaglandins, it is recommended that they are each given the abbreviation 'IsoP', with a prefix determined mainly by the location of the hydroxyl group in the side chain (5, 8, 12 or 15) that defines the structure further. Phytoprostanes from plants are named 'PhytoP-' and C22 neuroprostanes 'NeuroP-'. The basic structures of the four main F2‑IsoPs are illustrated.
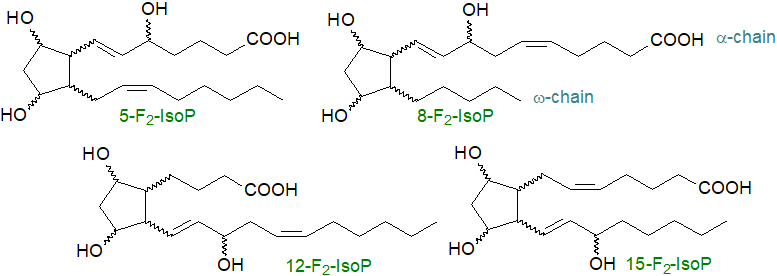 |
| Figure 1. Some basic isoprostane structures. |
Although large numbers of stereoisomers are possible in theory, stereochemical restraints imposed by the oxidation mechanisms restrict the numbers found in vivo, and often a single isomeric form or default structure tends to predominate. These mechanisms are described below, but in brief, an endoperoxide formed in an F-type cyclopentanediol in one of the first steps can only cleave to give two hydroxyl groups in a cis configuration, and they are by default depicted as having the α-configuration (or (S*)) by analogy to prostaglandins, while the default relative configuration of a side chain allylic hydroxy group is (S*), similarly. During cyclization, the mechanism ensures that that α- and ω‑chains are predominantly cis to each other. The latter structural feature for isoprostanes distinguishes them from prostaglandins, where the enzymatic reaction generates the trans configuration only. Some small proportion of the isoprostane products will have the same ring conformation as natural prostaglandins.
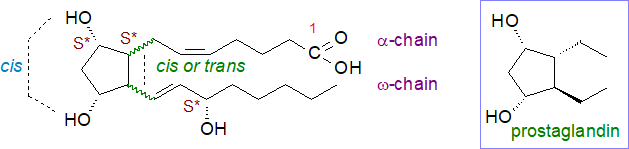 |
| Figure 2. Default structure for isoprostanes compared to natural prostaglandins (as proposed by Oger, C. et al. (2024); DOI with Corrigendum. |
The structures can be distinguished more precisely according to the cis- or trans-configurations of the side chain relative to the ring, and other aspects of stereochemistry by adding appropriate prefixes, when this information is known.
PGF2 isoprostanes: Isoprostanes have been found in very many animal tissues, including plasma and urine, although the basal levels vary appreciably among individuals and species, depending on the degree of oxidative stress. In the plasma of healthy humans, the level of F2‑isoprostanes is typically in the range of 20 to 30pg per ml, and this is roughly ten times greater than that of COX-derived PGF2α. In urine, the concentration of PGF2α (mainly unesterified) is forty times higher than that in plasma and most of this is derived from the isoprostane pathway (15-F2t-IsoP) as two enantiomers are present in equal amounts. The 5- and 15-series isoprostanes are most abundant because the precursors that lead to the 8- and 12-series can undergo further oxidation relatively easily. In plasma, F2‑isoprostanes are transported in esterified form mainly in the high-density lipoproteins (HDL), with some preference for the HDL3 sub-fraction. Isoprostanes have even been detected in sewage, suggesting that they might be used to monitor community-level metabolism.
Other omega-6 isoprostanes: Epoxy-isoprostanes formed in vivo have been detected in mildly oxidized LDL of atherosclerotic lesions. Dihomo-isoprostanes, including F2-dihomo-IsoPs and dihomo-isofurans, are related metabolites generated from adrenic acid (22:4(n‑6)), which is highly enriched in the adrenal glands and in the white matter of brain where it is associated with myelin. They are elevated in plasma of patients with Rett syndrome, a pervasive abnormality of development affecting females, and they are considered to be early markers of lipid peroxidation.
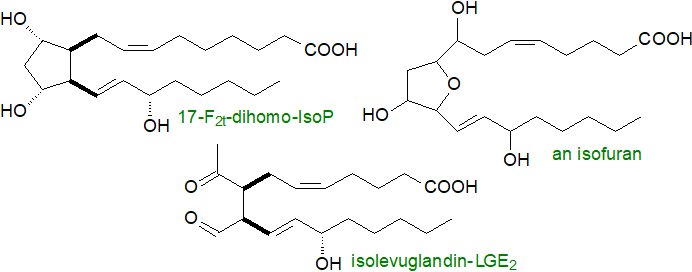 |
| Figure 3. Further isoprostanes derived from omega-6 fatty acids. |
Isofurans are oxidation products of arachidonate that contain substituted tetrahydrofuran rings (isomers with tetrahydropyran rings are known). Two mechanisms have been described for formation of these compounds, involving either cleavage of a cyclic peroxide intermediate or hydrolysis of an epoxide, and these can in theory lead to the formation of eight regioisomers in total as discussed below. Under conditions of high oxygen tension, production of these is favoured relative to isoprostanes as the final step is an attack of molecular oxygen rather than an intramolecular rearrangement.
Isolevuglandins (isoLGs) are a family of 4-ketoaldehydes, analogous to the levuglandins, which are generated from isoprostanes by opening of the carbon-carbon bond in the cyclopentane ring as opposed to the endoperoxide ring. They are distinguished from the levuglandins by their variable geometry in that eight isomers can be formed from each isoprostane. Isolevuglandins are highly reactive and were overlooked in tissue samples for many years until discovered as protein-adducts by an immunological approach, and as such, they can have a profound influence on tissue metabolism (see below).
Isothromboxanes have only been detected in significant amounts in animals subjected to severe oxidative stress, such as after oxidative injury caused by carbon tetrachloride administration, but the mechanism for their formation is still a matter of conjecture.
Omega-3-metabolites: While isoprostanes can be formed from any lipid with a 1,4,7-octatriene unit, the additional double bonds in eicosapentaenoic acid (20:5(n-3), EPA) and docosahexaenoic acid (DHA) mean that there is a much wider range of products, even if the general mechanisms are the same. Isoprostane-like compounds, i.e., F3, A3 and J3 isoprostanes, the last two with cyclopentenone rings, are formed from the oxidation of EPA in the heart muscle of mice in vivo; 192 F3-isoP stereoisomers are possible, but the 4- and 8-series are most abundant in vivo. Brain tissue contains relatively high proportions of DHA, and this gives rise to F4 isoprostane-like compounds that have been characterized and termed neuroprostanes; they are only produced by non-enzymatic mechanisms that do not utilize cyclooxygenases, which in fact they inhibit.
Although 256 possible isomers are possible in theory, stereochemical constraints in the cyclization process ensure that eight isomers predominate with the 4- and 20-series most abundant in vivo. Information on the metabolism and functions of these compounds is now emerging.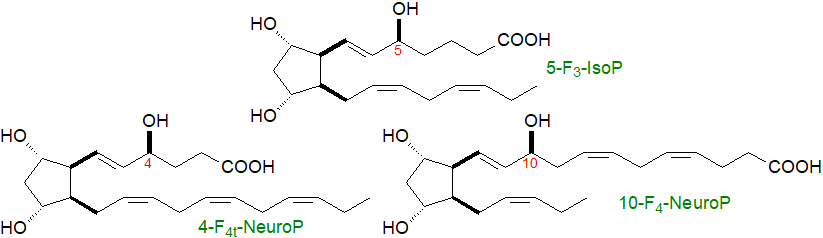 |
| Figure 4. Formulae of some representative F3-isoprostanes and neuroprostanes. |
In comparison to isoprostanes derived from omega-6 fatty acids, those obtained from the omega-3 family are less abundant in plasma (approximately 0.1-0.2 ng/ml versus 0.4-0.5 ng/ml under normal conditions), and they are not easy to quantify in plasma or urine other than during conditions of oxidative stress. They are more abundant in the brain. In plants, phytoprostanes are formed from α‑linolenic acid.
2. Synthesis in Vivo
Synthesis of isoprostanes in animal tissues in vivo is brought about by a series of free radical-catalysed reactions, most of which do not use enzymes, and any fatty acid with three or more double bonds can be a substrate. In order to commence isoprostane formation, there is a requirement for Reactive Oxygen Species (ROS) of which innumerable forms exist, and these can be derived by enzymatic or non-enzymatic means, such as superoxide anions, hydrogen peroxide, singlet oxygen, hydroxyl radicals, lipid hydroperoxides and peroxyl radicals (see our web pages on bioactive aldehydes and oxidized phospholipids).
In living systems, most intracellular ROS are derived from superoxide radicals (O2•-), produced mainly through the action of NADPH oxidases, xanthine oxidase and the mitochondrial electron-transport chain, and these are converted to hydrogen peroxide by the enzyme superoxide dismutase. Subsequent steps in the process of autoxidation of lipid molecules to hydroperoxides and thence to isoprostanes are largely non-enzymatic. This is often discussed in terms of two reactions, i.e., the Haber-Weiss and Fenton reactions, but the first of these is not now thought to be significant in vivo. The Fenton reaction generates highly toxic hydroxyl (OH•) or alkoxyl radicals from hydrogen peroxide and ferrous ions (Fe2+), with the latter oxidized to ferric ions (Fe3+) in the process. Under conditions of oxidative stress (defined below), hydroxyl radicals are produced in increasing amounts.
 |
| Figure 5. Fenton reaction - Generation of hydroxyl radicals. |
These radicals can abstract a hydrogen atom from bis-allylic methylene groups of polyunsaturated fatty acids bound to a glycerophospholipid under aerobic conditions in vivo in animals and plants to generate hydroperoxy-fatty acids. As this radical generation is not enzymatic, all methylene groups between two cis double bonds can potentially take part in the reaction, although not necessarily to the same degree. The main route thence to isoprostanes via an endoperoxide intermediate is illustrated below for the synthesis of 15‑F2‑IsoP isomers.
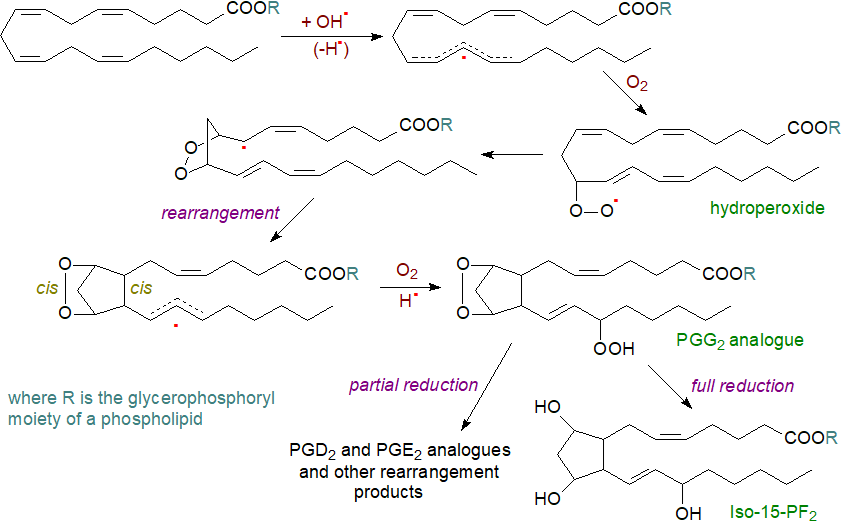 |
| Figure 6. Formation of isoprostanes via endoperoxides and 5-exo-cyclization. |
After hydrogen abstraction, the pentadienyl radical formed combines with an oxygen molecule to generate a racemic peroxy radical that has a propensity to rearrange to form equivalent amounts of α,α- and β,β-bicyclic endoperoxy radicals, which are configured almost exclusively cis with respect to the cyclopentane ring, the formation of which is triggered by 5-exo-cyclization which according to the Beckwith-Houk rules results predominantly in a relative cis-configuration of the α- and ω-chains. In the next step, the bicyclic endoperoxy radical reacts on either face of the side chain with a further oxygen molecule to produce a racemic hydroperoxy bicyclic endoperoxy radical. The chain reaction is terminated by abstraction of hydrogen from an appropriate donor molecule such as a polyunsaturated fatty acid, glutathione or α‑tocopherol, and some enzymatic reactions are possible at the last stages. This reaction can occur after hydrolysis from linkage to the phospholipid by phospholipase A2. The product is an Iso-PG, i.e., an analogue of PGG2, which can be reduced to the stable F2-IsoP. Isoprostanes with the hydroxyl group in positions 5, 8 or 12 are formed in an analogous manner and then can generate epoxides.
Isoprostanes of the Iso-PF series are produced in limited amounts only in vitro, but they are major metabolites in vivo through the reduction of Iso-PGs via natural endogenous reductants such as glutathione, hematin, lipoic acid, polyunsaturated fatty acids or glutathione peroxidase, the concentrations and accessibility of which may determine the pattern of final products. Thromboxane-like compounds can be sometimes be formed in vivo, and the catalyst in this instance is probably complexed iron, but PGI analogues are not produced.
As the G and H-ring endoperoxide structures are highly labile with a half-life of only a few minutes, they isomerize rapidly to give a variety of products, including analogues of PGE2 and PGD2. These are formed competitively with F2-IsoPs, and when cellular reducing agents, such as glutathione (GSH) or α-tocopherol, are depleted, formation of E2/D2-IsoPs is favoured. The latter can spontaneously dehydrate to form isoprostanes containing cyclopentenone rings, i.e., with a double bond and carbonyl group on the prostane ring and so are related to PGA2 and PGJ2. They are highly reactive electrophiles and readily form Michael adducts with thiols such as are found on cysteine residues in proteins and glutathione, and they are rapidly metabolized in vivo to water-soluble glutathione conjugates. At low oxygen concentrations, conventional isoprostane structure are produced mainly, but when the oxygen levels are higher, isofurans can be produced.
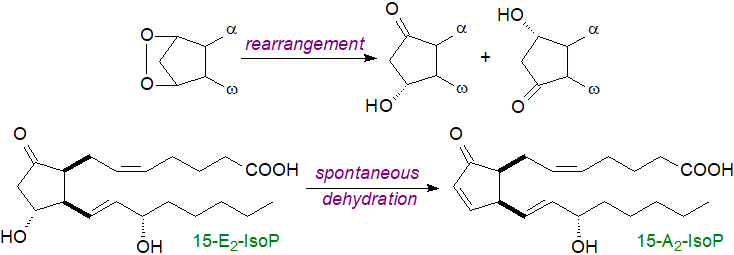 |
| Figure 7. Formation of A2/J2-isoprostanes |
Hydroperoxides: Polyunsaturated hydroperoxides do not serve only as precursors of isoprostanes, but can be reduced soon after they are formed to generate linear hydroperoxides like those generated enzymatically (but without the regio- and stereo-chemistry constraints), and these may also influence tissue metabolism. They can subsequently be precursors of aldehydes and oxidized phospholipids, including oxidatively truncated phospholipids (core aldehydes). Other products include dimer fatty acids linked carbon to carbon or via dioxygen bridges.
Antioxidants: Natural antioxidants limit the formation of hydroperoxides and thence of isoprostanes by mopping up free radicals. They include enzymatic antioxidants such as superoxide dismutase, catalase, glutathione peroxidase and thioredoxin, and non-enzymatic antioxidants such as vitamins A, C and E, coenzyme Q10 and selenium. The mechanism of antioxidant actions are discussed in more detail in our web page on tocopherols.
3. Catabolism of Isoprostanes
Isoprostanes are rendered inert or catabolized by the same enzymic mechanisms as those for the prostanoids. Thus, circulating unesterified F2‑isoprostanes are filtered in the kidney and emerge in the urine. They can also undergo metabolism in the liver to produce metabolites such as 2,3‑dinor-15-F2- and 2,3-dinor-5,6-dihydro-15-F2-isoprostanes, which are excreted in the urine. An alternative β‑oxidation process is present in rodents to produce 2,3,4,5-tetranor-15-F2-IsoP. D2/E2-IsoPs dehydrate in vivo to yield A2/J2-IsoPs, which can be further modified through rearrangement and dehydration to give first deoxy-A2- and deoxy-J2-IsoPs and then β-oxidation products of these. As well as in the form of the unesterified acids, they are secreted as taurine and glucuronide conjugates, and the latter are major metabolites of the F2-isoprostanes detected in urine.
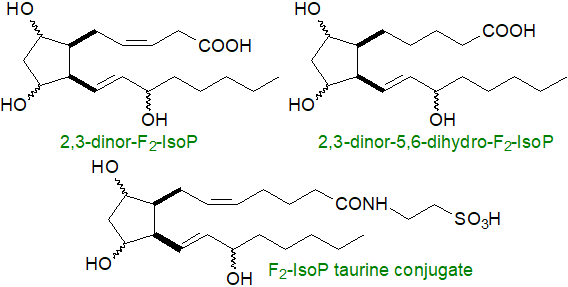 |
| Figure 8. Some products of isoprostane catabolism. |
4. Isoprostanes - Oxidative Stress and Other Metabolic Effects
Prostaglandin analogues: As the isoprostanes in animal tissues are formed from arachidonic acid predominantly in position 2 of phospholipids in membranes, they must be released by the action of phospholipase A2 (group IIA, V and X secretory), platelet-activating factor acetylhydrolases and related enzymes before they can exert their main physiological effects. In the unesterified form, they can circulate in the plasma and interact with membrane receptors such as kinase receptors (tyrosine and Rho) and to a lesser extent prostanoid receptors. However, they do have some functions while still linked to phospholipids (see below). There is an intriguing hypothesis that during evolution in primitive cells, isoprostane formation resulted from the increasing aerobic conditions, and these molecules were selected as a means of signalling more specific imbalances in the redox state of the cells. Only later did truly enzymatic pathways evolve to produce eicosanoids as signalling molecules, but isoprostane formation has been retained as a back-up system.
Isoprostanes are valuable indicators of oxidative stress in animal tissues, which has been defined as "a disturbance in the prooxidant–antioxidant balance in favour of the former", i.e., there is cellular oxidant injury and an excessive production of lipid peroxidation products, which may be factors in the development or exacerbation of cancer, and cardiovascular and neurological diseases, for example. The nature of the metabolites detected can be a marker for various disease states in humans. Although isoprostanes are most easily assayed non-invasively in urine, they can be measured in all tissues and bodily fluids analysed to date, including plasma, breath condensate, amniotic fluid and saliva. Measurement of isoprostanes has been termed by some to be the ‘gold standard’ by which oxidative damage and stress can be determined, a conclusion borne out by a multi-investigator study, termed the Biomarkers of Oxidative Stress (BOSS) Study, sponsored by the National Institute of Health in the USA. Towards this end, normal levels of isoprostanes in healthy humans have been defined, so that their influence on disease states and subsequent therapeutic intervention can be assessed.
 Increased levels of
urinary isoprostanes have been measured in many conditions that have been associated with excessive generation
of free radicals, such as poisoning with paraquat and carbon tetrachloride, smoking, alcoholism and cirrhosis of the liver.
From this data, they have been implicated in the pathophysiology of many human disease states, including obesity, brain degeneration
(especially Alzheimer's disease), kidney diseases, ischemia-reperfusion injury, atherosclerosis and diabetes.
There is good clinical evidence that the concentration of F2-IsoP in urine is an independent
and cumulative marker of coronary heart disease, reflecting high concentrations of this metabolite in atherosclerotic plaques,
where like the thromboxanes it may stimulate the TP receptor.
In a meta-analysis of data from many different studies, the occurrence of large increases in 8‑Iso‑PGF2α levels
have been established in diseases of the kidney, obstructive sleep apnoea, age-related macular degeneration, pre-eclampsia and respiratory
tract disorders, but not in hypertension, metabolic syndrome, asthma or perhaps surprisingly in tobacco smoking.
An increase in exhaled 8-isoP is observed in the breath of patient with chronic obstructive pulmonary disease (COPD).
A positive correlation between body-mass index (BMI) and urinary 8‑Iso‑PGF2α levels has been reported.
Increased levels of
urinary isoprostanes have been measured in many conditions that have been associated with excessive generation
of free radicals, such as poisoning with paraquat and carbon tetrachloride, smoking, alcoholism and cirrhosis of the liver.
From this data, they have been implicated in the pathophysiology of many human disease states, including obesity, brain degeneration
(especially Alzheimer's disease), kidney diseases, ischemia-reperfusion injury, atherosclerosis and diabetes.
There is good clinical evidence that the concentration of F2-IsoP in urine is an independent
and cumulative marker of coronary heart disease, reflecting high concentrations of this metabolite in atherosclerotic plaques,
where like the thromboxanes it may stimulate the TP receptor.
In a meta-analysis of data from many different studies, the occurrence of large increases in 8‑Iso‑PGF2α levels
have been established in diseases of the kidney, obstructive sleep apnoea, age-related macular degeneration, pre-eclampsia and respiratory
tract disorders, but not in hypertension, metabolic syndrome, asthma or perhaps surprisingly in tobacco smoking.
An increase in exhaled 8-isoP is observed in the breath of patient with chronic obstructive pulmonary disease (COPD).
A positive correlation between body-mass index (BMI) and urinary 8‑Iso‑PGF2α levels has been reported.
It has become apparent that isoprostane levels in plasma and tissues are highest during foetal and early neonatal life in comparison to adults, and that they may have roles in development and in the transition to postnatal life. The placenta is a major source of isoprostanes, and elevated levels have been recorded during pre-eclampsia relative to normal pregnancies.
Urinary isoprostane analysis has been used to assess the efficacy of antioxidants in vivo and to establish the value of antioxidant administration in clinical trials. For example, measurement of F2-IsoP levels has been employed to study the efficacy of vitamin E as an antioxidant, in demonstrating that doses of 1600 IU/day or greater of α-tocopherol are required. Surprisingly, supplements of vitamin C do not alter isoprostane levels in humans.
While isoprostanes have been observed to interact with innumerable physiological pathways in vitro, the extent of these in vivo is uncertain and controversial. Often it is not clear whether the isoprostanes are simply markers for oxidative events or are mediators. The properties of 15‑F2t‑IsoP, the first isoprostane to be available commercially, have been most studied, and following intravenous administration in many species, it has been shown to be a vasoconstrictor in the low nanomolar range in vascular beds, including those of kidney, blood vessels, lymphatic vessels, bronchi, gastrointestinal tract and uterus. In addition, it stimulates the induction of mitosis in certain vascular smooth muscle cells, and there is evidence that it inhibits pro-aggregation by thromboxanes via an interaction with the receptors for the latter.
In the lung, different tissues or cells respond to isoprostanes with excitatory or inhibitory effects, depending on the nature and concentration of each isoprostane isomer, as well as the type of cell and animal species. As in other tissues, oxidative stress is an important factor and isoprostanes are involved in various disease states of the lung. Indeed, it has been suggested that they are not merely markers for oxidative stress, but may be a novel class of inflammatory mediators, perhaps acting in the regulation of vascular smooth muscle tone, especially during foetal development. Similarly, isoprostanes have been implicated in oxidative damage to the liver, where they are markers or more controversially mediators.
 Lipid peroxidation
is a factor in many disease states associated with the brain,
where IsoPA2 and IsoPJ2 are usually considered to be the preferred products of the isoprostane pathway;
they have a potent impact upon neuronal apoptosis and exacerbate neurodegeneration caused by other insults at concentrations as low as 100 nM.
One reason for this is that the distinctive functional group of the cyclopentenone isoprostanes can react with the cysteine residue
of glutathione and with cysteine in cellular proteins with harmful consequences.
In other tissues, IsoPA2, IsoPJ2 and epoxy-IsoPJ2 may be anti-inflammatory.
They are discussed below in relation to their occurrence in esterified form.
Lipid peroxidation
is a factor in many disease states associated with the brain,
where IsoPA2 and IsoPJ2 are usually considered to be the preferred products of the isoprostane pathway;
they have a potent impact upon neuronal apoptosis and exacerbate neurodegeneration caused by other insults at concentrations as low as 100 nM.
One reason for this is that the distinctive functional group of the cyclopentenone isoprostanes can react with the cysteine residue
of glutathione and with cysteine in cellular proteins with harmful consequences.
In other tissues, IsoPA2, IsoPJ2 and epoxy-IsoPJ2 may be anti-inflammatory.
They are discussed below in relation to their occurrence in esterified form.
Arachidonate-derived isoprostanes and isofurans and neuroprostanes derived from docosahexaenoic acid have been shown to increase in concentration in diseased regions of brains from patients who have died from advanced Alzheimer's and Parkinson's diseases. The levels of these compounds increase in cerebrospinal fluid of patients with the early stages of these diseases, findings that are of diagnostic value and may assist in the evaluation of experimental therapies. As the levels of F2 isoprostanes derived from adrenic acid are markedly increased under conditions of oxidant stress, they may be a selective marker of white matter injury and for Rett syndrome and epilepsy. D2- and E2-isoprostanes have been detected in elevated concentrations in brain tissues affected by trauma.
Isolevuglandins: As the 4-oxoaldehyde unit is highly reactive towards primary amines, isolevuglandins form Schiff bases and pyrroles rapidly and irreversibly with the ε‑amino groups of lysyl residues in proteins, and these can undergo further oxidation with production of lactam and hydroxylactam end-products. The reaction is an order of magnitude faster than that of proteins with other aldehydes, such as 4‑hydroxynonenal, acrolein and malondialdehyde, as discussed in mechanistic terms in our web page on aldehydes. On the other hand, isolevuglandins do not react with thiols (e.g., cysteine) or indoles (e.g., histidine). Such isolevuglandin adducts disturb the behaviour of the proteins and inhibit their catabolism to influence various disease states. In cell membranes, analogous reactions can occur with the ethanolamine moiety of phosphatidylethanolamine to form pro-inflammatory adducts.
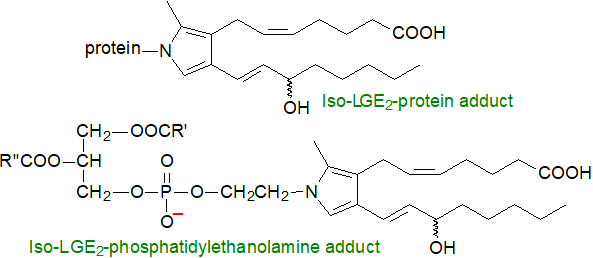 |
| Figure 9. Isolevuglandin adducts with proteins and phosphatidylethanolamine. |
Isolevuglandin-adduct formation has been linked to many different pathologies, including cardiovascular diseases, obesity, diabetes, cancer, neurodegeneration and eye diseases, as well as to ageing. By their influence on myocardial ferroptosis, i.e., iron-induced oxidation of membrane phospholipids, they may exacerbate the progression of atrial fibrillation. The chemistries of the various isomers are expected to be comparable because of the location of their reactive centres in a common core structure.
The concentrations of protein-adducts of isolevuglandins have been shown to increase greatly in plasma from patients with advanced atherosclerosis, where they can be harmful towards high-density lipoprotein structure and function. Such lipid-protein conjugates from both epithelial cells and plasma proteins, including apoprotein A1, may accumulate over a considerable time so could serve as a cumulative index for oxidative injury. Because of their irreversible reaction with proteins, the isolevuglandins together with the levuglandins are highly neurotoxic. These protein adducts have the potential to act as neoantigens and thus influence the immune system in dendritic cells, and an immune response may be responsible for the effects of isolevuglandin-protein adducts formed following stimulation by hypertensive factors such as excess dietary salt and catecholamines. Other conditions where increased amounts of isolevuglandins adducts have been detected include cancer, Alzheimer's disease, idiopathic pulmonary fibrosis, alcoholic liver disease and retinopathy.
Omega-3 metabolites: Isoprostanes derived from EPA and DHA are known to have an extensive influence on tissue metabolism, including some that have hitherto been ascribed to the unoxidized precursors. 15-F3t-IsoP from EPA does not induce platelet aggregation, and supplementation of the diet with eicosapentaenoic acid was found to reduce the levels of pro-inflammatory arachidonate-derived F2‑isoprostanes and thromboxanes by a substantial amount in experimental animals, possibly with a bearing on the reputed cardio-protective ability of eicosapentaenoic acid. Similarly, A3 and J3-IsoPs, the EPA-derived cyclopentenone isoprostanoids, are anti-inflammatory and indeed are antioxidants. In the new-born chicken and mouse, 15-E2-IsoP and 15-F2-IsoP have been shown to be potent vasoconstrictors of the ductus arteriosus, a vascular shunt between pulmonary artery and the aorta, so redirecting blood flow to the newly inflated lungs.
Neuroprostanes derived from DHA may promote apoptosis of cancer cells, while isoprostane analogues of the cyclopentenone neuroprostanes (A4- and J4‑NeuroPs) are potent anti-inflammatory mediators. F4-Neuroprostanes are promising biomarkers for a variety of neurodegenerative disorders, including Alzheimer's disease, autism, Rett syndrome, multiple sclerosis and Huntington's disease, and they are factors in traumatic brain injuries. It is apparent that some selectivity is observed in the nature of the isomers formed in each of these conditions, and the abundance of plasma 4F4t-NeuroP and 10F4t‑NeuroP are reported to be predictors of disease severity; the former is relatively abundant in the circulation, but not in urine, whereas the latter is clearly detectable in both.
Neuroprostanes such as 4R,S‑4F4t‑NeuroP, formed by peroxidation in cardiac membrane lipids, may be responsible for the anti-arrhythmic effects of DHA in the heart by counteracting the cellular stress caused by ROS; it is reported to be suitable for the treatment of acute myocardial infarction. One possible mechanism for these actions is that neuroprostanes activate peroxisome-proliferator-activated receptor PPARα and thence inhibit activation of NF-κB, a transcription factor that regulates the expression of pro-inflammatory molecules. Women with pre-eclampsia were found to have significantly elevated plasma isofurans and F4‑neuroprostanes, and the latter were greatly elevated in cord blood from their neonates, reflecting the oxidative challenge present at birth. Unfortunately, a lack of suitable standards and insufficient sensitivity in non-invasive analysis are hampering research.
Octadecanoids: Hydroperoxides of linoleate (18:2(n-6)), which is much more abundant in most animal tissues than any fatty acid with four or more double bonds, are formed by non-enzymatic oxidation in vivo under conditions of oxidative stress, and they can react to produce further oxidation/rearrangement products that have biological properties (see our web page on aldehydes). Although cyclic isoprostane-like products are formed to a limited extent, they have not been as extensively studied as the linear autoxidation products, and one such is epoxyketooctadecenoic acid (EKODE - one possible isomer illustrated), which is an electrophile and among its many metabolic properties has been shown to exacerbate colonic inflammation and colon tumorigenesis. Many other octadecanoids are produced enzymatically.
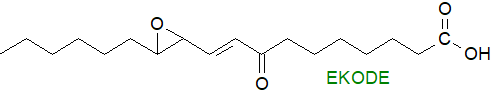
Comparable molecules are produced in plants from linoleate and α-linolenate (see our web page on phytoprostanes for further discussion), and they are formed in foods on storage and in greater amounts during cooking as in frying operations, and so can enter the food chain.
5. Functions of Esterified Isoprostanes - Oxidized Phospholipids
It should not be forgotten that isoprostanes are formed first as components of phospholipids in membranes rather than in the free form, and as long-lasting markers of oxidative damage, they enable the site of endogenous lipid peroxidation in cells to be identified. They may even induce signalling responses in an esterified state. Molecular models suggested that oxidized phospholipids, both of enzymatic and non-enzymatic origin, adopt highly twisted conformations and so disrupt membranes with direct impacts upon lipid-lipid interactions, ion gradients, and membrane fluidity and permeability. As they reorient in a lipid bilayer so that the oxidized chain moves towards the water/lipid head-group interface, the entire oxidized chain can protrude into the aqueous phase and so permit macrophage recognition. Likewise, sterol esters oxidized in this manner have undesirable properties.
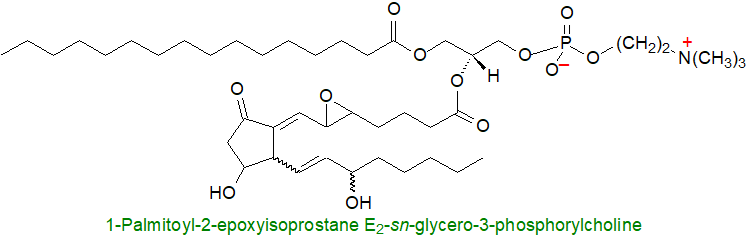
Each of the different oxidized phospholipids may affect a characteristic signalling pathway by acting as ligand to a specific receptor. In particular, 1‑palmitoyl-2-epoxyisoprostane E2-sn-glycero-3-phosphorylcholine, derived from the arachidonoyl analogue, has been shown to modulate the expression of a large number of genes in human aortic endothelial cells in vitro, and it is a potent activator of the PPARα at concentrations of less than 1 μg/mL, ten-fold lower than those observed in vascular cell walls. This lipid can be either pro- and anti-inflammatory via receptors in endothelial cells and macrophages, and there is a suggestion that it may have therapeutic potential for treatment of inflammatory diseases of the lung.
The cyclopentenone (A2) analogue and deoxy-A2/J2 isoprostanes in position sn-2 of phosphatidylcholine are potent pro-resolution mediators, like the protectins, resolvins and maresins. In these, the cyclopentenone motif is an electrophilic α,β‑unsaturated carbonyl moiety and can form covalent adducts with cysteine residues by Michael addition to affect the function of proteins. Phosphatidylethanolamine adducted to isolevuglandins has been found in tissues where it can induce the production of pro-inflammatory cytokines, for example. Studies with synthetic 1‑palmitoyl-2-15-deoxy-Δ12,14-prostaglandin J2‑sn‑glycero-3-phosphocholine found that it induced anti-inflammatory and antioxidant responses in macrophages through modulation of NF-κB, PPARγ and Nrf2 pathways. It may be beneficial towards atherosclerosis by inhibiting macrophage foam cell formation.
Among related observations, phospholipids that have been oxidatively cleaved to produce "core-aldehydes" have properties that resemble those of platelet-activating factor, while other oxidized phospholipids interact with receptors that are normally associated with the recognition of microbial pathogens, as discussed in separate web pages. Apoptosis (programmed cell death) can be initiated either by phosphatidylserine or cardiolipin containing oxidized fatty acids, although the mechanisms are somewhat different.
6. Analysis of Isoprostanes
Although lipid extracts containing isoprostanes are relatively stable, it is advisable to store plasma and tissue samples at −70 to −80°C to prevent artefact production. Gas chromatography allied to mass spectrometry (negative-ion chemical-ionization, GC/NICI-MS) with stable isotope dilution, after appropriate extraction and derivatization, is probably the most accurate and therefore definitive methodology for identifying and quantifying individual isoprostanes in plasma and urine, although HPLC (ideally with chiral phases) linked to tandem mass spectrometry is increasingly being used as there may then be no need for derivatization. Radioimmunoassay and ELISA kits are reputedly much less specific and therefore less reliable.
Suggested Reading
- Ahmed, O.S., Galano, J.-M., Pavlickova, T., Revol-Cavalier, J., Vigor, C., Lee, J.C.-Y., Oger, C. and Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: where are we now? Essays Biochem., 64, 463-484 (2020); DOI.
- Belik, J., González-Luis, G.E., Perez-Vizcaino, F. and Villamor, E. Isoprostanes in fetal and neonatal health and disease. Free Rad. Biol. Med., 48, 177-188 (2010); DOI.
- Bochkov, V.N., Oskolkova, O.V., Birukov, K.G., Levonen, A.L., Binder, C.J. and Stöckl, J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal., 12, 1009-1059 (2010); DOI.
- Davies, S.S. and Guo, L.L. Lipid peroxidation generates biologically active phospholipids including oxidatively N-modified phospholipids. Chem. Phys. Lipids, 181, 1-33 (2014); DOI.
- Davies, S.S., May-Zhang, L.S., Boutaud, O., Amarnath, V., Kirabo, A. and Harrison, D.G. Isolevuglandins as mediators of disease and the development of dicarbonyl scavengers as pharmaceutical interventions. Pharmacol. Therapeut., 205, 107418 (2020); DOI.
- Durand, T., Bultel-Ponce, V., Guy, A., Gros, V., Reversat, G., Vigor, C., Galano, J.M. and Oger, C. F4-neuroprostanes and F2-dihomo-isoprostanes: biomarkers and bioactive oxylipins. OCL, 30, 10 (2023); DOI.
- Friedli, O. and Freigang, S. Cyclopentenone-containing oxidized phospholipids and their isoprostanes as pro-resolving mediators of inflammation. Biochim. Biophys. Acta, Lipids, 1862, 382-392 (2017); DOI.
- Joumard-Cubizolles, L., Lee, J.C.Y., Vigor, C., Leung, H.H., Bertrand-Michel, J., Galano, J.M., Mazur, A., Durand, T. and Gladine, C. Insight into the contribution of isoprostanoids to the health effects of omega 3 PUFAs. Prostaglandins Other Lipid Mediators, 133, 111-122 (2017); DOI.
- Lee, Y.Y., Galano, J.M., Oger, C., Vigor, C., Guillaume, R., Roy, J., Le Guennec, J.Y., Durand, T. and Lee, J.C.Y. Assessment of isoprostanes in human plasma: technical considerations and the use of mass spectrometry. Lipids, 51, 1217-1229 (2016); DOI.
- Oger, C., Pavlicková, T., Bultel-Poncé, V., Guy, A., Galano, J.M., Jahn, U. and Durand, T. An update of isoprostanoid nomenclature. Prog. Lipid Res., 96, 101301 (2024); DOI - see also Corrigendum.
- Pérez-Sala, D. and Domingues, R. Lipoxidation targets: From basic mechanisms to pathophysiology. Redox Biol., 23, 101208 (2019); DOI - and many other articles in this special review volume.
- Revol-Cavalier, J., Quaranta, A., Newman, J.W., Brash, A.R., Hamberg, M. and Wheelock, C.E. The octadecanoids: synthesis and bioactivity of 18-carbon oxygenated fatty acids in mammals, bacteria, and fungi. Chem. Rev., 125, 1-90 (2025); DOI.
- and in relation to the history of the topic -
- Galano, J.-M., Lee, Y.Y., Oger, C., Vigor, C., Vercauteren, J., Durand, T., Martin Giera, M. and Leeb, J.C.-Y. Isoprostanes, neuroprostanes and phytoprostanes: An overview of 25 years of research in chemistry and biology. Prog. Lipid Res., 68, 83-108 (2017); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: October 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
