Sterols: 1. Cholesterol and Cholesterol Esters
In animal tissues, cholesterol (or cholest-5-en-3β-ol) is by far the most abundant member of a family of polycyclic lipids known as sterols, although it can be described as a polyisoprenoid or a triterpene from its biosynthetic origin. Cholesterol was first recognized as a component of gallstones as long ago as 1769, while the great French lipid chemist Chevreul isolated it from animal fats in 1815. However, it was well into the 20th century before the structure was fully defined by the German Chemist Heinrich Wieland, who in 1927, received the Nobel Prize in Chemistry for his work, the first of thirteen so honoured for research on cholesterol and its metabolism.
Cholesterol plays a vital role in animal life, and it is essential as a structural component of cell membranes, as a precursor of steroid hormones and bile acids in cells and for developmental signalling, transport processes, nerve conduction and the regulation of gene transcription. Every cell in vertebrates is able both to synthesise cholesterol and to metabolize it, and there is evidence that synthesis de novo is essential whatever the dietary intake. Indeed, this is vital in the brain. On the other hand, excess cholesterol can contribute to the pathology of various diseases, notably cardiovascular disease, neurodegenerative disorders and cancer, so cholesterol levels must be balanced in cells through synthesis, absorption, metabolic conversion and clearance to ensure an adequate but not excessive supply.
Here, the chemistry and biochemistry of cholesterols and its esters are described, but other documents in this website cover the related topics of plant sterols, oxysterols and other sterol derivatives, hopanoids and bile acids.
1. Cholesterol – Structure, Occurrence and Function in Membranes
 Cholesterol consists of a tetracyclic cyclopenta[a]phenanthrene structure with an iso-octyl side
chain at carbon 17.
The four rings (A, B, C, D) have trans ring junctions, and the side chain and two methyl groups (C-18
and C-19) are at an angle to the rings above the plane with β stereochemistry (as for the hydroxyl group on C-3);
there is a double bond between carbons 5 and 6.
Thus, the molecule has a rigid planar four-ring nucleus with a flexible tail.
Of the two recognized numbering systems in use, one originally described by Fieser and Fieser in 1959
and a second by IUPAC-IUB in 1989, the first is preferred by most current authors.
Cholesterol consists of a tetracyclic cyclopenta[a]phenanthrene structure with an iso-octyl side
chain at carbon 17.
The four rings (A, B, C, D) have trans ring junctions, and the side chain and two methyl groups (C-18
and C-19) are at an angle to the rings above the plane with β stereochemistry (as for the hydroxyl group on C-3);
there is a double bond between carbons 5 and 6.
Thus, the molecule has a rigid planar four-ring nucleus with a flexible tail.
Of the two recognized numbering systems in use, one originally described by Fieser and Fieser in 1959
and a second by IUPAC-IUB in 1989, the first is preferred by most current authors.
Cholesterol is a ubiquitous component of all animal tissues (and of some fungi) that is produced by every nucleated animal cell, where much of it is located in the membranes, although it is not evenly distributed. The highest proportion of unesterified cholesterol is in the plasma membrane (roughly 30-50% of the lipid in the membrane or 60‑80% of the cholesterol in the cell), while mitochondria and the endoplasmic reticulum have much less (~5% in the latter), and the Golgi contains an intermediate amount. Cholesterol is enriched in early and recycling endosomes, but not in late endosomes. It may surprise some to learn that the brain contains more cholesterol than any other organ, where it comprises roughly a quarter of the total free cholesterol in the human body, 70‑80% of which is in the myelin sheath, and it is the precursor of bioactive oxysterol metabolites. As a major component of synaptic vesicles in brain, it controls their shape and functional properties.
Of all the organic constituents of blood, only glucose is present in a higher molar concentration than cholesterol. In animal tissues, it occurs in the free form, esterified to long-chain fatty acids (cholesterol esters), in other covalent linkages, and in non-covalent forms that include the plasma lipoproteins. In plants, it tends to be a minor component only of a complex mixture of structurally related 'phytosterols', although there are exceptions, and it is nevertheless necessary as a precursor of some plant hormones.
 Animals in general
synthesise a high proportion of their cholesterol requirement while ingesting and absorbing appreciable amounts from foods.
On the other hand, many invertebrates, including insects, crustaceans and some molluscs cannot synthesise cholesterol
and must receive it from the diet; for example, spiny lobsters must obtain exogenous cholesterol to produce essential sex hormones.
Similarly, it must be supplied from exogenous sources to the primitive nematode Caenorhabditis elegans, where it may not have a major
role in membrane structure, other than perhaps in ion channels, although it is essential for the production of steroidal hormones required for
larval development;
its uptake is regulated by the novel lipid phosphoethanolamine glucosylceramide.
Some species can convert dietary plant sterols such as β-sitosterol to cholesterol.
Most prokaryotes lack cholesterol entirely with the exception of some pathogens that acquire it from eukaryotic hosts to ensure their intracellular
survival (e.g., Borrelia sp.), but one of marine origin is known to produce it de novo (see the web page on
plant sterols) and bacterial hopanoids are often considered to be
sterol surrogates.
Animals in general
synthesise a high proportion of their cholesterol requirement while ingesting and absorbing appreciable amounts from foods.
On the other hand, many invertebrates, including insects, crustaceans and some molluscs cannot synthesise cholesterol
and must receive it from the diet; for example, spiny lobsters must obtain exogenous cholesterol to produce essential sex hormones.
Similarly, it must be supplied from exogenous sources to the primitive nematode Caenorhabditis elegans, where it may not have a major
role in membrane structure, other than perhaps in ion channels, although it is essential for the production of steroidal hormones required for
larval development;
its uptake is regulated by the novel lipid phosphoethanolamine glucosylceramide.
Some species can convert dietary plant sterols such as β-sitosterol to cholesterol.
Most prokaryotes lack cholesterol entirely with the exception of some pathogens that acquire it from eukaryotic hosts to ensure their intracellular
survival (e.g., Borrelia sp.), but one of marine origin is known to produce it de novo (see the web page on
plant sterols) and bacterial hopanoids are often considered to be
sterol surrogates.
Cholesterol is vital for structural purposes in membranes and for lipid metabolism in general with an extraordinary diversity of roles, including cell signalling, morphogenesis, lipid digestion and absorption in the intestines, reproduction, stress responses, sodium and water balance, calcium and phosphorus metabolism, and the development and working of the central nervous system, but we can only touch on a few of these in this web page. It is a biosynthetic precursor of bile acids and steroid hormones (glucocorticoids, oestrogens, progesterones, androgens and aldosterone), and it is found in covalent linkage to specific membrane proteins or proteolipids ('hedgehog' proteins), which are vital for embryonic development. Ultraviolet light mediates cleavage of 7‑dehydrocholesterol, the last intermediate in the biosynthesis of cholesterol, to form vitamin D3. On the other hand, excess cholesterol in cells can be toxic, and a complex web of enzymes is essential to maintain the optimum concentrations. Because plasma cholesterol levels can be a major contributory factor to atherogenesis, media coverage has created what has been termed a ‘cholesterophobia’ in the population at large.
One of the main functions of cholesterol is to modulate the fluidity of membranes by interacting with their complex lipid components, specifically the phospholipids such as phosphatidylcholine and sphingomyelin. As an amphiphilic molecule, cholesterol is able to intercalate between phospholipids in lipid bilayers to span about half a bilayer. In its three-dimensional structure, it is in essence a planar molecule that can interact on both sides. The tetracyclic ring structure is compact and very rigid with the location of the hydroxyl group facilitating the orientation of the molecule in a membrane bilayer, while the positions of the methyl groups maximize interactions with other lipid constituents; the iso-octyl chain tends to align with saturated phospholipid chains but not necessarily with unsaturated chains.

As the α-face of the cholesterol nucleus (facing down) is 'smooth', it can make good contact with the saturated fatty-acyl chains of phospholipids down to about their tenth methylene group; the β-face (facing up) is made 'rough' by the projection of methyl groups from carbons 10 and 13. The interaction is mainly via van der Waals and hydrophobic forces with a contribution from hydrogen bonding of the cholesterol hydroxyl group to the polar head group and interfacial regions of the phospholipids, especially sphingomyelin. Intercalated cholesterol may disrupt electrostatic interactions between the ionic phosphocholine head groups of nearby membrane phospholipids, increasing their mobility. Indeed, there is evidence that cholesterol forms stoichiometric complexes with the saturated fatty acyl groups of sphingomyelin and to a lesser extent of phosphatidylcholine in the membrane microdomains known as rafts, and it has been even suggested that cholesterol may regulate the synthesis of sphingomyelin, especially those molecular species containing very-long-chain fatty acids.
Experiments with mutant cell lines and specific inhibitors of cholesterol biosynthesis suggest that an equatorial hydroxyl group at C-3 of sterols is essential for the growth of mammalian cells. The Δ5 double bond ensures that the molecule adopts a planar conformation, and this feature is also essential for cell growth as is the flexible iso-octyl side chain; the C-18 methyl group ensures the proper orientation of the sterol. While plant sterols are able to substitute for cholesterol in supporting many of the bulk properties of membranes in mammalian cells in vitro, cholesterol per se is essential for innumerable other purposes.
 In
the absence of cholesterol, a membrane composed of unsaturated lipids is in a fluid state that is characterized by a substantial degree of
lipid chain disorder, i.e., it constitutes a liquid-disordered phase, but cholesterol increases the degree of
order (cohesion and packing) in membranes, leading to formation of a liquid-ordered phase.
In contrast, it renders bilayers composed of more saturated lipids, which would otherwise be in a solid gel state, more fluid, so
cholesterol can promote and stabilize a liquid-ordered phase over a substantial range of temperatures and sterol concentrations.
Further, high cholesterol concentrations in membranes reduce their passive permeability to solutes.
These effects enable membranes to bend or withstand mechanical stresses, and they permit the fine-tuning of membrane lipid composition and
organization to regulate critical aspects of cell metabolism.
In
the absence of cholesterol, a membrane composed of unsaturated lipids is in a fluid state that is characterized by a substantial degree of
lipid chain disorder, i.e., it constitutes a liquid-disordered phase, but cholesterol increases the degree of
order (cohesion and packing) in membranes, leading to formation of a liquid-ordered phase.
In contrast, it renders bilayers composed of more saturated lipids, which would otherwise be in a solid gel state, more fluid, so
cholesterol can promote and stabilize a liquid-ordered phase over a substantial range of temperatures and sterol concentrations.
Further, high cholesterol concentrations in membranes reduce their passive permeability to solutes.
These effects enable membranes to bend or withstand mechanical stresses, and they permit the fine-tuning of membrane lipid composition and
organization to regulate critical aspects of cell metabolism.
Simplistically, the higher cholesterol concentrations in the plasma membrane support its barrier function by increasing membrane thickness and reducing its permeability to small molecules. In contrast, the endoplasmic reticulum has increased membrane flexibility because of its lower cholesterol concentrations and thus enables the insertion and folding of proteins in its lipid bilayer. While mitochondrial membranes have a low cholesterol content in total, this may be concentrated in nanodomains at regions of high curvature in the inner mitochondrial membrane with links to nucleoprotein complexes (nucleoids).
In comparison to other lipids, it has been reported that cholesterol can flip rapidly between the leaflets in a bilayer, although this does not appear to be accepted universally, leading to doubts as to the trans-bilayer distribution of cholesterol in some cellular membranes. Some recent evidence suggests that the concentration of cholesterol in the inner leaflet of the plasma membrane is much lower than that in the outer leaflet in a range of mammalian cells, but this is still debated. This distribution promotes negative curvature of membranes and may be a significant factor in bringing about membrane fusion as in the process of exocytosis. It may be relevant for the regulation of various cellular signalling processes at the plasma membrane.
Similarly, cholesterol has a role in the lateral organization of membranes and their free volume distribution, factors permitting more intimate protein-cholesterol interactions that may regulate the activities of membrane proteins, many of which bind strongly to cholesterol. A conserved region termed the ‘sterol-sensing domain’, which consists of 180 amino acids forming five transmembrane segments capable of binding sterol groups, is present in many membrane proteins, including several that take part in cellular cholesterol homeostasis or trafficking. Some proteins bind to cholesterol deep within the hydrophobic core of the membrane via binding sites on the membrane-spanning surfaces or in cavities or pores in the proteins, driven by hydrogen bond formation.
Cholesterol has an intimate interaction with G-protein-coupled receptors (GPCRs) to affect ligand binding and activation, either by direct high-affinity binding to the receptor, by changing their oligomerization state, or by inducing changes in the properties of the membrane. For example, it is essential for the stability and operation of the β2-adrenergic, oxytocin and serotonin receptors by increasing the agonist affinities, while rhodopsin is stabilized in an inert state until needed both through indirect effects on plasma membrane curvature and by a direct interaction between lipid and protein. The GPCR neurotransmitter serotonin1A receptor has ten closely bound cholesterol molecules, which control its organization and positioning; the receptor senses membrane cholesterol via a lysine residue in a so-called 'CRAC' motif in transmembrane helix 2. Ion pumps such as the (Na+-K+)-ATPase, which have specific binding sites for cholesterol molecules, consume a high proportion of the ATP in cells and are responsible for the ion gradients across membranes that are essential for cellular metabolism; depletion of cholesterol in the plasma membrane can switch off these ion pumps. In the nucleus of cells, cholesterol is intimately associated with chromatin.
The role of cholesterol together with sphingolipids in the formation of the transient nano-domains in the plasma membrane known as rafts (see this web page for detailed discussion) is crucial for cellular metabolism and signalling, while the interaction of cholesterol with ceramides is essential in skin for its barrier properties.
2. Cholesterol Biosynthesis
A complex series of at least thirty different enzymatic reactions is require for cholesterol biosynthesis, and these were unravelled in large measure by Konrad Bloch and Fyodor Lynen, who received the Nobel Prize for their work on the topic in 1964. When the various regulatory, transport and genetic studies of more recent years are considered, it would not be easy to treat this subject in depth here, so the bare bones only of mechanistic aspects of what is known as 'mevalonic acid (MVA) pathway' are described, but the references listed at the end of this document should serve as a guide to further study. Under hypoxia or UV stress in tissues such as the skin and gonads, some cholesterol synthesis occurs by a somewhat different route, the 2C-methyl-D-erythritol 4-phosphate (MEP) pathway with cycloartenol rather than lanosterol as the required intermediate, but this is much more important for sterol biosynthesis in plants so is described here.... Only a few bacteria can synthesise cholesterol, as discussed in our web page on plant sterols here...
Synthesis of cholesterol occurs mainly in the liver, although the brain (see below), peripheral nervous system and skin synthesise their own considerable supplies, and almost all nucleated cells have the capacity to synthesise their full complement. In the first steps, the key intermediate mevalonic acid is synthesised from acetyl-CoA and acetoacetyl-CoA, both of which are in fact derived from acetate, in two enzymatic reactions, the first of which is catalysed by 3-hydroxy-3-methyl-glutaryl (HMG)-CoA synthase in the cytosol. In subsequent reactions, the enzymes are membrane-bound and are located in the endoplasmic reticulum. The second enzyme HMG-CoA reductase is an important control point, and it is widely regarded as the rate-limiting step in the overall synthesis of sterols; it is regulated at the transcriptional level and by many more factors including a cycle of phosphorylation-dephosphorylation reactions. This enzyme is among the targets inhibited by the drugs known as ‘statins’, so that patients must then obtain much of their cholesterol from the diet via the circulation.
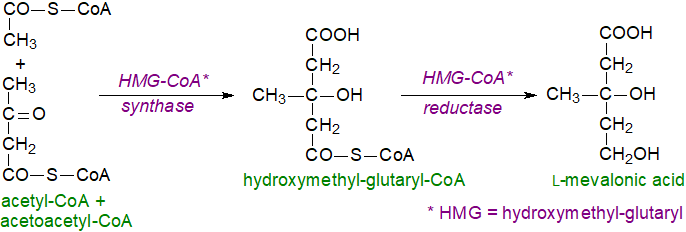 |
| Figure 1. Cholesterol biosynthesis - Step 1. L-mevalonic acid |
The next sequence of reactions begins with the phosphorylation of mevalonic acid by a mevalonate kinase to form the 5‑monophosphate ester, followed by a further phosphorylation to yield an unstable pyrophosphate, which is rapidly decarboxylated to produce 5-isopentenyl pyrophosphoric acid, the universal isoprene unit. An isomerase converts part of the latter to another intermediate 3,3-dimethylallyl pyrophosphoric acid.
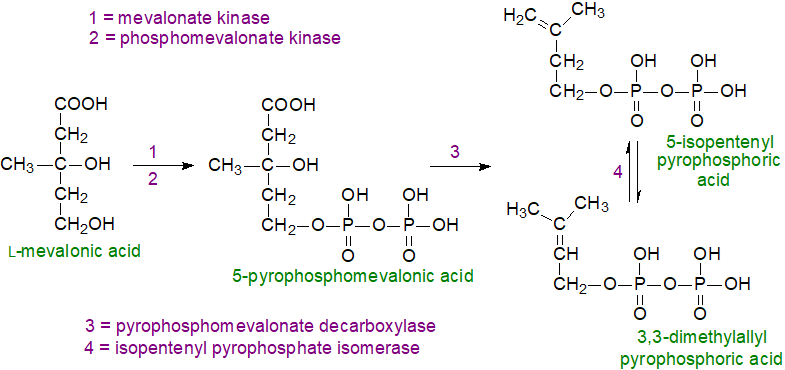 |
| Figure 2. Cholesterol biosynthesis - Step 2. 5-Isopentenyl pyrophosphate and 3,3-dimethylallyl pyrophosphate. |
5-Isopentenyl pyrophosphate is a nucleophile, but the isomerized product is electrophilic, facilitating the first step in the third series of reactions in which 5-isopentenyl pyrophosphate and 3,3-dimethylallyl pyrophosphate condense with the elimination of pyrophosphoric acid to form the monoterpenoid derivative geranyl pyrophosphate. This is reacted with another molecule of 5-isopentenyl pyrophosphate to produce the sesquiterpene derivative (C15) farnesyl pyrophosphate, two molecules of which are then condensed to yield presqualene pyrophosphate. In turn, this product is reduced by NADPH to produce a further critical intermediate squalene. The last two steps are catalysed by the enzyme squalene synthase, which regulates the flow of metabolites into either the sterol or non-sterol pathways (with farnesyl pyrophosphate as the branch point) and is considered to be the first committed enzyme in cholesterol biosynthesis.
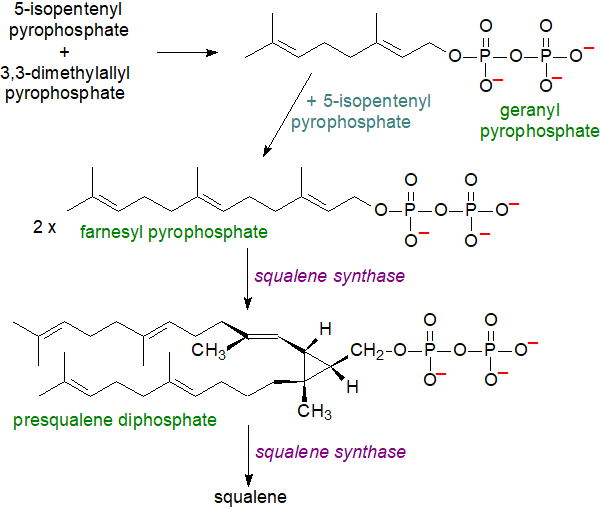 |
| Figure 3. Cholesterol biosynthesis - Step 3. Squalene. |
In the next reaction, squalene is oxidized by a squalene monooxygenase to squalene 2,3-epoxide, another control point in cholesterol biosynthesis (the rate-limiting enzyme of the committed cholesterol synthesis pathway), in which the oxygen atom is inserted to become the signature oxygen of the hydroxyl group in cholesterol. A truncated form of the enzyme is present in cancers and lipid droplets. The epoxide then undergoes cyclization catalysed by the enzyme squalene epoxide lanosterol-cyclase to form the first steroidal intermediate lanosterol (or cycloartenol in the pathway to phytosterols in some plants and other photosynthetic organisms). In this remarkable reaction, there is a series of concerted 1,2-methyl group and hydride shifts along the chain of the squalene molecule to bring about the formation of the four rings all under strict stereochemical control. As no intermediate compounds have been found, this is one of the most complex single enzymatic reactions ever to have been identified, although the enzyme is only 90 kDa in size. Again, the reaction takes place in the endoplasmic reticulum, but a cytosolic protein, sterol carrier protein 1 (SCP1), is required to bind squalene in an appropriate orientation in the presence of the cofactors NADPH, flavin adenine dinucleotide (FAD) and O2; the reaction is promoted by the presence of phosphatidylserine.
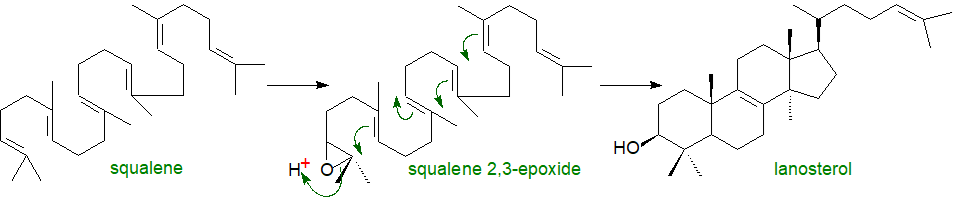 |
| Figure 4. Cholesterol biosynthesis - Step 4. Lanosterol. |
In subsequent steps, lanosterol is converted to cholesterol by a series of demethylations, desaturations, isomerizations and reductions in nineteen separate reactions. Thus, demethylation reactions produce zymosterol as an intermediate, and this is converted to cholesterol via a series of intermediates, all of which have been characterized, and by at least two pathways that utilize essentially the same enzymatic machinery but differ in the order of the various reactions, mainly at the point at which the Δ24 double bond is reduced. Desmosterol is the required intermediate in the so-called 'Bloch' pathway, while 7‑dehydrocholesterol is the immediate precursor in the 'Kandutsch-Russell' pathway, which avoids oxygen dependent steps although it is less efficient. While most tissues, including the liver, adrenal glands and testis, use the Bloch pathway mainly, the brain synthesises much of its cholesterol by the 'Kandutsch-Russell' pathway. This may enable production of other minor sterols for various purposes in different cell types/locations.
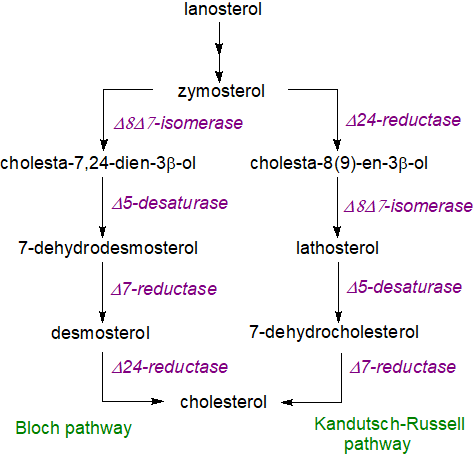 |
| Figure 5. Cholesterol biosynthesis - final steps. |
There is a high energy cost to this process, and synthesis of one cholesterol molecule consumes directly 18 ATPs, 16 NAD/NADPH, as well as acetyl-CoA and oxygen (11 molecules), i.e., roughly one hundred ATP equivalents.
Post synthesis, cholesterol is exported from the liver and transported to other tissues in the form of low-density lipoproteins (LDL) for uptake via their receptors, together with cholesterol obtained from the diet (mainly eggs and red meat) via intestinal absorption, so peripheral tissues can obtain their cholesterol either from the circulation or by synthesis de novo.
3. Regulation of Cholesterol Homeostasis
Although some cholesterol is converted to oxysterols and bile acids, it cannot be catabolized for energy purposes (see below), and its synthesis must be tightly regulated as it so expensive energetically. The rates of synthesis can vary over several hundred-fold depending on the availability of any external sources of cholesterol. Cholesterol homeostasis is a complex dynamic equilibrium process composed of regulated pathways of biosynthesis, uptake, efflux and esterification, and it involves a complex web of enzymes, transport proteins, membrane-bound transcription factors and epigenetic modifications.
Cholesterol concentration: Many factors are needed to maintain the large differences in cholesterol concentrations among the various membranes and organelles in cells within precise limits. To explain how cholesterol in the plasma membrane, where it is most abundant, can regulate cholesterol biosynthesis and uptake through enzymes in the endoplasmic reticulum, where it is least abundant, it has been suggested that there are three pools of cholesterol in the plasma membrane with their own distinct roles. The first of these is "accessible” to receptor proteins for transport to the endoplasmic reticulum, while the second pool is sequestered by sphingomyelin and can be released by the action of sphingomyelinase upon demand. The third residual pool of cholesterol is essential for membrane integrity. These correspond to about 16, 15 and 12 mol % of total plasma membrane lipids, respectively, in cholesterol-replete cells.
Simplistically, when cholesterol in the plasma membrane is in excess for any reason, e.g., after LDL uptake by receptor-mediated endocytosis, there is a rise in accessible (or 'active') cholesterol, some of which is transferred to the nascent HDL, but much is transported back to the endoplasmic reticulum to switch off cholesterol biosynthesis and expression of the LDL receptor. This process requires a host of regulatory proteins and mechanisms that can involve either vesicle formation or non-vesicular pathways, which utilize transport proteins, such as the ABC transporters and the Aster proteins (Aster-A, B and C). The latter are in a region of the endoplasmic reticulum in contact with the plasma membrane and facilitate the return of cholesterol.
The sterol regulatory element-binding proteins (mainly SREBP-1c and SREBP-2), which contain N-terminal transcription and C-terminal regulatory domains facing the cytosol with eight membrane-spanning helices, are essential to the maintenance of cholesterol levels. SREBP-1c is utilized mainly to regulate genes for fatty acid synthesis and energy storage and is inhibited by sterols, while SREBP-2 regulates the genes for cholesterol synthesis and metabolism. Each is synthesised as an inert precursor that is inserted into the endoplasmic reticulum where it can encounter an escort protein termed SREBP cleavage-activating protein (SCAP), which is the cellular cholesterol sensor and requires the amino acid glutamine for activation. Simplistically, when the latter recognizes that cellular cholesterol levels are inadequate, it binds to the regulatory domain of SREBP-2, and the SCAP-SREBP-2 complex then moves to the Golgi, where two proteases (designated site-1 and site-2 proteases) cleave the SREBP-2 to enable the C‑terminal regulatory domain to enter the nucleus. There it activates transcription factors, such as the nuclear liver X receptor (LXR), which stimulate the expression of the genes coding for the LDL receptor in the plasma membrane and for the biosynthetic enzyme HMG-CoA reductase to increase the rate of cholesterol uptake and synthesis. Conversely, when cholesterol in the endoplasmic reticulum exceeds a threshold, it binds to SCAP in such a way that it prevents the SCAP-SREBP-2 complex from leaving the membrane for the nucleus, cholesterol synthesis and uptake are thereby repressed, and cholesterol homeostasis is restored. In effect, cholesterol exerts feedback inhibition to suppress its own production by preventing the proteolytic cleavage and maturation of SREBP-2.
Ultimately, post-translational control of the many different biosynthetic enzymes provides a rapid means for modifying flux through the pathway in the endoplasmic reticulum; some are rapidly degraded in response to tissue levels of cholesterol and its intermediates, while others are controlled through phosphorylation or acetylation mechanisms. HMG-CoA reductase is recognized as the key enzyme in the regulation of the biosynthesis not only of cholesterol but also of many non-sterol isoprenoids, and this can be regulated by a feedback mechanism after binding of a characteristic element in the second transmembrane helix to Insig, a protein in the endoplasmic reticulum in humans encoded by the insulin induced gene 1. This makes the SCAP/SREBP complex stay longer in the ER preventing SCAP from carrying activated SREBP to the Golgi complex, causing a decreased expression of HMG-CoA-reductase and ultimately leading to ubiquitin-proteasomal degradation. Similarly, the second rate-limiting enzyme in cholesterol biosynthesis is squalene monooxygenase, which undergoes cholesterol-dependent proteasomal degradation when cholesterol is in excess, guided by a 12‑amino acid hydrophobic sequence on the enzyme that can serve as a degradation signal. When the cholesterol concentration in the endoplasmic reticulum is high, the degradation sequence detaches from the membrane and is exposed to provide the signal for the enzyme to be degraded.
Further regulation of cholesterol biosynthesis is exerted by sterol intermediates in cholesterol biosynthesis, such as lanosterol and 24,25‑dehydrolanosterol (dimethyl-sterols), and by cholesterol sulfate via accelerated degradation of the biosynthetic enzymes such as HMG-CoA reductase, while side-chain oxysterols like 25‑hydroxycholesterol interfere by suppressing the activation of SREBP-2 through binding to an oxysterol-sensing protein in the endoplasmic reticulum. It is noteworthy that ceramide down-regulates cholesterol synthesis – another link between cholesterol and sphingolipid metabolism. Several non-sterol isoprenoids are synthesised by the mevalonate pathway, including farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are necessary for protein prenylation, and their metabolism can be affected by the mechanisms for cholesterol homeostasis.
Cholesterol transport: Cholesterol in the endoplasmic reticulum is transferred to the Golgi and eventually to the plasma membrane by vesicular and non-vesicular transport mechanisms in part by soluble sterol transport proteins, including the so-called 'START' domain proteins, and partly by binding to those proteins that are intimately involved in the transport and metabolism of polyphosphoinositides such as phosphatidylinositol 4-phosphate (PI(4)P). In the latter mechanism, cholesterol is transported by binding to the ORD domain of oxysterol binding protein (OSBP) or Osh4 in yeast, before OSBP binds to PI(4)P in the plasma membrane to transfer its cargo. The explanation for this process is that cholesterol and PI(4)P are synthesised at two different locations, i.e., the endoplasmic reticulum for sterols and the trans-Golgi network and plasma membrane for PI(4)P, so the two lipids do not compete but rather can be exchanged. OSBP carries cholesterol in the forward direction to the trans-Golgi network and plasma membrane, while PI(4)P, which binds to a C‑terminal PH domain in the protein, is transported in the reverse direction. As this reaction is irreversible, a gradient of cholesterol along organelles of the secretory pathway is established. The subsequent hydrolysis of PI(4)P is the energy source for the reaction, and indeed, PI(4)P has been termed "lipid ATP".
OSBP is thus a lipid transfer protein that enables two organelles to exchange cholesterol rapidly between them at membrane contact sites in a cycle of reactions that include membrane tethering, cholesterol transport, PI(4)P counter transport and PI(4)P hydrolysis. In fact, OSBP is one of several such transport proteins, including ORP9 and GRAMD1s/Asters (GRAMD1a/GRAMD1b/GRAMD1c). There is a similar mechanism for the transport of phosphatidylserine from the endoplasmic reticulum to the inner leaflet of the plasma membrane.
Subsequently, the ATP binding cassette (ABC) transporters ABCA1 and ABCG1 in the plasma membrane, which contains much of the cellular cholesterol, export the excess. Nuclear factor (erythroid-derived 2)-like 1 or Nfe2L1/NRF1 in the endoplasmic reticulum binds directly to cholesterol and senses when its level is high to bring about a de-repression of genes that govern cholesterol removal with mediation by the liver X receptor.
 Within cells,
cholesterol derived initially from the lysosomal degradation of low-density lipoproteins is transferred first to the plasma membrane
and thence to the endoplasmic reticulum, the latter step by a mechanism involving proteins known as GRAMD1s embedded in the endoplasmic
reticulum membrane at sites in contact with the plasma membrane.
These have two functional domains: a START-like domain that binds cholesterol and a GRAM domain that binds anionic lipids, such as
phosphatidylserine, and so they can form a link between the two membranes that enables the transfer of cholesterol.
Within cells,
cholesterol derived initially from the lysosomal degradation of low-density lipoproteins is transferred first to the plasma membrane
and thence to the endoplasmic reticulum, the latter step by a mechanism involving proteins known as GRAMD1s embedded in the endoplasmic
reticulum membrane at sites in contact with the plasma membrane.
These have two functional domains: a START-like domain that binds cholesterol and a GRAM domain that binds anionic lipids, such as
phosphatidylserine, and so they can form a link between the two membranes that enables the transfer of cholesterol.
In peripheral tissues, excess cholesterol is exported to high-density lipoproteins (HDL) in the circulation, where much is esterified (see below) and eventually returned to the liver, a vital process discussed in more detail in our web page on lipoproteins and known as reverse cholesterol transport, and while the liver synthesises much of the cholesterol in the body, it is essential for its removal in bile as discussed in our web page on bile acids. Some lipoproteins are delivered to lysosomes by endocytosis with their content of cholesterol and cholesterol esters, which are hydrolysed by acid lipase, before the cholesterol is transported to the inner surface of the lysosomal membrane through the glycocalyx via a trans-glycocalyx tunnel with the aid of Niemann-Pick C1, C2 and other proteins, and thence via contact sites between membranes to other organelles again with the assistance of sterol transport proteins. In cellular membranes, cholesterol in excess of the stoichiometric requirement can escape back into the cell, where it may serve as a feedback signal to down-regulate cholesterol accumulation, while some is converted to the relatively inert storage form, cholesterol esters, and some is used for steroidogenesis.
Intestinal absorption: The intestines play a major part in cholesterol homeostasis via absorption of dietary cholesterol and faecal excretion of cholesterol and its metabolites. A transporter (Niemann-Pick C1-like 1 or NPC1L1) in the brush border membrane of enterocytes in the proximal jejunum of the small intestine takes up cholesterol from the intestinal contents, while the metabolism of sterols in the intestinal cells is controlled mainly by an acetyl-CoA acetyltransferase (ACAT2), which facilitates intracellular cholesterol esterification, and by the microsomal triglyceride transfer protein (MTTP), which is involved in the assembly of chylomicrons for export into lymph (see our web page on lipoproteins for further discussion). Some cholesterol can be transferred in the opposite direction (trans-intestinal cholesterol excretion), but the quantitative significance of this process is not clear. There is evidence that dietary cholesterol or that synthesised de novo within enterocytes is necessary to maintain intestinal integrity, as cholesterol derived from circulating lipoproteins is not sufficient for the purpose.
In the intestines and colon, the intestinal microflora can hydrogenate cholesterol from bile, the diet and desquamated cells to form coprostanol with an efficiency that is dependent on the nature of the microbial species. Coprastanol is not absorbed by the intestinal tissue to a significant extent, and it may inhibit the uptake of residual cholesterol. There are two mechanisms for this conversion in bacteria, one by direct reduction and another using cholestenone and coprostanone as intermediates, and as the relevant genes have now been identified, the therapeutic potential is under investigation.
Brain: There are substantial differences in cholesterol metabolism in brain and the central nervous system in comparison to the liver and peripheral tissues. As trace amounts only of cholesterol can cross the blood brain barrier from the plasma via transport in low-density lipoproteins, virtually all the cholesterol in brain must be synthesised de novo with the later steps by the Kandutsch-Russell pathway with the same enzymes and intermediates, mainly in astrocytes (glial cells). This synthesis is regulated independently of that in peripheral tissues, mainly by forms of the liver X receptor (LXR). During the perinatal and adolescent years, cholesterol is synthesised in large amounts to form the myelin that surrounds the axons, before this rate begins to decline to eventually reach about 10% of earlier values. The phospholipid bilayer of myelin sheaths contains ~70% of brain cholesterol, where it provides structural support and controls its fluidity and permeability; this is rate-limiting for developmental myelination. As a consequence of demyelinating insults as in multiple sclerosis, cholesterol is released from damaged myelin and can impede tissue regeneration.
Cholesterol in the brain is recycled between cells to prevent intracellular accumulation and to meet the varying cellular requirements, and it is exported from cells via the ABCA1, ABCG1 and ABCG4 transporters. Further transport to and from neurons occurs in the form of apo E complexes in discoidal HDL-like particles, which travel through the cerebrospinal fluid and deliver lipids to receptors, at least seven are known, which take up cholesterol from these lipoproteins. Apo E is synthesised in the brain, and its transcription is regulated by 24‑hydroxy-cholesterol concentrations. As cholesterol and oxysterols can provide neuroprotective effects and lower neuroinflammation, dysregulation of their concentrations has been noted in many neurodegenerative disorders. Most of the lipoproteins in cerebrospinal fluid differ from the nascent poorly-lipidated HDL secreted by astrocytes, suggesting that the latter are modified during maturation. Our web pages on oxysterols and on lipoproteins discuss cholesterol metabolism in the brain at greater length.
4. Cholesterol Catabolism
Cholesterol is not readily degraded in animal tissues so does not serve as a metabolic fuel to generate ATP. Only the liver possesses the enzymes to degrade significant amounts and then via pathways that do not lead to energy production. Cholesterol and oxidized metabolites (oxysterols) are transferred back from peripheral tissues in lipoprotein complexes to the liver for catabolism by conversion to oxysterols and bile acids under regulation by peroxisome proliferator‑activated receptors (PPARs), before the latter are exported into the intestines to aid digestion, while leading to some loss that is essential for cholesterol homeostasis (and is discussed in further web-pages on oxysterols, bile acids and lipoproteins).
As discussed briefly earlier, gut bacteria reduce the double bond in some of the cholesterol in the diet to form highly insoluble 5β-cholestan-3β-ol (coprostanol), which is excreted and can be used as a biomarker for sewage in the environment. Certain bacteria contain a 3β‑hydroxysteroid:oxygen oxidoreductase (EC 1.1.3.6), commonly termed cholesterol oxidase, a flavoenzyme that catalyses the oxidation of cholesterol to cholest-5-en-3-one, which is then rapidly isomerized to cholest-4-en-3-one as the first essential step in a more comprehensive catabolism of sterols in the environment. The enzyme is widespread in organisms that degrade organic wastes, and it is present in some pathogenic organisms where it influences the virulence of infections (see below). In biotechnology, it has been used in processes for manufacture of many steroids, and it is employed in clinical laboratories in a method for the determination of cholesterol levels in serum.
5. Cholesterol Esters
Cholesterol esters, i.e., with long-chain fatty acids linked to the hydroxyl group, are much less polar than free cholesterol, and they are the preferred form for transport in plasma and as a biologically inert storage or de-toxification form to buffer an excess. They do not contribute to membrane structures but are packed into intracellular lipid droplets and can accumulate in the fatty lesions of atherosclerotic plaques. Cholesterol esters are major constituents of the adrenal glands, where they are accompanied by esters of steroidal hormones and are concentrated in cytosolic lipid droplets adjacent to the endoplasmic reticulum; 17β-estradiol, the principal oestrogen in fertile women, is transported in lipoproteins in the form of a fatty acid ester.
Because of the mechanism of synthesis (see below), plasma cholesterol esters tend to contain relatively high proportions of the polyunsaturated components typical of phosphatidylcholine (Table 1). Arachidonic and "adrenic” (22:4(n-6)) acids can be abundant in cholesterol esters from the adrenal gland.
Table 1. Fatty acid composition of cholesterol esters (wt % of the total) from various tissues. |
|||||||
| Fatty acids | |||||||
|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | 20:4 | 22:4 | |
| Human | |||||||
| plasma | 12 | 2 | 27 | 45 | 8 | ||
| liver | 23 | 10 | 28 | 22 | 6 | ||
| Sheep | |||||||
| plasma | 10 | 2 | 27 | 35 | 7 | - | - |
| liver | 17 | 9 | 29 | 7 | 4 | 3 | - |
| adrenals | 13 | 7 | 35 | 18 | 2 | 4 | 2 |
| Data from - Christie, W.W. et al. Lipids, 10, 649-651 (1975); DOI. Nelson, G.J. Comp. Biochem. Physiol., 30, 715-725 (1969); DOI. Horgan, D.J. and Masters, C.J. Aust. J. Biol. Sci., 16, 905-915 (1963); DOI. Nestel, P.J. and Couzens, E.A. J. Clin. Invest., 45, 1234-1240 (1966); DOI. | |||||||
Biosynthesis: In most animal tissues, an enzyme acyl-CoA:cholesterol acyltransferase (ACAT) or sterol O-acyl-transferase (SOAT) synthesises cholesterol esters from CoA esters of fatty acids and cholesterol. ACAT exists in two forms, both of which are intracellular enzymes found in the endoplasmic reticulum and are characterized by multiple transmembrane domains and a catalytic histidine residue in a hydrophobic domain; they are members of the O‑acyltransferase (MBOAT) superfamily. ACAT1 is present in many tissues, but especially in macrophages and adrenal and sebaceous glands, which store cholesterol esters in the form of cytoplasmic lipid droplets. As it is responsible for the synthesis of cholesterol esters in arterial foam cells in human atherosclerotic lesions and may be a factor in the initiation and progression of cancer, it is seen as a target for therapy. ACAT2 is found only in the liver and small intestine, and it supplies cholesterol esters to the nascent lipoproteins. In yeast, there are analogous enzymes with ergosterol as the main sterol, but a different process occurs in plants (see our web page on plant sterols).
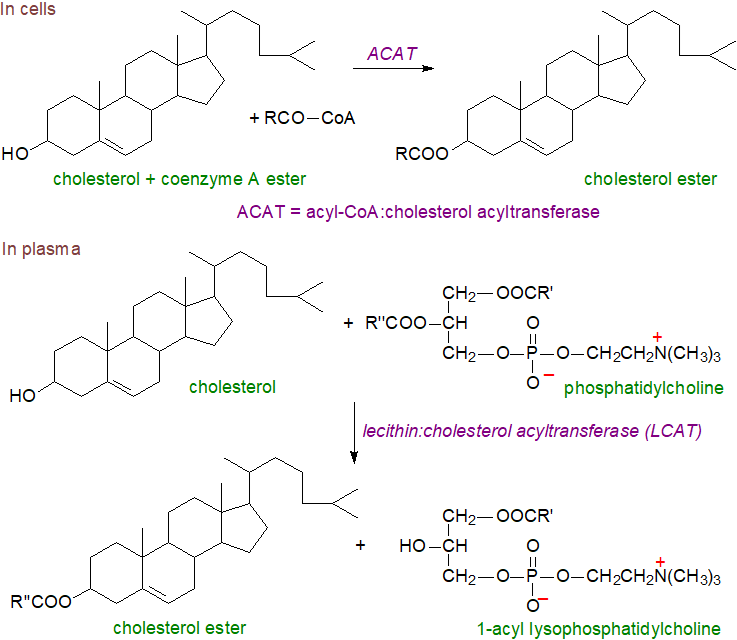 |
| Figure 6. Biosynthesis of cholesterol esters in cells and plasma. |
Cholesterol esterification in plasma occurs by a very different mechanism. In the high-density lipoproteins (HDL), cholesterol esters are synthesised largely by transfer of C18 fatty acids (18:1 and 18:2) to cholesterol from position sn-2 of phosphatidylcholine (‘lecithin’) catalysed by the enzyme lecithin:cholesterol acyl transferase (LCAT); the other product is 1‑acyl-lysophosphatidylcholine (see our web page on lipoproteins). The reaction occurs in several steps with first apoprotein A1 in the HDL acting to concentrate the lipid substrate near LCAT and present it in the optimal conformation, while at the same time, it opens a lid on the enzyme that activates it by exposing the site of transesterification. Then, cleavage of the sn-2 ester bond of phosphatidylcholine occurs via LCAT acting as a phospholipase to release of fatty acyl moiety, which is transacylated to the sulfur atom of a cysteine residue forming a thioester and ultimately is donated to the 3β‑hydroxyl group of cholesterol to form the cholesterol ester. Some LCAT has been detected in apolipoprotein B100-containing particles (β-LCAT as opposed to α‑LCAT with HDL).
It has been established that human LCAT is a relatively small glycoprotein with a polypeptide mass of 49 kDa, increased to about 60 kDa by four N‑glycosylation and two O-glycosylation moieties. Most of the enzyme is produced in the liver and circulates in blood while bound reversibly to HDL, where it is activated by the main protein component of HDL, apolipoprotein A1. As cholesterol esters accumulate in the lipoprotein core, cholesterol is removed from its surface thus promoting the flow of cholesterol from cell membranes into HDL. This in turn leads to morphological changes in HDL, which grow and become spherical. Subsequently, cholesterol esters are transferred to the other lipoprotein fractions LDL and VLDL, a reaction catalysed by cholesterol ester transfer protein. This process promotes the efflux of cholesterol from peripheral tissues (‘reverse cholesterol transport’), including macrophages in the arterial wall, for subsequent delivery to the liver. LCAT is the main driving force behind this process, and it is of great importance for cholesterol homeostasis and a suggested target for therapeutic intervention against cardiovascular and other diseases.
The stereospecificity of LCAT changes with molecular species of phosphatidylcholine containing arachidonic or docosahexaenoic acids, when the fatty acids from position sn-1 are transacylated and 2-acyl lysophosphatidylcholines are formed. This reaction may be relevant to the supply of these essential fatty acids to the brain in that such lysophospholipids cross the blood-brain barrier more readily than the free acids.
Oxidized Cholesterol Esters: All lipid classes containing polyunsaturated fatty acids are susceptible to oxidation. Under normal circumstances, cholesterol esters are relatively inert, but when they contain oxidized polyunsaturated fatty acids, their properties change, and they acquire biological activity (this applies also when the cholesterol moiety is oxidized as discussed on a separate web page here..). Such oxidized cholesterol esters may be formed both by a reaction with 15‑lipoxygenase and by free radical-induced lipid peroxidation, and they have been detected in lipoproteins, LDL mainly, in human blood and atherosclerotic lesions. Those oxidized cholesterol esters in plasma are trafficked into cells and metabolized by the same mechanisms as the corresponding unoxidized lipids.
Such 'minimally oxidized LDL' do not bind to CD36 but rather to CD14, a receptor that recognizes bacterial lipopolysaccharides. The result is stimulation of toll-like receptor 4 (TLR4), although the response differs from that to lipopolysaccharides. In addition, oxidized metabolites of cholesteryl arachidonate of this kind stimulate macrophages to express inflammatory cytokines of relevance to atherosclerosis among other effects. Oxidized cholesterol esters can be hydrolysed to release their fatty acids, which can then be incorporated into phospholipids with a different repertoire of actions. Our web page on oxidized phospholipids affords further information.
Hydrolysis of cholesterol esters: Cholesterol ester hydrolases in animals liberate cholesterol and free fatty acids upon demand for membrane and lipoprotein formation, and they provide cholesterol for hormone synthesis in adrenal cells. Many such hydrolases have been identified and include a carboxyl ester hydrolase, a lysosomal acid cholesterol ester lipase, hormone-sensitive lipase and hepatic cytosolic cholesterol ester hydrolase, which are located in many different tissues and organelles. A neutral cholesterol ester hydrolase has received most study, as it removes cholesterol esters from macrophages, so reducing the formation of foam cells and thence the development of fatty streaks within the arterial wall, a significant step in the progression of atherosclerosis.
6. Other Animal Sterols
Many sterols other than cholesterol occur in small amounts in tissues, most of which are intermediates in the biosynthetic pathway from lanosterol, and some have distinct functions of their own. Lanosterol, the first sterol intermediate in the biosynthesis of cholesterol, was first found in wool wax, both in free and esterified form, and this is still the main commercial source, as it is found at low levels only in most other animal tissues (typically 0.1% of the cholesterol concentration). As oxygen is required, lanosterol cannot be produced by primitive organisms, hence its absence from prokaryotes and leading to some speculation on its evolutionary significance as it is suggested that much greater possibilities opened for the evolution of eukaryotes when sterols became available. The production of cholesterol from lanosterol is seen as ‘molecular streamlining’ by evolution, removing protruding methyl groups that hinder the interaction between sterols and phospholipids in membranes.
Desmosterol (5,24-cholestadien-3β-ol), the last intermediate in the biosynthesis of cholesterol by the Bloch pathway, may be involved in the process of myelination, as it is found in relative abundance in the brains of young animals but not in those of adults other than in astrocytes. It is present in appreciable amounts in testes and spermatozoa together with another cholesterol intermediate, testis meiosis-activating sterol, and these and related sterols are essential for human reproduction. In addition, there is evidence that desmosterol stimulates certain genes for lipid biosynthetic enzymes in macrophages, and it may switch off others associated with the inflammatory response. In a rare genetic disorder, desmosterolosis, there is an impairment in the conversion of desmosterol to cholesterol with serious consequences in terms of mental capacity.
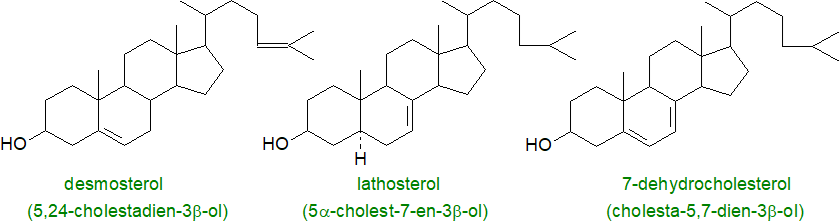 |
| Figure 7. Some other important animal sterols. |
In human serum, the levels of lathosterol (5α-cholest-7-en-3β-ol) were found to be inversely related to the size of the bile acid pool, and in general, the concentration of serum lathosterol is strongly correlated with the cholesterol balance under most dietary conditions. A further important animal sterol is 7-dehydrocholesterol (cholesta-5,7-dien-3β-ol), the final precursor to cholesterol in the Kandutsch-Russell biosynthesis pathway. In the skin, on irradiation with sunlight, this is converted to vitamin D3 (cholecalciferol), and in other tissues, it is the precursor for a family of 'spiral steroids', which regulate NaK-ATPases. It shields plasma and mitochondria membranes from phospholipid autoxidation and inhibits ferroptosis. In insects, cholesterol is converted back to 7‑dehydrocholesterol in the pathway to synthesise the developmental ecdysone hormones.
Marine invertebrates produce many novel sterols, with unusual cyclic structures and/or side chains, some derived from cholesterol and others from plant sterols or alternative biosynthetic intermediates. For example, at least 80 distinct sterols have been isolated from echinoderms and 100 from sponges.
Cholesterol will oxidize slowly in tissues or foods to form a range of different products with additional hydroperoxy, epoxy, hydroxy or keto groups, and these can enter tissues via the diet. From the standpoint of human health and nutrition, there is increasing interest in these oxo-sterols since accumulation in plasma is associated with inhibition of the biosynthesis of cholesterol and bile acids and with other abnormalities in plasma lipid metabolism. These and similar oxysterols produced in tissues by microsomal or mitochondrial oxidations are discussed in a further document on this web site. Sterols in membranes, and especially those with two double bonds in conjugation such as 7-dehydrocholesterol may be relevant to ferroptosis.
7. Cholesterol and Disease
Elevated cholesterol and cholesterol ester levels (hypercholesterolemia) are associated with the pathogenesis of cardiovascular disease (atherosclerotic plaques, myocardial infarctions and strokes), as is well known, and this is considered briefly on this website in relation to the metabolism of the plasma lipoproteins. The rate-limiting enzyme in the synthesis of cholesterol HMG-CoA reductase is the target of statins, but drugs that target other steps in the biosynthetic pathway, such as the squalene monooxygenase and lanosterol synthase, are under investigation. However, it should not be too controversial to suggest that healthy eating, perhaps by adherence to a Mediterranean type diet, ought to be the preferred option. Further discussion of such a complex and multifactorial nutritional and clinical topic is best left to others better qualified than me.
Cholelithiasis or the presence in the gallbladder or bile ducts of 'stones', which consist largely of crystalline deposits of cholesterol (~85%), is one of the most prevalent and costly digestive diseases in developed countries, and the primary cause is excessive excretion of cholesterol from the liver, although once more many other genetic and lifestyle factors can be involved.
At least eight different inherited disorders of cholesterol biosynthesis are recognized in humans that lead to congenital abnormalities in those afflicted, including developmental malformation and neurologic defects, while deficiencies in the enzymes responsible for the hydrolysis of cholesterol esters, such as the lysosomal acid lipase, occur in Wolman disease and cholesterol ester storage disease. As there is limited cholesterol transport across the placenta, the human foetus is highly dependent upon endogenous synthesis. While the molecular basis for the altered developmental pathways is not fully understood, impaired synthesis of the hedgehog family of signalling proteins, which incorporate covalently linked cholesterol, is involved in many cases. In others, there are confirmed enzyme defects, and the recessive Smith-Lemli-Opitz syndrome in infants born with a decreased concentration of the enzyme 7‑dihydrocholesterol reductase produces symptoms varying from mild autism to severe mental and often fatal physical problems. The effects are due to a lack of cholesterol and accumulation of 7‑dehydrocholesterol and its 27-hydroxy metabolite in the brain, as dietary cholesterol or that produced peripherally cannot be transported across the blood-brain barrier. In animal models, deficiencies in SREBP-2 and genes encoding sterol biosynthetic enzymes display embryonic lethality.
Niemann-Pick Type C disease is a rare and severe autosomal recessive disorder, i.e., a lysosomal storage disease in which an intracellular disruption of cholesterol transport causes accumulation of unesterified cholesterol and other molecules in the late endosomal/lysosomal compartments because of gene mutations mainly affecting the binding protein NPC1 (NPC2 to a lesser extent). This leads to damage and degeneration of various cells and tissues of the body, in particular the neurons in the central nervous system. Although some aberrant glycosphingolipid metabolism occurs, the disease is distinct from the Niemann-Pick Type A and B diseases, which are caused by defects in the storage of sphingomyelin in lysosomes, although there is then a secondary effect upon cholesterol storage. Excess accumulation of cholesterol is also associated with the metabolism of bis(monoacylglycero)phosphate in the lysosomal compartment.
Deficiencies in cholesterol transport and metabolism are factors in many forms of neurodegeneration, including conditions associated with old age and possibly Alzheimer’s and Huntington’s diseases, but this is discussed in our web page on oxysterols. Aberrant cholesterol metabolism has been noted in several diseases of the eye, including age-related macular degeneration, diabetic retinopathy and other common eye conditions such as cataracts, corneal diseases and dry eye syndrome.
Cholesterol and other sterols bind to several immune receptors in macrophages and T cells, and dynamic changes in cholesterol biosynthesis impact directly upon innate and adaptive immune responses with implications for health and disease. For example, cholesterol binds to the αβ T cell antigen receptor (αβTCR) via its trans-membrane domain where it is kept in an inert, non-signalling conformation until required to stimulate formation of receptor nanoclusters to increase their avidity for the antigen.
In cancer, there is a high demand for cholesterol to support the inherent nature of tumour cells to divide and proliferate,and perturbations of reverse cholesterol transport can have negative consequences. Dysregulation of cholesterol metabolism through enhanced synthesis, increased uptake and impaired efflux is associated with the development of malignant phenotypes, where crosstalk between SREBP2 and aberrant signalling pathways, which promote tumorigenesis, is a significant factor. Drugs that lower cholesterol levels in cancer cells by inhibiting the mevalonate pathway (including statins) are undergoing clinical trials. Also, over-expression of squalene epoxidase and ACAT1 are observed in some cancers, and inhibition of these enzymes has potential as targets for treatment.
When increased levels of sterols other than cholesterol are found in plasma, they usually serve as markers for abnormalities in lipid metabolism associated with disease states, such as premature atherosclerosis and xanthomatosis, which occur in two rare lipid storage diseases, cerebrotendinous xanthomatosis and sitosterolemia. In the former, cholestanol is present in all tissues, while in the latter, the dietary plant sterols campesterol and sitosterol accumulate in plasma and red blood cells. Inhibition of cholesterol biosynthesis may be associated with the appearance of some of the precursor sterols in plasma.
In infections with Mycobacterium tuberculosis, the organism uses host cholesterol in macrophages as the major carbon and energy source and thereby promotes persistent infection with appreciable effects on pathogenicity. It is able to degrade host cholesterol and incorporate the products, including oxysterol metabolites such as cholestenone, into its central carbon metabolism to generate cell envelope lipids. Similarly, Chlamydia trachomatis, a gram-negative obligate intracellular bacterium and a major cause of sexually transmitted infections, needs host cholesterol for growth. An HIV protein has a binding site for cholesterol, which it utilizes to facilitate the fusion with raft regions in membranes of the host cell, and this is one of many viruses that use cholesterol as part of their life cycle. Reduction in cellular cholesterol is sometimes seen as an anti-viral strategy, although this may not always be helpful.
8. Analysis
With animal tissues of clinical relevance such as plasma, the cholesterol content is often determined by using enzymatic methods from commercially available kits that are suited to routine analysis of large numbers of samples, though with less precision and selectivity than is achieved by chromatographic procedures. For the total cholesterol content, it is necessary to hydrolyse the cholesterol ester fraction first, and this usually needs more vigorous conditions than with glycerolipids. For more accurate or detailed analysis of animal and plant sterols, a sterol fraction is first isolated from lipid extracts by thin-layer or column chromatography, following hydrolysis if necessary. Individual components can then be determined by gas chromatography in the presence of an internal standard (e.g., epicoprostanol or betulin), often after conversion to trimethylsilyl ether derivatives to give sharper peaks. Mass spectrometry is invaluable for identification of individual components.
Sterol esters can be transmethylated for GC analysis of the fatty acid components, although the reaction may again be much slower than with glycerolipids, but intact sterol esters are best analysed by reversed-phase HPLC.
Recommended Reading
- Barrantes, F.J. The pleomorphic cholesterol sensing motifs of transmembrane proteins. Chem. Phys. Lipids, 266, 105460 (2025); DOI.
- Brown, A.J. and Sharpe, L.J. Cholesterol synthesis. In: Biochemistry of Lipids, Lipoproteins and Membranes (6th Edition). pp. 328-358 (edited by N.D. Ridgway and R.S. McLeod, Elsevier, Amsterdam) (2016) - see Science Direct.
- Cardoso, D. and Perucha, E. Cholesterol metabolism: a new molecular switch to control inflammation. Clin. Sci., 135, 1389-1408 (2021); DOI.
- Cerqueira, N.M.F.S.A., Oliveira, E.F., Gesto, D.S., Santos-Martins, D., Moreira, C., Moorthy, H.N., Ramos, M.J. and Fernandes, P.A. Cholesterol biosynthesis: a mechanistic overview. Biochemistry, 55, 5483-5506 (2016); DOI.
- Cui, D.X., Yu, X.Q., Guan, Q.Y., Shen, Y., Liao, J.J., Liu, Y. and Su, Z.G. Cholesterol metabolism: molecular mechanisms, biological functions, diseases, and therapeutic targets. Mol. Biomed., 6, 72 (2025); DOI.
- Dunina-Barkovskaya, A.Y. Cell membrane cholesterol and regulation of cellular processes: new and the same old thing. Biochemistry (Moscow) A, Membrane Cell Biol., 18, 224-240 (2024); DOI.
- Faulkner, R. and Jo, Y. Synthesis, function, and regulation of sterol and nonsterol isoprenoids. Front. Mol. Biosci., 9, 1006822 (2022); DOI.
- Garçon, D., Berger, J.M., Cariou, B. and Le May, C. Transintestinal cholesterol excretion in health and disease. Curr. Atheroscl. Rep., 24, 153-160 (2022); DOI.
- Gonen, A. and Miller, Y.I. From inert storage to biological activity-in search of identity for oxidized cholesteryl esters. Front. Endocrin., 11, 602252 (2020); DOI.
- Hai, Q.M. and Smith, J.D. Acyl-coenzyme A: cholesterol acyltransferase (ACAT) in cholesterol metabolism: from its discovery to clinical trials and the genomics era. Metabolites, 11, 543 (2021); DOI.
- Ikonen, E. and Zhou, X. Cholesterol transport between cellular membranes: A balancing act between interconnected lipid fluxes. Developmental Cell, 56, 1430-1436 (2021); DOI.
- Jakubik, J. and El-Fakahany, E.E. Allosteric modulation of GPCRs of class A by cholesterol. Int. J. Mol. Sci., 22, 4 (2021); DOI.
- Jiang, W., Jin, W.L. and Xu, A.M. Cholesterol metabolism in tumor microenvironment: cancer hallmarks and therapeutic opportunities. Int. J. Biol. Sci., 20, 2044-2071 (2024); DOI.
- Juste, C. and Gerard, P. Cholesterol-to-coprostanol conversion by the gut microbiota: what we know, suspect, and ignore. Microorganisms, 9, 1881 (2021); DOI.
- Kunnen, S. and Van Eck, M. Lecithin:cholesterol acyltransferase: old friend or foe in atherosclerosis? J. Lipid Res., 53, 1783-1799 (2012); DOI.
- Luo, J., Yang, H.Y. and Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol., 21, 225-245 (2020); DOI.
- Meng, Y., Heybrock, S., Neculai, D. and Saftig, P. Cholesterol handling in lysosomes and beyond. Trends Cell Biol., 30, 452-466 (2020); DOI.
- Pfrieger, F.W. The Niemann-Pick type diseases - A synopsis of inborn errors in sphingolipid and cholesterol metabolism. Prog. Lipid Res., 90, 101225 (2023); DOI.
- Rao, S.R. and Fliesler, S.J. Cholesterol homeostasis in the vertebrate retina: biology and pathobiology. J. Lipid Res., 62, 100057 (2021); DOI.
- Savulescu-Fiedler, I. and others. The cross-talk between the peripheral and brain cholesterol metabolisms. Curr. Issues Mol. Biol., 47, 115 (2025); DOI.
- Sharpe, L.J., Coates, H.W. and Brown, A.J. Post-translational control of the long and winding road to cholesterol. J. Biol. Chem., 295, 17549-17559 (2020); DOI.
- Schumacher, M.M. and DeBose-Boyd, R.A. Posttranslational regulation of HMG CoA reductase, the rate-limiting enzyme in synthesis of cholesterol. Annu. Rev. Biochem., 90, 659-679 (2021); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: November 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
