Fatty Acids: Polyunsaturated with
Methylene-Interrupted Double Bonds
1. Structure and Nomenclature
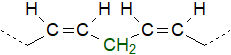 The
lipids of all higher organisms contain appreciable quantities of polyunsaturated fatty acids ('PUFA')
with methylene-interrupted double bonds, i.e., with two or more double bonds of the cis/Z-configuration
separated by a single methylene group; the term ‘homo-allylic’ is also used to describe this molecular feature.
In higher plants, the number of double bonds in fatty acids seldom exceeds three,
but in algae and animals, there can be up to six (very rarely more in some marine organisms).
Most bacteria of terrestrial origin, other than Cyanobacteria, cannot produce polyunsaturated fatty acids,
but many bacterial families of marine origin can do so.
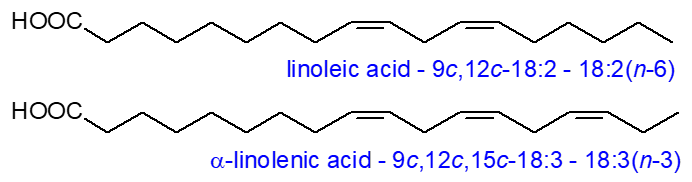
Two principal families of polyunsaturated fatty acids occur in nature that are derived biosynthetically from
linoleic (octadeca-9Z,12Z-dienoic) and
α-linolenic (octadeca-9Z,12Z,15Z-trienoic) acids.
The
lipids of all higher organisms contain appreciable quantities of polyunsaturated fatty acids ('PUFA')
with methylene-interrupted double bonds, i.e., with two or more double bonds of the cis/Z-configuration
separated by a single methylene group; the term ‘homo-allylic’ is also used to describe this molecular feature.
In higher plants, the number of double bonds in fatty acids seldom exceeds three,
but in algae and animals, there can be up to six (very rarely more in some marine organisms).
Most bacteria of terrestrial origin, other than Cyanobacteria, cannot produce polyunsaturated fatty acids,
but many bacterial families of marine origin can do so.
Two principal families of polyunsaturated fatty acids occur in nature that are derived biosynthetically from
linoleic (octadeca-9Z,12Z-dienoic) and
α-linolenic (octadeca-9Z,12Z,15Z-trienoic) acids.

In the shorthand nomenclature, these precursor fatty acids are designated 9Z,12Z-18:2 and 9Z,12Z,15Z-18:3, respectively; the number before the colon specifies the number of carbon atoms and that after the colon, the number of double bonds. Biochemists and nutritionists find it useful to denote the position of the terminal double bond in the form (n-x), where n is the chain-length of the fatty acid and x is the number of carbon atoms from the last double bond, with the assumption that all the other double bonds are methylene-interrupted. Thus, linoleate and α-linolenate are 18:2(n‑6) and 18:3(n‑3), respectively (18:2ω6 and 18:3ω3 in the older literature), and they are the precursors for the omega-6 or (n-6) and omega-3 or (n-3) families of polyunsaturated fatty acids, respectively.
Both parent fatty acids can be synthesised in plants, but not in the tissues of higher animals, and they are therefore essential nutrients or 'Essential Fatty Acids (EFA)' that are required for health. Polyunsaturated fatty acids of both families can be found in most lipid classes but mainly as constituents of the phospholipids, where they confer distinctive properties on the membranes, for example to decrease their rigidity. In both animals and plants, they are precursors of many classes of oxylipin with vital signalling and metabolic functions. Dysregulation of their metabolism is seen in chronic diseases, including cardiovascular disorders, diabetes, cancer, neurodegenerative conditions and depression. Sphingolipids are exceptions as they rarely contain polyunsaturated fatty acids in other than trace amounts.
2. The n-6 Family of Polyunsaturated Fatty Acids
Linoleic acid is a ubiquitous component of plant lipids and of all the seed oils of commerce, and corn, sunflower and soybean oils usually contain over 50% of linoleate, while safflower oil contains up to 75%. Although all the linoleate in tissues of higher animals must be acquired from the diet, it is usually the most abundant dienoic fatty acid in mammals (and in most glycerolipid classes), typically at levels of 15 to 25% of the total, although it can amount to as much as 75% of the total fatty acids of heart cardiolipin. While it is a significant component of fish oils, fatty acids of the n-3 family tend to predominate in this instance. Linoleic acid is the precursor for a family of oxylipins, the octadecanoids, which act as lipid mediators.
As exceptions to this general rule, linoleate and its longer-chain metabolites can be synthesised by some primitive invertebrates, including many species of insects (e.g., cockroaches and termites), nematodes (Caenorhabditis elegans) and pulmonates (air-breathing slugs and snails).
 The remaining members of the n-6 family of fatty acids are synthesised from linoleate in animal
and plant tissues by a sequence of elongation and desaturation (cis/Z) reactions as described below, and these too act as essential fatty
acids.
Shorter-chain components may be produced by alpha or beta-oxidation.
The remaining members of the n-6 family of fatty acids are synthesised from linoleate in animal
and plant tissues by a sequence of elongation and desaturation (cis/Z) reactions as described below, and these too act as essential fatty
acids.
Shorter-chain components may be produced by alpha or beta-oxidation.
γ‑Linolenic acid ('GLA' or octadeca-6Z,9Z,12Z-trienoic acid or 18:3(n-6)) is usually a minor component of animal tissues in quantitative terms (<1%), as it is rapidly converted to higher metabolites. It is found in a few seed oils, and those of evening primrose, borage and blackcurrant are produced commercially. Evening primrose oil (ca. 10% GLA) is widely used both as a nutraceutical and a medical or veterinary product, and it has been established that GLA in maternal milk is crucial for cardiomyocyte maturation in the human infant by a mechanism that involves activation of retinoid X receptors (transcription factors).
Eicosa-11Z,14Z-dienoic acid (20:2(n-6)) is a common minor component of animal tissues. Eicosa-8Z,11Z,14Z-trienoic acid (dihomo-γ-linolenic acid or 20:3(n-6)) is the immediate precursor of arachidonic acid and of a family of eicosanoids (PG1 prostaglandins), but it does not accumulate to a significant extent in animal tissue lipids and is typically about 1-2% of the phospholipid fatty acids.
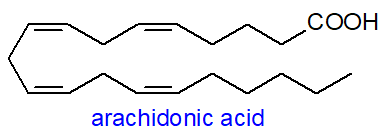
 Arachidonic acid
(eicosa-5Z,8Z,11Z,14Z-tetraenoic acid or 20:4(n-6)) is the most important metabolite of linoleic
acid in animal tissues, both in quantitative and biological terms, with meat as the main dietary source in humans.
It is often the most abundant polyunsaturated component of the phospholipids in membranes,
and it can comprise as much as 40% of the fatty acids of phosphatidylinositol.
As such, it has an obvious role in regulating the physical properties of membranes, but the free acid takes part in the mechanism
by which apoptosis is regulated, and it has other signalling functions in cells, especially in the central nervous system.
While some arachidonate is found in all fish oils, polyunsaturated fatty acids of the n-3 family tend to be present
in much larger amounts.
Arachidonic acid is sometimes detected as a constituent of mosses, liverworts, lichens and ferns,
but there appears to be only one definitive report of its occurrence in a higher plant (Agathis robusta).
The fungus Mortierella alpina is a commercial source of arachidonate via a fermentation process.
Arachidonic acid
(eicosa-5Z,8Z,11Z,14Z-tetraenoic acid or 20:4(n-6)) is the most important metabolite of linoleic
acid in animal tissues, both in quantitative and biological terms, with meat as the main dietary source in humans.
It is often the most abundant polyunsaturated component of the phospholipids in membranes,
and it can comprise as much as 40% of the fatty acids of phosphatidylinositol.
As such, it has an obvious role in regulating the physical properties of membranes, but the free acid takes part in the mechanism
by which apoptosis is regulated, and it has other signalling functions in cells, especially in the central nervous system.
While some arachidonate is found in all fish oils, polyunsaturated fatty acids of the n-3 family tend to be present
in much larger amounts.
Arachidonic acid is sometimes detected as a constituent of mosses, liverworts, lichens and ferns,
but there appears to be only one definitive report of its occurrence in a higher plant (Agathis robusta).
The fungus Mortierella alpina is a commercial source of arachidonate via a fermentation process.
Several families of eicosanoids (oxylipins) are derived from arachidonate, including prostaglandins (PG2 series), thromboxanes, leukotrienes and lipoxins, with phosphatidylinositol being the primary source. These are necessary for innumerable purposes in tissues that are discussed in elsewhere in these web pages. Although 2-arachidonoylglycerol and anandamide (N‑arachidonoylethanolamine) are noteworthy as endocannabinoids with signalling functions, they are minor lipids in tissues in quantitative terms.
4,7,10,13,16-Docosapentaenoic acid (22:5(n-6)) is usually a relatively minor component of animal lipids, but it is the main C22 polyunsaturated fatty acid in the phospholipids of testes, where it can amount to 70% of the fatty acids of lysobisphosphatidic acid in this tissue. In this instance, C22 fatty acids of the n-3 family are present at relatively low levels, in contrast to most other lipids of reproductive tissues.
Other fatty acids of the n-6 family that are found in animal tissues include 22:3(n-6) and 22:4(n-6), and the latter (7,10,13,16-docosatetraenoic or adrenic acid) is a significant component of the phospholipids of the adrenal glands and testes. Tetra- and pentaenoic fatty acids of the n-6 family from C24 to C30 have been found in testes, where they are essential for male fertility and sperm maturation, while even longer homologues occur in retina. In ceramides and sphingomyelin of rat testes, lipid classes that do not normally contain PUFA, 28:4(n‑6) and 30:5(n‑6) fatty acids are the major very-long-chain (VLC)-PUFAs found, while 32:3(n‑6) and 32:4(n‑6) are abundant in the lipids of human spermatozoa. VLC fatty acids (VLCFA) of this type were first reported from human brain in patients with the rare inherited disorder, Zellweger's syndrome, but it is now established that such fatty acids with up to 38 carbon atoms and with from 3 to 6 methylene-interrupted double bonds are present at low levels in the brains of normal young humans, with 34:4(n‑6) and 34:5(n‑6) tending to predominate.
The most highly unsaturated fatty acid of the n-6 family to have been characterized are 28:7(n-6) (4,7,10,13,16,19,22-octacosaheptaenoate), which has been found in the lipids of marine dinoflagellates and herring muscle, and 4,7,10,13,16,19,22,25,28-tetratriacontanonaenoic acid (34:9(n‑6)) from the freshwater crustacean Bathynella natans.
3. The n-3 Family of Polyunsaturated Fatty Acids
α-Linolenic acid (18:3(n-3)) is a major component of the leaves and especially of the photosynthetic apparatus of algae and higher plants, where most of it is synthesised. It can amount to 65% of the total fatty acids of linseed oil, where its relative susceptibility to oxidation has practical commercial value in paints and related products. In contrast, soybean and rapeseed oils have up to 7% of linolenate, and this reduces the value of these oils for cooking purposes because of their propensity to autoxidize and turn rancid. In animal tissue lipids, α-linolenic acid tends to be a minor component (<1%), the exception being grass-eating non-ruminants such as the horse or goose, where it can amount to as much as 10% of the adipose tissue lipids. α‑Linolenic acid is the biosynthetic precursor of oxylipins such as the jasmonates in plants, which behave in ways that can be compared to those of the eicosanoids in animals. As with linoleate, the longer chain members of the n-3 family of fatty acids are synthesised from α‑linolenate in animal and plant tissues by a sequence of elongation and desaturation reactions as described below, while shorter-chain components may be produced by alpha or beta-oxidation. These too are essential fatty acids in animal nutrition.
Stearidonic acid (6,9,12,15-octadecatetraenoic or 18:4(n-3)) is occasionally found in plants as a minor component, such as Echium sp., and it occurs in algae and fish oils. The first elongation product 11,14,17-eicosatrienoic acid (20:3(n‑3)) can usually be detected in the phospholipids of animal tissues but rarely at above 1% of the total, although somewhat higher concentrations can occur in fish oils. 8,11,14,17-Eicosatetraenoic acid (20:4(n-3)) is found in most fish oils and as a minor component of animal phospholipids. It is frequently encountered in algae and in terrestrial bryophytes (mosses, liverworts and hornworts), together with other long-chain polyunsaturated fatty acids of the n‑3 family, but there are few definitive reports in higher plants. 3,6,9,12,15-Octadecapentaenoic acid or 18:5(n-3) is only rarely detected but can be a significant component of the lipids of dinoflagellates and microalgae, and it may enter the marine food chain from this source.
 5,8,11,14,17-Eicosapentaenoic acid
('EPA' or 20:5(n-3)) occurs widely in algae and in fish oils, which are major commercial sources, but there are few definitive reports
of its occurrence in higher plants.
In animal tissues, it is a major constituent of the phospholipids and it is the precursor of the PG3 series of
prostaglandins and of E-series resolvins, which are anti-inflammatory,
but it may have biological activities of its own, including anti-cancer effects by promoting the expression of genes that suppress tumours.
There is currently some interest in the role of EPA in alleviating the symptoms of neurological disorders such as schizophrenia.
5,8,11,14,17-Eicosapentaenoic acid
('EPA' or 20:5(n-3)) occurs widely in algae and in fish oils, which are major commercial sources, but there are few definitive reports
of its occurrence in higher plants.
In animal tissues, it is a major constituent of the phospholipids and it is the precursor of the PG3 series of
prostaglandins and of E-series resolvins, which are anti-inflammatory,
but it may have biological activities of its own, including anti-cancer effects by promoting the expression of genes that suppress tumours.
There is currently some interest in the role of EPA in alleviating the symptoms of neurological disorders such as schizophrenia.
7,10,13,16,19-Docosapentaenoic acid ('DPA' or 22:5(n-3)) is relatively abundant in fish oils, and it is usually present in animal phospholipids at a level of 2 to 5%. As the most second most abundant n-3 polyunsaturated fatty acid in the brain, it may be beneficial for the elderly and for early-life development. While it has received relatively little study, it is known that it can be retro-converted to EPA and that it reacts with lipoxygenases to form distinctive oxylipins, such as the specialized pro-resolving mediators utilized in the resolution of inflammation.
 4,7,10,13,16,19-Docosahexaenoic acid
('DHA' or 22:6(n-3)) is usually the end point of α-linolenic acid metabolism in animal tissues.
It is a major component of fish oils (tuna eyeballs are a rich source) and of animal phospholipids, with those of brain synapses and retina
containing particularly high proportions.
Indeed, there is some evidence that increased levels of this fatty acid are correlated with improved cognition and
behaviour in the development of the human infant, although this is controversial.
DHA is not present in higher plants, but it is found in high concentrations in many species of algae,
especially those of marine origin, which may be the primary source of the DHA and EPA in fish.
Via the food chain, fish contribute substantially to the levels of these fatty acids in terrestrial animals including humans.
4,7,10,13,16,19-Docosahexaenoic acid
('DHA' or 22:6(n-3)) is usually the end point of α-linolenic acid metabolism in animal tissues.
It is a major component of fish oils (tuna eyeballs are a rich source) and of animal phospholipids, with those of brain synapses and retina
containing particularly high proportions.
Indeed, there is some evidence that increased levels of this fatty acid are correlated with improved cognition and
behaviour in the development of the human infant, although this is controversial.
DHA is not present in higher plants, but it is found in high concentrations in many species of algae,
especially those of marine origin, which may be the primary source of the DHA and EPA in fish.
Via the food chain, fish contribute substantially to the levels of these fatty acids in terrestrial animals including humans.
As with the n-6 family, very-long-chain fatty acids (C24 to C38) of the n-3 family occur in the retina, brain, skin, testes and sperm, where they are derived biosynthetically by elongation of the C20 and C22 polyunsaturated precursors by the elongase ELOV4. 20:5(n-3) in retina is preferentially elongated in comparison to 22:6(n‑3). Although DHA is esterified mainly to position sn-2 of phospholipids, the longer-chain analogues are esterified to position sn-1 of these lipids in retina. They are precursors of the oxylipins known as elovanoids (protectin analogues) in brain and retina.
Other fatty acids of the n-3 family that are found in nature include 22:3(n-3) from animal tissues and 16:3(n-3), which is a common constituent of leaf lipids (see our web pages on mono- and digalactosyldiacylglycerols). 16:4(n-3), 16:4(n-3), 21:5(n-3), 24:5(n-3) and 24:6(n-3) are occasionally present in marine organisms, including fish. Trace levels of highly unsaturated fatty acids of the n-3 family (suggested to be 38:7(n-3) to 44:12(n-3)) have been reported from the brains of patients with genetic impairments of peroxisome metabolism, but the most highly unsaturated fatty acids of the n‑3 family to have been characterized from samples of normal origin are 4,7,10,13,16,19,22,25-octacosaoctaenoate (28:8(n-3)) from marine dinoflagellates and 34:10(n-3) from a fish oil concentrate.
When platinum salts (e.g., cisplatin) are administered to cancer patients, synthesis of hexadeca-4,7,10,13-tetraenoic acid or 16:4(n‑3) is induced and confers systemic resistance to this and a broad range of other DNA-damaging chemotherapeutic agents. The mechanism involves binding to the G protein-coupled receptor 120 (GPR120 or FFAR4) on splenic macrophages by the free acid to produce chemo-protective lysophosphatidylcholines. Like 16:4(n-3), EPA, DHA and probably other polyunsaturated fatty acids of the n‑3 family activate the GPR120 receptor/sensor and are broadly anti-inflammatory in macrophages. When stimulated by this means, GPR120 mediates potent insulin sensitizing and anti-diabetic effects in vivo by repressing macrophage-induced tissue inflammation. While n-3 fatty acids are special in this context, other fatty acids, including palmitoleate and linoleate, are agonists for this receptor and the precise molecular mechanisms behind its actions remain to be elucidated.
Of the fatty acids of the n-3 family, DHA is special, both as a precursor of other metabolites and in esterified form as a component of membrane lipids. It is not a substrate for the prostaglandin synthase-cyclooxygenase enzymes, and indeed it inhibits them, but via the action of lipoxygenases, it is the precursor of docosanoids such as the resolvins, protectins and maresins (or specialized pro-resolving mediators), which are analogous to the eicosanoids but have potent anti-inflammatory and immuno-regulatory actions. DHA per se may ameliorate autoimmune inflammation directly, for example by inducing the GPR120 signalling pathway in dendritic cells. It has been demonstrated to be beneficial towards inflammatory disorders of the intestine and in reducing the risk of colon cancer, possibly mediated by associations with signalling proteins in membranes, while it affects gene transcription to regulate many proteins for fatty acid synthesis and desaturation.
 The concentration
of DHA in tissues has been correlated with several human disease states, and while it is vital for many functions of the brain,
all organs require it for some purpose.
Particular attention has been given to its role in the retina, where it is a major component of the phospholipids in the
photoreceptor outer segment membranes and is necessary for optimum retinal efficiency.
In this instance, a primary role is to maintain the disc shape in photoreceptor cells, as cellular membranes containing DHA in the
phospholipids are more flexible than those containing arachidonic acid and other fatty acids,
and they may increase the stability and efficiency of rhodopsin.
In some cases, sight defects have been ameliorated with DHA supplementation.
The preferred substrate for uptake by the brain and retina is DHA esterified as
lysophosphatidylcholine, as this can cross the blood-brain barrier more easily than the free acid
with the aid of a receptor/transporter known as the sodium-dependent LPC symporter 1 (MFSD2A); the same is true for EPA.
DHA is intimately involved with phosphatidylserine metabolism in neuronal tissue.
Similarly, N‑docosahexaenoylethanolamine or 'synaptamide' is a significant
metabolite in brain tissue and is a signalling molecule that induces neurogenesis, neuritogenesis and synaptogenesis in developing neurons,
a further mechanism by which DHA promotes brain development and metabolism.
The concentration
of DHA in tissues has been correlated with several human disease states, and while it is vital for many functions of the brain,
all organs require it for some purpose.
Particular attention has been given to its role in the retina, where it is a major component of the phospholipids in the
photoreceptor outer segment membranes and is necessary for optimum retinal efficiency.
In this instance, a primary role is to maintain the disc shape in photoreceptor cells, as cellular membranes containing DHA in the
phospholipids are more flexible than those containing arachidonic acid and other fatty acids,
and they may increase the stability and efficiency of rhodopsin.
In some cases, sight defects have been ameliorated with DHA supplementation.
The preferred substrate for uptake by the brain and retina is DHA esterified as
lysophosphatidylcholine, as this can cross the blood-brain barrier more easily than the free acid
with the aid of a receptor/transporter known as the sodium-dependent LPC symporter 1 (MFSD2A); the same is true for EPA.
DHA is intimately involved with phosphatidylserine metabolism in neuronal tissue.
Similarly, N‑docosahexaenoylethanolamine or 'synaptamide' is a significant
metabolite in brain tissue and is a signalling molecule that induces neurogenesis, neuritogenesis and synaptogenesis in developing neurons,
a further mechanism by which DHA promotes brain development and metabolism.
During phospholipid biosynthesis in brain, retina and spermatids, DHA is first converted to the CoA ester by long chain acyl-CoA synthetase 6 (ACSL6) before utilization by lysophosphatidic acid acyltransferase 3 (LPAAT3) to form a phosphatidic acid precursor for other phospholipid classes (both of these enzymes are specific for DHA). As a phospholipid constituent, it profoundly affects all membranes, modulating their structure and function, because in such an environment, DHA adopts a more compact conformation than more saturated chains with an average length of 8.2Å at 41°C compared to 14.2Å for oleic chains. This is the result of the adoption of a conformation with pronounced twists of the chain, which reduce the distance between the ends; the methyl group with its extra bulk is located in the interior region of a bilayer. In mixed-chain phospholipids, a further consequence is a marked increase in the conformational disorder of the saturated chain.
There is an incompatibility between the rigid structure of cholesterol and the highly flexible chains of DHA, promoting the lateral segregation of membranes into PUFA-rich/cholesterol-poor and PUFA-poor/cholesterol-rich regions, with the latter ultimately becoming the membrane microdomains known as rafts. Although PUFA-rich/cholesterol-poor membrane microdomains are technically less easy to study than rafts, they may contain crucial proteins. It has been proposed that changes in the conformation of signalling proteins when they move between these very different domains may have the potential to modulate cell metabolism in a manner that may explain some of the health benefits of dietary consumption of DHA.
4. The (n-9) and Other Families of Polyunsaturated Fatty Acids
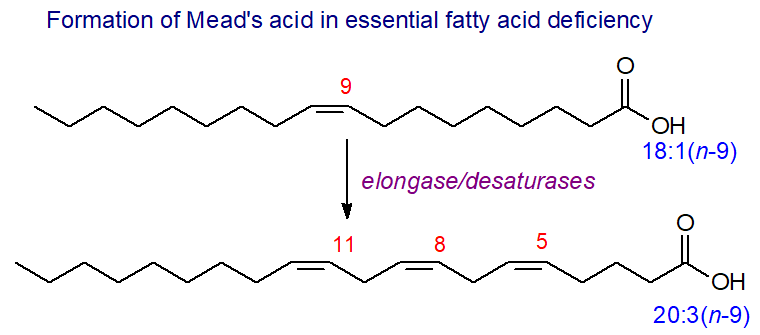
Oleate can be chain elongated and desaturated in animal tissues with 5,8,11-eicosatrienoic acid (20:3(n-9) or 'Mead's acid') as an end product, although this only accumulates in tissues when the animals are suffering from essential fatty acid deficiency (see below). As with all polyunsaturated fatty acids, the new double bonds are introduced between the carboxyl group and an existing double bond, while the terminal part of the molecule is unchanged. In this condition, 18:2(n‑9), 20:2(n‑9) and 22:3(n‑9) are other fatty acids of the n-9 family that may be found at low levels. Mead's acid can be converted to oxylipins in vitro at least, and it has been argued that these might influence some disease states.
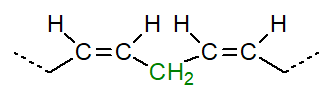 |
| Figure 1. Biosynthesis of Mead's acid in EFA deficiency. |
9,12-Hexadecadienoic acid (16:2(n-4)) is found in marine microorganisms and is presumably the biosynthetic precursor of other fatty acids with an n‑4 terminal structure, i.e., 18:2(n-4), 20:2(n-4), 16:3(n-4) and 18:3(n-4). Fatty acids of an n-1 family present in marine organisms may be derived biosynthetically by further desaturation (Δ15) of 6,9,12-hexadecatrienoic acid (16:3(n-4)). The main naturally occurring fatty acids of this type are 16:4(n-1) and 18:4(n-1), but 18:5(n-1) has been detected. Trace amounts of polyunsaturated fatty acids that may be of an n-7 family are occasionally encountered in marine organisms and they have been detected in RAW macrophages; they are presumably metabolites of 9‑16:1. 5,8-Octadecadienoic acid (‘sebaleic’ acid, n-10), a unique component of human skin wax, is derived by elongation and desaturation of 6-16:1. Odd-chain PUFAs of an n-8 family are produced in rats deficient in essential fatty acids and fed supplements of pentadecanoic acid.
5. Biosynthesis of Linoleic, Linolenic and Higher Polyunsaturated Acids in Plants
Linoleic and α-linolenic acids are synthesised in plant tissues from oleic acid by the introduction of cis-double bonds between the existing double bond and the terminal methyl group by the sequential action of Δ12- (FAD2) and Δ15-desaturases (FAD3, FAD7 and FAD8). FAD7 and 8 are in the plastids, while FAD3 is an extraplastidial enzyme and is phyllogenetically distinct from the other two.
 |
| Figure 2. Biosynthesis of alpha-linolenic acid in plants. |
This depiction of the process is over simplistic as many of the steps occur when the fatty acids are linked to glycerolipids, and they require transfer across membranes between different cellular compartments. As described in our web page dealing with saturated fatty acids, most fatty acid synthesis occurs in the plastids with palmitoyl-ACP and then stearoyl-ACP as the primary products of fatty acid synthases. The latter is desaturated to form oleoyl-ACP, which must be hydrolysed to oleic acid (see our web page on monoenes) and then converted to oleoyl-CoA for transport across the plastid envelope. 1‑Acyl-2-oleoyl-phosphatidylcholine (PC) is formed by an acyltransferase, and this is the main substrate for the membrane-bound Δ12‑desaturase (FAD2) in the endoplasmic reticulum with formation of 1‑acyl-2-linoleoyl-PC. Some 1‑acyl-2-linolenoyl-PC is formed from this by the action of FAD3. How the diacylglycerol moiety of 1‑acyl-2-linoleoyl-PC is transferred back into the plastid in not yet known; it may be that a phospholipid transfer protein is able to transport the intact PC across the membrane, or hydrolysis to form diacylglycerols (DAG) may occur for transport. The next certain product in the plastid is a linoleoyl-monogalactosyldiacylglycerol (MGDG), and this is the substrate for the Δ15-desaturases (FAD7/8) with formation of α‑linolenoyl-MGDG.
 |
| Figure 3. Biosynthesis of linoleate and linolenate in leaves of plants. |
Two distinct desaturases (multiple isoforms) have been characterized that can insert the Δ12-double bond, i.e., a plastidial enzyme (FAD6), which uses the terminal methyl group as a reference point and is an ω6-desaturase as it introduces the double bond six carbons from the terminal carbon. The second is an extra-plastidial oleate Δ12‑desaturase (FAD2), which is selective for C12,13-desaturation independently of chain length and is closely related to an enzyme in the seeds of castor oil (Ricinus communis) that converts oleate to 12R‑hydroxyoleate (ricinoleate). Indeed, whether the product is a hydroxyl group or a double bond may depend on the nature of only four amino acid residues. Although less is known of the desaturases (FAD3/7/8) that convert linoleate to α-linolenate, it is argued that FAD3 at least should be considered as an ω3- rather than as a Δ15‑enzyme; it has much in common with hydroxylase enzymes. In plastids, lipid-bound fatty acids synthesised in this way are hydrolysed to the free fatty acids for transport out of this organelle to the endoplasmic reticulum where they are rapidly esterified for incorporation into other membranes.
As more plants are investigated, further desaturases with variations in their structures, substrates and sub-cellular distributions continue to be discovered. Those plants that produce significant amounts of 16:3(n-3) add further complications, and it is evident that much remains to be learned of the overall process. Analogues of linoleic acid with trans/E-double bonds have been found in a few seed oils, and 9Z,12E-18:2 is reported from Dimorphotheca and Crepis sp. while 9E,12E-18:2 is found in Chilopsis linearis, but nothing appears to be known of their biosynthesis.
Infrequently in plants, a double bond is inserted between an existing double bond and the carboxyl group as in the biosynthesis of γ‑linolenic acid in evening primrose and borage seed oils, in which the double bond in position 6 is inserted after those in positions 9 and 12. Such Δ6‑desaturases, sometimes termed 'front-end' desaturases, contain the required donor of reduced equivalents, cytochrome b5, physically fused to the N‑terminus. Biosynthesis of plant fatty acids with conjugated double bond systems or more than one methylene groups between double bonds are described here..
 |
| Figure 4. Biosynthesis of gamma-linolenic acid in plants. |
Algae and lower organisms: C20 and C22 polyunsaturated fatty acids of both the n-6 and n-3 families are synthesised in algae, which contain a much wider range of desaturases and elongases than are present in higher plants, and it is apparent that these are distributed between plastids and extra-plastid cellular compartments with various lipid substrates. Both eukaryotic-like (C20/C20, C18/C18) and prokaryotic-like (C18/C16, C20/C16) species of complex lipids are formed (see our web page on plant galactolipids). In marine microalgae, such as Emiliania huxleyi, octadecapentaenoic acid (18:5(n-3)) is synthesised in the plastids and is present only in the glycoglycerolipids, i.e., mono- and digalactosyldiacylglycerols, while DHA is synthesised outside the plastids and is found only esterified to phospholipids. Plastids convert stearic acid to 20:5 by an aerobic mechanism through sequential desaturations with a last step of Δ3 desaturation, but oleic acid is not produced outwith the plastids, and linoleic and linolenic acids must be provided for conversion to DHA by an anaerobic pathway catalysed by a PUFA synthase.
Longer-chain polyunsaturated fatty acids can be synthesised by the fungus Mortierella alpina and some mosses. In Cyanobacteria, an acyl-lipid desaturase first inserts a double bond at C-9 of stearic acid linked to position sn-1 of a phospholipid, to form oleate before a series of acyl-lipid desaturases react sequentially to produce 9,12-18:2, 6,9,12-18:3 and 6,9,12,15-18:4. In particular, the Δ15 desaturase 'counts' from the carboxyl end to introduce a double bond between carbons 15 and 16, i.e. it is not an n-3 desaturase. These enzymes are highly specific for the substrate, which is always a fatty acyl chain esterified to the position sn-1, as well as for the position of insertion of the double bond in the acyl chain.
6. Biosynthesis of the n-6 Family of Polyunsaturated Fatty Acids in Animals
In the tissues of higher animals, additional double bonds can only be inserted between an existing double bond and the carboxyl group, as illustrated for Mead's acid above. The linoleic acid, which is the primary precursor molecule for the n-6 family of fatty acids, must come from the diet. Biosynthesis of polyunsaturated fatty acids occurs mainly in the liver with a sequence of desaturation and chain elongation steps, as illustrated below, and the various enzymes require the acyl-CoA esters as substrates not intact lipids (unlike plants).
 |
| Figure 5. Biosynthesis of the n-6 family of polyunsaturated fatty acids. |
The first step is rate limiting with the introduction of a double bond in position 6 by the desaturase FADS2 to form γ‑linolenic acid (6,9,12-18:3 or 18:3(n-6) or GLA), before chain elongation by a two-carbon unit gives 20:3(n‑6), which is converted to arachidonic acid by a Δ5‑desaturase (FADS1). This is the main end-product of the process, but two further chain-elongation steps can sometimes occur and yield first 22:4(n‑6) and then 24:4(n‑6), which can be further desaturated by a Δ6‑desaturase to 24:5(n-6). At least three elongases, designated ELOVL2, 4 and 5, have been characterized of which ELOVL4 is most active in the retina and ELOVL5 in liver. ELOVL4 is responsible for the formation of very-long-chain polyunsaturated fatty acids (up to C38) in brain, retina and testes, and in marine animals (see our web pages on saturated fatty acids for a more detailed discussion of elongases). All the enzymes to this stage are located in the endoplasmic reticulum of the cell, but 22:4(n‑6) must be transferred to the peroxisomes for retro-conversion (β‑oxidation) to 22:5(n-6). While a recent study reports that direct desaturation of 22:4(n-6) to 22:5(n-6) by a desaturase produced by the FADS2 gene can occur in human cells in vitro, the relative contributions of the two pathways are not known.
The marine parasitic protozoon Perkinus marinus and at least three other unrelated unicellular organisms synthesise arachidonic acid by an alternative pathway in which elongation of linoleic to 11,14-eicosadienoic acid is followed by sequential desaturation by Δ8- and Δ5‑desaturases. These enzymes are now known to be present in mammals, although the extent of their participation in synthesis of polyunsaturated fatty acids in vivo is uncertain.
Desaturases: The Δ5- and Δ6-desaturases, FADS1 and FADS2, respectively, are membrane bound enzymes with a cytochrome b5-like domain fused physically to the N‑terminus and with three histidine-enriched boxes, characteristic of membrane desaturases, and two membrane spanning regions. They use molecular oxygen and an electron transport system as described in our web page dealing with the biosynthesis of monoenoic fatty acids. FADS2 consists of 444 amino acids with a molecular weight of 52.2 kDa, and it is expressed in the brain, liver, lungs, heart and other tissues in humans. It is the rate-limiting enzyme in the biosynthesis of polyunsaturated fatty acids, but it is also responsible for the biosynthesis of sapienic acid (6-16:1) in skin. Mice modified genetically to lack this enzyme can produce 5Z,11Z,14Z-eicosatrienoic (sciadonic) acid instead of arachidonic acid.
While FADS2 is vital for healthy human metabolism, it is expressed abnormally in many different malignant cancers, and this expression is significantly correlated with tumour proliferation, cell migration and invasion, together with a poor prognosis for the progression of the disease. Membrane fluidity and the transmission of signals is affected, and the production of proinflammatory mediators such as the eicosanoids is promoted to the detriment of health. In humans, the FADS1 and FADS2 genes are located adjacent to each other on chromosome 11, and reduced expression of these, especially in relation to DHA biosynthesis (next section), has been associated with symptoms of bipolar disorder in an animal model.
7. Biosynthesis of the n-3 family of Polyunsaturated Fatty Acids in Animals
Dietary α-linolenic acid is the primary precursor molecule for the n-3 family of fatty acids in tissues of higher animals, and the main pathway to the formation of docosahexaenoic acid (22:6(n-3)) requires a sequence of chain elongation and desaturation steps (Δ5 and Δ6 desaturases), as illustrated below, with acyl-CoA esters as substrates. Thus, α-linolenic acid is sequentially desaturated and elongated, with double bonds being inserted between existing double bonds and the carboxyl group, as far as 24:6(n-3), starting again with a Δ6‑desaturase as illustrated.
 |
| Figure 6. Biosynthesis of the n-3 family of polyunsaturated fatty acids. |
The final step of what has been termed the ‘Sprecher’ pathway (after Prof. Howard Sprecher of Ohio State University) is retro-conversion of 24:6(n‑3), i.e., removal of the first two carbon atoms by a process of β-oxidation, and takes place in the peroxisomes of the cell to generate 22:6(n-3). As with the n-6 family, a recent study reports direct desaturation of 22:5(n-3) to 22:6(n-3) by a Δ4‑desaturase produced by the FAD2 gene in human cells in vitro, with evidence suggesting that the process may occur in mitochondria; this enzyme can also act as a Δ6- or Δ8‑desaturase depending on the substrate. Once more, the relative contributions of the two pathways for the synthesis of 22:6(n-3) in rodents and humans have still to be determined. The reverse reaction, i.e., synthesis of 24:6(n-3) from 22:6(n-3), occurs in rats, as does retroconversion of 22:6(n-3) to 20:5(n-3).
A single gene in the rabbitfish codes for an enzyme that performs both Δ5 and Δ4 desaturation to synthesize 22:6(n-3), and Δ4-, Δ5- and Δ8‑desaturases have been found in certain micro-algae of marine origin (e.g., Pavlova salina), suggesting that a more direct route to DHA may exist in these organisms that includes desaturation of 22:5(n-3). Thraustochytrium, a unicellular protist, used as a commercial source of DHA, has both a Δ4‑desaturation-dependent pathway (aerobic) and a polyketide synthase-like pathway (anaerobic - see below). On the other hand, marine invertebrates such as molluscs, bivalves and cephalopods lack some of the expected desaturases and elongases, and they produce polyunsaturated fatty acids of both the (n-3) and (n-6) families by unconventional routes that include the use of methyl-end desaturases.
All the various intermediates may be found in tissues, especially those of fish, but eicosapentaenoic (20:5(n-3)), docosapentaenoic (22:5(n-3)) and docosahexaenoic (22:6(n-3)) acids tend to be by far the most abundant. In human tissues, the measured rates of conversion of α-linolenic acid to longer-chain metabolites in some experiments are very low, suggesting that a high proportion of the latter must come from the diet (meat, eggs and fish) in normal circumstances, and it has been argued that dietary supplementation with DHA is crucial to maintain sufficient levels in brain and retina. On the other hand, as there are allegedly inconsistencies in the experimental data and vegans do not suffer any deficiency symptoms, there is a school of thought that DHA is utilized rapidly for various purposes and that the actual rate of synthesis may be higher than has been reported. Much of the α-linolenic acid in the diet is directed to long-term storage in adipose tissue and may be made available only slowly for DHA synthesis, thus leading to misleading experimental data. During the natural processes of turnover and renewal of cell membranes in retinal cells, mechanisms exist to ensure that DHA is conserved.
Efficient use of DHA in brain and reproductive tissues for synthesis of phospholipids to support membrane structures requires a specific acyl-coA synthetase (ACLS6) and 1-acylglycerol-3-phosphate O-acyltransferase 3 (AGPAT3), which catalyses synthesis of DHA-containing phospholipids. Over-accumulation of phospholipids containing DHA is prevented when an excess of unsaturated fatty acids inhibits ubiquitin regulatory X domain-containing protein 8 (UBXD8), which activates AGPAT3. Much of the DHA synthesised in the liver is esterified initially to phosphatidylethanolamine, but then it is exported via the plasma triacylglycerols to the brain. In mice, the fatty acid elongase ELOVL2 takes part in synthesis of endogenous DHA in the liver by elongating EPA to the C22 and C24 metabolites, it is indispensable for photoreceptors in the retina, and it may be a factor in the control of lipogenesis de novo and many other aspects of lipid metabolism. In humans, genome-wide DNA methylation analyses have identified DNA methylation of the ELOVL2 promoter as one of the most robust and reliable molecular biomarkers for chronological age.
As with the n-6 family, the fatty acid elongase designated ELOVL4 is responsible for the biosynthesis of the very-long-chain polyunsaturated fatty acids (up to C38) of the n-3 family found in the retina, brain, testis and skin, and in marine animals. There is a report that an increase in concentration of these fatty acids is a factor in neurodegeneration in frontotemporal dementia.
The whitefly, Bemisia tabaci, an agricultural pest that feeds on phloem sap of many plants, has acquired a plant Δ12 desaturase gene family BtFAD2 by horizontal transfer, which enables it to produce linoleic acid and thence further polyunsaturated fatty acids and prostaglandins required for reproduction de novo. Similarly, in contrast to higher plants and mammals, the nematode C. elegans and its relatives possess all the enzymes required for the synthesis of 20:4(n‑6) and 20:5(n‑3) fatty acids de novo, and this is true for Collembola (springtails, tiny hexapod arthropods), which are believed to have acquired the required enzymes by horizontal transfer from a marine microbe. With nematodes, an unusual bifunctional Δ12/Δ15 desaturase is utilized in the synthesis of the linoleate/linolenate precursors. Many primitive marine invertebrates have an omega-3 desaturase, and so they can synthesise omega‑3 polyunsaturated fatty acids from omega-6 precursors, including arachidonic acid. The marine copepod Tigriopus californicus, possesses all the desaturases (including Δ4) and elongases necessary for the biosynthesis of docosahexaenoic acid de novo, while polychaetes (annelid worms) of both marine and fresh-water origin have the enzymatic machinery to synthesise arachidonic acid and EPA.
8. Biosynthesis of Polyunsaturated Fatty Acids by a Polyketide Route
With acetyl-CoA as the primary precursor, the synthesis of 22:6(n-3) by the route described above uses approximately 30 distinct enzymes and 70 reactions, but a very different and much simpler pathway by polyketide synthases has been found in some marine bacteria such as Shewanella sp. The conventional view of polyketides is of secondary metabolites consisting of multiple building blocks of ketide groups (–CH2–CO–) that form polar cyclic structures, but other endpoints are possible. The polyketide enzymes for the synthesis of polyunsaturated fatty acids have a modular organization like that of fatty acid synthases with which they have some structural homology, and in marine bacteria such as Moritella marina, the polyketide synthase is a mega-enzyme complex containing at least four proteins designated PfaA, PfaB, PfaC and PfaD. Like the fatty acid synthase in bacteria, this enzyme system uses acyl carrier protein as a covalent attachment for chain synthesis and proceeds in iterative cycles adding C2 units and double bonds, but in contrast to the elongation-desaturation pathway, the double bonds are introduced during the process of fatty acid synthesis. Thus, aerobic desaturation is not required for introducing double bonds into the existing acyl chain, and this is sometimes termed an ‘anaerobic’ pathway, although a similar pathway has been found in some aerobic organisms such as the micro alga Schizochytrium sp.
As the chain elongates, the ketones groups formed initially are reduced to hydroxyls, and this is followed by dehydration reactions to introduce the double bonds. For this purpose, six catalytic centres are required: 3-ketoacyl synthase, 3-ketoacyl-ACP-reductase, dehydrase, enoyl reductase, dehydratase/2-trans 3-cis isomerase, dehydratase/2-trans and 2-cis isomerase. These take part in a sequence of reactions that include chain elongation, trans-double bond formation and isomerization to the cis-conformation to produce fatty acids of both the omega-3 and omega-6 families, ultimately of (DHA or 22:6(n-3)) and docosapentaenoic (DPA or 22:5(n-6)) acids as shown.
 |
| Figure 7. Polyketide pathways for biosynthesis of omega-3 and omega-6 fatty acids. |
In marine Gammaproteobacteria, the polyketide/fatty acid synthase mechanism is encoded by a set of five genes, which are regulated by a novel transcriptional regulator. Much remains to be learned of this process in relation to EPA and DHA synthesis, but it is evident that it proceeds via different intermediates from the well-established aerobic pathway, so that analyses of fatty acid compositions can suggest which pathway is involved as illustrated.

Comparable reactions occur is some terrestrial bacteria such as the myxobacterial genus Aetherobacter, but the biosynthetic pathways differ somewhat from those in marine organisms in terms of gene organization and the structures of the enzyme components of the PUFA synthases.
By re-arranging the order and combinations of the various enzymes, such bacteria can utilize polyketide biosynthesis pathways to modify the intermediates in the growing polyketone chain to produce many different final products including antibiotics, toxins and pigments.
9. Catabolism
Polyunsaturated fatty acids of all families are broken down in animal tissues to produce energy by a multi-step process of β-oxidation, as discussed in our web page on carnitines. Some very-long-chain fatty acids are oxidized in peroxisomes or 'microbodies, mainly in the kidney and liver, and the products are medium-chain fatty acids, which are transported to mitochondria for further oxidation. In plants, glyoxysomes in germinating seeds can break down fatty acids rapidly to acetyl-CoA, while β-oxidation occurs in leaves mainly in peroxisomes but also in mitochondria.
All polyunsaturated fatty acids with methylene-interrupted double bonds are susceptible to autoxidation through the action of Reactive Oxygen Species (ROS) in tissues, the higher the degree of unsaturation the greater the reactivity. In this process, hydroperoxides are formed initially, and these can degrade further, often by spontaneous reactions. For reasons of practical convenience, this process is discussed in a number of different contexts in this site, and our web page Introduction to oxylipins has links to the relevant pages. Polyunsaturated fatty acids can be converted enzymatically to various oxygenated derivatives by many different oxidases, and they can be catabolized eventually in this form. While autoxidation is an appreciable factor in non-biological conditions, such as during cooking or storage of foods, these web pages are devoted to metabolism in living tissues.
10. Essential Fatty Acids (EFA)
Most fatty acids have some properties that are not easily replaced and can be vital for some aspect of metabolism. Many different fatty acids in the unesterified (free) state, including the polyunsaturated components, interact with multiple G protein-coupled receptors for free fatty acids (FFAR) on cell surfaces and have roles in the regulation of nutrition (see our web page on free fatty acids), but many other functions of specific fatty acids, both saturated and unsaturated, require an esterified state. As discussed above, linoleic and α‑linolenic acids cannot be synthesised in the tissues of higher animals and must be obtained from the diet, i.e., mainly from plants via the food chain (some invertebrates are an exception as discussed above). There is an absolute requirement for these 'essential fatty acids' for growth, reproduction and good health. On a global scale, the longer-chain polyunsaturated fatty acids are produced primarily from photosynthetic marine microalgae, heterotrophic protists and bacteria, before entering the food chain of fish and thence of other animals.
Linoleic and α‑linolenic acids from plant sources serve as precursors of the longer-chain metabolites in animals, but whether the rate of synthesis of EPA and DHA from α-linolenic acid is sufficient in healthy humans has been debated. As pointed out above, there is no evidence that vegans suffer from any deficiency, although dietary supplements of EPA and DHA may still confer health benefits. Young animals deprived of essential fatty acids in the diet rapidly display adverse symptoms, including diminished growth, liver and kidney damage, and dermatitis, and these can result eventually in death. One key biochemical parameter is the 'triene-tetraene' ratio, i.e., the ratio of 20:3(n‑9) to 20:4(n‑6) fatty acids in plasma; levels greater than 0.4 reflect essential fatty acid deficiency. While it takes longer for the effects to become apparent in older animals, which may have substantial stores of essential fatty acids in their body fats, symptoms do appear eventually, and EFA deficiency has been observed in adults on parenteral nutrition as well as in human infants. It can be seen in certain genetic disorders, including cystic fibrosis, although the mechanism is not understood.
The absolute requirements for essential fatty acids are dependent on several factors, including species and sex (females have a higher requirement for n-3 fatty acids), but they are usually considered to be a minimum of 1 to 2% for linoleate and somewhat less for α‑linolenate. In contrast, the requirement for α‑linolenate in fish is higher than for linoleate. For some years it was believed that cats lacked a Δ6‑desaturase and had an absolute requirement for arachidonic acid in their diet, i.e., they were obligate carnivores, but this now known not to be true although the enzyme activity can be low.
 It has
sometimes been argued that linoleate and α‑linolenate per se may in fact be less important than the longer-chain
polyunsaturated fatty acids in animal biology, but there is an absolute requirement for linoleate as a component of
ceramides in skin, and octadecanoid oxylipins
derived from linoleate are critical in skin, adipose tissue and other organs, while γ‑linolenic acid in maternal milk
is reported to drive cardiac metabolic maturation.
On the other hand, it is possible that the essentiality of α‑linolenic acid may reside only in the long-chain
polyunsaturated fatty acids formed from it, as octadecanoid oxylipins derived from this fatty acid are rarely identified in vivo,
and there appears to be little research on the topic.
However, studies are underway with transgenic cattle with either a Fat-1 gene from C. elegans or the Fad3 gene from flax,
which can insert double bonds in the n‑3 position, in the hope of producing beef with greater nutritional value
(and some interesting biochemistry).
It has
sometimes been argued that linoleate and α‑linolenate per se may in fact be less important than the longer-chain
polyunsaturated fatty acids in animal biology, but there is an absolute requirement for linoleate as a component of
ceramides in skin, and octadecanoid oxylipins
derived from linoleate are critical in skin, adipose tissue and other organs, while γ‑linolenic acid in maternal milk
is reported to drive cardiac metabolic maturation.
On the other hand, it is possible that the essentiality of α‑linolenic acid may reside only in the long-chain
polyunsaturated fatty acids formed from it, as octadecanoid oxylipins derived from this fatty acid are rarely identified in vivo,
and there appears to be little research on the topic.
However, studies are underway with transgenic cattle with either a Fat-1 gene from C. elegans or the Fad3 gene from flax,
which can insert double bonds in the n‑3 position, in the hope of producing beef with greater nutritional value
(and some interesting biochemistry).
It is evident that arachidonic, eicosapentaenoic, docosahexaenoic acids and other polyunsaturated fatty acids of the n-6 and n-3 families each have distinct functions, some of which are discussed briefly above, that are vital for healthy animal metabolism. Simplistically, fatty acids of the n-3 family are considered protective by reducing inflammation, improving metabolic function, and lowering disease risk, while the n-6 family can exacerbate inflammation when consumed in excess. They are precursors of eicosanoids and other oxylipins, including prostaglandins (PG1, PG2 and PG3 series), thromboxanes and leukotrienes, and docosanoids, including resolvins, protectins and maresins (specialized pro-resolving mediators), and of the endocannabinoids, all of which have vital properties. n-3 Polyunsaturated fatty acids per se may be beneficial by regulatory actions in signalling processes, for example in T-cells, by modulating membrane receptors or by influencing gene transcription. As DHA is the most unsaturated of these acids, it is highly susceptible to autoxidation, and there is an intriguing suggestion that it may be a master regulator of the antioxidant defences that preserve cellular redox status by promoting the transcriptional regulation of cellular antioxidants through stimulation of nuclear factor erythroid 2-related factor 2 (Nrf2).
In the skin disease psoriasis, the expression of fatty acid desaturase 2 (FADS2), the rate-limiting enzyme in the biosynthesis of polyunsaturated fatty acids, is consistently reduced in keratinocytes from patients with the condition and in mouse models with a resulting impairment of DHA biosynthesis. Peroxisome proliferator-activated receptor alpha (PPARα) is an upstream transcriptional activator of FADS2, and pharmacological activation of this has been shown to alleviates psoriatic inflammation in a FADS2-dependent manner, suggesting a potential therapeutic strategy.
Polyunsaturated fatty acids confer distinctive attributes on the complex lipids that may be required in membranes, where their highly flexible nature affects membrane biophysics, including their fluidity, flexibility and thickness, and this may in turn influence innumerable metabolic events. On the other hand, there are suggestions that excessive amounts of polyunsaturated fatty acids in membranes and tissues, including those of the n-3 family, have the potential to cause harm, because the propensity of all such fatty acids for oxidation can lead to potentially toxic levels of hydroperoxides in tissues, as is discussed in relation to lipid classes in other web pages on this website, such as that on oxidized phospholipids.
Although the actual requirement for polyunsaturated fatty acids is relatively low, general nutritional advice for the human diet until recently was that they should comprise a substantial part of the daily intake. Over the last 30 years, there has been a large increase in the consumption of linoleic acid because of an increased use of vegetable oils rich in this fatty acid in the diet, while by comparison, the intake of n-3 polyunsaturated fatty acids has been reduced because of a relative decrease in the consumption of fish and vegetables. The consequence is that the ratio of n‑6 to n‑3 fatty acids in the diet can be of the order of 20:1, whereas it was probably closer to 2:1 in historical times in developed countries. High linoleate diets have been correlated with an increased risk of cancer of the colon, and this may be a consequence of the production of excessive amounts of epoxy octadecenoic acids by the cytochrome P450 monooxygenase pathway. On the other hand, 10-hydroxy-12Z-octadecenoic acid produced from linoleate by intestinal microorganisms is anti-inflammatory and is beneficial towards obesity induced by high fat diets in mice.
It is now generally considered that the relative proportion of n‑3 to n‑6 polyunsaturated fatty acids in the diet should be increased, if not the total amounts. There is evidence that dietary supplements of fish oils are beneficial to brain development in young children but less so in the management of neurodegenerative diseases and the onset of dementia in the elderly (unless supplement are started early). However, there is enhanced bioavailability when EPA or DHA is supplemented in the form of lysophosphatidylcholine, and there is hope that this may lead to a beneficial treatment towards cognitive decline.
An expert nutritional panel has recommended that infant formulae should contain both arachidonic and docosahexaenoic acids at levels of at least 0.5 to 0.64% of the total calories each, although the optimum concentrations are not known. Indeed, it is not sufficient to consider simply the minimum levels required, as these fatty acids are necessary for innumerable aspects of the healthy development of the neonate and infant; suboptimal levels can impact the development of the immune system and increase the risk of allergic diseases and respiratory illness. Surprisingly, the European Food Standard Agency (2014) rejected recommendations that arachidonic acid should be included in infant formulae, although there is ample evidence that it is required for development. The Mediterranean diet, which is now often recommended by nutritionists, contains high proportions of oleic acid (18:1(n-9)) to balance out the polyunsaturates. Detailed discussion of such contentious topics is best left to nutritional experts of whom I am not one.
11. Analysis
Provided that adequate precautions are taken to prevent autoxidation during extraction, derivatization and storage, gas chromatography (GC) of the methyl esters is the most convenient method for the routine analysis of polyunsaturated fatty acids. Other derivatives can be better for definitive identifications, as discussed in our mass spectrometry pages.
Recommended Reading
- Burdge, G.C. α-linolenic acid interconversion is sufficient as a source of longer chain ω-3 polyunsaturated fatty acids in humans: An opinion. Lipids, 57, 267-287 (2022); DOI.
- Cerone, M. and Smith, T.K. Desaturases: Structural and mechanistic insights into the biosynthesis of unsaturated fatty acids. IUBMB Life, 74, 1036-1051 (2022); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Crawford, M.A. and others. The imperative of arachidonic acid in early human development.. Prog. Lipid Res., 91, 101222 (2023); DOI.
- Djuricic, I. and Calder, P.C. Pros and cons of long-chain omega-3 polyunsaturated fatty acids in cardiovascular health. Annu. Rev. Pharm. Toxic., 63, 383-406 (2023); DOI.
- Drouin, G., Rioux, V. and Legrand, P. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie, 159, 36-48 (2018); DOI.
- Kawashima, H. and Yoshizawa, K. The physiological and pathological properties of Mead acid, an endogenous multifunctional n-9 polyunsaturated fatty acid. Lipids Health Dis., 22, 172 (2023); DOI.
- Koletzko, B. and 26 others. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr., 111, 10-16 (2020); DOI.
- Kyselová, L., Vítova, M. and Řezanka, T. Very long chain fatty acids. Prog. Lipid Res., 87, 101180 (2022); DOI.
- Lee-Okada, H.C., Xue, C.X. and Yokomizo, T. Recent advances on the physiological and pathophysiological roles of polyunsaturated fatty acids and their biosynthetic pathway. Biochim. Biophys. Acta, Lipids, 1870, 159564 (2025); DOI.
- Li-Beisson, Y., Neunzig, J., Lee, Y. and Philippar, K. Plant membrane-protein mediated intracellular traffic of fatty acids and acyl lipids. Curr. Opinion Plant. Biol., 40, 138-146 (2017); DOI.
- Monroig, Ó., Shu-Chien, A.C., Kabey, N., Tocher, D.R. and Castro, L.F.C. Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: From genes to functions. Prog. Lipid Res., 86, 101157 (2022); DOI.
- Mora, I., Arola, L., Caimari, A., Escoté, X. and Puiggròs, F. Brain Structured long-chain omega-3 fatty acids for improvement of cognitive function during aging. Int. J. Mol. Sci., 23, 3472 (2022); DOI.
- Petermann, A.B., Reyna-Jeldes, M., Ortega, L., Coddou, C. and Yevenes, G.E. Roles of the unsaturated fatty acid docosahexaenoic acid in the central nervous system: molecular and cellular insights. Int. J. Mol. Sci., 23, 5390 (2022); DOI.
- Qiu, X., Xie, X. and Meesapyodsukac, D. Molecular mechanisms for biosynthesis and assembly of nutritionally important very long chain polyunsaturated fatty acids in microorganisms. Prog. Lipid Res., 79, 101047 (2020); DOI.
- Wassall, S.R. and Stillwell, W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem. Phys. Lipids, 153, 57-63 (2008); DOI.
- Xu, R.C., Molenaar, A.J., Chen, Z. and Yuan, Y. Mode and mechanism of action of omega-3 and omega-6 unsaturated fatty acids in chronic diseases. Nutrients, 17, 1540 (2025); DOI.
- Yeboah, G.K., Lobanova, E.S., Brush, R.S. and Agbaga, M.-P. Very long chain fatty acid-containing lipids: a decade of novel insights from the study of ELOVL4. J. Lipid Res., 62, 100030 (2021); DOI.
I recommend the Chapter on fatty acid biosynthesis in the book - Gurr, M.I., Harwood, J.L., Frayn, K.N., Murphy, D.J. and Michell, R.H. Lipids: Biochemistry, Biotechnology and Health (6th Edition). (Wiley-Blackwell) (2016).
- and of interest from a historical standpoint-
- Holman, R.T. George O. Burr and the discovery of essential fatty acids. J. Nutr., 118, 535-540 (1988); DOI.
- Martin, S.A., Brash, A.R. and Murphy, R.C. The discovery and early structural studies of arachidonic acid. J. Lipid Res., 57, 1126-1132 (2016); DOI.
- Spector, A.A. and Kim, H.Y. Discovery of essential fatty acids. J. Lipid Res., 56, 11-21 (2015); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: October 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
