Leukotrienes, Lipoxins and Related Eicosanoids
The oxygenated metabolites or oxylipins derived from arachidonic and related fatty acids are produced through a series of complex interrelated biosynthetic pathways often termed the 'eicosanoid cascade'. Here, the di-substituted eicosanoids derived from arachidonic acid with 5-lipoxygenase of primary importance are described, including the leukotrienes, lipoxins, eoxins and hepoxilins, together with their manifold biological activities. Of these, the most studied are the leukotrienes, a group of inflammatory mediators that are synthesised primarily by leukocytes in response to a variety of stimuli, including antigens, immune complexes, complement, cytokines, osmotic challenges and pollutants. The lipoxins may differ from the others in that they support the resolution of inflammation, although this is currently the subject of debate.
The prostanoids (prostaglandins, thromboxanes and prostacyclins) have distinctive ring structures in the centre of the molecule and are discussed on their own web page, as are the linear mono-hydroxyeicosatetraenes (HETE) and related lipids. Any discussion of these oxylipins must take into account the other specialized pro-resolving mediators, i.e., the resolvins, protectins and maresins, derived from the (n-3) family of polyunsaturated fatty acids, but for practical reasons these have their own web page. The balance between these various oxylipins can be critical for health.
1. Leukotrienes
The term ‘leukotriene’ was coined because these eicosanoids, first discovered by Samuelsson and colleagues in the white blood cells derived from bone marrow, i.e., the leukocytes, have three double bonds in conjugation (though they have four in total), resulting in characteristic absorbance peaks in their UV spectra (at 270, 280 and 290 nm). They are produced from arachidonic acid mainly by immune cells and are known to produce a wide range of effects in tissues, most of which involve some form of signalling like that of short-lived paracrine reagents. Leukotrienes can be classified according to whether or not they are linked covalently to cysteine, and the structures and basic mechanism for biosynthesis of these oxylipins are described below. The 3- and 5‑series leukotrienes are less studied and have 5,8,11-eicosatrienoic and 5,8,11,14,17-eicosapentaenoic acids, respectively, as the precursors.
Certain types of reaction are common to the synthesis from essential fatty acids (EFA) of both the pro-inflammatory lipid mediators, such as the leukotrienes and those with pro-resolution properties, such as the specialized pro-resolving mediators. First, there is an antarafacial hydrogen abstraction at the C3 position in cis,cis-1,4-diene moieties. This is followed by a stereo-selective insertion of molecular oxygen to form a carbon-oxygen bond in a hydroperoxide intermediate, and then there is a second hydrogen abstraction with an intramolecular nucleophilic attack by the oxygen atom in this intermediate to produce an epoxide. The final step is hydrolase-assisted nucleophilic addition of water to a cis-double bond in the epoxide intermediate (not illustrated).
 |
| Figure 1. Common reactions for production of lipid mediators. |
5-Lipoxygenase - a key enzyme: The biosynthetic precursor of the leukotrienes is arachidonic acid released mainly from phosphatidylinositol and its phosphorylated forms by the action of phospholipase A2 (cPLA2α), and this is acted upon by enzymes located at the endoplasmic reticulum (ER) or nuclear membrane, each of which has a high stereospecificity, starting with 5-lipoxygenase (5‑LOX, ALOX5) (see our web page on mono-hydroxyeicosatetraenes for a discussion of lipoxygenases in general).
In humans, 5-LOX is expressed mainly in cells of myeloid origin (neutrophils, eosinophils, monocytes-macrophages, mast cells and dendritic cells) and in foam cells of atherosclerotic tissue (in other cells, synthesis is blocked by DNA methylation). In resting cells, 5‑LOX occurs either in the cytosol or in the nucleus as a soluble enzyme, depending on the cell type, but in response to cell activation, it co-migrates with phospholipase A2 to the endoplasmic reticulum and perinuclear membranes where the lipase liberates arachidonic acid from phospholipids for metabolism. 5-LOX is a dioxygenase similar in structure to other lipoxygenases and contains an α‑helix catalytic domain that contains non-haeme iron and an N-terminal domain, which bind to calcium and zwitterionic phosphatidylcholine in membranes (but not cationic phospholipids) and are indispensable for its activity. In contrast to the prostaglandins, an increase in free arachidonic acid alone is not sufficient to induce leukotriene synthesis. Little leukotriene synthesis occurs in resting cells, but this is stimulated by cellular events that raise the level of calcium ions, while the enzyme is regulated by phosphorylation at three serine residues by kinases. The enzyme is regenerated after each catalytic cycle by either a mono-oxygenase or the LTA4 synthase mechanism.
Leukotriene biosynthesis: In the first step of a two-stage concerted reaction in leukotriene biosynthesis, 5‑LOX embedded in the membrane generates 5S‑hydroperoxy-6t,8c,11c,14c-eicosatetraenoic acid (5‑HPETE) from arachidonic acid by a dioxygenation reaction, i.e., the incorporation of one molecule of oxygen at the C-5 position.
To work properly in the second step, 5-LOX requires the presence of two accessory proteins embedded in the membrane - five‑lipoxygenase activating protein (FLAP) and coactosin-like protein (CLP). FLAP forms a trimer with each monomer consisting of four transmembrane α-helices connected by two loops on each of the cytosolic and luminal sides together with a binding pocket for arachidonic acid, from which the latter can interact with the 5-LOX catalytic domain and enable transfer to this site. It may promote coupling of phospholipase A2 (cPLA2) to 5-LOX at the membrane (both cPLA2 and 5-LOX are Ca2+-dependent). 5‑HPETE can be released as such and reduced to 5S-hydroxy-eicosatetraenoic acid (5‑HETE), but with the aid of FLAP and CLP, 5-LOX is able to catalyse the transformation of 5‑HPETE into 5,6‑epoxy-7E,9E,11Z,14Z-eicosatetraenoic acid or leukotriene A4 (LTA4), which is the first of the leukotrienes. Although LTA4 is highly unstable with a half-life of only a few seconds at pH 7.4 in vitro, it is stabilized to some extent in cells by binding to albumin or other proteins that remove water from the immediate environment of the epoxide structure. While it appears to have no biological functions of its own, it is a pivotal intermediate in the synthesis of other leukotrienes and of lipoxins (see below).
 |
| Figure 2. Biosynthesis of leukotrienes. |
In humans, the enzymic reactions leading to the dihydroxy acid LTB4 or to the cysteinyl-leukotrienes, e.g., LTC4, are more important from a system standpoint, and their synthesis is controlled by the location of the enzymes for each product in certain types of cell. Hydrolysis of LTA4 is catalysed by LTA4 hydrolase (LTA4H), a zinc-dependent metallo-protein, which has a dual functionality as an aminopeptidase and is widely expressed in almost all mammalian cells. Unlike most other enzymes of in the 'leukotriene cascade', it is present in the cytosol of the cell so there must be some mechanism to ensure that it is close to the nuclear membrane where the other steps in the process occur. The product is LTB4 or 5S,12R‑dihydroxy-6,8,10,14-(Z,E,E,Z)-eicosatetraenoic acid. LTA4H has a high specificity for its substrate LTA4, and it undergoes suicide inactivation during catalysis. It has yet to be determined how such a labile molecule as LTA4 is transferred from 5-LOX to LTA4H and how the product LTB4 is transported to the plasma membrane for export, but it is presumed to be protected in a hydrophobic pocket in a protein such as albumin. LTB4 and its synthesising enzymes (5-LOX, FLAP and LTA4H) are located in intracellular multivesicular bodies, which release their content as exosomes upon stimulation.
 The alternative pathway
for LTA4 metabolism is prominent in cells expressing the enzyme LTC4 synthase (LTC4S or glutathione-S-transferase),
which is found on the nuclear envelope of cells and adds the tripeptide glutathione (γ‑glutamyl-cysteinyl glycine) to carbon-6 to yield
peptido-leukotriene C4 (LTC4, 5S,6R-S-glutathionyl-7,9,11,14-(E,E,Z,Z)-eicosatetraenoic acid),
the first of the 'cysteinyl leukotrienes'; FLAP is again essential to the reaction.
Although this synthase is found in mainly in immune cells, such as mast cells, eosinophils and monocytes,
it is also present in platelets and epithelial cells but is suppressed by stimulation of PKC-dependent phosphorylation.
LTC4 can be transported out of cells by the multidrug resistance protein 1 (MRP1), which is an ATP-binding cassette (ABC) transporter.
A second glutathione-S-transferase (MGST2) is a versatile enzyme that is a LTC4 synthase, a general glutathione transferase
and a peroxidase.
The alternative pathway
for LTA4 metabolism is prominent in cells expressing the enzyme LTC4 synthase (LTC4S or glutathione-S-transferase),
which is found on the nuclear envelope of cells and adds the tripeptide glutathione (γ‑glutamyl-cysteinyl glycine) to carbon-6 to yield
peptido-leukotriene C4 (LTC4, 5S,6R-S-glutathionyl-7,9,11,14-(E,E,Z,Z)-eicosatetraenoic acid),
the first of the 'cysteinyl leukotrienes'; FLAP is again essential to the reaction.
Although this synthase is found in mainly in immune cells, such as mast cells, eosinophils and monocytes,
it is also present in platelets and epithelial cells but is suppressed by stimulation of PKC-dependent phosphorylation.
LTC4 can be transported out of cells by the multidrug resistance protein 1 (MRP1), which is an ATP-binding cassette (ABC) transporter.
A second glutathione-S-transferase (MGST2) is a versatile enzyme that is a LTC4 synthase, a general glutathione transferase
and a peroxidase.
A subsequent reaction of LTC4 with γ-glutamyl-transpeptidase (GGT1), attached to the plasma membrane, removes the glutamic acid residue to yield LTD4, and this is acted upon by the ubiquitous dipeptidase (DPEP), which is bound to the plasma membrane and anchored through glycosyl-phosphatidylinositol, to produce LTE4.
A natural isomer of arachidonic acid, 8,11,14,17-eicosatetraenoic acid (20:4(n-3)), undergoes a comparable series of reactions to produce LTB4 analogues, i.e., 8‑hydroxy-9,11,14,17-eicosatetraenoic and 8,15-dihydroxy-9,11,13,17-eicosatetraenoic acids, and peptido-conjugates that are related structurally to the cysteinyl-leukotrienes are produced from protectins, resolvins and maresins.
Lysophosphatidylinositol formed when arachidonic acid is released for leukotriene biosynthesis can be reacylated at the endoplasmic reticulum by the lysophospholipid acyltransferase LPLAT11 (MBOAT7) to enter the phosphatidylinositol cycle, whereby the lipid components are replenished and phosphatidylinositol 4,5‑bisphosphate is synthesised at the plasma membrane. Ultimately, the arachidonic acid content of the phosphatidylinositol in the perinuclear membranes is renewed by this means.
Surprisingly, the red alga Gracilaria vermiculophylla produces a novel oxylipin, 5R,8S-diHETE, which rearranges via a 1,8-diol-forming mechanism to produce an enantiomeric mixture of LTB4 isomers. The same organism synthesises prostaglandins.
Trans-cellular biosynthesis. It has become apparent that some of these reactions can occur in one cell type (donor cell) before the intermediate is passed to a second cell type (acceptor cell) to complete the conversion into the lipid mediator, so mechanisms must exist to transport the eicosanoid intermediate between cells and across phospholipid membrane barriers. As some cell types do not have all the required enzyme systems for production of the full range of LTA4 metabolites, they can synthesise them by such trans-cellular mechanisms. LTA4 synthesised in neutrophils is released to neighbouring acceptor cells such as erythrocytes or platelets that lack 5-LOX but possess the enzyme LTA4 hydrolase and are then able to produce leukotriene LTB4, while LTA4 generated and released from neutrophils is acted upon in acceptor cells such those in the vascular wall by a second LTC4 synthase (microsomal glutathione S‑transferase 2 or MGST2) to produce LTC4. Despite the chemical instability of LTA4, these processes can be highly efficient and have the potential to generate elevated concentrations of cysteinyl-leukotrienes at the local level to affect organ function. Trans-cellular mechanisms are likewise critical for the synthesis of lipoxins (see below).
Catabolism. If LTA4 is not metabolized quickly, it can be transformed by non-enzymic hydrolysis of the epoxide ring into relatively inert dihydroxy acids (all four stereoisomers of LTB4). LTB4 is catabolized in the liver first by ω-oxidation by a cytochrome P450 enzyme, followed by β-oxidation from the ω‑carboxyl position to produce 18‑carboxy-dinor-LTB4. At this point, the pathway established for prostanoids and lipoxins (below) operates. An alternative pathway in macrophages involves oxidation of the 12‑hydroxyl group to produce inert 12‑oxo-LTB4 and eventually 10,11-dihydro-LTB4. Catabolism of LTC4 occurs after conversion to LTE4, which can then be subjected to ω-oxidation.
 |
| Figure 3. Leukotriene catabolism. |
Functions: As pro-inflammatory mediators at concentrations in the low nanomolar range, leukotrienes stimulate cellular responses that are quick in onset but do not last long, such as smooth muscle contraction, phagocyte chemotaxis and increased vascular permeability, all of which are mediated via G‑protein coupled receptors. They are strongly implicated in immuno-metabolic disorders (allergies).
 Leukotriene B4 is one of the most potent chemotactic agents known and is important
in the inflammatory process as one of the first signals that attract innate immune cells such as leukocytes to a site of insult.
Its action is mediated via two cell surface G protein-coupled receptors, BLT1 and BLT2 (chemoattractant-like).
Of these, BLT1 is the high-affinity receptor for LTB4 and is expressed primarily in leukocytes.
The second receptor BLT2 has a lower affinity for LTB4 and is expressed relatively ubiquitously in human tissues
but mainly in mast cells and in epithelial cells of intestine and skin.
Both receptors are seen as potential drug targets against inflammatory diseases.
Leukotriene B4 is one of the most potent chemotactic agents known and is important
in the inflammatory process as one of the first signals that attract innate immune cells such as leukocytes to a site of insult.
Its action is mediated via two cell surface G protein-coupled receptors, BLT1 and BLT2 (chemoattractant-like).
Of these, BLT1 is the high-affinity receptor for LTB4 and is expressed primarily in leukocytes.
The second receptor BLT2 has a lower affinity for LTB4 and is expressed relatively ubiquitously in human tissues
but mainly in mast cells and in epithelial cells of intestine and skin.
Both receptors are seen as potential drug targets against inflammatory diseases.
LTB4 causes neutrophils to adhere to vascular endothelial cells and enhances the rate of migration of neutrophils into extra-vascular tissues, while triggering several responses for host defence, including the secretion of lysosomal enzymes, the activation of NADPH oxidase, nitric oxide formation and phagocytosis. It induces such intracellular signalling events as the mobilization of calcium, activation of phospholipases, the production of diacylglycerols and phosphoinositides, and the release of either anti- or pro-inflammatory agents, depending on circumstances. Beneficial properties are evident in that it is involved in the elimination of pathogenic viruses (including influenza and COVID‑19), bacteria, fungi, protozoan parasites and helminths, while during fungal infections, transcellular biosynthesis of LTB4 orchestrates neutrophil swarming, a defence mechanism triggered by targets larger than the capacity of a single neutrophil to phagocytose.
However, excessive LTB4 can induce acute and chronic inflammation that results in various inflammatory diseases. For example, 5‑lipoxygenase and LTB4 have been implicated in the chronic inflammation that is part of the pathophysiology of asthma, rheumatoid arthritis, inflammatory bowel disease, metabolic disease, ophthalmic diseases, atherosclerosis and certain cancers. The enzymes and receptors of the 5-LOX pathway can be upregulated in adipose tissue, and LTB4 has a key role in adipose tissue inflammation while promoting liver steatosis and insulin resistance in muscle and adipose tissue, factors that are relevant to obesity. Elevated levels of LTB4 and of the enzyme LTA4 hydrolase have been detected in several cancers, suggesting that the latter might be a therapeutic target, but further complications arise from reports that the BLT1 receptor is an anti-tumour agent while BLT2 accelerates chemotherapy resistance and tumour promotion. Treatment with blockers of the BLT1 receptor reduced rheumatoid arthritis and bronchial asthma in mice, but BLT2 deficiency facilitated several diseases in the small intestine and skin.
In contrast, leukotriene B5 or 5S,12S-dihydroxy-6Z,8E,14Z,17Z-eicosapentaenoic acid, derived from eicosapentaenoic acid (20:5(n‑3) or EPA) strongly inhibits the pro-inflammatory effects of LTB4, while 8,15-dihydroxy-9,11,13,17-eicosatetraenoic acid derived from 20:4(n‑3) is similarly anti-inflammatory. In relation to atherosclerotic plaques, it has been reported that the deleterious effects of leukotriene LTB4 resulting from an excessive inflammatory response are countered by the presence of specialized proresolving mediators derived from (n‑3) polyunsaturated fatty acids, especially resolvin D1 (RvD1), suggesting a new therapeutic approach to promote plaque stability and alleviate many other inflammatory conditions resulting from leukotriene exposure.
Perhaps surprisingly, 12S‑hydroxyheptadeca-5Z,8E,10E–trienoic acid (12-HHT), a C17 metabolite of prostaglandin H (PGH2), is a high affinity ligand for BLT2. Relatively large amounts of this oxylipin are produced by activated platelets during skin injury, and interaction with this receptor on epidermal keratinocytes has several benefits that include accelerating skin wound healing by enhancing cell migration. Via the receptor BLT1, LTB4 stimulates the migration of dendritic cells into the skin to bring the immune system into play. It has been suggested that some side effects of non-steroidal anti-inflammatory drugs, such as delayed wound healing, may be caused by reduced 12-HHT production rather than diminished production of prostaglandins. On the other hand, 12-HHT and its oxo-metabolite may induce resistance to a broad spectrum of anti-cancer chemotherapeutic agents.
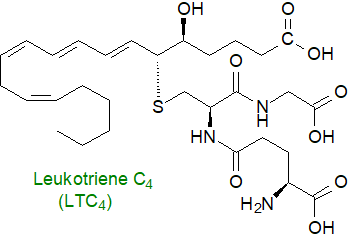 Leukotriene C4, together with LTD4 and
LTE4 (the cysteinyl-leukotrienes, which jointly comprise the 'slow-acting substance of anaphylaxis',
recognized but not identified in the 1930s), are known to be pro-inflammatory, including by constriction of the airways and
vascular smooth muscle, increasing plasma exudation and oedema, and enhanced mucus secretion.
They are harmful in asthma, a chronic allergy disease, and in other inflammatory conditions,
including atherosclerosis and myocardial infarction, cancer, and gastrointestinal, skin (atopic dermatitis) and immune disorders.
The levels of LTB4 and LTC4 in the circulation are elevated in patients with cerebral ischemia, while patients with
peripheral artery disease and some respiratory diseases have elevated levels of LTE4 in urine.
Leukotriene C4, together with LTD4 and
LTE4 (the cysteinyl-leukotrienes, which jointly comprise the 'slow-acting substance of anaphylaxis',
recognized but not identified in the 1930s), are known to be pro-inflammatory, including by constriction of the airways and
vascular smooth muscle, increasing plasma exudation and oedema, and enhanced mucus secretion.
They are harmful in asthma, a chronic allergy disease, and in other inflammatory conditions,
including atherosclerosis and myocardial infarction, cancer, and gastrointestinal, skin (atopic dermatitis) and immune disorders.
The levels of LTB4 and LTC4 in the circulation are elevated in patients with cerebral ischemia, while patients with
peripheral artery disease and some respiratory diseases have elevated levels of LTE4 in urine.
They act through receptors, mainly cysteinyl-leukotrienes type 1 to 3 (CysLT1R, CysLT2R and CysLT3R) at the plasma membrane and nuclear membrane, while GPR99 is a high-affinity receptor for LTE4, and a purinergic receptor, P2Y12, mediates LTE4‑dependent pulmonary inflammation. These are quite distinct from the BLT receptors, and 5‑lipoxygenase inhibitors and antagonists to cysteinyl-leukotrienes receptors are both proving useful in treatment of asthma and rhinitis. LTD4 acting through CysLT1R is a highly potent bronchoconstrictor so is of special importance to asthma, and antagonists to the receptor are reported to be highly effective in treating allergic rhinitis and for long-term control of asthma. Now, more selective drugs are being sought, and recent clinical evidence suggests such inhibitors/antagonists may be of value for the treatment of cardiovascular diseases and possibly even for obesity, as the CysLT1 receptor is present in adipose tissue. In particular, 'montelukast' is reported to be an effective and orally active leukotriene receptor antagonist, especially as an adjuvant to low-dose inhaled corticosteroids for treatment of asthma, and is being trialled for many other conditions, although there can be serious side effects in some patients. Inflammation caused by cysteinyl leukotrienes in the lung are countered by cysteinyl maresins. In atopic and allergic-contact dermatitis, cysteinyl leukotrienes are potent itch inducers as a result of coupling of LTC4 with its receptor CysLT2R in peripheral sensory neurons.
Food allergy is an incurable chronic condition that arises when the immune system mounts an adaptive response to food antigens to release factors that trigger immunoglobulin E and its receptor in intestinal mast cells to cause allergic symptoms ranging from mild local reactions to life-threatening anaphylaxis. It has been determined that cysteinyl leukotrienes are important drivers of oral anaphylaxis, and they stimulate gut absorption of food allergens to promote anaphylaxis in mice. Blocking their synthesis may be a new therapeutic approach for treatment of this illness.
LTD4 and LTE4 are overexpressed in several types of cancer, including cancer of the pancreas, colon, stomach, prostate, ovaries and lung, and they are considered to be tumorigenic. They may modulate the initiation, progression and metastasis of tumours through regulating the proliferation, apoptosis, migration and invasion of cancer cells, while promoting resistance to immunotherapy. As up‑regulation of the expression of their receptors has been observed in several human cancers, there is great interest in drugs that might inhibit these lipids by acting as agonists to their receptors. Apart from leukotriene biosynthesis, 5-LOX can influence gene expression by modulating miRNA processing and transcription factor shuttling in cancers. Cysteinyl-leukotrienes have been implicated in disorders of the central nervous system that include cerebral ischemia, multiple sclerosis, Parkinson's disease and Alzheimer's disease. They play a role in the pathophysiology of the last of these by binding to their receptors in the brain to stimulate the release of cytokines, which lead directly to the formation of insoluble amyloid senile plaques and neuronal damage. LTE4 has been reported to upregulate the prostaglandin synthase COX-2 through the PPARγ receptor in mast cells.
While the general view is that leukotrienes are harmful in relation to the immune system and allergic diseases, such as asthma, there are suggestions that they may be beneficial in that they stimulate the body’s innate immunity against pathogenic bacterial, fungal and viral infections, by promoting the expression of mediators and receptors for immune defence. For example, LTB4 can trigger the release of antimicrobial agents.
2. Lipoxins and Related Compounds
Lipoxins are trihydroxy-eicosatetraenoic acids derived from arachidonic acid by dioxygenation mechanisms, with the four double bonds in conjugation, that have been identified in studies with cell preparations in vitro. They were initially claimed to be the first lipid mediators to be discovered to take part in the resolution phase of inflammation and so were specialized pro-resolving mediators (SPMs) together with the resolvins, protectins and maresins, which are derived from the omega-3 family of polyunsaturated fatty acids (not the omega-6 family). As few cell types have both the required lipoxygenases, they must be formed mainly by trans-cellular pathways. It should be noted that many of the claims regarding these oxylipins can no longer be fully substantiated, as forensic analyses have been unable to detect their presence in tissues in vivo. However, there seems little doubt that synthetic lipoxins have potential as therapeutic agents.
There are at least three routes to the biosynthesis of lipoxins identified by studies in vitro that differ among cell types, but a common feature is the insertion of molecular oxygen at two sites in arachidonic acid by lipoxygenases. As illustrated for the biosynthesis of those designated lipoxins A4 (LXA4) and B4 (LXB4) in human mucosal cells (airway epithelial cells, gastrointestinal tract and monocytes), the first step is the formation of 15S‑hydroperoxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (15S‑HPETE) by a 15‑lipoxygenase (ALOX12, ALOX15, ALOX15B).
 |
| Figure 4. Biosynthesis of lipoxins. |
In the second step, 15S-HPETE or the reduced form 15S-HETE is acted upon by a 5‑lipoxygenase in polymorphonuclear neutrophils to form first an epoxy intermediate, i.e., 5S,6S‑epoxy-15S-hydroxy-ETE and then, depending on the cell type, by hydrolases to form either 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid (LXA4) or 5S,14R,15S-trihydroxy-6Z,8E,10Z,12Z-eicosatetraenoic acid (LXB4). The stereochemistry of the 15S‑hydroxyl group is retained in both products. This pathway not only leads to lipoxin biosynthesis but may also reduce leukotriene formation.
In a similar series of reactions in blood vessels in vitro, a trans-cellular interaction between leukocytes and platelets occurs via the same epoxy intermediate as in the first mechanism. The initial step is the action of a 5-lipoxygenase with the aid of FLAP in leukocytes to form leukotriene A4 (LTA4), which is secreted into the plasma and is available for reaction with a 12‑lipoxygenase (ALOX12) in platelets to form lipoxins (platelets are not able to produce lipoxins directly from arachidonate).
A third mechanism has been discovered that produces lipoxins of different stereochemistry, i.e., the epi-lipoxins, sometimes termed the aspirin-triggered lipoxins (‘ATL’), as the reaction is initiated by aspirin and requires the cyclooxygenase COX‑2 in the first step. As discussed in our web page on prostaglandins, COX-2 is induced in endothelial and epithelial cells in response to a variety of stimuli, and aspirin acetylates the enzyme to switch it from prostanoid biosynthesis to production of 15R-HETE rather than the S‑enantiomer. This is in turn converted to 5S,6S-epoxy-15R-hydroxy-ETE, as described above for lipoxins, by the action of the 5‑lipoxygenase in leukocytes and thence to epi-lipoxins, i.e., epi-LXA4 (5S,6S,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid) and epi-LXB4 (5S,14R,15R-trihydroxy-6E,8Z,10E,12E-eicosatetraenoic acid), the latter with 15R‑stereochemistry. 15R-HETE produced by the action of a cytochrome P450 enzyme in the absence of aspirin can be converted to 15‑epi‑lipoxins in the same manner.
 |
| Figure 5. Biosynthesis of epi-lipoxins. |
In contrast to the trans-cellular pathway, both steps can occur in macrophages alone, i.e., in a single cell type. A complex sequence of reactions begins with activation of toll-like receptor 4 (TLR4), a receptor for endotoxins, which leads to accumulation of the cyclooxygenase-2-derived lipoxin precursor 15-HETE esterified to the membrane phospholipids, especially phosphatidylinositol, a reaction that can be enhanced by aspirin treatment. It may be stored in this inert form until needed for anti-inflammatory purposes, when P2X7, a purinergic receptor for extracellular ATP, is activated with the result that efficient hydrolysis of the phospholipid-bound 15‑HETE by group IVA cytosolic phospholipase cPLA2α takes place followed by conversion of the unesterified 15-HETE to lipoxins by 5‑LOX.
Catabolism. Lipoxins are rendered inert by the actions of 15-hydroxyprostaglandin dehydrogenase and prostaglandin reductase with production of 13,14‑dihydro-15-hydroxy-LXA4 and its 15-oxo metabolite; omega-oxidation (CYP3A) has a similar effect. The epi-lipoxins have a two-fold longer half-life than the lipoxins as they are catabolized less efficiently, possibly because of the uncommon 15R‑stereochemistry.
Functions: Lipoxins were the first eicosanoids to be discovered that are 'switched on' to limit acute and chronic inflammation by increasing cytosolic calcium (Ca2+) levels to facilitate neutrophil clearance from sites of infection and reduce the release of pro-inflammatory mediators from macrophages where they have anti-apoptotic effects. They are chemo-attractants for monocytes, i.e., cells that are required for wound healing, while LXA4 reduces the production of hydrogen peroxide and of reactive nitrogen species in primary neutrophils. Lipoxins inhibit the production and action of chemokines while simultaneously stimulating anti-inflammatory cytokines. It has been argued that to produce such effects in vitro they have been often been administered experimentally at very high concentrations, much higher indeed than likely to occur physiologically, so it is too early to say how some of the reported functions described here will stand the test of time. Nonetheless, it is likely that synthetic lipoxin analogues will have therapeutic value as drugs.
 LXA4 is believed to act mainly by binding to a G-protein-coupled receptor complex (ALX or ALX/FPR2),
but this is not agreed universally.
An alternative suggestion is that its metabolite 15-oxo-LXA4, an electrophilic α,β‑unsaturated ketone, alkylates
nucleophilic amino acids and can modulate redox-sensitive and enzyme function, for example to activate erythroid related factor 2 expression
and thence anti-inflammatory and repair genes and to inhibit NF-κB-regulated pro-inflammatory mediator expression,
i.e., the mechanism is not dependent on a specific receptor.
LXA4 is believed to act mainly by binding to a G-protein-coupled receptor complex (ALX or ALX/FPR2),
but this is not agreed universally.
An alternative suggestion is that its metabolite 15-oxo-LXA4, an electrophilic α,β‑unsaturated ketone, alkylates
nucleophilic amino acids and can modulate redox-sensitive and enzyme function, for example to activate erythroid related factor 2 expression
and thence anti-inflammatory and repair genes and to inhibit NF-κB-regulated pro-inflammatory mediator expression,
i.e., the mechanism is not dependent on a specific receptor.
The lipoxin structures are conserved across species, and all the observed reactions are highly stereo-selective in terms of double bond geometry and the chirality of the hydroxyl groups. As leukotriene LTA4 is the precursor of all other leukotrienes as well as being a direct precursor of lipoxins, it has been suggested that as inflammation advances and LTA4 levels rise, it starts feeding lipoxin synthesis with subsequent initiation of resolution. Together with the protectins, resolvins and maresins, lipoxins could control the inflammatory response in such pathogenic conditions as asthma, periodontitis, arthritis, cardiovascular disorders, cancer, and gastrointestinal, periodontal, kidney and pulmonary diseases. They are reported to be neuro-protective in retinal astrocytes and the optic nerve, while dysregulation of lipoxin synthesis could be a factor in Alzheimer's and other neurological diseases. Thus, they oppose LTC4 and inhibit bronchial spasms. Like lipoxins, the aspirin-triggered epi-lipoxins have potent anti-inflammatory actions, and this may provide further explanation for the efficacy of aspirin as a drug; it not only inhibits the synthesis of pro-inflammatory mediators but also induces the synthesis of others that are anti-inflammatory (pro-resolution).
Both the lipoxins and epilipoxins, together with the docosahexaenoic acid metabolite resolvin D1, are reported to function in much the same way in vitro. For example, resolvin D1 has been shown to decrease the ratio of proinflammatory leukotriene B4 to proresolving lipoxin A4 via a kinase signalling pathway by means of which the nuclear:cytoplasmic ratio of 5‑lipoxygenase, the common enzyme in the biosynthesis of these two eicosanoids, is lowered. This shift in 5‑LOX location dampens LTB4 production.
Lipoxins may have a regulatory role in the immune response to infection by parasitic pathogens, such as Toxoplasma gondii and Mycobacterium tuberculosis. LXB4 and epi‑LXB4 are effective treatments both by oral administration and topical applications. The possibilities for therapeutic intervention with lipoxins to reduce the adverse effects of inflammation in many different disease states are being explored with animals and humans, although lipoxins per se are not yet available in sufficient quantity for clinical study. Synthetic lipoxin mimetics with greatly improved chemical stability, i.e., benzo-LXA4, is undergoing clinical trials for human periodontal diseases, and it may have potential benefits in diseases of the kidney. Other lipoxin analogues are in the development stage with fourth-generation synthetic LXA4 mimetics containing imidazole- and oxazole-rings (e.g., 'AT-01-KG').
3. Hemiketal eicosanoids
Two isomeric hemiketals, designated HKE2 (illustrated) and HKD2, are formed in epithelial cells by reaction of 5S‑hydroxytetraenoic acid (5S-HETE) with COX-2 with the reaction proceeding via an unstable di-endoperoxide. HKE2 promotes angiogenesis by enhancing activation of the vascular endothelial growth factor receptor 2 (VEGFR2).
 |
| Figure 6. Biosynthesis of the hemiketal eicosanoid HKE2. |
4. Eoxins
Eoxins are the C14,15 counterparts of the cysteinyl-leukotrienes and are products of the 12/15‑lipoxygenase (15-LOX-1, ALOX15) of human eosinophils and mast cells. The primary metabolite of the lipoxygenase, 15-HPETE, reacts with the enzyme further to produce the 14,15‑epoxide, designated eoxin A4, and then by analogy with leukotriene biosynthesis, this in turn is conjugated with glutathione to produce eoxin C4 and thence eoxin D4 (linked to Cys-Glc) and eoxin E4 (linked to Cys only).
 |
| Figure 7. Biosynthesis of eoxins. |
Like the cysteinyl-leukotrienes, the eoxins are potent pro-inflammatory agents. They have been implicated in inflammation of the airways in asthma patients and in those with Hodgkin lymphoma, a malignant disorder with many characteristics of an inflammatory illness. They have been implicated in cancer growth in skin. Ethanolamides of EXC4 and EXD4, termed 'eoxamides', and analogues of anandamide are produced in human cell preparations in vitro.
5. Hepoxilins
Hepoxilins are short-lived monohydroxy-epoxy eicosanoids produced in several organs or cell types but mainly the epidermis in humans. They are derived from unesterified arachidonic acid first by the action of a 12‑lipoxygenase, of which the spinal eLOX3 isoform is most important, followed by two divergent pathways to produce 12S‑hydroperoxy-5c,8c,10t,14c-eicosatetraenoic acid (12S‑HPETE), which can either be reduced to the hydroxy compound (12S-HETE) or can be acted upon by a hepoxilin synthase (isomerase) to cause isomerization of the hydroperoxide group to produce an epoxide. The relative rates of the two pathways are controlled by the reducing potential of the cell.

Hepoxilins contain both hydroxyl and epoxy groups, the latter across the C11-C12 double bond, and unlike the leukotrienes and lipoxins, none of the double bonds are in conjugation. Two have been characterized, i.e., 8S/R-hydroxy-11S,12S-trans-epoxyeicosa-5Z,9E,14Z-trienoic acid (hepoxilin A3 or HXA3) and 10S/R‑hydroxy-11S,12S-trans-epoxyeicosa-5Z,9Z,14Z-trienoic acid (hepoxilin B3 or HXB3), but only HXA3 is thought to be necessary in tissues. The epoxide ring is labile and can be opened by an epoxide hydrolase to yield trihydroxy metabolites termed ‘trioxilins’, which may have some function in tissues. Analogous compounds derived from eicosapentaenoic and docosahexaenoic acids have been described.
In skin, the epidermal lipoxygenase 12R-LOX generates the fatty acid hydroperoxide R-HPODE from linoleate in esterified form (complex ceramides), before eLOX3 acting as a hydroperoxide isomerase produces hepoxilin-like compounds such as hepoxilin A illustrated. Hydrolysis of the epoxide ring produces octadec-9R,10S,13R-trihydroxy,11E-enoate (tri-HODE), while the action of an NAD+-dependent dehydrogenase generates octadec-9,10-trans-epoxy,13-oxo,11E-enoate. These induce changes to the unique structural ceramides in this tissue by promoting covalent linkages to epidermal proteins (see our web page on skin ceramides for further discussion).
 |
| Figure 8. Formation of hepoxilin-like compounds from linoleate in the epidermis. |
Hepoxilins have pro-inflammatory properties in the skin, especially in psoriatic lesions, but they are anti-inflammatory in neutrophils. Most of observations suggest an association with mobilization of calcium and potassium ions within cells or across membranes. In addition, hepoxilin A3 is now known to be a regulator of mucosal inflammation in response to infection by bacterial pathogens, such as that responsible for Lyme disease. Although oxylipin production by lipoxygenases in brain tissues tends to be low, there is significant biosynthesis of hepoxilins in the pineal gland, which may take part in the regulation of melatonin production. It contributes to increased sensitivity to pain during inflammation by interaction with TRPV1 and TRPA1 receptors. Stable synthetic analogues of hepoxilins are useful treatments in animal models of lung fibrosis, cancer, thrombosis and diabetes.
Recommended Reading
- Gilbert, N.C., Newcomer, M.E. and Werz, O. Untangling the web of 5-lipoxygenase-derived products from a molecular and structural perspective: The battle between pro- and anti-inflammatory lipid mediators. Biochem. Pharm., 193, 114759 (2021); DOI.
- Haeggström, J.Z. and Newcomer, M.E. Structures of leukotriene biosynthetic enzymes and development of new therapeutics. Annu. Rev. Pharm. Toxic., 63, 407-428 (2023); DOI.
- Häfner, A.-K., Kahnt, A.S. and Steinhilber, D. Beyond leukotriene formation - The noncanonical functions of 5-lipoxygenase. Prostaglandins Other Lipid Mediators, 142, 24-32 (2019); DOI.
- Horn, T., Adel, S., Schumann, R., Sur, S., Kakularam, K.R., Polamarasetty, A., Redanna, P., Kuhn, H. and Heydeck, D. Evolutionary aspects of lipoxygenases and genetic diversity of human leukotriene signalling. Prog. Lipid Res., 57, 13-39 (2015); DOI.
- Koudelka, A. and others. Lipoxin A4 yields an electrophilic 15-oxo metabolite that mediates FPR2 receptor-independent anti-inflammatory signaling. J. Lipid Res., 66, 100705 (2025); DOI.
- Lee, M., Boyce, J.A. and Barrett, N.A.Cysteinyl leukotrienes in allergic inflammation. Annu. Rev. Pathol., Mechanisms Disease., 20, 115-141 (2025); DOI.
- Nakamura, M. and Shimizu, T. Therapeutic target of leukotriene B4 receptors, BLT1 and BLT2: Insights from basic research. Biochimie, 215, 60-68 (2023); DOI.
- Okuno, T. and Yokomizo, T. Metabolism and biological functions of 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid. Prostaglandins Other Lipid Mediators, 152, 106502 (2021); DOI.
- Pace-Asciak, C.R. Pathophysiology of the hepoxilins. Biochim. Biophys Acta, Lipids, 1851, 383-396 (2015); DOI.
- Parchem, K. and others. Oxylipin profiling for clinical research: Current status and future perspectives. Prog. Lipid Res., 95, 101276 (2024); DOI.
- Powell, W.S. Eicosanoid receptors as therapeutic targets for asthma. Clin. Sci., 135, 1945-1980 (2021); DOI.
- Rådmark, O., Werz, O., Steinhilber, D. and Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease Biochim. Biophys. Acta, Lipids, 1851, 331-339 (2015); DOI - and many other articles in this journal issue.
- Saier, L. and Peyruchaud, O. Emerging role of cysteinyl LTs in cancer. Brit. J. Pharm., 179, 5036-5055 (2022); DOI.
- Saqib, U. and others. Lipoxins as modulators of diseases. Cells, 14, 1244 (2025); DOI.
- Serezani, C.H., Divangahi, M. and Peters-Golden, M. Leukotrienes in innate immunity: still underappreciated after all these years? J. Immun., 210, 221-227 (2023); DOI.
- Teder, T., Rådmark, O., Haeggström, JZ. and Lohelaid, H. Animal models in leukotriene research: Current insights into complex pathways and therapeutic intervention. Pharmacol. Therapeut., 276, 108944 (2025); DOI.
- Tyrrell, V.J. and others. Lipidomic and transcriptional analysis of the linoleoyl-omega-hydroxyceramide biosynthetic pathway in human psoriatic lesions. J. Lipid Res., 62, 100094 (2021); DOI.
- Wan, M., Tang, X., Stsiapanava, A. and Haeggström, J.Z. Biosynthesis of leukotriene B4. Seminars Immunol., 33, 3-15 (2017); DOI - and other articles in this journal issue.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
