Plant and Fungal Oxylipins
While plants lack an immune system in the sense that it exists in animals, they do possess mechanisms that are comparable in that they recognize potential pathogens and stress vectors and then initiate defence responses. They have recognition receptors in plasma membranes that identify pathogen-associated molecule patterns and respond rapidly following identification of pathogen-secreted effector proteins by the intracellular receptors by triggering basal immunity against entire classes of pathogens. The first line of defence is the plant cell wall and cuticular barrier, which not only provides a protective shield against physical damage by animals, insects, abiotic stresses (e.g., freeze-thawing) and attack by bacteria and fungi that is not simply passive but transmits danger signals within the plant as immunogenic factors.
Then, various types of oxygenated fatty acids, collectively termed ‘oxylipins’, are synthesised to transmit the signals, among which the jasmonates have a special importance and are present ubiquitously in land plants. In many ways, these lipid mediators resemble the eicosanoids derived from arachidonate in animals, which have so many varied functions but mainly in inflammatory processes, but they are also phytohormones, which govern the growth and development of plants. Some plant oxylipins act directly by being distasteful to insect predators, some are sufficiently volatile that they can alert neighbouring plants, while others can communicate the information on cell damage over long distances within a plant to coordinate a comprehensive response. These operate in tandem with many other lipids, including conventional fatty acids when unesterified, and lipid enzymes such as lipases. Related oxylipins are present in fungi, yeasts and mosses.
1. Introduction to Plant Oxylipin Biosynthesis
There are few definitive reports of the presence of arachidonic acid in higher plants, although it is present in some algae and lower plants, so plant oxylipins are octadecanoids derived from linoleic acid (18:2(n-6)) or more often α‑linolenic acid (18:3(n-3)), released from their lipid bonds by acyl hydrolases (lipases) of various kinds. In Arabidopsis thaliana, which has been the model plant for many studies of oxylipin biosynthesis and metabolism, the core phospholipase A family consists of ten members, some of which can hydrolyse both phospholipids and galactolipids.
In brief, an initial step in the biosynthesis of almost all plant oxylipins is the action of lipoxygenases, while cytochrome P450 and pathogen-induced oxygenases have subsidiary roles. Depending on the source of the enzyme, lipoxygenases (EC 1.13.11.12) (LOX) catalyse the oxidation of α‑linolenic acid into either 9- or 13‑hydroperoxy-octadecatrienoic acid or a mixture of both, which are highly reactive and are quickly metabolized by various enzymes into series of oxylipins, as summarized in the figure below, each of which mediate a range of metabolic events.
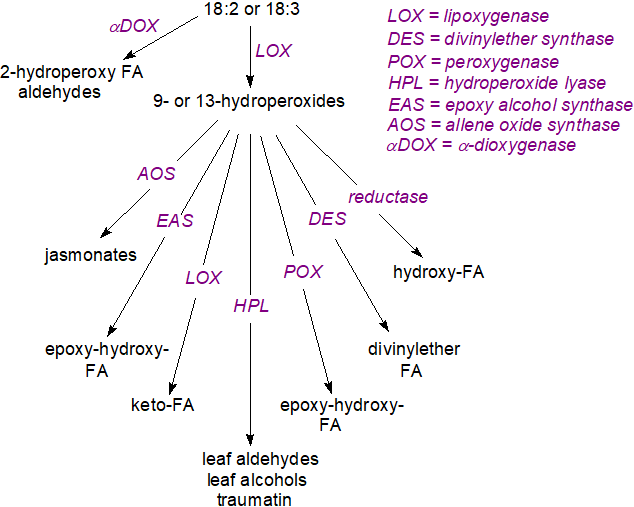 |
| Figure 1. The main enzymes for oxylipin production from linoleate and linolenate in plants. |
Those enzymes and their products listed are ubiquitous, and of these, the allene oxide synthase generates short-lived intermediates that rearrange in various ways, for example by cyclizing to form compounds such as oxo-phytodienoic acid and thence the jasmonates, i.e., most of the non-volatile oxylipins in plants, while closely related hydroperoxide lyases produce the fragmented and volatile fatty acid/aldehyde derivatives. The jasmonates in particular are viewed as central to plant development and responses to biotic and abiotic stresses, although there is extensive crosstalk with other oxylipins and plant hormones. Although some red algae such as Gracilaria contain prostaglandins and are known to have a cyclooxygenase gene, it is not known why these oxylipins are synthesised in the organisms. Some other enzymes not listed can be specific to certain plants. In view of the numerous enzymes and reactions, it is not surprising that new plant oxylipins and their functions continue to be identified.
While many lipid mediators result from these enzymatic processes, others such as phytoprostanes and aldehydes are produced adventitiously when they encounter reactive oxygen species (ROS), and together, they are a redox regulatory network that couples ROS generation with subsequent stress signalling pathways in plants.
2. Plant Lipoxygenases and Hydroperoxide Formation
Hydroperoxides are generated in plants primarily by the action of lipoxygenases, i.e., non-heme iron-containing dioxygenases that are widely distributed in fungi, plants and animals. That from soybean was among the first to be studied in detail, including its three-dimensional structure, and the knowledge gained assisted greatly our understanding of the analogous animal enzymes. Plant LOXs consist of a single polypeptide chain with a molecular mass of 94 to 104 kDA with the carboxy-terminal domain harbouring the catalytic site of the enzyme, which contains a non-heme iron atom that is coordinated with five amino acids - three histidines, one asparagine and the carboxyl group of the carboxy-terminal isoleucine; the amino-terminal domain may take part in membrane or substrate binding. Lipoxygenases in general are discussed in our web page dealing with hydroxyeicosatetraenes (HETE), but here the plant enzymes only are described.
From amino acid-sequence studies of enzymes from many plant sources, it is evident that there are two main families of lipoxygenases, designated ‘type-1’ (mainly extra-plastidial) and ‘type-2’ (mainly plastidial), and in A. thaliana, lipoxygenases are located mainly in the plastidial envelope and stroma of leaf chloroplasts. Many different iso-enzymes exist that are dependent on plant species, and they include both soluble cytoplasmic and membrane-bound enzymes, with eight different isoforms known of soybean lipoxygenase, for example. There are suggestions that in different subcellular regions, these may provide different pools of hydroperoxy fatty acids, which serve as substrates for alternative metabolic or physiological pathways.
Lipoxygenases catalyse the addition of molecular oxygen to polyunsaturated fatty acids containing a (cis,cis)-1,4-pentadiene system to yield an unsaturated fatty acid hydroperoxide. In brief, the first and rate-limiting step is the abstraction of a hydrogen atom by non-heme ferric iron (Fe(III)), involving a proton-coupled electron transfer in which the electron is transferred directly to the iron and the proton is acquired simultaneously by the hydroxide ligand in a concerted mechanism to give a substrate radical, while the iron atom is reduced to the ferrous state (Fe(II)). Oxygen can be added to either end of the pentadiene system with high stereospecificity, and in the case of linoleic and α‑linolenic acids, this leads to either the 9S- or 13S-hydroperoxy derivatives or both depending on the iso-form of the enzyme; A. thaliana contains two genes encoding for 9-LOXs and four that encode 13‑LOXs. Physiological conditions can affect this positional specificity (regiospecificity), and though normally the 13S isomer predominates, under conditions of low oxygen concentration, the soybean LOX‑1 yields equal amounts of the two isomers. Photosynthetic tissues tend to generate mainly 13S‑hydroperoxides, but 9‑LOX metabolites are more abundant in potato.
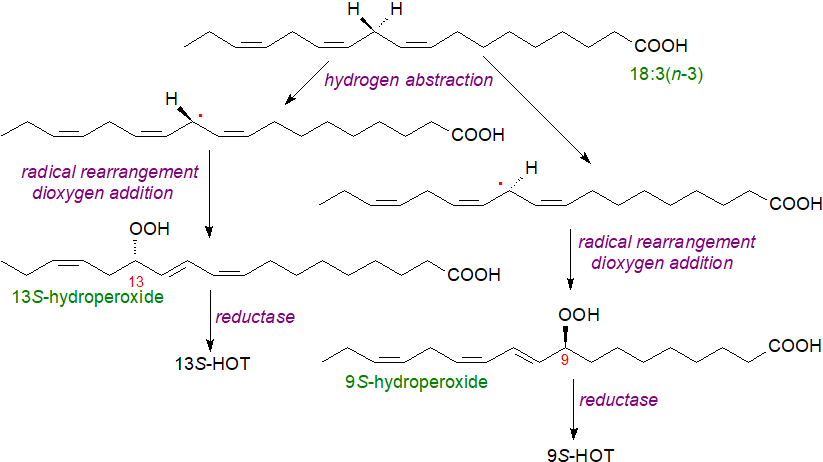 |
| Figure 2. The action of lipoxygenase on α-linolenic acid. |
Free acids are the preferred substrates for 9-LOX, and under conditions of stress in plants, lipases or unspecific acyl-lipid hydrolases are activated that rapidly break down the complex lipids, another analogy with animal systems, and initiate oxylipin formation, but it is evident that 13-LOX can react with esterified fatty acids in lipids also and perhaps disrupt the cellular membranes as in stress situations. The reaction then proceeds in three stages as illustrated above for α‑linolenic acid, with the first step a stereospecific abstraction of a hydrogen atom from the methylene group between the double bonds. The resulting delocalized free radical undergoes an allylic rearrangement to trans-cis conjugated diene systems before the oxygen molecule adds to form the hydroperoxides, which are then reduced by hydroperoxide reductases to the corresponding hydroxides, i.e., the hydroxytrienes (9S-HOT and 13S-HOT). Both of the latter can elicit separate and specific defence responses in plants either directly or via their metabolites.
 Further steps for the wide range of oxylipins found in plants, such as the jasmonates, differ between
the 9- and 13‑LOX products.
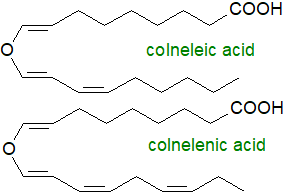
Among other oxylipins that could be described, colneleic and colnelenic acids originating from 18:2- and 18:3-derived
hydroperoxides, respectively, via 9‑LOX only are divinyl ether fatty acids produced subsequently by divinyl ether synthases of the CYP74
family in leaves of potato plants infected by bacteria, fungi or viruses.
They have a defensive role against potato blight (Phytophthora infestans) especially, and they are
found both in free and esterified states and are toxic to many other plant pathogens.
Tomato plants, which like potato are of the Solanum family, generate these oxylipins as a defence response to attack by nematodes,
such as the root-knot Meloidogyne javanica.
Other plants can make stereo-isomers of colnelenic acid or a related oxylipin from oleate ((11Z)-etheroleic acid).
No comparable 13S products result, but the 13S-LOX pathway only is used for the synthesis of jasmonates (next section).
Further steps for the wide range of oxylipins found in plants, such as the jasmonates, differ between
the 9- and 13‑LOX products.
Among other oxylipins that could be described, colneleic and colnelenic acids originating from 18:2- and 18:3-derived
hydroperoxides, respectively, via 9‑LOX only are divinyl ether fatty acids produced subsequently by divinyl ether synthases of the CYP74
family in leaves of potato plants infected by bacteria, fungi or viruses.
They have a defensive role against potato blight (Phytophthora infestans) especially, and they are
found both in free and esterified states and are toxic to many other plant pathogens.
Tomato plants, which like potato are of the Solanum family, generate these oxylipins as a defence response to attack by nematodes,
such as the root-knot Meloidogyne javanica.
Other plants can make stereo-isomers of colnelenic acid or a related oxylipin from oleate ((11Z)-etheroleic acid).
No comparable 13S products result, but the 13S-LOX pathway only is used for the synthesis of jasmonates (next section).
Algae differ from higher plants in that they can contain significant amounts of higher polyunsaturated fatty acids such as eicosapentaenoic (EPA or 20:5(n‑3)) and docosahexaenoic (DHA or 22:6(n-3)) acids, and hydroxy-EPA derivatives, such as 15‑HEPE, are products of lipoxygenases in marine algae as a defence mechanism against bacteria and other predators (see Part 10 below).
3. Jasmonates and Related Compounds
The jasmonates are 12-carbon cyclic fatty acids and their derivatives or precursors, which are produced from α-linolenic acid and are signalling mediators in algae and higher plants for plant stress responses and hormones for growth and development. A key structural feature is a cyclopentanone ring resembling that in mammalian prostaglandins (surely no coincidence), but the stereochemistry of the active species has until recently been a cause of confusion. Upon stimulation by a stress condition, biosynthesis is initiated by the release of α‑linolenic acid from position sn-1 of the galactolipids (or phospholipids) of plastid membranes by a galactolipase (glycerolipase or phospholipase A1), though which enzyme is involved may depend on the nature and site of the stimulus (16:3(n-3) can be a jasmonate precursor in some species). The unesterified α-linolenic acid is then acted upon by a 13‑lipoxygenase to yield 13S‑hydroperoxy-9c,11t,15c-octadecatrienoic acid as the first intermediate (1) as illustrated below.
Allene oxide synthase is one of a family of related cytochrome P450 mono-oxygenases collectively termed the CYP74 subfamily, which are not typical of the common P450 monooxygenases in that they do not require molecular oxygen nor NAD(P)H-dependent cytochrome P450-reductase. Instead, the new carbon–oxygen bonds are created by using an acyl hydroperoxide both as substrate and oxygen donor, in essence acting as a dehydratase. This enzyme catalyses the next step (2), and the product is the allene oxide 12,13S‑epoxy-9c,11t,15c-octadecatrienoic acid. As this compound is highly unstable, it can cyclize spontaneously to form 12-oxo-10,15c-phytodienoic acids (‘OPDA’), i.e., with a prostaglandin-like structure, in two of the four possible stereoisomers (or it can be hydrolysed rapidly to α- and γ-ketols). However, reaction with the enzyme allene oxide cyclase (3) produces only 12‑oxo‑9S,13S-phytodienoic acid (the cis-(+)-enantiomer - 12‑oxo‑PDA), which is the primary isomer of biological relevance. Not only is this the precursor of the jasmonates and a component of the arabidopsides (see below), but it has signalling functions of its own.
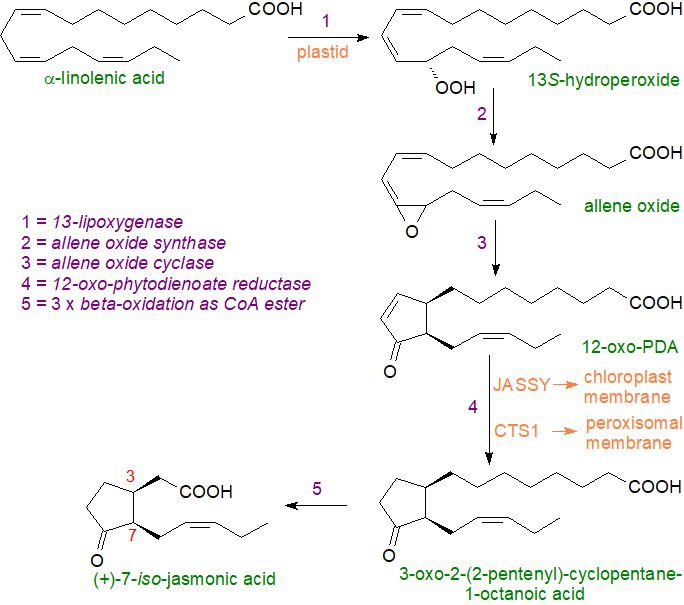 |
| Figure 3. Biosynthesis of jasmonic acid. |
Both the allene oxide synthase and the cyclase are located in the chloroplasts, and they probably operate in concert; they may even be linked physically in some type of complex, as suggested by the high stereochemical purity of the product given the instability of the intermediates. Some fungi have a novel allene oxide cyclase that can utilize linoleic acid to form jasmonate analogues lacking the double bond in the alkyl chain (via 12-OPEA).
Further reactions occur in peroxisomes, cytosol, endoplasmic reticulum and vacuoles. A protein designated JASSY transfers 12‑oxo‑PDA from the chloroplast outer membrane into the cytosol, before this intermediate is transferred by an ATP-binding cassette transfer protein (designated 'CTS1' or 'PAX1') from the cytosol to the peroxisomes, where a flavin-dependent 12‑oxo-phytodienoate reductase (OPR3) reduces the double bond in position 10, i.e., in the cyclopentenone ring, to produce 3-oxo-2-(pent-2'-enyl)-cyclopentane-1-octanoic acid. This is a necessary step in directing the metabolism towards jasmonic acid, as this compound only (after conversion to its CoA ester) can undergo three cycles of β‑oxidation catalysed by the multifunctional enzyme complex acyl-CoA oxidase to give the 12‑carbon 7‑iso‑jasmonic acid. Biosynthesis of jasmonic acid is blocked by several inhibitors, including acetylsalicylic acid (aspirin), which directly inhibits the expression of the relevant genes.
While (-)-7-iso-jasmonic acid is the main isomer isolated from plant tissues and was long thought to be the key metabolite, it is now recognized that (+)‑7‑iso-jasmonic acid or cis-(epi)-jasmonic acid is in fact the active isomer. As both side chains are on the same side of the 3R,7S-cyclopentanone ring and the keto group at C-6 can tautomerize to an enol, the more thermodynamically stable (3R,7R)-isomer (-)- or trans-jasmonic acid is usually isolated as the main product (90% of the equilibrium mixture) during analysis.
 After transfer of the jasmonic acid to the cytosol,
(3R,7S)-jasmonoyl-L-isoleucine is then formed rapidly from jasmonic acid,
and this is now known to be a central element in hormone signalling by jasmonates.
The enzyme jasmonoyl isoleucine conjugate synthase 1 (JAR1) in the cytosol catalyses the final step, although at least one alternative enzyme
to JAR1, designated GH3.10, is present in leaf tissue (but 12‑hydroxy-jasmonic acid is its preferred substrate).
As with the free acid, the common isomer (‑)‑7‑jasmonoyl-L-isoleucine is not
the active isomer, but rather the much less abundant (+)-7-epimer.
pH changes promote conversion of the (+)-7- to the inert (-)-7-epimer, suggesting that this may be a simple if minor mechanism to
regulate jasmonate metabolism.
cis-(+)-12-Oxo-phytodienoic acid (cis‑(+)‑OPDA), the biosynthetic precursor of jasmonic acid,
has also been found in conjugation with isoleucine and 2‑mercaptoethanol, and as an α‑monoacylglycerol.
After transfer of the jasmonic acid to the cytosol,
(3R,7S)-jasmonoyl-L-isoleucine is then formed rapidly from jasmonic acid,
and this is now known to be a central element in hormone signalling by jasmonates.
The enzyme jasmonoyl isoleucine conjugate synthase 1 (JAR1) in the cytosol catalyses the final step, although at least one alternative enzyme
to JAR1, designated GH3.10, is present in leaf tissue (but 12‑hydroxy-jasmonic acid is its preferred substrate).
As with the free acid, the common isomer (‑)‑7‑jasmonoyl-L-isoleucine is not
the active isomer, but rather the much less abundant (+)-7-epimer.
pH changes promote conversion of the (+)-7- to the inert (-)-7-epimer, suggesting that this may be a simple if minor mechanism to
regulate jasmonate metabolism.
cis-(+)-12-Oxo-phytodienoic acid (cis‑(+)‑OPDA), the biosynthetic precursor of jasmonic acid,
has also been found in conjugation with isoleucine and 2‑mercaptoethanol, and as an α‑monoacylglycerol.
A further 11 classes of jasmonate metabolites are formed by methylation, reduction, decarboxylation and glucosylation, including 12‑hydroxy-jasmonoyl-isoleucine, cis-jasmone and methyl jasmonate, and many of these have functions of their own, both as isoleucine conjugates and in the free state after hydrolysis of the conjugate. On the other hand, some metabolites produced by hydroxylation of the pentenyl side chain, e.g., sulfation of the hydroxylated derivatives, are inert so switch off jasmonic acid signalling. An ω‑oxidation pathway, in which jasmonoyl-isoleucine is oxidized by cytochrome P450 enzymes, mainly CYP94 isoforms, to a 12-hydroxy metabolite and then further via a 12‑aldehydo intermediate to dicarboxy-jasmonoyl-isoleucine, together with deconjugation by amidohydrolases, is now recognized as a major route for catabolism and deactivation of the hormone, although the hydroxy intermediate can itself cause weak and sustainable expression of certain JA-responsive genes until further oxidation occurs. A comparable mechanism operates with jasmonoyl-phenylalanine.
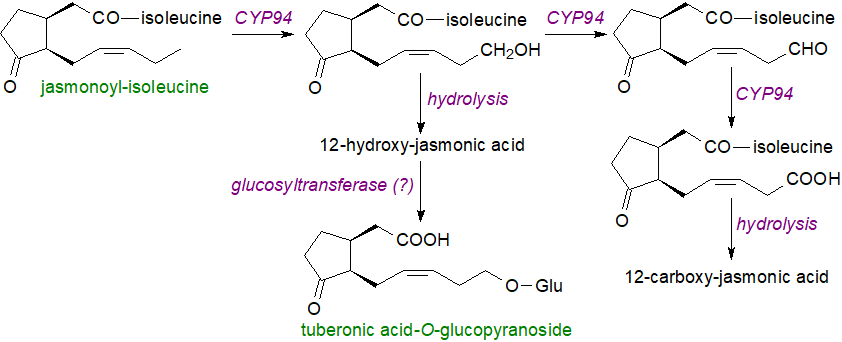 |
| Figure 4. Oxidation and further metabolism of jasmonoyl-isoleucine. |
The glucopyranosyl derivative of tuberonic acid, derived from jasmonic acid after hydroxylation at C-12, is synthesised in the leaves of potatoes and transported down to the stolons where it induces tuber formation indirectly by influencing gibberellic acid signalling. Another derived signalling mediator is methyl jasmonate in a reaction catalysed by S-adenosyl-L-methionine:jasmonic acid carboxyl methyltransferase, while decarboxylation is the route to the volatile cis-jasmone.
As well as being a primary precursor of (-)-jasmonic acid, (+)-12-oxo-phytodienoic acid (cis-OPDA) regulates growth processes and certain jasmonate-responsive genes in plants, and it induces and fine-tunes defence and stress responses (see below), as does a C20 analogue of OPDA detected in a liverwort. OPDA amino acid conjugates are utilized for temporary storage of OPDA in stress responses but do not take part in signalling.
Dinor-oxo-phytodienoic acid (dinor-OPDA) is a metabolite related to jasmonic acid but derived from hexadecatrienoic acid (16:3(n-3)),
and it has its own signalling functions, such as the response to wounding.
In bryophytes and lycophytes, an unsaturated isomer of this Δ4-dinor-iso-OPDA, i.e. with altered chemistry at the ring,
is the main signalling hormone.
 In a separate metabolic pathway in maize, 9‑lipoxygenase generates hydroperoxides from linoleate that are cyclized with
the ultimate production of jasmonate-like molecules, such as 10‑oxo‑11-phytoenoic acid (10-OPEA) and metabolites,
which have been termed "death acids".
These are induced by fungal infections, such as southern leaf blight, and are an important element in plant defence.
In the roots of cereals, the allene oxide synthase pathway produces a range of novel oxylipins termed "graminoxins", i.e. dicarboxylic acids with
one of the carboxyl groups located centrally in the alkyl chain.
In a separate metabolic pathway in maize, 9‑lipoxygenase generates hydroperoxides from linoleate that are cyclized with
the ultimate production of jasmonate-like molecules, such as 10‑oxo‑11-phytoenoic acid (10-OPEA) and metabolites,
which have been termed "death acids".
These are induced by fungal infections, such as southern leaf blight, and are an important element in plant defence.
In the roots of cereals, the allene oxide synthase pathway produces a range of novel oxylipins termed "graminoxins", i.e. dicarboxylic acids with
one of the carboxyl groups located centrally in the alkyl chain.
4. Other Products of Enzymes of the CYP74 Subfamily
The CYP74B subfamily of fatty acid hydroperoxide-transforming cytochromes P450 enzymes, including the allene oxide synthase, generate several further types of oxidation product. Once more, most start with the 13S‑hydroperoxy intermediate from the lipoxygenase reaction, and oxylipins of various kinds are synthesised in plants as summarized in the figure below. These reactions all proceed first via free radical and then unstable epoxide intermediates, which can either rearrange spontaneously or undergo diverse enzymic reactions, though many aspects of the mechanisms require clarification or confirmation. It is of interest from an evolutionary standpoint, that two key enzymes in this family, hydroperoxide lyase and divinylether synthase, differ only in a few amino acid residues, and there are other close relationships between enzymes of the CYP74 family. Analogous enzymes are found in bacteria and lower animals.
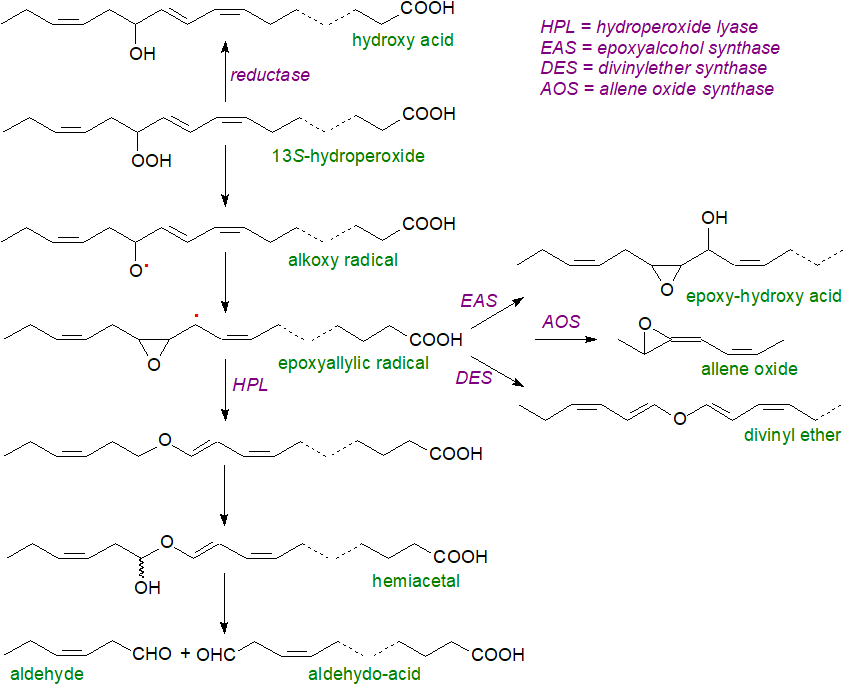 |
| Figure 5. Oxylipins generated by the CYP74 family of monooxygenases. |
After the formation of an epoxyallylic radical, the enzyme hydroperoxide lyase, which should be more accurately termed a hemiacetal synthase, converts the epoxy intermediate into a vinyl ether and thence to a short-lived hemiacetal, which spontaneously decomposes into two short chain fragments, i.e., cis-3-hexenal and 12‑oxo-cis-9-dodecenoic acid, though the positions and geometry of the double bonds may change by chemical or enzymatic isomerization. The enzyme is found in various subcellular locations, depending on plant species. The unsaturated aldehydes, such as hex-3-enal, are volatile and have potent effects on cellular metabolism (as discussed briefly below, although bioactive aldehydes in general have their own web page). Hexenyl acetate is a major product of wounding in Arabidopsis.
In alternative pathways, the 13S-lipoxygenase product can be reduced to a hydroxy acid, while a peroxygenase or pathogen-induced oxygenase pathway is another route to the biosynthesis of epoxy-hydroxy fatty acids. The epoxyallylic radical intermediate is a precursor for further oxylipins, and for example, it can be converted by an epoxyalcohol synthase into an epoxyhydroxy fatty acid (allene oxides are products of a separate enzymic reaction). 9-Hydroxy-10-oxo-12Z,15Z-octadecadienoic acid (KODA) is synthesised from α-linolenic acid via 9-lipoxygenase (9‑LOX) and allene oxide synthase; although the amount is usually low, it aids plants to recover from stress. In a few plants, the enzyme divinylether synthase converts fatty acid 9-hydroperoxides into divinyl ethers such as colneleic, colnelenic or etheroleic acids as discussed above.
5. Dioxygenase Products and Other Oxylipins
In response to fungal infections, additional oxidation reactions occur in leaf oil bodies. An α-dioxygenase, two forms of which exist (in tobacco and Arabidopsis) that are distinct from the lipoxygenases (and related structurally to mammalian COX enzymes), works in concert with caleosins, i.e., Ca2+‑binding proteins associated with lipid bodies, to generate various 2R‑hydroperoxy fatty acids and then the hydroxide, e.g., 2‑hydroxy-linolenic acid (illustrated). Alternatively, the hydroperoxide can decay spontaneously to produce the long-chain aldehyde one carbon atom shorter as part of the α-oxidation process, while a further route to such aldehydes is catabolism of phytosphingosine-1-phosphate. Both types of product may be involved in plant defensive reactions against fungi and other pathogens.
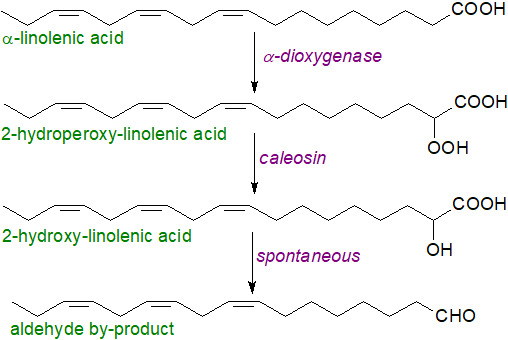 |
| Figure 6. Biosynthesis of 2R-hydroxy-linolenate and long-chain aldehydes in oil bodies. |
Caleosins can react directly with α-linolenic acid to introduce an epoxide group at any of the double bonds, and they can react with the 13‑lipoxygenase metabolite to produce a 13-hydroxy,15,16-epoxy-octadecadienoic acid. Persin or 1-acetoxy-2R-hydroxy-12Z,15Z-heneicosadien-4-one from avocado leaves is a powerful deterrent to animal predation and is toxic to lactating livestock. Fungal dioxygenases are discussed below.
Di- and trihydroxy-octadecanoids are synthesised in some plants, and phaseolic or 2-oxo-5,8,12-trihydroxy-dodecanoic acid was first found in bean seeds and has been shown to stimulate the growth of pea stem segments, to induce α‑amylase synthesis in barley endosperm, and to retarded senescence in barley leaf segments.

6. Phytoprostanes (Plant Isoprostanes)
 In plants subjected to biotic and abiotic stresses, α-linolenic acid is the precursor of oxygenated fatty acids and
C18‑isoprostanoids (dinor-isoprostanes or phytoprostanes) via non-enzymatic, free radical-catalysed pathways as for
isoprostane synthesis in animals but with singlet oxygen
generated in chloroplasts during photosynthesis as the most important reactive oxygen species (ROS).
As phytoprostanes are derived from C18 precursors, they differ from the animal isoprostanes in the number of double bonds and
the lengths of the side chains.
The phytoprostane PPE1 is illustrated as an example.
In plants subjected to biotic and abiotic stresses, α-linolenic acid is the precursor of oxygenated fatty acids and
C18‑isoprostanoids (dinor-isoprostanes or phytoprostanes) via non-enzymatic, free radical-catalysed pathways as for
isoprostane synthesis in animals but with singlet oxygen
generated in chloroplasts during photosynthesis as the most important reactive oxygen species (ROS).
As phytoprostanes are derived from C18 precursors, they differ from the animal isoprostanes in the number of double bonds and
the lengths of the side chains.
The phytoprostane PPE1 is illustrated as an example.
The reaction is initiated by the abstraction of a hydrogen radical and is followed by addition of oxygen to give a cyclic endoperoxide, before addition of a further oxygen molecule forms the hydroperoxide. Abstraction of the hydrogen atom at C‑14 generates phytoprostanes G1 (PPG1) of type I, while hydrogen abstraction at C-11 yields PPG1 of type II. The endoperoxide group is highly unstable, and PPG1 molecules rearrange spontaneously or are reduced to D1, E1 and F1 ring analogues of the animal prostaglandins (and named from these with an additional 'P'), with further dehydration and isomerization to the J1 and other types.
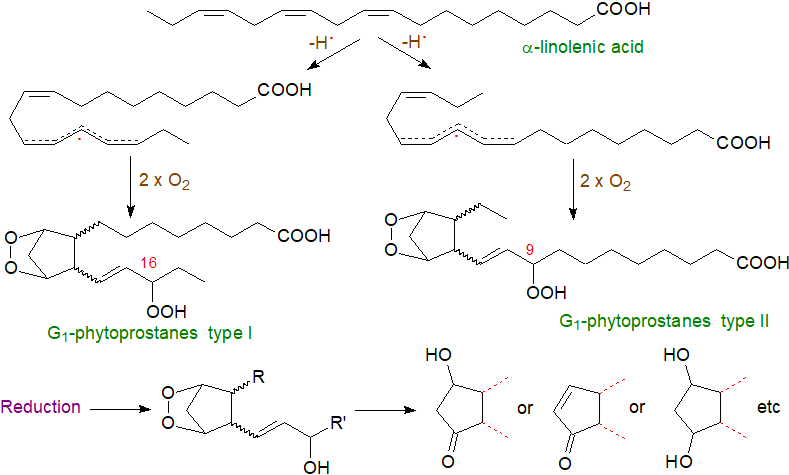 |
| Figure 7. Formation of type G phytoprostanes from α-linolenic acid in plants. |
Whereas lipoxygenases insert oxygen only at C-9 or C-13 of the precursor fatty acids with defined stereochemistry, ROS can make racemic hydroperoxides at C‑10 and C-12 of linoleate and at C-15 and C-16 of linolenate. Indeed, in the twenty or so plants analysed to date, various regioisomers of free phytoprostanes of the A1, B1, D1, E1, F1, G1 and deoxy-J1 series have been detected, but the series with the hydroxyl group in positions 9 and 16 tend to be most abundant in vivo, especially PPD1 and PPF1. Isofurans derived from linolenate and analogous to those found in animal tissues have also been detected in plants. Like the isoprostanes, phytoprostanes are generated mainly in a lipid-bound state in membranes, but they can be released as the free acids, while glutathione conjugates of PPA1 accumulate after pathogen infection in A. thaliana. In red and brown macro- and micro-algae, analogous phytoprostanes occur together with isoprostanes derived from endogenous arachidonic and eicosapentaenoic acids.
Such non-enzymatically derived lipids are synthesised continuously in healthy plant tissues in the range for each component of 0.01 to 6.7 nmol/g dry weight in tomato leaves. Indeed, the concentrations of esterified phytoprostanes can be an order of magnitude higher than those of the equivalent free jasmonates and two to three orders of magnitude higher than of analogous isoprostanes in animal tissues. Plants respond to environmental stresses by enhancing the production of ROS as signalling molecules to initiate a robust defence, but they must manage excessive ROS levels by means of a sophisticated antioxidative defence system with both enzymatic and non-enzymatic components, which work together to alleviate any deleterious effects upon biomolecules.
In evolutionary terms, phytoprostanes are likely to have been developed before oxylipins from enzymatic reactions, and they are still required for many purposes in plants. The cyclopentenone-phytoprostanes PPA and PPB up-regulate gene expression for enzymes that respond to challenges by foreign organisms or external conditions, while they down-regulate genes for cell division and growth. Like the jasmonates, they trigger phytoalexin production (see below). Such studies are at an early stage, and little is known of the response to differing regioisomers.
Phytoprostanes may be good biomarkers for oxidative degradation of plant foods such as occurs during improper storage. As they are present in vegetable oils and have been detected in plasma, the properties of phytoprostanes in terms of potential responses in human cells are under investigation in vitro at least, and the initial reports are that they may be useful anti-inflammatory agents. On the other hand, F1‑phytoprostanes, which are present in remarkably high concentrations in pollen, may stimulate pro-inflammatory agents associated with allergic responses.
7. Aldehydes
Other natural products of fatty acid oxidation that operate in plant tissues include unsaturated aldehydes (alkenals), such as trans‑3-hexenal and trans‑3-hexenol - sometimes termed the 'leaf aldehyde and alcohol', respectively. The primary precursors are hydroperoxides of lipid-bound fatty acyl moieties, and these can be generated by lipoxygenases or non-enzymatically (autoxidation) by the action of ROS, which include hydrogen peroxide (H2O2), superoxide anions (O2•–), hydroxyl radicals (OH•), nitric oxide (NO•) and peroxyl radicals (LOO•) generated by enzymic and other means under conditions of oxidative stress in plant cells, together with singlet oxygen (1O2 or O=O) in photosynthetic tissues.
Fission of hydroperoxides can then occur spontaneously or by the action of the enzyme hydroperoxide lyase (see part 4 above), and the products can be isomerized or further oxidized to yield many different bioactive compounds, including the volatile aldehydes, as described in part 5 above. The biochemistry and physiology of such aldehydes in mammalian systems are discussed in greater detail in a separate web page, although mechanistic aspects of their formation in plants and their reactions with proteins are very similar. There is a more restricted range of unsaturated fatty acid precursors in plants in comparison to animals, and as α-linolenate is the main fatty acid in leaf tissue, C6 aldehydes predominate (rarely C3), although C9 aldehydes are products of linoleate mainly.
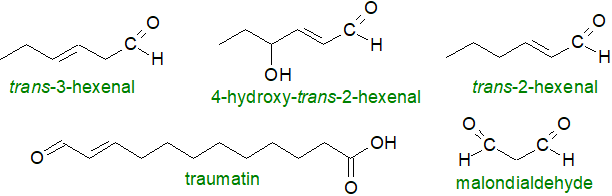 |
| Figure 8. Some aldehydes produced by oxidative fission in plants. |
The other fission products, aldehydo-acids, remain attached to the glycerolipid precursors, and such 'oxidatively truncated lipids' can disrupt membranes to increase their permeability directly and through signalling actions. In this manner, traumatin or 12‑oxo‑trans-10-dodecanoic acid, a plant wound hormone, results from isomerization of 10‑oxo-cis-9-octadecanoic acid, and this can in turn undergo autoxidation to yield 'traumatic' acid, i.e., 10E-dodeca-1,12-dicarboxylic acid. The latter is a wound healing agent in plants that stimulates cell division near a trauma site to create a protective callus over the damaged tissue, but it is a growth hormone in algae. Fatty acid fragments such as pimelic and especially azelaic acids (C7 and C9 dicarboxylic acids, respectively) are generated from hydroperoxy fatty acids of the 9-LOX pathway with aldehydes, and these may be involved in systemic acquired resistance and the defence against pathogens.
As high concentrations of such aldehydes can cause irreversible damage to plant cells membranes and ultimately lead to cell death, the damage caused and the mechanisms of detoxification are of appreciable importance. Defence mechanisms include enzymes such as aldehyde dehydrogenases, aldo/keto reductases, 2‑alkenal reductases and glutathione transferases, together with the presence of antioxidants, such as tocopherols and carotenoids.
Several unsaturated aldehydes, but especially 2E-hexenal, have antimicrobial properties and are defensive towards fungi, bacteria and arthropods to which they are toxic; they can reduce the fecundity of insect pests. When leaves are wounded, this volatile compound is formed rapidly and is able to diffuse rapidly through damaged or infected tissues before release into the air. It can then participate in a complex signalling system as a warning to other plants and insect predators in the vicinity. 2E‑4‑Hydroxynonenal produced from 3Z‑nonenal in plants acts as an anti-fungal agent. By inducing the expression of stress-associated genes, volatile aldehydes are involved in abiotic stress responses. Malondialdehyde generated in leaves non-enzymatically from hydroperoxides is an indicator of cellular damage when in excess, and it may interact directly with plant proteins and modify gene expression to induce regulatory genes that provide cellular protection under conditions of oxidative stress.
Other effects result from lipoxidation, i.e., covalent binding of electrophilic aldehydes to proteins, or glutathione and nucleic acids, via the reaction of α,β‑unsaturated carbonyl structures with free thiol (illustrated) or amine groups via the Michael addition reaction. Unsaturated hydroperoxides and the carbonyl groups of the other cleavage product 12‑oxo‑phytodienoic acid may react in the same way. In this manner, they may be signalling mediators by reacting with redox-regulated proteins, but they can act as damage/signalling agents in many different physiological situations, including root injury, programmed cell death, senescence, stomata response to abscisic acid, and root response to auxin.
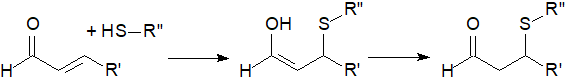 |
| Figure 9. Reaction of 2-alkenals with free thiol groups. |
8. Esterified Oxylipins
The phytoprostanes and other non-enzymatic oxylipins are created when esterified to galactolipids and phospholipids, and it has long been known that plant oxylipins synthesised by enzymatic means are often present in the esterified state. For example, ester-linked 13S‑hydroxy-9Z,11E-octadecadienoic acid is formed during germination of cucumber cotyledons, suggesting a role in plant development. New lipidomic methodologies have now simplified the analysis of intact lipids containing oxylipins and stimulated research into the topic, although knowledge of how such lipids interact with plant processes remains limited.
The best known of these new complex lipids are the arabidopsides. Thus, a number of different mono- and digalactosyldiacylglycerols containing 12‑oxo‑phytodienoic acid and/or dinor-oxo-phytodienoic acids in positions sn-1 and/or sn-2 (and even linked to the carbohydrate moiety) and termed 'arabidopsides A to G' have been isolated from stressed plants of A. thaliana after leaf wounding, during the hypersensitive response to bacterial pathogens, or under cold stress (two representatives illustrated). In plants challenged by a bacterial pathogen, a monogalactosyldiacylglycerol containing two 12‑oxo‑phytodienoate and one dinor-oxo-phytodienoate acyl chain (arabidopside E) was found to accumulate in amounts up to 8% of the total lipids, up to 150 times more than in the free state, and it was shown to have anti-bacterial properties in vitro. Not only do they accumulate in stressed leaves of A. thaliana but, to a limited extent, in distal non-wounded leaves of the same plant, as well as at trace levels in normal plants. It is now apparent that fatty acids esterified to galactolipids, as well as the free acids, may be substrates for biosynthesis of OPDA, and both lipoxygenase 2 and the allene oxide synthase are able to oxidize lipid-bound fatty acids.

Arabidopsides are synthesised in the chloroplasts and are found mainly in the thylakoid membrane, although it is possible that they could be trafficked to the plasma membrane and beyond as they are known to influence root growth. It seems probable that they are a storage pool, which upon stimulation could release OPDA for direct signalling or as a substrate for production of jasmonic acid, although there are suggestions that the intact lipids have antibacterial and antifungal properties and may be a factor in senescence.
Although these intriguing lipids may not have a widespread distribution in the Brassica family, more lipids of this kind are being reported from other genera, and other oxylipins are known to be present in the esterified state in plants subject to damage by pathogens or freeze/thawing. Oxylipins including 12‑oxo‑phytodienoate linked to phosphatidylinositol and phosphatidylglycerol have been detected in stressed Arabidopsis, and lipoxygenase products have been found esterified to various phospholipids in other plants. In addition, it has been established that acylation of the head-group of monogalactosyldiacylglycerol is a common stress response in plants, with the nature of the additional fatty acyl group, which can include OPDA, varying with species and the stress applied.
Colneleic and colnelenic acids, produced by the action of 9-LOX, occur esterified to the sn-2 position of phospholipids in potato, suggesting they are present in a pool that, like the arabidopsides, would be immediately available in response to challenge by pathogens, for example to inhibit germination of fungal spores. Mono- and digalactosyldiacylglycerols containing divinyl ether fatty acids and termed 'linolipins' occur in leaves of flax plants (Linum usitatissimum L.) subjected to damage by freezing-thawing and in those inoculated with cells of the phytopathogenic bacterium Pectobacterium atrosepticum, and related lipids have been found in damaged leaves of the meadow buttercup (Ranunculus acris).
Plant N-acylethanolamides, in which the fatty acid moiety is a lipoxygenase product of linoleic acid, have been detected in seeds of A. thaliana, where they act synergistically with abscisic acid to modulate the transition from embryo to seedling.
9. Functions of Plant Oxylipins
By coordinating the patterns of gene expression within and between cells, oxylipins together with other plant hormones, such as salicylic acid, ethylene, auxin, brassinosteroids, gibberellic acid and abscisic acid, orchestrate intricate networks that regulate plant growth, development, and responses to environmental stress, e.g., insect attack, pathogen infection, wounding, UV light, salts, and drought. They control vital physiological processes such as leaf senescence, tuber formation, photosynthesis, reproduction, seed germination and growth, while eliciting generation of secondary metabolites including flavonoids, phytoalexins, terpenoids anthraquinones, anthocyanin, xanthonoid, and more. Through cross-talking with other genes in plants, e.g., the gibberellin, auxin, and phytochrome signalling pathways, oxylipins mediate trade-offs between growth and defence - too complex a topic to be discussed here (but see the reading list below).
When plants are attacked by bacterial or fungal pathogens, lipases are activated that release unsaturated fatty acids and trigger the synthesis of a range of oxylipins with diverse roles. Some of these have direct antimicrobial or anti-insect properties, while others, such as the jasmonates and their precursors the oxo-phytodienoic acids, are potent regulators of defence mechanisms upon which much recent research has been concentrated. Simplistically, hydroperoxide lyase and allene oxide synthase compete for substrates within the lipoxygenase pathway with the latter mediating plant defence responses directly by forming jasmonic acid, whereas the volatile products of the hydroperoxide lyase pathway operate indirectly by attracting the natural enemies of plant invaders. Most organelles in plants have the capacity to make oxylipins, but it is increasingly being recognized that lipid droplets in which triacylglycerols are stored have a role in mediating stress responses as well as supplying substrates and sequestering potentially toxic products. These are synthesised in the endoplasmic reticulum and thylakoid membranes (plastoglobules) and are associated with a range of biosynthetic enzymes (see our web page on triacylglycerol biosynthesis).
Jasmonates: 9S,13S-12-Oxo-phytodienoic (OPDA) acid and jasmonic acid metabolites like (+)-7-jasmonoyl-L-isoleucine (JA-Ile), are phytohormones that regulate growth and developmental processes, secondary metabolism, defence against attack by insects and pathogens, and protection from most other abiotic stresses. In response to changes in the environment and transient endogenous signalling events, jasmonates can induce stomatal opening, inhibit photosynthetic CO2 assimilation, and affect the uptake of nitrogen and phosphorus and the transport of organic nutrients such as glucose to balance plant growth and defence. Long-distance transmission of jasmonate signals within the plant occurs via the vascular bundle.
Jasmonoyl-isoleucine is the primary jasmonate known to act at the molecular level at the nucleus via a receptor complex that contains a 'Coronatine Insensitive 1 or COI1' protein. At low JA-Ile levels, the transcription of JA-responsive genes is repressed because of the accumulation of transcriptional repressor proteins (so-called ‘JAZ’ proteins). This repressed state is then de-repressed by elevated JA-Ile levels in response to environmental and developmental cues, which promote the binding of JA-Ile with JAZ in the COI1 coreceptor and initiate ubiquitination and the subsequent degradation of JAZ by proteasomes. A co-receptor complex between COI1 and a JAZ protein in the presence of JA-Ile with inositol pentakisphosphate is a cofactor. A transporter (JAT1) acts as a JA-Ile carrier into the nucleus and a jasmonic acid carrier out of the cytoplasm and across the plasma membrane. 12‑Hydroxy-JA-Ile synthesised in the endoplasmic reticulum differentially stimulates a subset of the JA‑Ile co-receptors to control and/or modulate some jasmonate-dependent responses that improve plant resilience.
In relation to fertility and growth, jasmonates are necessary for pollen maturation, and in such varied processes as seed germination, fruit ripening, root growth, flower development (including sex determination), flowering time, senescence and tendril coiling; plants defective in jasmonate biosynthesis or signalling are male-sterile. They act by mechanisms that are as yet poorly understood to initiate signalling pathways both intra- and inter-cellularly that modulate the expression of a number of genes and thence the synthesis of many key proteins often by interacting with abscisic acid, gibberellin and light. With many aspects awaiting clarification, the picture emerging is a highly complex one, and there are metabolic differences among plants that have yet to be explained.
Jasmonates are pivotal for root regeneration by signalling to induce cell proliferation and restore the root meristem by stimulating certain transcription factors to promote callus formation and shoot regeneration. They may down-regulate genes for the core metabolism of the plant simultaneously by interacting with gibberellins to decide whether defence or growth should have priority. As well as inducing tuber production, tuberonic acid is a leaf-closing factor that induces leaf movement in motor cells of certain plants.
 Each of the various
jasmonate derivatives, i.e., the free acid, methyl ester and conjugates with amino acids, has its own influence on plant metabolism,
often in defence mechanisms.
Wound response is one of the most-studied pathways of jasmonates in signal transduction with the tomato often as the experimental model.
Jasmonic acid levels in undamaged leaves of mature plants are barely detectable, but wounding induces a rapid synthesis of jasmonic acid
and jasmonoyl-isoleucine in both local and systemic tissues.
In brief, local wounding results in breakdown of cells and release of fatty acids, and at the same time, cleavage of the octadecapeptide
systemin is initiated from prosystemin to stimulate jasmonic acid biosynthesis with various jasmonic acid conjugates.
This is a signal that leads to systemic expression of genes encoding proteinase inhibitors and anti-feedant and poisonous compounds,
which deter insect herbivores by inhibiting their digestive capabilities and 'immunizing' the plant against further herbivore attacks,
while promoting the biosynthesis of protective surface waxes.
In partnership with other defence mechanisms including those based on phytohormones, they participate the responses to excessive cold and heat
stress in plants in vivo while reducing cold injury in fruits and vegetables during storage,
Each of the various
jasmonate derivatives, i.e., the free acid, methyl ester and conjugates with amino acids, has its own influence on plant metabolism,
often in defence mechanisms.
Wound response is one of the most-studied pathways of jasmonates in signal transduction with the tomato often as the experimental model.
Jasmonic acid levels in undamaged leaves of mature plants are barely detectable, but wounding induces a rapid synthesis of jasmonic acid
and jasmonoyl-isoleucine in both local and systemic tissues.
In brief, local wounding results in breakdown of cells and release of fatty acids, and at the same time, cleavage of the octadecapeptide
systemin is initiated from prosystemin to stimulate jasmonic acid biosynthesis with various jasmonic acid conjugates.
This is a signal that leads to systemic expression of genes encoding proteinase inhibitors and anti-feedant and poisonous compounds,
which deter insect herbivores by inhibiting their digestive capabilities and 'immunizing' the plant against further herbivore attacks,
while promoting the biosynthesis of protective surface waxes.
In partnership with other defence mechanisms including those based on phytohormones, they participate the responses to excessive cold and heat
stress in plants in vivo while reducing cold injury in fruits and vegetables during storage,
Volatile jasmonate metabolites, such as cis-jasmone and methyl jasmonate, may regulate the behaviour of some insects by deterring herbivores or attracting their predators. Methyl jasmonate can influence other plants at a distance by inducing transcription of defensive genes encoding proteins and secondary compounds such as anthocyanins and alkaloids. In a study of the mechanism of this interaction with the model bryophyte Marchantia polymorpha, it has been demonstrated that airborne methyl jasmonate is metabolized to jasmonic acid and 12‑hydroxyjasmonic acid, while inducing increased expression of biosynthetic genes for jasmonates.
Jasmonates are part of the defence system against viruses carried by insects, and they take part in the defence against bacterial pathogens such as those that feed on necrotic tissue or cells undergoing apoptosis. The bacterial phytotoxin coronatine exerts is toxic effects by binding to the receptor for jasmonoyl-isoleucine and by interfering with the response to salicylic acid. While certain soil-borne microorganisms are beneficial by enhancing the defensive capacity of plants, with jasmonic acid as a regulator of the process, others can hijack jasmonate synthesis to cause hormonal signalling imbalances and enhance infection. Jasmonates promote the beneficial interactions between certain mycorrhizal fungi and nitrogen-fixing bacteria and plants, and they may even enable communication between plants.
12-Oxo-phytodienoic acid (cis-OPDA), a biosynthetic precursor of jasmonic acid, controls a set of genes that are independent of the latter in the regulation of seed germination, the development of the embryo and plant defence. Working together with salicylic acid, it recognizes the bacterial quorum-sensing lipoamino acid N‑3‑oxo-tetradecanoyl-L-homoserine lactone and primes the plant for an enhanced defence by a mechanism that is separate from the jasmonic acid signalling cascade. Aside from local defence, OPDA may be involved in long-distance signalling to facilitate the systemic propagation of immunity. There may also be crosstalk between OPDA and the phytohormone abscisic acid. While no receptor for OPDA has yet been discovered, it does bind to cyclophilin in plastids and thence induces a signalling pathway. OPDA and dinor-OPDA are present in nonvascular land plants (e.g., mosses and liverworts), but jasmonoyl-isoleucine is not.
Although they do not occur naturally in animal tissues, some jasmonate metabolites and methyl jasmonate in particular have been shown to be highly cytotoxic towards human cancer cell lines in vitro and may have therapeutic potential for this and other human diseases. Many other plant oxylipins, including aldehydes, oxy-, epoxy- and hydroxy-derivatives of fatty acids, phytoprostanes and phytofurans are reported to have benefits for human health.
Other oxylipins: Many of the activities of other lipoxins are discussed in earlier sections above, and aside from acting as precursors of jasmonates, most oxylipins derived from the lipoxygenase pathway have characteristic activities of their own. Even the primary 9S- and 13S‑hydroperoxides and hydroxides have antifungal and anti-microbial properties, and for example, in Arabidopsis, LOX1-derived fatty acid hydroxides regulate stomatal closure in an abscisic acid-independent way and are an element of the defence reactions against bacterial pathogens. 2-Hydroxy-linolenic acid from leaf oil bodies and the epoxy and hydroxy derivatives of linoleic acid resulting from the peroxygenase pathway are toxic to fungal pathogens, while 9-keto-10E,12Z,15Z-octadecatrienoic acid, produced from linoleic acid by the action of 9-LOX, is a potent inhibitor of the plant pathogen Pseudomonas syringae pv. tomato. They may be part of a short-term local response, while the jasmonates operate over a longer time scale.
10. Oxylipins from Fungi, Mosses and Algae
 In unicellular algae, aldehydes derived
from C20 fatty acids are formed as a defence response on wounding, while multicellular algae produce oxylipins from C18
and C20 polyunsaturated fatty acids.
Marine algae use lipoxygenase (LOX)-mediated oxidative pathways and polyunsaturated fatty acids to generate hydroperoxide intermediates,
which subsequently undergo regio- and stereospecific transformations to yield a wide range of oxygenated metabolites, including several with
conserved vinyl-α-substituted cyclopentenyl moieties, as signalling or defence molecules.
Marine diatoms synthesise many distinctive oxylipins, some of which have even been found in prokaryotes.
The functions of most of these compounds have yet to be determined, although similar molecules in fungi are known to act as hormone-like signals
that regulate such processes as asexual and sexual spore development and toxin production.
In unicellular algae, aldehydes derived
from C20 fatty acids are formed as a defence response on wounding, while multicellular algae produce oxylipins from C18
and C20 polyunsaturated fatty acids.
Marine algae use lipoxygenase (LOX)-mediated oxidative pathways and polyunsaturated fatty acids to generate hydroperoxide intermediates,
which subsequently undergo regio- and stereospecific transformations to yield a wide range of oxygenated metabolites, including several with
conserved vinyl-α-substituted cyclopentenyl moieties, as signalling or defence molecules.
Marine diatoms synthesise many distinctive oxylipins, some of which have even been found in prokaryotes.
The functions of most of these compounds have yet to be determined, although similar molecules in fungi are known to act as hormone-like signals
that regulate such processes as asexual and sexual spore development and toxin production.
Mosses are of interest in that they are intermediate between unicellular algae and flowering plants in evolutionary terms. As the complete genome of the moss Physcomitrella patens is available, it makes an ideal model organism for oxylipin study. It makes a range of C18 and C20 oxylipins, including 12‑hydroperoxy-eicosatetraenoic acid (12-HPETE) and further metabolites from arachidonate, which makes up 30% of the total fatty acids in the organism, but it does not synthesise jasmonic acid.
Fungi and yeasts synthesise a variety of oxylipins from saturated and unsaturated fatty acids that include oleate through to arachidonic and eicosapentaenoic acids, which can occur naturally in these organisms. In addition, fungi generate many endogenous oxylipins from the fatty acids of host plants and animals during infections by the action of linoleate diol synthases, cytochrome P450 dioxygenases and lipoxygenases, and enzymes with a LOX domain fused to a cytochrome P450 enzyme at the C-terminal end are known. Many filamentous fungi pathogens contain dioxygenases with homology to human cyclooxygenases, and the prostaglandin metabolites PGF2 and PGF2-lactone, derived from arachidonic acid, which does not occur in plants have been detected in yeasts of the Lipomycetaceae family, while PGE2 is produced by Candida albicans and some others. The latter organism is a pathogen that utilizes host arachidonic acid for this purpose and as a first step in β‑oxidation to generate 3‑hydroxy-eicosanoids, which stimulate its growth and virulence. 2-(14,15-Epoxyeicosatrienoyl) glycerol, i.e., a 2-monoacylglycerol, has been detected in tomato roots as a product of fungal symbiosis. The mechanisms of fungal-plant interactions with C20 polyenoic fatty acids are now being unravelled, and they may engage plant signalling networks to induce resistance to the pathogens. They elicit a cascade of reactions, including an oxidative burst and transcription of genes for the hypersensitive response.
On the other hand, octadecanoids are more common, most with an 8R‑hydroxyl group though some 5S-, 9R-, 9S-, 10R or 13S-substituents occur, and dihydroxy- and hydroxy,epoxy-metabolites are known. 8R‑Hydroxy-octadeca-9,12-dienoic and 5S,8R-dihydroxy-octadec-9-enoic acids are products of Aspergillus sp., and comparable oxylipins can be formed from oleate. In ascomycetes, the linoleic acid-derived oxylipin 13S-hydroxy-9Z,11E-octadecadienoic acid (13S-HODE) is utilized for the transcriptional reprogramming required for asexual development.

While fungal pathogens can synthesise jasmonates by much the same pathway as in plants and then exploit host oxylipins to increase their own virulence, oxylipins such as the jasmonates in plants act in the opposite sense to resist the attack of fungal pathogens. Volatile C8 oxylipins, 1‑octen-3-ol, 3‑octanone, 3-octanol and others are synthesised in fungi from the longer-chain oxylipins, and they participate in the regulation of the germination of fungal spores and the subsequent developmental processes. They have antibacterial and antifungal properties and act as signalling molecules during the interactions with higher plants, nematodes and insects. Incidentally, they are responsible for the characteristic smell of mushrooms.
Various fungal dioxygenases (DOX) are known that differ in the positional and stereospecificity of the hydroperoxide products from linoleate, i.e., 8S-DOX, 9S-DOX, 8R-DOX, 9R-DOX and 10R-DOX (in addition to the α-DOX - see above). They are of interest because they are produced by devasting fungal pathogens of wheat and other crops and they participate in other plant-fungal interactions and the reproduction of fungi.
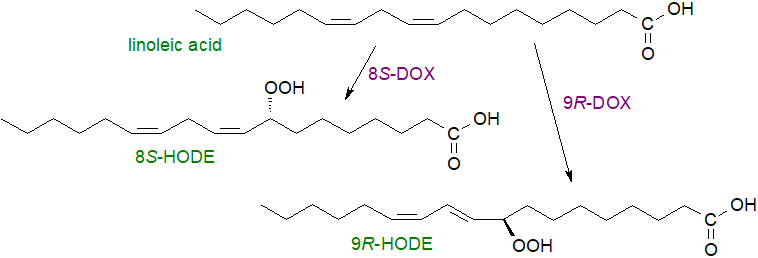 |
| Figure 10. Two of the products of the reaction of fungal dioxygenases with linoleic acid. |
Some bacteria have lipoxygenase enzymes that can oxidize host unsaturated fatty acids during infections of plants and animals to create oxylipins that signal to mediate virulence, and for example, the opportunistic bacterial pathogen Pseudomonas aeruginosa uses a diol synthase to catalyse the stereospecific oxygenation of oleic acid to 10-HOME and 7,10-diHOME.
11. Analysis
As with the eicosanoids, gas chromatography-mass spectrometry or high-performance liquid chromatography allied to electrospray tandem mass spectrometry are preferred for the analysis of the plant oxylipins, with HPLC and UV detection as a useful complementary technique. Internal standards labelled with stable isotopes, such as O18, are essential for quantification.
Recommended Reading
- Ahmed, O.S., Galano, J.-M., Pavlickova, T., Revol-Cavalier, J., Vigor, C., Lee, J.C.-Y., Oger, C. and Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: where are we now? Essays Biochem., 64, 463-484 (2020); DOI.
- Beccaccioli, M., Pucci, N., Salustri, M., Scortichini, M., Zaccaria, M., Momeni, B., Loreti, S., Reverberi, M. and Scala, V. Fungal and bacterial oxylipins are signals for intra- and inter-cellular communication within plant disease. Front. Plant Sci., 13, 823233 (2022); DOI.
- Genva, M., Akong, F.O., Andersson, M.X., Deleu, M., Lins, L. and Fauconnier, M.L. New insights into the biosynthesis of esterified oxylipins and their involvement in plant defense and developmental mechanisms. Phytochem. Rev., 18, 343-358 (2019); DOI.
- Kataria, P., Guy, A., Durand, T. and Oger, C. The vinyl-α-substituted cyclopentyl oxylipins. Biochimie, 239, 41-50 (2025); DOI.
- Knieper, M., Viehhauser, A. and Dietz, K.-J. Oxylipins and reactive carbonyls as regulators of the plant redox and reactive oxygen species network under stress. Antioxidants, 12, 814 (2023); DOI.
- Kuźniak, E. and Gajewska, E. Lipids and lipid-mediated signaling in plant-pathogen interactions. Int. J. Mol. Sci., 25, 7255 (2024); DOI.
- Loreto, F. and D'Auria, S. How do plants sense volatiles sent by other plants? Trends Plant. Sci., 27, 29-38 (2022); DOI.
- Lu, J.H., Xu, Y., Wang, J.L., Singer, S.D. and Chen, G.Q. The role of triacylglycerol in plant stress response. Plants-Basel, 9, 472 (2020); DOI.
- Nishizato, Y., Okumura, T., Matsumoto, K. and Ueda, M. Recent advances in the chemistry and biology of plant oxylipin hormones. Nat. Prod. Rep., 42, 1175-1194 (2025); DOI.
- Oliw, E.H. Catalytic and structural comparisons of fatty acid dioxygenases related to cyclooxygenases and peroxidases. Arch. Biochem. Biophys., 773, 110574 (2025); DOI.
- Ponce de León, I., Hamberg, M. and Castresana, C. Oxylipins in moss development and defense. Front. Plant Sci., 6, 483 (2015); DOI.
- Revol-Cavalier, J., Quaranta, A., Newman, J.W., Brash, A.R., Hamberg, M. and Wheelock, C.E. The octadecanoids: synthesis and bioactivity of 18-carbon oxygenated fatty acids in mammals, bacteria, and fungi. Chem. Rev., 125, 1-90 (2025); DOI.
- Savchenko, T., Degtyaryov, E., Radzyukevich, Y. and Buryak, V. Therapeutic potential of plant oxylipins. Int. J. Mol. Sci., 23, 14627 (2022); DOI.
- Seth, T., Asija, S., Umar, S. and Gupta, R. The intricate role of lipids in orchestrating plant defense responses. Plant Sci., 338, 111904 (2024); DOI.
- Toporkova, Y.Y., Smirnova, E.O., Gorina, S.S. and Bhatia, M. Epoxyalcohol synthase branch of lipoxygenase cascade. Curr. Iss. Mol. Biol., 46, 821-841 (2024); DOI.
- Vu, H.S., Roth, M.R., Tamura, P., Samarakoon, T., Shiva, S., Honey, S., Lowe, K., Schmelz, E.A., Williams, T.D. and Welti, R. Head-group acylation of monogalactosyldiacylglycerol is a common stress response, and the acyl-galactose acyl composition varies with the plant species and applied stress. Physiol. Plant., 150, 517-528 (2014); DOI.
- Wang, M.L., Fan, X.L. and Ding, F. Jasmonate: a hormone of primary importance for temperature stress response in plants. Plants-Basel, 12, 4080 (2023); DOI.
- Wasternack, C. and Song, S.S. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot., 68, 1303-1321 (2017); DOI - part of a special issue of the journal with other relevant reviews.
- Yadav, M., Satija, S., Awasthi, S., Singh, I.K. and Singh, A. Lipoxygenases: The gatekeepers in plant resilience against biotic stress. Plant Gene, 45, 100557 (2026); DOI.
- Yi, R., Li, Y.R. and Shan, X.Y. OPDA/dn-OPDA actions: biosynthesis, metabolism, and signaling. Plant Cell Rep., 43, 206 (2024); DOI.
- Zheng, C., Chen, J.P., Wang, X.W. and Li, P. Reactive oxygen species in plants: metabolism, signaling, and oxidative modifications. Antioxidants., 14, 617 (2025); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: January 2026 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
