Sterols 3. Sterols and their Conjugates
from Plants and Lower Organisms
1. Plant Sterols - Structures and Occurrence
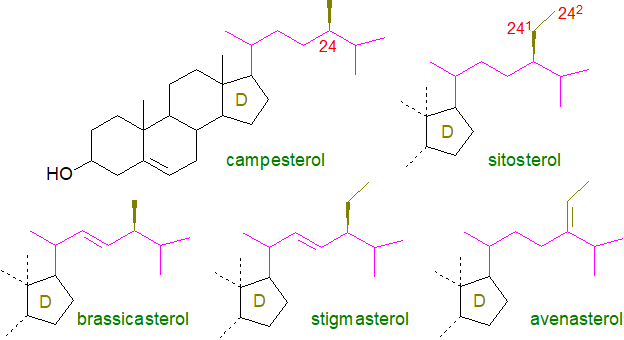 Plants, algae and fungi contain a rather different range of sterols from those in animals.
Like cholesterol, to which they are related structurally and biosynthetically,
plant sterols form a group of triterpenes with a tetracyclic cyclopenta[a]phenanthrene structure and a side chain at carbon 17,
sometimes termed the C30H50O triterpenome.
The four rings (A, B, C, D) have trans ring junctions, and the side chain and two methyl groups (C-18 and C-19)
are at an angle to the rings above the plane with β stereochemistry (as for a hydroxyl group commonly located on C-3).
The basic sterol from which other sterol structures are defined is 5α-cholestan-3β-ol with the numbering scheme recommended by IUPAC.
Plants, algae and fungi contain a rather different range of sterols from those in animals.
Like cholesterol, to which they are related structurally and biosynthetically,
plant sterols form a group of triterpenes with a tetracyclic cyclopenta[a]phenanthrene structure and a side chain at carbon 17,
sometimes termed the C30H50O triterpenome.
The four rings (A, B, C, D) have trans ring junctions, and the side chain and two methyl groups (C-18 and C-19)
are at an angle to the rings above the plane with β stereochemistry (as for a hydroxyl group commonly located on C-3).
The basic sterol from which other sterol structures are defined is 5α-cholestan-3β-ol with the numbering scheme recommended by IUPAC.
The phytosterols (as opposed to zoosterols) include campesterol, β-sitosterol, stigmasterol and Δ5‑avenasterol, some of which are illustrated. These more common plant sterols have a double bond in position 5 and a definitive feature – a one- or two-carbon substituent with variable stereochemistry in the side chain at C-24, i.e., a new chiral centre is present and C-24 epimeric pairs can occur (e.g., campesterol/22-dihydrobrassicasterol), and this is preserved during subsequent metabolism. For example, campesterol is a 24-methylsterol, while β-sitosterol and stigmasterol are 24‑ethylsterols. Occasionally, there is a double bond in this chain that can be of the cis or trans configuration as in stigmasterol (at C22) or fucosterol (C24), the main sterol in green algae.
Phytosterols can be further classified on a structural or biosynthetic basis as 4‑desmethyl sterols (i.e., with no substituent on carbon‑4, e.g., sitosterol), 4α‑monomethyl sterols (s (e.g., gramisterol) and 4,4‑dimethyl sterols (e.g., lanosterol). The most abundant group are the 4‑desmethyl sterols, which may be subdivided into Δ5-sterols (illustrated above), Δ7‑sterols (e.g., α-spinasterol) and Δ5,7-sterols depending on the position of the double bonds in the B ring. As the name suggests, brassicasterols (24‑methyl-cholesta-5,22-dien-3β-ol and related sterols) are best known from the brassica family of plants, but they are also common constituents of marine algae (phytoplankton). Phytostanols (fully saturated) are normally present at trace levels only in plants, but they are relatively abundant in cereal grains.
Many different sterols may be present in photosynthetic organisms, and the amounts and relative proportions are dependent on the species; more than 250 different phytosterols have been recorded with 60 in corn (maize) alone. Most published data are from seed oils as these are major components of the human diet, but all plant tissues contain sterols and the compositions of these in leaves and pollen have been shown to differ and be specific to each plant species. As a rough generality, a typical plant sterol mixture would be 70% sitosterol, 20% campesterol and 5% stigmasterol (or >70% 24-ethyl-sterols and <30% 24-methyl-sterols), although this will vary with the stage of development and in response to stress. Table 1 contains data on the main components from some representative commercial seed oils.
Table 1. Composition of sterols (free plus esterified) in some commercial seed oils (mg/Kg). |
|||||||
| corn | cottonseed | olive | palm | rapeseed | soybean | sunflower | |
|---|---|---|---|---|---|---|---|
| campesterol | 2005 | 333 | 17 | 139 | 2930 | 571 | 410 |
| stigmasterol | 677 | 50 | - | 95 | - | 577 | 337 |
| β‑sitosterol | 6457 | 4018 | 1303 | 426 | 4198 | 1734 | 2653 |
| Δ5‑avenasterol | 104 | 194 | 443 | 33 | 1109 | 135 | 432 |
| Data adapted from Verleyen, T. et al. J. Am. Oil Chem. Soc., 79, 117-122 (2002); DOI. | |||||||
Cholesterol is usually only a minor component of plant sterols (<1%), but it is unwise to generalize too much, as it can be the main sterol component of red algae and of some families of higher plants such as in the Solanaceae, Liliaceae and Scrophylariaceae. It can also be a significant sterol constituent of chloroplasts, shoots, pollen and leaf surface lipids in other plant families; wheat roots contain 10% and cells of Arabidopsis thaliana contain 19% of the sterols as cholesterol. Biosynthesis of the toxic glycoalkaloid solanine of potatoes requires cholesterol as an intermediate. Brassinosteroids, ecdysteroids (phytoecdysteroids) and withanolides are polyhydroxylated plant sterols that can occur in appreciable amounts in some plant species. In yeasts and fungi, ergosterol is usually the main sterol (see below). Sterols are found in some bacterial groups but not in archaea, and hopanoids in bacteria are considered to be functional triterpenoid counterparts.
Sterols can occur in plants in the 'free' state, i.e., in which the sterol hydroxyl group is not linked to any other moiety, and as conjugates with the hydroxyl group covalently bound via an ester bond to a fatty acid, i.e., as sterol esters, or via a glycosidic linkage to glucose (and occasionally other sugars), i.e., as steryl glycosides.
2. Plant Sterols - Biosynthesis
The biosynthetic route to plant sterols resembles that to cholesterol in many aspects in that it follows an isoprenoid biosynthetic pathway with isopentenyl pyrophosphate, derived primarily from mevalonate, as the key building block in the cytoplasm (but not plastids although some of the isoprene units produced can be transferred from the cytoplasm to plastids). The main pathway for the biosynthesis of isopentenyl pyrophosphate and dimethylallyl pyrophosphate, the isoprene units, is described in our web page on cholesterol and so need not be repeated here. It is known as the 'mevalonic acid (MVA) pathway' and functions in the cytosol, endoplasmic reticulum and mitochondria.
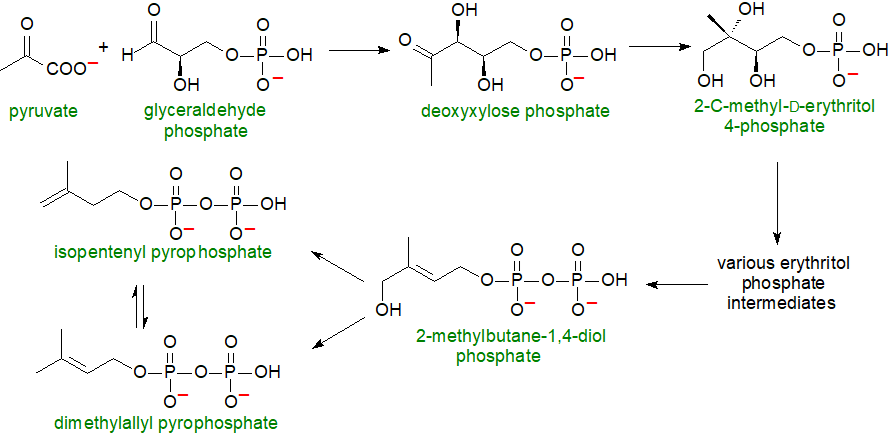
An alternative pathway that does not use mevalonic acid as a precursor was established first for bacterial hopanoids, but has since been found in plant chloroplasts, algae, cyanobacteria, eubacteria and some parasites (but not in animals). This route is variously termed the ‘non-mevalonate’, ‘1‑deoxy-D-xylulose-5-phosphate’ (DOXP) or better the 2C-methyl-D-erythritol 4‑phosphate (MEP) pathway as the last compound is presumed to be the first committed intermediate in sterol biosynthesis by this route. In the first step, pyruvate and glyceraldehyde phosphate are combined to form deoxyxylose phosphate, which is in turn converted to 2C‑methyl-D-erythritol 4-phosphate. The pathway then proceeds via various erythritol intermediates until isopentenyl pyrophosphate and dimethylallyl pyrophosphate are formed. In much of the plant kingdom, both the MVA and MEP pathways operate in parallel, but green algae use the MEP pathway only. Thereafter, sterol biosynthesis continues via squalene and (3S)-2,3-oxidosqualene.
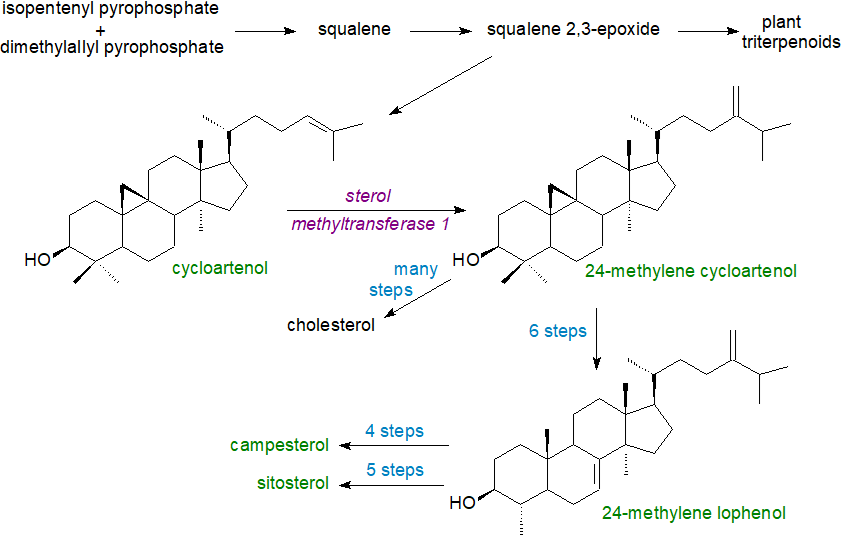
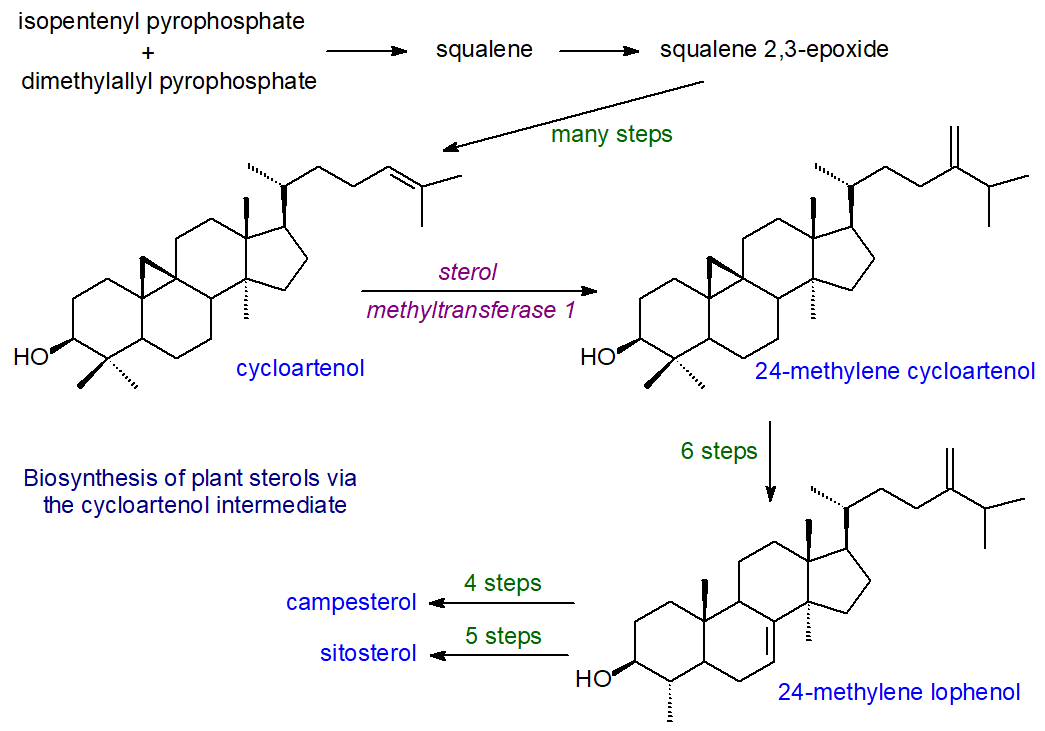
In photosynthetic organisms (as opposed to yeast and fungi), the subsequent steps in the biosynthesis of plant sterols differ from that for cholesterol in that the important intermediate in the route from squalene via 2,3-oxidosqualene is cycloartenol, rather than lanosterol, and this is produced by the action of a 2,3(S)‑oxidosqualene-cycloartenol cyclase (cycloartenol synthase). Then, the enzyme sterol methyltransferase 1 converts cycloartenol to 24-methylene cycloartenol as the first step in introducing the methyl group onto C-24, while the enzyme cyclopropyl sterol isomerase is required to open the cyclopropane ring. Animals lack the sterol C24-methyltransferase gene. While this pathway is in essence linear up to the synthesis of 24-methylene lophenol, a bifurcation then occurs that results in two alternative pathways, one of which leads to the synthesis of sitosterol and stigmasterol and the other to that of campesterol. In fact, there are more than thirty enzyme-catalysed steps in the overall process of plant sterol biosynthesis, each associated with membranes, and detailed descriptions are available from the reading list below. Although the 4,4‑dimethyl- and 4α‑methylsterols are part of the biosynthetic pathway, they are only minor if ubiquitous sterol components of plants. New biosynthetic pathways are now being discovered by genome analysis that reveal the complexity of sterol biosynthesis in different plant species.
Dinoflagellates produce a characteristic 4-methylsterol termed dinosterol and others like gorgosterol via lanosterol as precursor. Protozoans synthesise many different sterols related to those in plants, and some species of Acanthamoeba and Naegleria produce both lanosterol and cycloartenol, but only the latter is used for synthesis of other sterols including ergosterol.
Cholesterol in plants is produced from cycloartenol as the required intermediate with the Sterol Side Chain Reductase 2 (SSR2) as the key enzyme (or a functional related enzyme in some species) together with distinct sterol demethylases, e.g., CYP51. It is now established that the cholesterol biosynthetic pathway in tomato plants comprises 12 enzymes acting in 10 steps. Of these, half evolved through gene duplication and divergence from phytosterol biosynthetic enzymes, whereas others act reciprocally in both cholesterol and phytosterol metabolism. Algae can produce cholesterol in a multi-step process from cycloartenol and many more sterols via 24‑methylene lophenol as an intermediate. It is hoped that genetic manipulation of these enzymes will lead to plants that synthesise high value steroidal products.
Oxidation: Phytosterols can be subjected to non-enzymatic oxidation with formation of oxysterols in the same way as cholesterol in animals, resulting in ring products such as hydroxy-, keto-, epoxy- and triol-derivatives, and further enzymic reactions can oxidize the side chain. Photosensitized oxidation is more common in plants and is much faster (>1500 times); it starts with the ene-addition of singlet oxygen (1O2) on either side of the double bond in the B ring to generate 5α-/6α-/6β-hydroperoxy-sterols, of which 5α-OOH is the most abundant and rearranges to form the more stable 7α‑OOH isomer. In foods stored under LED lighting in food retailers, this is the main reaction.
3. Plant Sterols - Function
Like cholesterol, plant sterols are amphiphilic and are vital constituents of all membranes but especially of the plasma membrane, the mitochondrial outer membrane and the endoplasmic reticulum. The three-dimensional structure of the plant sterols is such that there are planar surfaces at both the top and the bottom of the molecules, which permit multiple hydrophobic interactions between the rigid sterol and the other components of membranes. Indeed, they must determine the physical properties of membranes to an appreciable extent. It is believed that campesterol, β-sitosterol and 24‑methylcholesterol (in this order) can regulate membrane fluidity and permeability in plant membranes by restricting the mobility of fatty acyl chains in a similar manner to cholesterol in mammalian cells, but stigmasterol has much less effect on lipid ordering and no effect on the permeability of membranes. In the plasma membrane, plant sterols associate with the glycosphingolipids such as glucosylceramide and glycosylinositolphosphoceramides (and the sterol glycosides) in raft-like sub-domains, analogous to those in animal cells, and these support the membrane location and activities of many proteins with crucial functions in plant cells.
Sterols (and their conjugates) are involved in how plant membranes adapt to changes in temperature and other biotic and abiotic stresses. For example, β‑sitosterol is a precursor of stigmasterol via the action of a C22-sterol desaturase, and the ratio of these two sterols is a factor in the resistance of A. thaliana plants to low and high temperatures. Plant sterols can modulate the activity of membrane-bound enzymes, and stigmasterol and cholesterol regulate the activity of the Na+/K+-ATPase in plant cells, probably in a manner analogous to that of cholesterol in animal cells. Stigmasterol may be required specifically for cell differentiation and proliferation. As well as being the precursor of plant steroidal hormones, campesterol is a signalling molecule that regulates growth, development and stress adaptation.
4. Steroidal Plant Hormones and Related Metabolites
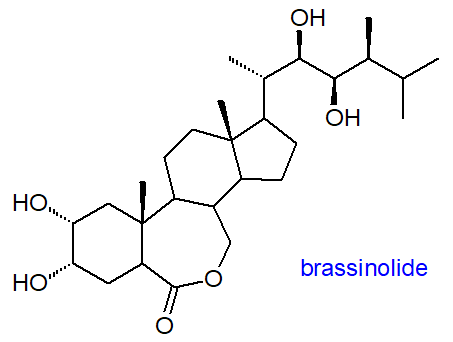 Campesterol
is the precursor of a family of nearly 70 polyhydroxy steroids that occur in minute amounts in plants from algae to angiosperms and act as growth
hormones; they are collectively named brassinosteroids, including brassinolide illustrated, as
they were first detected in Brassica sp.
They are essential for plant growth processes, including cell elongation, division, differentiation,
immunity and development of reproductive organs, and they are involved in the regulation of innumerable aspects of metabolism.
Via signal transduction pathways, they interact with transcription factors through phosphorylation cascades to regulate the expression
of target genes.
Brassinosteroids are also signalling molecules in abiotic stress responses such as drought, salinity, high temperature, low temperature
and heavy metal stresses.
Outwith plants, they may have biomedical applications as anticancer drugs for endocrine-responsive cancers to induce apoptosis
and inhibit growth.
Some plant species produce small amounts of steroid hormones that are often considered to be of animal origin only,
including progesterone and testosterone, and these may have physiological roles in plants.
Campesterol
is the precursor of a family of nearly 70 polyhydroxy steroids that occur in minute amounts in plants from algae to angiosperms and act as growth
hormones; they are collectively named brassinosteroids, including brassinolide illustrated, as
they were first detected in Brassica sp.
They are essential for plant growth processes, including cell elongation, division, differentiation,
immunity and development of reproductive organs, and they are involved in the regulation of innumerable aspects of metabolism.
Via signal transduction pathways, they interact with transcription factors through phosphorylation cascades to regulate the expression
of target genes.
Brassinosteroids are also signalling molecules in abiotic stress responses such as drought, salinity, high temperature, low temperature
and heavy metal stresses.
Outwith plants, they may have biomedical applications as anticancer drugs for endocrine-responsive cancers to induce apoptosis
and inhibit growth.
Some plant species produce small amounts of steroid hormones that are often considered to be of animal origin only,
including progesterone and testosterone, and these may have physiological roles in plants.
The phytoecdysteroids, polyhydroxylated ketosteroids that resemble androgens and insect-moulting hormones in structure, are perhaps surprisingly derived from cholesterol (in some species from lathosterol). They offer protection against plant-eating insects by causing them to moult, undergo metabolic destruction and eventually die. The most common of these is 20-hydroxyecdysone, but more than 400 related molecules are known, with functions in growth regulation as well as defence (they may have pharmaceutical applications). Withanolides are complex plant oxysterols built on an ergostane skeleton, which are believed to be defence compounds against insect herbivores.
5. Sterol Esters in Higher Plants
Sterol esters are present in all plant tissues, but they are most abundant in tapetal cells of anthers, pollen grains, seeds and senescent leaves. In general, they are minor components relative to the free sterols other than in leaf surface waxes. Usually, the sterol components of sterol esters are similar to the free sterols, although there may be relatively less of stigmasterol. The fatty acid components tend to resemble those of the other plant tissue lipids, but there can be significant differences on occasion. Sterol esters are presumed to serve as inert storage forms of sterols, as they are often enriched in the intermediates of sterol biosynthesis and can accumulate in lipid droplets within the cells. Although they have been found in some membranes, e.g., microsomes and mitochondrial preparations, their function there is uncertain. They may have a role in transport within cells and between tissues as they can be present in the form of soluble lipoprotein complexes.
Biosynthesis of sterol esters in A. thaliana is known to occur in the endoplasmic reticulum by the action of a phospholipid:sterol acyltransferase, which catalyses transfer of a fatty acyl group to the sterol from position sn-2 of phospholipids - mainly phosphatidylethanolamine; the enzyme is very different from those in animals and yeasts. However, an acyl CoA:sterol acyltransferase closer in structure to the animal enzyme has been characterized that prefers saturated fatty acyl-CoAs as acyl donors and cycloartenol as the acceptor molecule. The enzymes responsible for the hydrolysis of sterol esters in A. thaliana are not yet known.
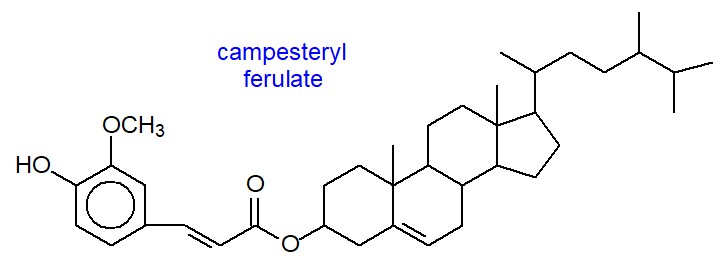
Certain distinctive phytosterol esters occur in the aleurone cells of cereal grains, including trans-hydroxycinnamate, ferulate (4-hydroxy-3-methoxycinnamate) and p‑coumarate esters. Rice bran oil is a rich source of esters of ferulic acid and a mixture of sterols and triterpenols, termed 'γ-orizanol'’, and a representative compound is illustrated. This is sold as a health food supplement, because of claimed beneficial effects, including cholesterol-lowering and antioxidant activities, enhanced muscle growth and sports performance, none of which have been confirmed by rigorous clinical testing.
6. Sterol Glycosides
Leaf and other tissue in plants contain a range of sterol glycosides and sterol acyl-glycosides in which the hydroxyl group at C3 on the sterol is linked to the sugar by a glycosidic bond. Other than in the genus Solanum, where they can represent up to 85% of the sterol fraction in tomato fruit, they tend to be minor components relative to other lipids. Typical examples (glucosides of β‑sitosterol) are illustrated below. Most of the common plant sterols occur in this form, but Δ5 sterols are usually preferred (Δ7 in some genera). Glucose is the most common carbohydrate moiety, but galactose, mannose, xylose and arabinose can be present depending on plant species, and occasionally, complex carbohydrates with up to five hexose units linked in a linear fashion are detected. Algae contains sterol glycosides with a wide range of sterol and carbohydrate components. In plant, animal, fungal and most bacterial steryl glycosides, there is a β‑glycosidic linkage, but in a few bacterial species there is an α-linkage.
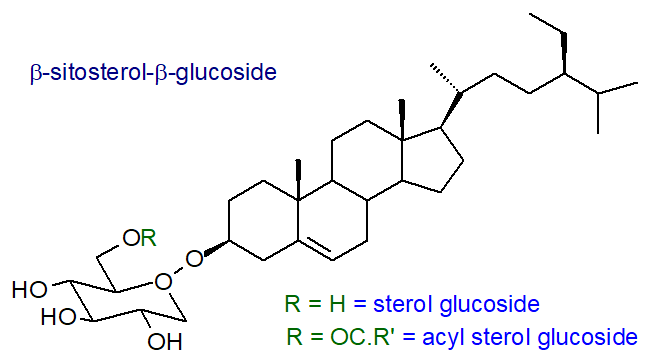
Usually, the sterol acyl-glycosides are present at concentrations that are two- to tenfold greater than those of the non-acylated forms, and they are known to be located in the plasma membrane, tonoplasts and endoplasmic reticulum. The nature of the fatty acid component in the acyl sterol glycosides can vary as well as the hydroxyl group to which they are linked, although it is most often position 6 of the glucose moiety. In potato tubers, the 6'-palmitoyl-β-D-glucoside of β-sitosterol is the major species, while the corresponding linoleate derivative predominates in soybeans.
Biosynthesis involves reaction of free sterols with a glucose unit catalysed by a sterol glycosyltransferase, or by reaction of the sterol with uridine diphosphoglucose (UDP-glucose) and UDP-glucose:sterol glucosyltransferase on the cytosolic side of the plasma membrane. The acyl donor for acyl sterol glycoside synthesis is not acyl-coenzyme A but is believed to be a glycerolipid. Although steryl β‑D‑glycoside hydrolases have been characterized from plants that reverse this reaction, no fatty acyl hydrolase activity for sterol acyl-glycosides is yet known.
 The
functions of sterol glycosides and sterol acyl-glycosides are slowly being revealed, and they are believed to be significant components
of the plasma membrane that associate with sphingolipids in raft-like domains.
In a lipid bilayer, it seems probable that sterol glycosides are oriented with the sterol moiety buried in the hydrophobic core
with the sugar located in the plane of the polar head groups of the membrane, while with sterol acyl-glycosides both the sterol moiety
and the fatty acid chain are embedded in the hydrophobic core.
The esterified form may be involved in the adaptation of plant membranes to low temperatures and other stresses.
It is possible that sterol glycosides have a role in signal transmission through membranes, and they are reported to be beneficial in the
response to pathogens.
Sitosterol-β-D-glucoside in the plasma membrane is believed to be the primer molecule for cellulose synthesis in plants,
as in cotton (Gossypium arboreum) fibre, where it may be required for the initiation of glucan polymerization.
Eventually, the sterol is removed from the polymer by a specific cellulase enzyme (the multimeric cellulase synthase is believed to be stabilized
by sterols in the plasma membrane).
One route to the biosynthesis of glucosylceramides in plants involves transfer of the glucose
moiety of sterol glycosides to ceramide.
The
functions of sterol glycosides and sterol acyl-glycosides are slowly being revealed, and they are believed to be significant components
of the plasma membrane that associate with sphingolipids in raft-like domains.
In a lipid bilayer, it seems probable that sterol glycosides are oriented with the sterol moiety buried in the hydrophobic core
with the sugar located in the plane of the polar head groups of the membrane, while with sterol acyl-glycosides both the sterol moiety
and the fatty acid chain are embedded in the hydrophobic core.
The esterified form may be involved in the adaptation of plant membranes to low temperatures and other stresses.
It is possible that sterol glycosides have a role in signal transmission through membranes, and they are reported to be beneficial in the
response to pathogens.
Sitosterol-β-D-glucoside in the plasma membrane is believed to be the primer molecule for cellulose synthesis in plants,
as in cotton (Gossypium arboreum) fibre, where it may be required for the initiation of glucan polymerization.
Eventually, the sterol is removed from the polymer by a specific cellulase enzyme (the multimeric cellulase synthase is believed to be stabilized
by sterols in the plasma membrane).
One route to the biosynthesis of glucosylceramides in plants involves transfer of the glucose
moiety of sterol glycosides to ceramide.
Sterol glycosides appear to be necessary for the pathogenicity of certain fungi, and ergosterol glycosides such as ergosterol 3β‑D‑glucoside are troublesome components of plant fungal pathogens. They enable the organisms to resist oxidative and pH stresses, and they have functions in cell recycling and membrane dynamics.
Sterol glycosides have only rarely been reported from organisms other than plants and fungi, although some bacteria such as the gram-negative bacterium Helicobacter pylori and Borrelia burgdorferi, the causative agent of Lyme disease produce cholesterol glucoside from host cholesterol during infection. On the other hand, cholesteryl glucoside has been found as a natural component of a few animal tissues, and through acting as immunoadjuvants, sterol glycosides are reported to be efficacious in protecting animal hosts against lethal Cryptococcal infections. In the human diet, sterol glycosides have potential benefits in that like free sterols they inhibit the absorption of cholesterol from the gut and reduce the plasma cholesterol levels. The fatty acids are removed from sterol acyl-glycosides by enzymes in the intestine.
Many plant species contain complex steroidal (or terpenoid) saponins, which consist of an aglycone often based on a triterpenoid furostanol or spirostanol skeleton derived from cholesterol or a steroidal alkaloid (in which nitrogen atoms replace one or more carbon atoms) and an oligosaccharide chain of two to five hexose or pentose moieties, attached to the 3β-hydroxyl group of the sterol. α‑Solanine is the well-known steroidal glycoalkaloid in potato and consists of the trisaccharide solanose linked to the alkaloid solanidine. These can interact with cholesterol in plant membranes to form insoluble complexes, which increase membrane permeability, while a further unusual property is that they are poisonous to fish.
7. Ergosterol and Other Sterols in Yeasts and Fungi
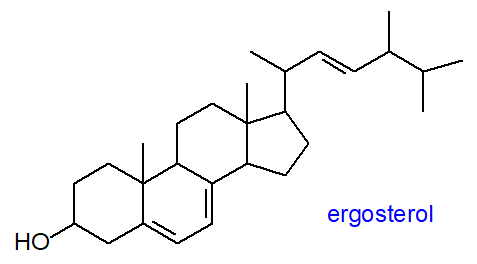 Yeasts
and fungi together with microalgae and protozoa can contain a wide range of different sterols.
Of these, ergosterol ((22E)‑ergosta-5,7,22-trien-3β-ol) is the most common and is accompanied
by other sterols not normally abundant in higher plants including cholesterol, 24‑methyl cholesterol, 24-ethyl cholesterol and
brassicasterol, depending upon species.
In Saccharomyces cerevisiae, which is widely studied as a model species of yeasts, ergosterol is the most abundant sterol (ca. 12% of all
lipids), with the highest levels in the plasma membrane (up to 40% of the lipids or 90% of the total cell sterols).
Yeasts
and fungi together with microalgae and protozoa can contain a wide range of different sterols.
Of these, ergosterol ((22E)‑ergosta-5,7,22-trien-3β-ol) is the most common and is accompanied
by other sterols not normally abundant in higher plants including cholesterol, 24‑methyl cholesterol, 24-ethyl cholesterol and
brassicasterol, depending upon species.
In Saccharomyces cerevisiae, which is widely studied as a model species of yeasts, ergosterol is the most abundant sterol (ca. 12% of all
lipids), with the highest levels in the plasma membrane (up to 40% of the lipids or 90% of the total cell sterols).
Like cholesterol and in contrast to the plant sterols, it is synthesised in the endoplasmic reticulum via lanosterol as an intermediate and then zymosterol, but the pathway diverges at this stage to produce fecosterol on the way to ergosterol (see the reading list below for further details). Ergosterol is transported to other organelles within the cell in a non-vesicular manner by two families of evolutionarily conserved sterol-binding proteins - 'Osh' and 'Lam', which can optimize the sterol composition of cell membranes rapidly under conditions of stress. As in humans, a Niemann-Pick protein NCR1 integrates sterols into the lysosomal membrane prior to further distribution as part of the mechanism of sterol homeostasis. Some antifungal drugs are targeted against ergosterol, either by binding to it to cause damaging cellular leakage or to prevent its synthesis from lanosterol.
Many mutants defective in ergosterol biosynthesis have been isolated, and these have yielded a great deal of information on the features of the sterol molecule required for its structural role in membranes of yeast and fungi. Whereas ergosterol stabilizes the liquid-ordered phase in the same manner as cholesterol and forms raft microdomains with sphingolipids in membranes, lanosterol does not. Ergosterol has a multiplicity of functions in the regulation of yeast growth in that it regulates membrane trafficking and polarized cell growth in filamentous fungi, it is essential for sexual reproduction and pheromone sensing, and it has numerous roles in pathogenicity and antifungal resistance.
Under some conditions, especially those that retard growth, a high proportion of the sterols in yeasts can be in esterified form, where they are stored in the organelles know as lipid droplets. Ergosterol esters are synthesised in yeast by enzymes (ARE1 and ARE2), which are related to ACAT-1 and ACAT-2 that perform this function in animals, and both transfer an activated fatty acid to the hydroxyl group at the C3-position of a sterol molecule. Specific sterol ester hydrolases that catalyse the reverse reaction have been characterized from yeasts, two in lipid droplets and one at the plasma membrane. Many fungal species and slime moulds contain steryl glycosides (ergosteryl β‑monoglucopyranosides in the former), but they are present at very low levels only in the widely studied yeast Saccharomyces cerevisiae.
In a reaction that seems to be unique among eukaryotes, most fungi can conjugate the 3β-hydroxyl group of ergosterol with aspartate in an RNA-dependent reaction catalysed by an ergosteryl-3β-O-L-aspartate synthase, with the reverse reaction using a dedicated hydrolase; ergosteryl-3β-O-glycine has been detected in one species. A phylogenomic study has shown that this pathway is conserved across higher fungi (except S. cerevisiae), including pathogens, and it has been suggested that these reactions constitute a homeostasis system with a potential impact upon membrane remodelling, trafficking, antimicrobial resistance and pathogenicity.
8. Bacterial Sterols
Hopanoids take the place of sterols in many species of bacteria, but it has long been recognized that some bacteria take up cholesterol and other sterols from host animals during infections for use as membrane constituents. Indeed, an external source of sterols is required for growth in species of Mycoplasma. While there have been several reports of biosynthesis of sterols by various bacterial species, a high proportion of these appear now to have been discounted because of fungal contamination, and in particular, the possibility of sterol biosynthesis in cyanobacteria has been controversial although Calothrix sp. NIES-4105 can demethylate sterols at the C-4 position.
That said, there is good evidence that a few species of prokaryotes at least have the capacity to synthesise sterols de novo, and there is definitive evidence that the marine myxobacterium Enhygromyxa salina produces cholesterol by a biosynthetic pathway that appears to be largely homologous to that in eukaryotes. The main exception is that demethylation at C-4 occurs through unique bacterial proteins, so distinguishing bacterial from eukaryotic synthesis. Among the eubacteria, certain methylotrophs (Methylobacterium and Methylosphaera species) produce mono- and dimethyl sterols, including lanosterol, while some soil bacteria produce 4‑desmethylsterols. Methylococcus capsulatus, which produces several unique Δ18(14)-sterols and is known to utilize a squalene epoxidase and a lanosterol-14-demethylase. It has now been established from gene sequence studies that a few bacteria contain enzymes of the sterol biosynthesis pathway such as oxidosqualene cyclase, but as these have no obvious evolutionary link, it seems probable that they were acquired via lateral transfer from eukaryotes.
9. Plant Sterols in the Human Diet and Health
The absorption of dietary plant sterols and stanols in humans is low (0.02-3.5%) compared to cholesterol (35-70%), although there are comparable amounts in an average Western diet. The explanation is believed to be that the Niemann-Pick C1-like protein 1 (NPC1L1), which is responsible for cholesterol absorption in enterocytes, does not take up plant sterols efficiently, while two transporters (ABCG5 and ABCG8) redirect any that are absorbed back into the intestinal lumen. In some rare cases, increased levels of plant sterols in plasma serve as markers for an inherited lipid storage disease (phytosterolemia) caused by mutations in the enterocyte transporters. Among many symptoms, accelerated atherosclerosis is often reported although the reasons for this are not clear. There is evidence that while plant sterols can substitute for cholesterol in maintaining membrane function in mammalian cells, they can exert harmful effects by disrupting cholesterol homeostasis with relevance to the brain as phytosterols are able to cross the blood-brain barrier but cannot be oxidized enzymatically because of the alkyl moiety on C24.
Substantial amounts of phytosterols are available as by-products of the refining of vegetable oils and of tall oil from the wood pulp industry. As it appears that they can inhibit the uptake of cholesterol from the diet and thereby reduce the levels of this in the plasma low-density lipoproteins, there is now a substantial use of such commercial sources of plant sterols as "nutraceuticals" in margarines and other foods, although a drug based on plant sterols (Cytellin, Eli Lilly Company) has been available in the U.S.A. since the 1950s. Hydrogenated phytosterols or "stanols" are also used in manufactured foods, and studies suggest they are as effective as sterols in reducing LDL cholesterol. The consensus amongst experts in the field (including the FDA in the USA) is that such dietary supplements do indeed have these effects, and such claims can be used in advertising of commercial products, with the important caveat that there are no randomized, controlled clinical trial data that establish ensuing benefits to health and cardiovascular disease.
Of the common plant sterols, β-sitosterol is reportedly of benefit for human pharmacology with no significant toxicity. It has value for the treatment of malignant cancers (breast, prostate, colon, lung, stomach and leukaemia) by triggering apoptosis in tumour cells through the regulation of the PI3K/Akt signalling pathway and by the generation of mitochondrial reactive oxygen species; it may form a template for the development of novel anti-tumour drugs. In addition, it interferes with multiple cell signalling pathways, and many other pharmacological effects are under investigation with reported beneficial effects for the development of the human foetus and new-born and for the treatment of non-alcoholic steatohepatitis, inflammatory bowel disease and allergic asthma, although improvements to the delivery systems appear to be required if its promise is to be fulfilled. Similar claims are now being made for stigmasterol, and this is also used for commercial production of the female hormone, progesterone.
It is not clear whether oxy-phytosterols are generated in animal tissues, but those produced by enzymatic or non-enzymatic means in plants can enter the food chain, especially when they are produced during cooking. Although they are not efficiently absorbed, 7‑keto‑sitosterol and 7‑keto‑campesterol have been detected in human plasma and have the potential to exert a variety of biological effects, as they have pro-atherogenic and pro-inflammatory properties in animal models.
10. Analysis
In the analysis of animal and plant sterols, sterol fractions are first isolated from lipid extracts by thin-layer or column chromatography. Alkaline hydrolysis procedures will cleave the bonds in sterol esters easily to release the free sterols, but it is more difficult to break glycosidic bonds, which require strong acid, and this can result in artefact formation; enzymatic hydrolysis methods involving glycosylases are now available that do not suffer from such problems. Individual sterol components can then be determined by gas chromatography in the presence of an internal standard (e.g., epicoprostanol or betulin), often after conversion to trimethylsilyl ether derivatives to give sharper peaks, although mass spectrometry is usually required for identification of individual components. Analysis of the minor oxysterols that may be found in plasma or foods is a rather specialized task because they tend to be present at rather low levels, and there is a danger of further oxidation or side reactions during the analytical process.
Sterol esters are trans-methylated for GC analysis of the fatty acid components, although the reaction may again be much slower than with glycerolipids, while intact sterol esters are best separated by reversed-phase HPLC, ideally linked to mass spectrometry. Analysis of sterol glycosides can be concerned as much with carbohydrate as with lipid chemistry.
Recommended Reading
- Barkas, F., Bathrellou, E., Nomikos, T., Panagiotakos, D., Liberopoulos, E. and Kontogianni, M.D. Plant sterols and plant stanols in cholesterol management and cardiovascular prevention. Nutrients., 15, 2845 (2023); DOI).
- Cassim, A.M., Gouguet, P., Gronnier, J., Laurent, N., Germain, V., Grison, M., Boutté, Y., Gerbeau-Pissot, P., Simon-Plas, F. and Mongrand, S. Plant lipids: Key players of plasma membrane organization and function. Prog. Lipid Res., 73, 1-27 (2019); DOI.
- Darnet, S., Blary, A., Chevalier, Q. and Schaller, H. Phytosterol profiles, genomes and enzymes - an overview. Front. Plant Sci., 12, 665206 (2021); DOI.
- Das, N., Mishra, S.K., Bishayee, A., Ali,E.S. and Bishayee, A. The phytochemical, biological, and medicinal attributes of phytoecdysteroids: An updated review. Acta Pharm. Sinica B, 11, 1740-1766 (2021); DOI.
- Evtyugin, D.D., Evtuguin, D.V., Casal, S. and Domingues, M.R. Advances and challenges in plant sterol research: fundamentals, analysis, applications and production. Molecules, 28, 6526 (2023); DOI.
- Ferrer, A., Altabella, T., Arró, M. and Borona, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res., 67, 27-37 (2017); DOI.
- Khan, Z. and others. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chemico-Biol. Interact., 365, 110117 (2022); DOI.
- Lee, A.K., Wei, J.H. and Welander, P.V. De novo cholesterol biosynthesis in bacteria. Nature Commun., 14, 2904 (2023); DOI.
- Lipko, A. and Swiezewska, E. Isoprenoid generating systems in plants - A handy toolbox how to assess contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthetic process. Prog. Lipid Res., 63, 70-92 (2016); DOI.
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev., 111, 6423-6451 (2011); DOI.
- Nolan, T., Chen, J. and Yin, Y. Cross-talk of brassinosteroid signaling in controlling growth and stress responses. Biochem. J., 474, 2641-2661 (2017); DOI.
- Rogowska, A. and Szakiel, A. The role of sterols in plant response to abiotic stress. Phytochem. Rev., 19, 1525–1538 (2020); DOI.
- Rohmer, M. From molecular fossils of bacterial hopanoids to the formation of isoprene units: discovery and elucidation of the methylerythritol phosphate pathway. Lipids, 43, 1095-1107 (2008); DOI.
- Schlag, S., Huang, Y.I. and Vetter, W. GC/EI-MS method for the determination of phytosterols in vegetable oils. Anal. Bioanal. Chem., 414, 1061-1071 (2022); DOI.
- Sokolov, S.S., Trushina, N.I., Severin, F.F. and Knorre, D.A. Ergosterol turnover in yeast: an interplay between biosynthesis and transport. Biochemistry (Moscow), 84, 346-357 (2019); DOI.
- Sonawane, P.D., Pollier, J., Panda, S. and 19 others. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nature Plants, 3, 16205 (2016); DOI.
- Vanmierlo, T., Husche, C., Schött, H.F., Pettersson, H. and Lütjohann, D. Plant sterol oxidation products - analogs to cholesterol oxidation products from plant origin? Biochimie, 95, 464-472 (2013); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: March 6th, 2024 | ||
