Fatty Acids: Straight-Chain Monoenoic
 Straight- or normal-chain (even-numbered),
monoenoic fatty acids, i.e., with one double bond, make up a high proportion of the total in most natural lipids.
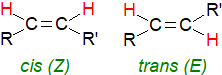
Normally, the double bond is of the cis- or Z-configuration, although some fatty acids occur occasionally in nature with
trans- or E‑double bonds (Z from "zusammen", E from "entgegen" in German - depending on whether two substituent groups of
higher priority are on the same or opposite sides, respectively, of the double bond).
Straight- or normal-chain (even-numbered),
monoenoic fatty acids, i.e., with one double bond, make up a high proportion of the total in most natural lipids.
Normally, the double bond is of the cis- or Z-configuration, although some fatty acids occur occasionally in nature with
trans- or E‑double bonds (Z from "zusammen", E from "entgegen" in German - depending on whether two substituent groups of
higher priority are on the same or opposite sides, respectively, of the double bond).
1. Structure and Nomenclature
The most abundant monoenoic fatty acids in animal and plant tissues have straight (unbranched) chains with 16 or 18 carbon atoms, but analogous fatty acids with 10 to 36 carbon atoms occur naturally in an esterified state, and fifty structurally distinct monoenoic fatty acids have been identified in plants alone. They are named systematically from the saturated hydrocarbon with the same number of carbon atoms, the final 'ane' being changed to 'enoic'. Thus, the fatty acid with 18 carbon atoms, one double bond of the Z-configuration in position 9 relative to the carboxyl group, and the structural formula -

- is systematically named 9Z-octadecenoic acid, although it is more usual to see the trivial name oleic acid in the scientific literature, while in shorthand nomenclature, it is often simply designated '18:1' (or 9Z-18:1, (Z)-9-18:1, Δ9-18:1 or 9c-18:1). Double bonds in fatty acids are often assumed to be of the Z-configuration when there is no definitive information, and the older terms cis and trans remain useful to describe related structural groups.
The position of the double bond can also be denoted as (n‑x), where n is the chain-length of the fatty acid and x is the number of carbon atoms from the double bond to the terminal carbon atom of the molecule of the molecule, i.e., oleic acid is 18:1(n-9) (or often 18:1n-9, or in the early literature 18:1ω9). Although this contradicts the convention that the position of substituents should be related to that of the carboxyl carbon, it is of great convenience to lipid biochemists. Animal and plant lipids frequently contain families of monoenoic fatty acids with similar terminal structures, but with different chain-lengths that may arise from a common precursor either by chain-elongation or by beta-oxidation, and the (n‑x) nomenclature helps to point out such relationships.
A list of a few monoenoic fatty acids together with their systematic and trivial names and their shorthand designations is given in Table 1, although I prefer to avoid trivial names for all but the most common of these.
Table 1. The common monoenoic fatty acids |
||
| Systematic name | Trivial name | Shorthand designation |
| 9Z-tetradecenoic | myristoleic | 9-14:1 or 14:1(n-5) |
| 6Z-hexadecenoic | sapienic | 6-16:1 or 16:1(n-10) |
| 7Z-hexadecenoic | hypogeic | 7-16:1 or 16:1(n-9) |
| 9Z-hexadecenoic | palmitoleic | 9-16:1 or 16:1(n-7) |
| 6Z-octadecenoic | petroselinic | 6-18:1 or 18:1(n-12) |
| 9Z-octadecenoic | oleic | 9-18:1 or 18:1(n-9) |
| 11Z-octadecenoic | cis-vaccenic | 11-18:1 or 18:1(n-7) |
| 11Z-eicosenoic | gondoic | 11-20:1 or 20:1(n-9) |
| 13Z-docosenoic | erucic | 13-22:1 or 22:1(n-9) |
| 15Z-tetracosenoic | nervonic | 15-24:1 or 24:1(n-9) |
| 3E-hexadecenoic | 3E-18:1 | |
| 9E-octadecenoic | elaidic | 9E-18:1 |
| 11E-octadecenoic | vaccenic | 11E-18:1 |
A cis-double bond in a fatty acid introduces a 30° bend in the alkyl chain, and this tends to result in looser packing in membranes or crystal structures. Very long-chain (20:1 upwards) cis-monoenoic fatty acids have relatively high melting points, but the more common C18 monoenes are liquid at room temperature as are triacylglycerols (or oils and fats) containing high proportions of monoenoic fatty acids. Analogous fatty acids with trans double bonds are normally higher melting. Monoenoic fatty acids with branched chains have been identified in some bacteria and marine invertebrates (c.f., here..).
2. Occurrence
cis-18:1 Isomers
Oleic acid (9Z-18:1 or 18:1(n-9)) is by far the most abundant monoenoic fatty acid in plant and animal tissues, both in structural lipids and in depot fats. It can comprise 30 to 40% of the total fatty acids in adipose fats of animals and 20 to 80% of the seed oils of commerce. Olive oil contains up to 78% of oleic acid and may be of special nutritional value as part of the Mediterranean diet. Indeed, this acid has many noteworthy biological activities discussed in relation to animal metabolism below, both in the free and esterified state with amides such as oleamide and oleoylethanolamine having their own metabolic properties. In plants, it is reported to influence defence signalling against bacterial and fungal pathogens by upregulating the expression of genes that regulate nitric oxide production. Oleic acid is the biosynthetic precursor of a family of fatty acids with the (n‑9) terminal structure and with chain-lengths of 20 to 24 or more.
cis-Vaccenic acid (11Z-18:1 or 18:1(n-7)) is a common monoenoic fatty acid of bacterial lipids, and it is usually present as a minor component of most plant and animal tissues. It is occasionally a more abundant constituent of those plants containing appreciable amounts of its biosynthetic precursor, palmitoleate (9Z-16:1 or 16:1(n-7)). Elongation of palmitoleate is the source of 11Z‑18:1 in animal tissues, but vaccenic acid per se, from the Latin 'vacca' meaning cow and abundant in ruminant fats, is the trans or E isomer.
Petroselinic acid (6Z-18:1) occurs up to a level of 50% or more in seed oils of the Apiaceae (Umbelliferae) family, including carrot, celery, parsley and coriander, and Thunbergia sp. contain up to 92% of this acid. It is reported to have antidiabetic, antibacterial, and antifungal properties. 10‑18:1 (with 8-16:1) is considered specific for methane-oxidizing bacteria, but apart from these, monoenoic isomers with a double bond in an even-numbered position are only occasionally encountered in nature. Other cis-octadecenoic acids, such as 5, 7, 13 and 15Z-18:1, are occasionally seen in lipids of fish or marine invertebrates, while 5-18:1 is a minor component of the seed oil of meadowfoam and of a few other plants.
cis-10:1 to 17:1 Isomers
2Z-Monoenoic acids (C10 to C16), occasionally with methyl branches or further double bonds, are present in Gram-negative bacteria and are known as 'diffusible signalling factors'. They influence microbial virulence as well as cell adhesion, biofilm formation and cell motility.
9Z-Decenoic acid together with 9-12:1 and 9-14:1 are trace component of cow's milk fat, and the last of these is a minor but common constituent of marine oils, and it is occasionally reported from seed oils and bacteria. 4-Decenoic acid is present in seed oils of the Lauraceae (as has 4‑12:1 and 4‑14:1), while 5- and 7-14:1 have been found in some lipids of bacterial or marine origin. Other medium-chain monoenoic isomers have been detected in body fluids from beta-oxidation of longer-chain fatty acids and in microbial lipids, while various 15:1 and 17:1 isomers are described from time to time, mostly in microbial lipids or fish oils.
 9Z-Hexadecenoic acid (palmitoleic acid, 9-16:1 or 16:1(n-7)) is a ubiquitous but normally minor
component of animal lipids, but it can be much more abundant in fish oils such as cod-liver oil, when it may be accompanied by the 7- and
occasionally the 11-isomer.
It is a major constituent of a few plant oils such as macadamia nuts or the pulp of sea buckthorn fruit.
In mice, it has recently been found to be a lipokine, a newly coined word to define a lipid hormone, i.e.,
it is an adipose tissue-derived signalling molecule, which amongst other effects stimulates
the action of insulin in muscle (see below), and it is an essential covalent modifier of
Wnt proteins.
As there is so little in a normal diet, it has been suggested that palmitoleic acid may serve as a marker for lipogenesis
de novo from glucose.
In plants, it is protective against certain fungal pathogens.
The isomeric 7Z-hexadecenoic acid (7-16:1 or 16:1(n‑9)) is reported to be enriched in the lipids of foamy monocytes
and is anti-inflammatory; it may be a biomarker for early detection of cardiovascular disease.
9Z-Hexadecenoic acid (palmitoleic acid, 9-16:1 or 16:1(n-7)) is a ubiquitous but normally minor
component of animal lipids, but it can be much more abundant in fish oils such as cod-liver oil, when it may be accompanied by the 7- and
occasionally the 11-isomer.
It is a major constituent of a few plant oils such as macadamia nuts or the pulp of sea buckthorn fruit.
In mice, it has recently been found to be a lipokine, a newly coined word to define a lipid hormone, i.e.,
it is an adipose tissue-derived signalling molecule, which amongst other effects stimulates
the action of insulin in muscle (see below), and it is an essential covalent modifier of
Wnt proteins.
As there is so little in a normal diet, it has been suggested that palmitoleic acid may serve as a marker for lipogenesis
de novo from glucose.
In plants, it is protective against certain fungal pathogens.
The isomeric 7Z-hexadecenoic acid (7-16:1 or 16:1(n‑9)) is reported to be enriched in the lipids of foamy monocytes
and is anti-inflammatory; it may be a biomarker for early detection of cardiovascular disease.
6Z-Hexadecenoic acid (6-16:1 or 16:1(n-10) or ‘sapienic’ acid) is the single most abundant component in human sebum lipids and may be biocidal, but it is rarely found elsewhere in nature. In sebum, it is accompanied by an elongation and desaturation product 5,8‑octadecadienoic acid. It has been detected in plasma and erythrocytes with increased levels in morbidly obese patients, and although its role in these tissues is not known, it is present in macrophages in mice as well as humans, together with the two other 16:1 isomers. 6-16:1 occurs in some seed oils of the Umbelliferae and in Thunbergia species, and the 4-isomer has been detected in some seed oils and various marine sources. A further isomer, 10Z‑hexadecenoic acid (10‑16:1), is a component of triacylglycerols in Mycobacterium vaccae, a soil-derived bacterium, and is reported to have anti-inflammatory, immunoregulatory and stress resilience properties.
cis-20:1 to 32:1 Isomers
Very-long-chain monoenoic fatty acids of the (n-9) family occur in a variety of natural sources, often accompanied by analogous fatty acids of the (n‑7) family) in animal tissues, and those from 20:1 to 26:1 are normal constituents of sphingolipids from both animals and plants. Odd-numbered very-long-chain monoenes (23:1 upwards) from brain belong to the (n‑8) and (n-10) families, presumably because they are synthesised by chain-elongation of 9-17:1 (17:1(n-8)) and 9‑19:1 (19:1(n‑10)), respectively (see below). An even wider range of chain-lengths is found in monoenes from plant waxes and sponge lipids, and some of these fatty acids may contain methyl branches as well as the double bond. Monoenes of the (n-5) family (16:1 to 24:1) are utilized for the biosynthesis of the anacardic acids in plants.
11Z-Eicosenoic acid is a common if minor constituent of animal tissues and fish oils, often accompanied by the 13-isomer. In plants, it is present in rapeseed oil and seed oils of other Brassicas, while 5Z-20:1 can amount to 67% of the total fatty acids in meadowfoam oil.
Erucic acid (13Z-22:1) occurs naturally in fish oils and in small amounts in the phospholipids of animal tissues (often with some 15-22:1), but it is probably best known as the major component (up to 66%) of the total fatty acids in native rapeseed oil, where its presence is generally considered harmful to consumers (discussed below); edible cultivars lacking erucic acid have been developed. 5-22:1 is a component of meadowfoam oil.
15Z-Tetracosenoic acid (nervonic) is present in small amounts in phospholipids and especially sphingolipids of animal tissues; the trivial name derives from its occurrence in brain myelin sphingolipids, where it is indispensable for sustaining the normal function and structural integrity of nerve cells. It is a major constituent of some seed oils, such as Brassica and Lunaria species. 17‑26:1 is found in sphingolipids of animals (including sponges), as well as a few seed oils (e.g., Tropaeolum and Ximenia).
Trans fatty acids
Although monoenoic fatty acids with double bonds of the trans- or E-configuration are relatively rare in nature, tissues of ruminant animals, such as cows, sheep and goats, can contain a number of different 18:1 isomers (and those of 14:1, 16:1 and 17:1) of both the cis and trans-configuration as shown in Table 2. 11E‑18:1 (vaccenic) makes up 50% of the trans-monoenes, which can comprise 10 to 15% of the total monoenes or 3 to 4% of the total fatty acids, but there are appreciable amounts of other isomers from 7E- to 16E-18:1. With the cis-isomers, 9Z- and 11Z-18:1 predominate as might be expected. 9E-16:1 is found in animal tissues after β‑oxidation of dietary vaccenic acid. These trans-monoenes are metabolites from the biohydrogenation of linoleic and linolenic acids during digestion of herbage by rumen microorganisms (see below) before they are taken up into the tissues of the animal and find their way into meat and dairy foods.
Table 2. Distribution of double bonds in cis and trans‑octadecenoates from bovine adipose tissue (wt % of the total in each class). |
||
| Double bond position | cis-18:1 | trans-18:1 |
|---|---|---|
| 7 | 1 | 1 |
| 8 | 1 | 2 |
| 9 | 85 | 5 |
| 10 | 2 | 12 |
| 11 | 9 | 47 |
| 12 | 2 | 6 |
| 13 | 7 | |
| 14 | 7 | |
| 15 | 6 | |
| 16 | 8 | |
| Hay, J.D. and Morrison, W.R. Lipids, 8, 94-95 (1973); DOI. | ||
In addition, monoenoic fatty acids with E-configurations have been found in the membrane lipids of some aerobic bacteria, such as Pseudomonas putida and P. aeruginosa, where they are derived from isomerization of existing double bonds of the Z-configuration by an isomerase (Cti), a periplasmic heme-c enzyme, using phospholipids as substrates without a need for protein partners. As trans-isomers have higher melting points than the cis-equivalents, this enables the organisms to modulate membrane fluidity rapidly to make them more rigid in response to external stresses that impede their growth, including exposure to antibiotics. trans-18:1 isomers are often present in refined seed oils in commerce as a by-product of industrial hydrogenation of the polyunsaturated components to reduce their propensity to oxidation in foods.
3E-Hexadecenoic acid is a normal constituent of the photosynthetic tissues (chloroplasts) of plants, where it is located characteristically in the phosphatidylglycerol fraction, and it is presumed to have some as yet undefined minor function, as Arabidopsis mutants that lack this fatty acid appear to grow normally. 9E- and 3E‑18:1 are occasionally reported from seed oils. Some bacteria contain 9E-16:1, which results from isomerization of the cis-isomer, while 6E-18:1 has been detected in several organisms of marine origin.
3. Biosynthesis of Monoenoic Fatty Acids
In nearly all higher organisms, including many bacteria, yeasts, algae, protozoa, plants and animals, double bonds are introduced into fatty acids by aerobic desaturation mechanisms that utilize preformed fatty acids as the substrates and desaturase enzymes, which are oxygenases that can remove two protons from adjacent carbon atoms in a hydrocarbon chain to create a carbon-carbon double bond. In an exploration of 56 eukaryotic genomes, 275 desaturase homologs were identified with four subfamilies introducing double bonds at distinct locations. A different mechanism for biosynthesis of monoenoic fatty acids is present in simple proteobacteria as discussed below.
Animals and Yeasts: Depending on species, there are many membrane-bound stearoyl-CoA Δ9-desaturase (SCD) isoforms, the predominant type, which share considerable sequence homology and have overlapping but characteristic tissue specificities. In humans, there are two isoforms with SCD1 expressed in adipose tissue, liver, lungs, brain and heart predominantly, while SCD5 is expressed mainly in the brain and pancreas. The latter shares limited homology with rodent isoforms (four in mice, SCD1 to 4) and was once thought to be unique to primates but has now been found in other vertebrates, including ruminants, pigs, dogs and birds. Human SCD1 can utilize saturated acyl-CoAs with an acyl chain length of 14 to 19 carbons as substrates but favours octadecanoyl-CoA (stearoyl or 18:0-CoA) over others. In mice, SCD2 is expressed ubiquitously and is the main isoform in the brain, although it is induced by high-fat diets in adipose tissue, lung and kidney; it synthesises palmitoleoyl-CoA and oleoyl-CoA, while SCD3 prefers palmitoyl-CoA as substrate. In other animals, isoforms of desaturases can have differing fatty acyl specificities.
The CoA ester of octadecanoic acid is converted directly to oleoyl-CoA by these enzymes via a concerted removal of hydrogen atoms from carbons 9 and 10 (D‑stereochemistry in each instance). There are three components to the desaturase complex: a flavoprotein, i.e., NADH cytochrome b5 reductase, a haem-containing protein, i.e., cytochrome b5, and the desaturase itself, and they are believed to be situated next to each other in membranes. From X-ray studies of human SCD1 in complex with its substrate stearoyl-CoA in the endoplasmic reticulum, it has been shown that there is a cytosolic domain containing a di‑metal catalytic centre consisting of two iron cations that are coordinated by nine conserved histidine residues and one water molecule, while four trans-membrane alpha-helices present a hydrophobic core. Acyl-CoA substrates bind to the surface of the cytoplasmic domain both by hydrogen bonding and by ionic interactions between the phosphates of CoA and the positively charged surface of the enzyme that enable the acyl-chain to enter a hydrophobic tunnel, which extends to the interface of the cytoplasmic and transmembrane domains with its shape determining the positional specificity of the enzyme and the cis-conformation of the product. Molecular oxygen and a reduced pyridine nucleotide (NADH or NADPH) are required cofactors with the oxygen activated at the di-iron centre and reduced to water, while the ferrous catalytic centre is regenerated by transfer of electrons from cytochrome b5.
 |
| Figure 1. Desaturation of stearoyl-coA to oleoyl-CoA in animals. |
Inhibition of the desaturase by its product is possible, but normally this is rapidly removed in vivo by membrane-bound acyltransferases so this is not an issue. In the yeast, Saccharomyces cerevisiae, the Δ9-desaturase Ole1 is a component of a super-complex with some of the acyltransferases responsible for glycerolipid synthesis.
In animals, SCD1 is known to be tightly regulated by hormones and many other cellular and dietary factors, and for example, this isoform is induced by insulin and repressed by leptin, the hormone derived from adipocytes that suppresses appetite and regulates energy homeostasis and many aspects of lipid metabolism. In RAW macrophages, SCD1 is the source of minor amounts of odd-chain monounsaturated fatty acids with n-6, n-8, and n-10 double bonds, as well as n-5, n-7, and n-9 families of even-chain isomers. At the transcriptional level, it is regulated mainly by sterol responsive element binding protein SREBP-1c, which also induces the expression of other enzymes for fatty acid biosynthesis de novo, i.e., Δ5, Δ6 and Δ9 desaturases and ELOVL6. Other transcription factors include the liver X receptor and peroxisomal proliferator-activated receptors (PPARs), while SCD1 is controlled by rapid proteolytic cleavage. Stearoyl‑CoA desaturases in turn control many aspects of lipid metabolism as is discussed below.
Palmitoleate is synthesised from palmitate by a similar mechanism via the stearoyl-CoA desaturase, but sapienic acid (6-16:1) results from the action of a different enzyme, Δ6 desaturase (FADS2), normally associated with desaturation of linoleic acid (see our web page on polyunsaturated fatty acids). There are tissue-specific mechanisms in the human sebaceous gland to enable FADS2 to act in this way, including a reduction in competing desaturase activity. In certain cancer cells, 8-18:1 can be produced by this mechanism when uptake of fatty acids of exogenous origin is inhibited, while a Δ11 double bond can be introduced similarly into odd-chain fatty acids in RAW macrophages.
Chain Elongation: Subsequently, oleic and other monoenoic fatty acids can be chain elongated by two carbon atoms to give longer-chain fatty acids of the (n-9) family, while palmitoleate (9-16:1 or 16:1(n-7)) is the precursor of the (n-7) family of fatty acids. In mammalian systems, the elongases differ from those for the biosynthesis of longer-chain polyunsaturated fatty acids, and elongases (ELOVL proteins) in general are discussed in our web page dealing with saturated fatty acids. Of the seven elongation iso-enzymes, ELOVL1, 3, 4, 6 and 7 participate in the elongation of monounsaturated fatty acids but with differing tissue locations and substrate requirements; ELOVL4 is responsible for the C24 and longer-chain fatty acids in sphingolipids. In contrast, alpha- and beta-oxidation can occur to give shorter chain fatty acids of the two monoene families, with the former yielding odd-chain homologues.
 |
| Figure 2. Biosynthesis of the n-9 and n-7 families of monoenoic fatty acids. |
Plants: There are two unrelated types of fatty acid desaturase (with multiple isoforms) in higher plants that are involved in the synthesis of monoenoic fatty acids, i.e., soluble acyl-acyl carrier protein (ACP) desaturases and membrane-bound desaturases, both of which are di‑iron-oxo enzymes with a dimeric quaternary structure. Those most studied are the soluble enzymes that occur mainly in the plastid and use preformed fatty acids bound to ACP, rather than to CoA, as the substrate. The crystal structure of the enzyme from the castor plant (Ricinus communis) has been characterized by spectroscopy and molecular biology methods and consists of two identical monomers, each containing a catalytic site with a di‑iron-oxo cluster and a potential substrate-binding region, i.e., a hydrophobic pocket traversing the protein. The iron is reduced by ferredoxin, and molecular oxygen is bound to it to enable removal of hydrogens and electrons in a stepwise manner from carbons 9 and 10 of stearoyl-ACP to create a double bond. After modelling studies, it has been suggested that the stearoyl substrate fits into the hydrophobic pocket particularly well if it adopts a gauche conformation at the C9–C10 positions in the region adjacent to the di-iron core, thus facilitating regio-selective syn‑dehydrogenation to generate oleate (sometimes described as "a textbook example of a lock-and-key type of binding site"). Newly synthesised monoenoic acids are converted to the CoA esters and transferred to the endoplasmic reticulum for esterification and conversion to linoleic and linolenic acids (see our web page on polyunsaturated fatty acids).
The structures of other soluble plant desaturases that differ in the positional specificity of double bond insertion and the chain-length of the substrate have been determined, and these are responsible for the biosynthesis of the many unusual fatty acids found in plants. As they are similar to the stearoyl ACP Δ9‑desaturase in amino acid sequences and in the di-iron binding amino acid motifs, it has been suggested that changes to as few as four amino acid locations can change the regiospecificity of desaturation, probably by altering the presentation of the substrate to the catalytic site. For example, five residues substituted from the castor sequence into the corresponding positions in the Thunbergia sequence converted the Δ6-16:0-ACP desaturase of the latter into a Δ9‑18:0‑ACP desaturase. A mechanistic link between desaturases and hydroxylases has been observed in some plants.
Those plant desaturases located in membranes are very different from cytosolic desaturases, and that in the endoplasmic reticulum uses cytochrome b5 reductase as the flavoprotein with cytochrome b5 as the electron carrier. They can often utilize substrate fatty acids when esterified to lipids (other than the CoA or ACP esters), a factor that can influence regiospecificity, as the position of desaturation obtained with a bifunctional 7/9‑16:0 desaturase is reportedly controlled by its subcellular targeting to the precursor fatty acid as a component of different glycerolipids in each organelle. Some cyanobacteria have Δ9 desaturases (Des or acyl-lipid desaturases) that act upon stearic acid esterified to position sn-1 of glycerophospholipids, while others require the substrate in positions sn-2. With membrane desaturases, it seems probable that the catalytic site differs fundamentally in structure from soluble desaturases, perhaps by having a cleft, which substrates enter laterally, rather than a deep binding cavity. These desaturases in cyanobacteria and plants participate in remodelling lipids to change membrane fluidity in response to temperature variation and other abiotic stresses.
A Δ6-stearoyl-acylcarrier protein (18:0-ACP) desaturase from Thunbergia laurifolia in plastids responsible for the biosynthesis of petroselinic acid (6‑18:1) has been characterized and shown to differ from the archetypal Δ9-18:0-ACP desaturase by mutations in key amino acids. In seed oils of the Umbelliferae, a different mechanism operates in which this fatty acid is synthesised by a desaturase that removes hydrogens from position 4 of palmitoyl-ACP before the resulting 4-16:1 is elongated by two carbon atoms to give 6-18:1. After release of the free acid by a thioesterase, it is transferred to the endoplasmic reticulum and converted to the CoA ester for incorporation into triacylglycerols. Sequential two-carbon elongation of monoenoic acids by elongases in this manner is a common method of altering the positional distribution of double bonds relative to the carboxyl group in hydrocarbon chains in plants as in animals.
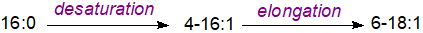 |
| Figure 3. Biosynthesis of petroselinic acid in the Umbelliferae. |
Little is known of the mechanism of biosynthesis of 3E-16:1 in plants, other than it requires molecular oxygen, while palmitic acid esterified to position sn-1 of phosphatidylglycerol is the probable substrate for the presumed desaturase.
Bacteria: Several routes to the generation of monoenoic fatty acids in bacteria are known, but most synthesise these by anaerobic mechanisms that utilize the fatty acid synthetase II (FAS II), in which the various enzymes in the process are dissociated (as discussed in greater detail in our web page on saturated fatty acids). In brief, there are four enzymatic reactions in each iterative cycle of chain elongation, and in the first step, 3‑ketoacyl-ACP synthase I (FabB) or II (FabF) adds a two-carbon unit from malonyl-ACP to the growing acyl-ACP, before the resulting keto ester is reduced by a NADPH-dependent 3-ketoacyl-ACP reductase (FabG). The elements of water are removed by a 3‑hydroxyacyl-ACP dehydratase (FabA or FabZ), before the last step in which an enoyl-ACP reductase (FABI or FabK) generates the saturated acyl-ACP. To introduce a double bond, this process is interrupted at critical points, depending on the organism
In the much-studied facultative anaerobe Escherichia coli, the double bond in monoenes is generated at a branch point in fatty acid synthesis at the dehydratase step during the fourth cycle of chain elongation. Instead of chain elongation proceeding as normal, an isomerase converts the 2E‑decenoyl-ACP to 3Z-decenoyl-ACP. Of the two hydratases, FabZ can catalyse dehydration only, while FabA is bifunctional and carries out both dehydration and isomerization. 3Z-Decenoyl-ACP is not a substrate for the enoyl-ACP reductase, but it can be further elongated eventually to an 11Z‑18:1 fatty acid. Different isoforms of the condensing enzyme exist with FabF being the most common, while FabB is important for the introduction of the double bond. FabF is responsive to temperature and may regulate the degree of unsaturation and thence fluidity of membranes in an organism, a process known as homeoviscous adaptation. The reaction is terminated by acyltransferases, such as the glycerol-3-phosphate acyltransferase, which transfers the fatty acyl group to a complex lipid.
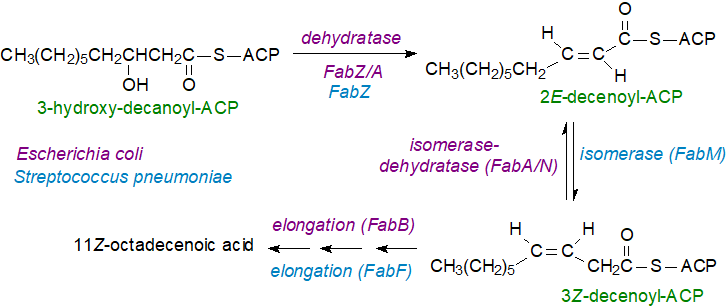 |
| Figure 4. Biosynthesis of monoenoic fatty acids in bacteria. |
For many years, this was thought to be the characteristic pathway for biosynthesis of unsaturated fatty acids in all bacteria, but it is now recognized that this precise mechanism is restricted to a few proteobacteria, such as E. coli. While the detailed mechanisms and enzymes for most bacteria have yet to be adequately characterized, Gram-positive bacteria are known to utilize a variation in the mechanism in which a dedicated 2E,3Z‑decenoyl-ACP isomerase acts after the dehydration step. Streptococcus pneumoniae introduces the double bond by means of a such an enzyme (FabM), which bears no structural similarity to FabA, although it utilizes the same substrate. In Aerococcus viridans, the pathway for synthesis of monounsaturated fatty acids branches at the 3-hydroxydodecanoyl-ACP stage.
Aerobic mechanisms exist in some bacteria, and Pseudomonas aeruginosa has been shown to have two aerobic desaturase enzymes as well as an anaerobic system. One of these is a membrane-associated Δ9‑desaturase, which is capable of regioselective cis-dehydrogenation through activation of molecular oxygen with a di-iron cluster at the catalytic site and introduces a double bond into the Δ9‑position of fatty acyl chains attached to the sn-2 position of existing glycerophospholipids; the other enzyme reacts in the same way with acyl-CoA from exogenous saturated fatty acids. Bacillus subtilis has a Δ5-acyl desaturase, which is an integral membrane protein with a di-iron unit held by three histidine clusters and utilizes ferredoxin and flavodoxins as electron donors.
In some bacteria, including Pseudomonas aeruginosa, a common pathogen, there is a cis-trans isomerase (Cti), i.e., a periplasmic haeme-c enzyme, which catalyses cis-trans isomerization in phospholipid-bound fatty acids in order to rapidly modulate membrane fluidity in response to stresses that impede bacterial growth.
Rumen metabolism: Dietary fatty acids such as linoleic acid are subjected to biohydrogenation by bacteria in the rumen of cows, goats and sheep (ruminant animals) to remove one (or more) double bond and generate trans isomers, including monoenes. Many different organisms are present and create many different metabolites by mechanisms that are still poorly understood (possibly including a cis-trans isomerase (Cti)), but a major route involves the isomerization of the cis double bond in position 12 to form conjugated octadec-9Z,11E-dienoic (rumenic) acid (this step is discussed further in our web page on conjugated fatty acids), which undergoes biohydrogenation to yield octadec-11E-enoic (vaccenic) acid. Both fatty acids are taken up by the host animals and find their way into meat and dairy foods.
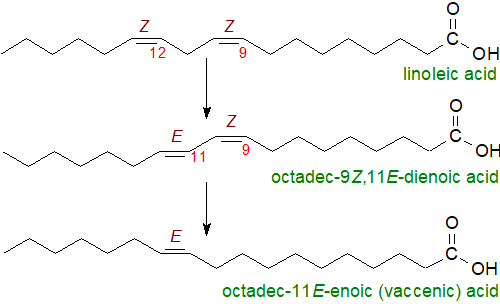 |
| Figure 5. Biohydrogenation of linoleic acid by rumen microorganisms. |
Catabolism: Most unsaturated fatty acids are broken down in animal tissues for energy by the multi-step process of β‑oxidation, as discussed in our web page on carnitines.
4. Chemical Reactivity
Monounsaturated fatty acids are much less reactive than polyunsaturated toward autoxidation, a process discussed in relation to tocopherols and elsewhere in these pages, but they are not immune. There is an initial abstraction of a hydrogen atom during initiation to form a radical at either of the allylic positions, which correspond to carbons 8 and 11 of oleic acid, to yield (Z)-peroxyl radicals by reaction with oxygen. Both allylic radicals have resonance states that can generate (E)‑peroxyl radicals, i.e., some geometrical and positional isomerization of the double bond occurs. Propagation of the reaction and eventual termination can then take place as in more unsaturated systems.
 |
| Figure 6. Chemical reactivity of monoenoic fatty acids with oxidizing agents. |
Isomerization of isolated double bonds from the Z to E configuration can occur rapidly in the presence of thiyl radicals, without double bond migration, and the same reaction can occur more slowly with nitrogen radicals. With the latter, there can be some addition to the double bond to synthesise nitro fatty acids.
5. Nutritional and Metabolic Aspects
The relative proportion of saturated to monounsaturated fatty acids is an important aspect of phospholipid composition and this ratio regulates overall cell function, growth, and survival with an influence on such disease states as cardiovascular disease, obesity, diabetes, liver dysfunction, intestinal inflammation, neuropathological conditions and cancer. In human metabolic disease, for example, there are increased ratios of 18:1 and 16:1 fatty acids relative to the saturated precursors, while in diabetes, monoenes have been shown to have cyto-protective actions in pancreatic β‑cells. cis‑Monoenoic acids have desirable physical properties for membrane lipids in that they are liquid at body temperature yet are relatively resistant to oxidation. They are now recognized by nutritionists as being beneficial in the human diet, and oleic acid comprises a high proportion of the fatty acids of olive oil, a major fat component of the ‘Mediterranean diet’, which is generally considered to be a healthy one with a diminished incidence of cardiovascular disease and cancer. However, there are differences in metabolism between dietary oleate and that synthesised de novo in human tissues.
One exception among the monoenes is erucic acid (13-22:1) because studies with laboratory rats in the 1970s appeared to show that it could adversely affect the metabolism of the heart. As rapeseed oil was a major dietary source for humans, low erucic oils such as 'Canola' were developed to circumvent the problem. There have apparently been no studies to confirm the toxicity of erucic acid in humans or other primates, and it has since been argued that the rat studies were flawed. Meantime, erucic acid has been used as a major component of 'Lorenzo's oil' to treat adrenoleukodystrophy with no apparent harm. There are reports that dietary nervonic acid (15-24:1) may be of benefit in neurodegenerative diseases such as Alzheimer’s disease, multiple sclerosis and Parkinson’s disease; it has anti-inflammatory properties and attenuates the progression of atherosclerosis.
Although monoenoic fatty acids are abundant in the diet, stearoyl-CoA desaturase (SCD1) controls the level of oleic acid biosynthesis de novo in animals and determines body adiposity and lipid partitioning. It is a critical regulator of innumerable physiological processes, including energy homeostasis, development, autophagy, tumorigenesis and inflammation. Aberrant transcriptional and epigenetic activation of SCD1 promotes proteins that cause anomalous lipid accumulation thus enhancing the progression of obesity, non-alcoholic fatty liver disease, diabetes and cancer. When the activity of the enzyme is high, fat storage is favoured, but when it is low, metabolic pathways that promote the burning of fat are induced together with decreased lipid synthesis in adipose tissue and liver. A lipidomic study of the impact of SCD1 deficiency revealed substantial changes in lipid levels and increased accumulation of saturated fatty acids in both the liver and plasma together with elevated hepatic and plasma acylcarnitine levels. This is especially important for milk production, as SCD1 orchestrates metabolic adaptations during lactation to ensure adequate milk synthesis to support the rapidly growing neonates.
In brown adipose tissue, SCDI activity is significantly increased during long-term cold exposure, and by stimulating the release of norepinephrine, this sustains thermogenesis. Insulin-signalling components are upregulated in SCD1 deficiency with effects upon glycogen metabolism in insulin-sensitive tissues. SCD1 stimulates AMP-activated protein kinase, an enzyme that phosphorylates and deactivates acetyl-CoA-carboxylase, which regulates both fatty acid synthesis and oxidation in a reciprocal manner, i.e., by promoting fatty acid synthesis but decreasing oxidation. In the heart, loss of SCD1 is protective under conditions of a high-fat diet as it reduces fatty acid oxidation and increases glucose oxidation, but it can predispose individuals to atherosclerosis under conditions of hyperlipidemia.
 The range and sequence of cellular
events are complex, but the anti-obesity hormone leptin inhibits the expression of the gene for stearoyl-CoA desaturase so that levels of this
enzyme fall, leading to inactivation of acetyl-CoA carboxylase and thence to promotion of fatty acid oxidation and inhibition of fatty
acid synthesis.
Genetically modified mice that are deficient in the SCD1 isoform are protected from cellular lipid accumulation and obesity,
and they have increased insulin sensitivity.
It has been reported that in hepatocytes, but not adipocytes, oleic acid feeding increases the number and size of lipid droplets while
simultaneously inhibiting lipogenesis.
The range and sequence of cellular
events are complex, but the anti-obesity hormone leptin inhibits the expression of the gene for stearoyl-CoA desaturase so that levels of this
enzyme fall, leading to inactivation of acetyl-CoA carboxylase and thence to promotion of fatty acid oxidation and inhibition of fatty
acid synthesis.
Genetically modified mice that are deficient in the SCD1 isoform are protected from cellular lipid accumulation and obesity,
and they have increased insulin sensitivity.
It has been reported that in hepatocytes, but not adipocytes, oleic acid feeding increases the number and size of lipid droplets while
simultaneously inhibiting lipogenesis.
Oleic is known to interact with a wide range of receptors, including GPR40 (FFAR1), GPR120 (FFAR4), PPARγ, LXR and EGFR, and it is apparent that as a non-chiral molecule with rotatable bonds, it can adopt a range of diffuse conformations when bound to different macromolecules to influence their behaviour. For example, unesterified oleic acid is an endogenous ligand for the orphan nuclear receptor TLX/NR2E1, which governs neural stem and progenitor cell self-renewal and proliferation, and as a result, it is essential for the survival of neural stem cells. This fatty acid can also block the calcium-activated chloride channel TMEM16A/ANO1 in membranes with the potential for a beneficial impact on the cardiovascular system; in brown adipose tissue, it is a ligand for GPR3, which promotes thermogenesis.
It has long been known that cancer cells and tumours synthesise lipids at a high rate with an aberrant pattern of fatty acids, including a preponderance of saturated and monoenoic species, and constitutive activation of fatty acid synthesis with Δ9-desaturation de novo is a metabolic hallmark in most cancer cells. This is driven by a requirement for lipids, especially phospholipids, for new membranes and is accomplished by a dynamic sequence of reactions utilizing all the enzymes for fatty acid synthesis including SCD1. In part, this may because oleate activates phospholipase D by S-acylation to modulate the dynamics of membrane microdomains. SCD1 is also a factor in the molecular pathways of cell survival, especially towards autophagy and apoptotic and non-apoptotic cell death. As it is a central regulator of the complex metabolic and signalling events that control the development of cancer cells, it has been suggested that the development of selective inhibitors might be an alternative treatment for various types of cancer such as the chemo-resistant malignancies. So far, no trials of experimental inhibitors have advanced beyond the preclinical stage.
Likewise, pharmaceutical companies are seeking drugs that will inhibit SCD1 with the hope of producing anti-obesity effects in patients, and there are potential benefits for intervention in the treatment of non-alcoholic fatty liver disease and diabetes. SCD1 is a factor in autoimmunity, and by suppressing the differentiation of regulatory T cells, it is hope that pharmacological inhibitors of SCD1 may have value for inhibition of autoimmunity and to reduce neuroinflammation, although there are instances where this may not be helpful.
SCD2 is crucial for mouse development and metabolism, but influences the pathology of obesity, chronic kidney disease and various neurological diseases. The gene for the synthesis of SCD5 is located on a different chromosome from that for SCD1, so presumably there is some different requirement. Palmitic acid may be the preferred substrate in brain, but not in all tissues, and SCD5 may be a special factor for brain development in infants; there is evidence of some involvement in disease states. High expression of oleoyl-ACP hydrolase is a major factor in life-threatening respiratory viral diseases, including influenza, COVID-19 and respiratory syncytial virus.
Among esterified lipids containing oleate, certain amide derivatives, such as oleamide and oleoylethanolamine, have well defined functions in animal tissues, while 2‑oleoylglycerol is a signalling molecule in the intestines, as discussed elsewhere on this site. 1,2-Dioleoyl-phosphatidylinositol is signalling lipid, derived from the action of the stearoyl-CoA desaturase (SCD1), and acts as a lipokine to respond to stresses that promote protein degradation, apoptosis and autophagy.
From studies largely with mice, it has been suggested that adipose tissue uses lipokines such as palmitoleic acid (9Z-16:1) to communicate with distant organs and regulate metabolism throughout the body. One proposal is that palmitoleic acid synthesised in adipose tissue stimulates muscle insulin action and suppresses hepatic lipogenesis in steatosis (or 'fatty liver') by the inhibition of SCD1 and triacylglycerol synthesis. Higher concentrations in plasma from exogenous administration are strongly associated with insulin sensitivity, independently of age, sex and adiposity in healthy humans, and they may be associated with decreased obesity depending upon dietary conditions; its concentration is elevated in plasma of obese patients. An alternative suggestion is that palmitoleic acid regulates lipid metabolism by its influence upon diacylglycerol metabolism. Under a basal diet, low-doses of this fatty acid were found to inhibit the conversion of diacylglycerols to triacylglycerols and reduce hepatic lipid accumulation, while medium-to-high doses redirected the flux of diacylglycerols toward phospholipid metabolism pathways to lower body weight and the adiposity index. Somewhat different results were obtained with high fat diets.
In macrophages, palmitoleate generation is reported to alleviate lipotoxicity-induced stress in the endoplasmic reticulum with a reduction in apoptosis and benefits towards the progression of atherosclerosis, and it protects against lipopolysaccharide-induced inflammation and inflammasome activity. Oral supplements reportedly improve the skin barrier. It may be relevant that palmitoleic acid is linked to a conserved serine residue in the Wnt family of proteins necessary for tissue development, and it is essential if they are to carry out their role in tissues. Its elongation product, cis-vaccenic acid, is reported to have its own functions, and other 16:1 isomers are reported to be beneficial to consumers.
The current nutritional view is that dietary trans-monoenoic fatty acids, especially those from industrial hydrogenation processes, should be considered as harmful and in the same light as saturated fatty acids, possibly because they enhance intracellular signalling pathways that induce inflammation and cell death under conditions of metabolic stress. That said, there is a school of thought that natural trans-fatty acids such as those found in ruminant meat and dairy foods are broadly neutral towards health, possibly because the main isomer, vaccenic acid (11E-18:1) can be converted in tissues to conjugated linoleic acid (9Z,11E-18:2). Similarly, trans-palmitoleic acid (9E-16:1) from retroconversion of vaccenic acid is reported to be protective towards the risk of type 2 diabetes. Detailed discussion of this topic is best left to nutritional experts of whom I am not one.
Recommended Reading
- Chatgilialoglu, C., Ferreri, C., Melchiorre, M., Sansone, A. and Torreggiani, A. Lipid geometrical isomerism: from chemistry to biology and diagnostics. Chem. Rev., 114, 255-284 (2014); DOI.
- Cronan, J.E. Unsaturated fatty acid synthesis in bacteria: Mechanisms and regulation of canonical and remarkably noncanonical pathways. Biochimie, 218, 137-151 (2024); DOI.
- Galanty, A., Grudzinska, M., Pazdziora, W. and Pasko, P. Erucic acid-both sides of the story: a concise review on its beneficial and toxic properties. Molecules, 28, 1924 (2023); DOI.
- Ghosh, S., Caceres, R., Congrove, S., Ntambi, J.M. and Dasgupta, B. Stearoyl-CoA desaturase in development and disease. Prog. Lipid Res., 101, 101374 (2026); DOI.
- Guo, Q., Li, T., Qu, Y., Liang, M., Ha, Y., Zhang, Y. and Wang, Q. New research development on trans fatty acids in food: Biological effects, analytical methods, formation mechanism, and mitigating measures. Prog. Lipid Res., 89, 101199 (2023); DOI.
- Guo, Z.Q., Bergeron, K.F., Lingrand, M. and Mounier, C. Unveiling the MUFA-cancer connection: insights from endogenous and exogenous perspectives. Int. J. Mol. Sci., 24, 9921 (2023); DOI.
- Huang, W.W. and others. Cis-Palmitoleic acid regulates lipid metabolism via diacylglycerol metabolic shunting. Foods, 14, 2504 (2025); DOI.
- Igal, R.A. and Sinner, D.I. Stearoyl-CoA desaturase 5 (SCD5), a Δ9 fatty acyl desaturase in search of a function. Biochim. Biophys. Acta, Lipids., 1866, 158840 (2021); DOI.
- Kalyesubula, M. and others. Stearoyl-CoA desaturase-1 is vital for milk lipid synthesis: Deletion impairs mammary gland and neonatal development. J. Lipid Res., 66, 100941 (2025); DOI.
- Kazaz, S., Miray, R., Lepiniec, L. and Baud, S. Plant monounsaturated fatty acids: Diversity, biosynthesis, functions and uses. Prog. Lipid Res., 85, 101138 (2022); DOI.
- Koeberle, A., Löser, K. and Thürmer, M. Stearoyl-CoA desaturase-1 and adaptive stress signaling. Biochim. Biophys. Acta, Lipids, 1861, 1719-1726 (2016); DOI.
- Kyselová, L., Vítova, M. and Řezanka, T. Very long chain fatty acids. Prog. Lipid Res., 87, 101180 (2022); DOI.
- Li, Y.H., Gao, X.L., Gao, Z.A., Cao, Z.H., Xiong, P. and Liu, X.T. Sources and biological functions of nervonic acid: Advances and perspectives. Chem. Phys. Lipids, 273, 105554 (2025); DOI.
- Los, D.A. and Leusenko, A. 50 years since the concept of homeoviscous adaptation. Biochimie, 231, 98-103 (2025); DOI.
- Mauger, M., Ferreri, C., Chatgilialoglu, C. and Seemann, M. The bacterial protective armor against stress: The cis-trans isomerase of unsaturated fatty acids, a cytochrome-c type enzyme. J. Inorg. Biochem., 224, 111564 (2021); DOI.
- O'Neill, L.M., Miyazaki, M., Bond, L.M., Lewis, S.A., Ding, F., Liu, Z. and Ntambi, J.M. Fatty acid desaturation and elongation in mammals. In: Biochemistry of Lipids, Lipoproteins and Membranes, 7th Edition. pp. 201-226 (edited by N.D. Ridgway and R.S. McLeod, Elsevier, Amsterdam) (2021) - see Science Direct.
- Sun, Q. and others. SCD1 is the critical signaling hub to mediate metabolic diseases: Mechanism and the development of its inhibitors. Biomed. Pharmacoth., 170, 115586 (2024); DOI.
I can also recommend the chapter on fatty acid biosynthesis in the book - Gurr, M.I., Harwood, J.L., Frayn, K.N., Murphy, D.J. and Michell, R.H. Lipids: Biochemistry, Biotechnology and Health (6th Edition). (Wiley-Blackwell) (2016).
For tutorials on mass spectral analysis of monoenoic and other fatty acids - see our mass spectrometry pages.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: February 2026 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
