Phosphatidylinositol and Related Phosphoinositides
Although it had long been recognized that phosphatidylinositol or 1,2-diacyl-sn-glycero-3-phospho-(1'-myo-inositol) was a key membrane constituent, it was something of a surprise when the manifold biological activities of this lipid and then of the derived phosphatidylinositol phosphates and their hydrolysis products were discovered in animals, plants and microorganisms. Many years after the initial discoveries in the 1950s, these lipids continue to be a major focus for research efforts around the world with considerable relevance to human health as metabolic dysfunction contributes to many disease states.
Phosphatidylinositol and its various metabolites, collectively termed 'phosphoinositides', and relevant enzymes can be located and operate within different organelles in cells, and they form part of what have been termed phosphoinositide and phosphatidylinositol cycles, their versatility stemming largely from the myo-inositol head group, a hexahydroxy six-carbon ring, which and can be reversibly phosphorylated on the 3, 4 and 5 positions. Myo-inositol is a sugar alcohol with two of the hydroxyls (linked to carbons 2 and 5) lying above the respective hydrogens relative to the mean plane of the ring in a chair-like conformation.

In addition to their structural role in membranes, phosphoinositides are intimately involved in innumerable aspects of membrane trafficking and signalling through interactions with specific effector proteins in eukaryotic cells that are essential to cell growth and metabolism. Only a brief overview of such a highly complex topic is possible here.
1. Phosphatidylinositol
Structure and Occurrence: Phosphatidylinositol is both a membrane constituent and a crucial participant in metabolic processes in most life forms, both directly and via its metabolites. It is an acidic (anionic) phospholipid that in essence consists of a phosphatidic acid backbone linked via the phosphate group to position 1 of inositol. In general, myo-D-inositol is by far the commonest stereochemical form and the most stable, although other isomers, scyllo and chiro, have been found on occasion in plants with the latter in liver and skeletal muscle as phosphoglycans. It is synthesised in many human tissues, but the kidney makes about two grams per day from glucose. The sn‑1‑stearoyl-2-arachidonoyl molecular species, which has a unique place in animal metabolism, is illustrated.

Phosphatidylinositol is abundant in brain tissue where it can amount to 10% of the phospholipids, but it is present in all tissues, cell types and membranes at relatively low levels in comparison to many other phospholipids. In rat liver, it amounts to 1.7 micromoles/g., i.e., less than phosphatidylcholine, phosphatidylethanolamine and phosphatidylserine. Under normal conditions, it is present entirely in the inner leaflet of the erythrocyte membrane and of the plasma membrane in nucleated cells. Other than in Mycobacteria and Actinomycetales, phosphatidylinositol per se is only occasionally found in prokaryotes, although the thermophilic α-proteobacterium Rhodothermus marinus contains dialkylether glycerophosphoinositides, while Archaea produce alkyl-glycero-analogues with an alternative stereochemistry of the glycerol moiety.
The fatty acid composition of phosphatidylinositol is rather distinctive as shown in Table 1, and in almost all animal tissues, the characteristic feature is a high content of stearic and arachidonic acids. All the stearic acid is linked to position sn-1 and all the arachidonic acid to position sn-2, and as much as 70% of the total lipid may consist of the single species sn-1-stearoyl-sn-2-arachidonoyl-glycerophosphorylinositol (see Table 2 below). Although 1‑alkyl- and alkenyl- forms of phosphatidylinositol are known, they tend to be much less abundant than the diacyl form. In plant phosphatidylinositol, e.g., Arabidopsis thaliana, palmitic acid is the main saturated fatty acid in position sn-1, while linoleic and linolenic acids are the main unsaturated components in position sn-2. Palmitic acid is in position sn-1 with oleic and palmitoleic acids in position sn-2 predominantly in yeasts; the Amoebozoa have a C16 alkyl group in position sn-1 and cis-vaccenic acid in position sn-2.
Table 1. Fatty acid composition of phosphatidylinositol (wt % of the total) in animal and plant tissues. |
||||||||||
| Tissue | Fatty acids | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | 20:3 | 20:4 | 22:3 | 22:5 | 22:6 | |
| Bovine brain [1] | 8 | 38 | 10 | 1 | - | 5 | 34 | 2 | tr. | 1 |
| Bovine liver [2] | 5 | 32 | 12 | 6 | 1 | 7 | 23 | 4 | 3 | 5 |
| Rat liver [3] | 5 | 49 | 2 | 2 | 4 | 35 | 1 | |||
| A. thaliana [4] | 48 | 3 | 2 | 24 | 24 | |||||
| [1] = Holub, B.J. et al. J. Lipid Res.., 11, 558-564 (1970);
DOI. [2] = Thompson, W. and MacDonald, G., J. Biol. Chem., 250, 6779-6785 (1975); DOI. [3] = Wood, R. and Harlow, R.D. Arch. Biochem. Biophys., 135, 272-281 (1969); DOI. [4] = Browse, J. et al. Biochem. J., 235, 25-31 (1986); DOI. |
||||||||||
At its location at the cytoplasmic leaflet, phosphatidylinositol is a major contributor to membrane asymmetry, and as the inositol headgroup is relatively bulky because of hydration and hydrogen bond formation of the six hydroxyl groups and is larger than the cross-sectional area of the acyl chains, this leads to less crowded packing of the acyl chains. Increased water penetration of the bilayer and changes to membrane fluidity result that are important for metabolism, signalling and transport of transmembrane proteins.
Biosynthesis: The basic mechanism for biosynthesis of phosphatidylinositol and phosphatidylglycerol is sometimes termed a branch point in phospholipid synthesis, as phosphatidylcholine and phosphatidylethanolamine are produced by a somewhat different route.
Most eukaryotes can synthesise the precursor inositol de novo via glucose-6-phosphate where among other organs, the human kidney makes about two grams per day (with more coming from the diet). As with phosphatidylglycerol (and thence cardiolipin), phosphatidylinositol is formed biosynthetically from phosphatidic acid via the intermediate cytidine diphosphate diacylglycerol, which is produced by the action of a CDP-diacylglycerol synthase, the rate-limiting enzyme in phosphatidylinositol biosynthesis. Then, the enzyme CDP-diacylglycerol inositol phosphatidyltransferase ('phosphatidylinositol synthase' or 'PIS') catalyses a reaction with myo-inositol to produce phosphatidylinositol with cytidine monophosphate (CMP) as other product of the reaction.
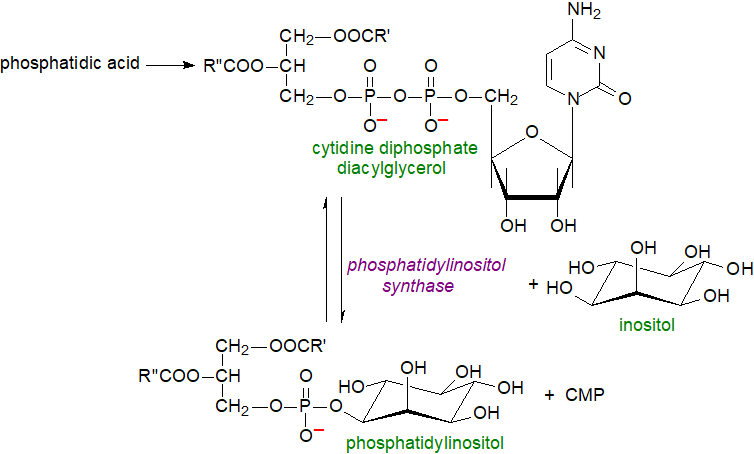 |
| Figure 1. Biosynthesis of phosphatidylinositol in eukaryotes. |
Only one isoform of PIS exists in mammals, and it is located in the endoplasmic reticulum (ER), in part in a sub-compartment of this associated with mitochondria (mitochondria-associated membranes - MAM) and not in mitochondria per se. Indeed, it is reported that PIS is present in a mobile ER-derived sub-compartment that makes transient contacts with other organelles, including the plasma membrane, and facilitates distribution of phosphatidylinositol. As PIS can catalyse the reverse reaction, the rate of phosphatidylinositol synthesis is determined by the relative concentrations of the precursors and product, and for the reaction to continue, the latter must be transported away from the site of synthesis via lipid exchange proteins and vesicular or tubular carriers. Much of the phosphatidylinositol is delivered to other membranes by vesicular transport, but a family of soluble phosphatidylinositol transfer proteins (PITPα, PITPβ and PITPNC1) provides phosphatidylinositol from the ER to kinases for phosphorylation to produce phosphatidylinositol phosphates (see below).
Molecular species specificity: The phosphatidylinositol synthase does not produce the fatty acyl distributions observed in the final product (see Table 2 below), but earlier in the biosynthetic process 1‑stearoyl-2-arachidonoyl species of diacyl-sn-glycerols are converted preferentially into phosphatidic acid by the epsilon isoform of diacylglycerol kinase (DGKε), anchored to the membrane via its N-terminal hydrophobic helix segment; ATP is the phosphate donor. One of the CDP-diacylglycerol synthases (CDS2) has similar specificity in the generation of the immediate precursor CDP-diacylglycerols from phosphatidic acid.
Alternatively, lysophosphatidylinositol formed as a by-product of eicosanoid formation (see below) or as an intermediate as part of the normal cycle of deacylation-acylation of phosphatidylinositol in tissues may be remodelled to give the final distinctive composition (the Lands cycle - see our web page on phosphatidylcholine for a fuller discussion of this process). For this purpose, a membrane-bound O-acyltransferase (LPLAT11 or MBOAT7), which acts at position sn‑2 of lysophosphatidylinositol with a marked preference for arachidonoyl-CoA, is ubiquitously expressed in animal tissues. Structural studies of the enzyme demonstrate that arachidonoyl-CoA and lysophosphatidylinositol access the catalytic centre through a twisted tunnel from the cytosol and lumenal sides, respectively. Deficiencies in this enzyme and a reduction in the operation of the Lands' cycle have been correlated with various disease states in the liver such as those in which there is a failure of glucose homeostasis together with systemic insulin resistance. Incidentally, this may be one means by which free arachidonic acid and eicosanoid levels are regulated.
Lysocardiolipin acyltransferase (LYCAT; also known as LCLAT1 or ALCAT1) exhibits a preference for lysophosphatidylinositol and lysophosphatidylglycerol over other phospholipids in vitro and incorporates 18:0 rather than shorter chain fatty acids into position sn-1 of phosphatidylinositol and other phosphoinositides, mainly phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3-phosphate; this enzyme may be located adjacent to the phosphatidylinositol synthase in the endoplasmic reticulum. Some of the phosphatidylinositol in membranes is derived from re-cycling of polyphosphoinositides via the phosphatidylinositol cycle, and this could influence the molecular composition (see below). For example, during agonist stimulation, highly unsaturated polyphosphoinositides are replenished much faster than more saturated forms. In macrophages subjected to inflammatory stimuli, phosphatidylinositol containing two molecules of arachidonate is produced by remodelling reactions, and there is evidence that it is a novel molecule regulating innate immune responses in these cells.
A further species, 1,2-dioleoyl-phosphatidylinositol, is a signalling lipid derived in part from the action of the stearoyl-CoA desaturase (SCD1), and it acts as a lipokine to respond to stresses that trigger protein degradation, apoptosis and autophagy. In activated human monocytes, palmitoleic acid is transferred from phosphatidylcholine to phosphatidylinositol by remodelling mechanisms with possible signalling consequences. The highly characteristic distribution of fatty acids on the glycerol moiety of phosphatidylinositol breaks down in those cancer cells with a mutation on the transcription factor p53 gene, one of the most highly mutated genes in cancers.
Plants and bacteria: In contrast to animals, plants have two phosphatidylinositol synthase isoforms, PIS1 and PIS2, which utilize particular species of the CDP-diacylglycerol substrate. PIS1 generates phosphatidylinositol with saturated or monounsaturated fatty acids preferentially, while PIS2 utilizes polyunsaturated fatty acids, the two forms possibly required for different cellular purposes. In yeasts, some biosynthesis may occur on the cytosolic side of the plasma membrane. In protozoan parasites, such as Trypanosoma brucei, the site of phosphatidylinositol synthase may be the lumen of the endoplasmic reticulum and Golgi, and there is evidence for two separate pools of product, the bulk membrane form derived from inositol imported from the environment and a second for the synthesis of GPI anchors, which uses myo-inositol synthesised de novo. Similarly, apicomplexans, e.g., Toxoplasma gondii and Plasmodium spp., which are obligate intracellular parasites and causative agents of disease in humans, synthesise phosphatidylinositol.
Few bacteria contain phosphatidylinositol, although Mycobacteria are an exception, and it is present in some of the Bacteroidetes and other proteobacteria. The Actinomycetes contain complex lipophosphoglycans of which phosphatidylinositol is a component. In these and many other bacteria, CDP-diacylglycerols and inositol phosphate are combined by a phosphatidylinositol-phosphate synthase to generate phosphatidylinositol phosphate, which is subsequently dephosphorylated to generate phosphatidylinositol. Archaeal ether lipids include analogues of phosphatidylinositol, and these are synthesised by a comparable mechanism, i.e., by reaction of inositol 1-phosphate with CDP-archaeol to form archaetidylinositol 3‑phosphate and thence archaetidylinositol (see our web page on Archaeal lipids). In contrast, in the thermophilic bacterium Rhodothermus marinum, L-myo-inositol-1-phosphate cytidylyltransferase catalyses the formation of CDP-inositol from inositol-1-phosphate and CTP, before a synthase catalyses the transfer of the inositol-1-phosphate group from CDP-inositol to dialkylether glycerols to produce phosphoinositol ether lipids; this differs from all other pathways, which use activated forms of the precursor lipids as intermediates.
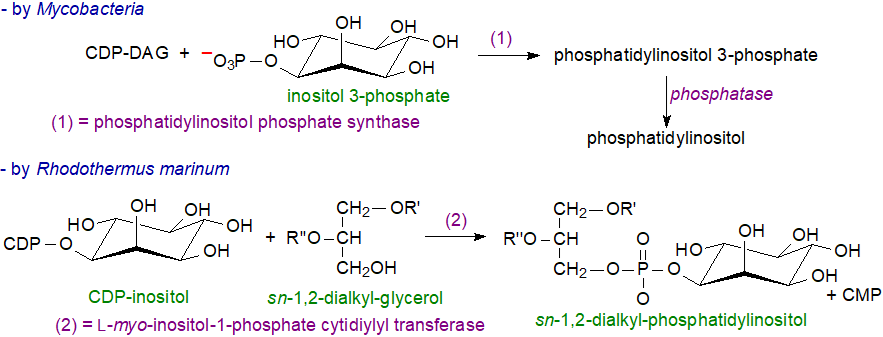 |
| Figure 2. Biosynthesis of phosphatidylinositol in bacteria. |
Function: As well as being negatively charged building blocks of membranes, the inositol phospholipids (including the phosphatidylinositol phosphates or 'polyphosphoinositides' discussed below) are crucial to interfacial binding of proteins and for the regulation of protein function at the cell interface. As phosphoinositides are polyanionic, they can be very effective in non-specific electrostatic interactions with proteins, but they are most efficient in binding to pleckstrin homology (PH) domains of proteins involved in intracellular signalling or as constituents of the cytoskeleton (see below). At least three phosphatidylinositol molecules are present in the crystal structure of human erythrocyte glycophorin, for example, and they influence binding to other proteins via their head groups. The lipid is an essential structural element of yeast cytochrome bc1.
In animal tissues, phosphatidylinositol is the primary source of the arachidonic acid for biosynthesis of eicosanoids, including prostaglandins, via the action of the enzyme phospholipase A2, which releases the fatty acids from position sn-2. 2‑Arachidonoylglycerol, an endogenous ligand for the cannabinoid receptor, may be a further product of phosphatidylinositol metabolism.
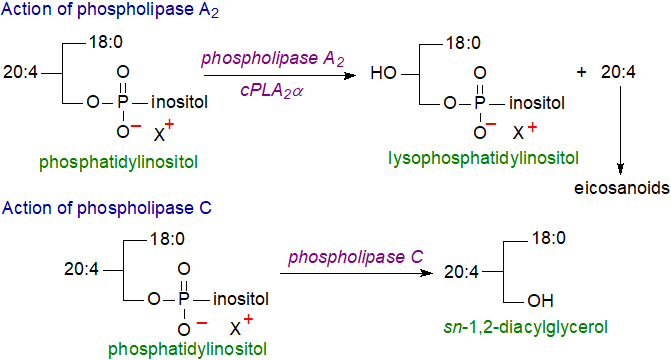 |
| Figure 3. Actions of phospholipase A2 and C on phosphatidylinositol. |
Similarly, phosphatidylinositol and the phosphatidylinositol phosphates are the main source of sn-1,2-diacylglycerols that serve as signalling molecules in animal cells via the action of a family of enzymes collectively known as phospholipase C (see below and our web pages on diacylglycerols for further discussion). In brief, diacylglycerols regulate a group of at least a dozen related enzymes known as protein kinase C, which in turn control many cellular processes, including differentiation, proliferation, metabolism and apoptosis. While signalling by phosphoinositide-specific phospholipase C is involved in various stress and developmental responses in plants, diacylglycerols do not act directly but are precursors for inositolpolyphosphates and phosphatidic acid.
2. Phosphatidylinositol Phosphates (Polyphosphoinositides) in Animals
Structure and Occurrence: The pioneering work of Mable and Lowell Hokin in the 1950s led to the discovery that phosphatidylinositol was converted to polyphosphoinositides with vital signalling and other properties, including cell communication via signal transduction, cell survival and proliferation, membrane trafficking and modulation of gene expression. Phosphatidylinositol is now known to be phosphorylated by substrate-selective kinases that place the phosphate moiety on positions 3, 4 and/or 5 of inositol with the balance among them maintained by phosphatases and phospholipases. Seven different isomers are known (mono-, bis- and tris-phosphorylated), which are produced in a tightly coordinated manner, and all of these are required for characteristic purposes with each turning over much more rapidly than the parent phosphatidylinositol molecule. Although the most significant in quantitative and possibly biological terms were long thought to be phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5‑bisphosphate, it is now recognized that phosphatidylinositol 3‑phosphate and its metabolites may be as important.
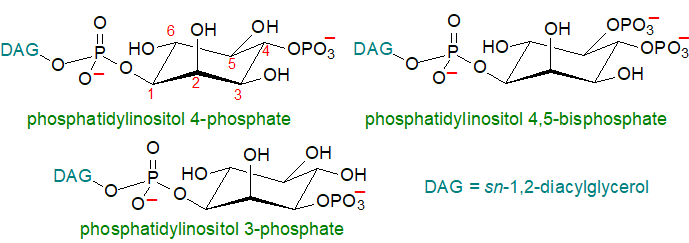
These lipids are usually present at low levels only in tissues, typically at about 0.5 to 1% of the total lipids of the inner leaflet of the plasma membrane, so they are unlikely to have an appreciable structural role. On the other hand, static measurements of lipids that turn over very rapidly do not provide a meaningful assessment of their cellular effects. The positional distributions of fatty acids in the phosphatidylinositol, phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate of ox brain are listed in Table 2. In each the saturated fatty acids are concentrated in position sn-1 and polyunsaturated, mainly arachidonate, in position sn-2; there are few differences among the three lipids in this tissue.
Table 2. Distribution of fatty acids (mol % of the total) in positions sn‑1 and sn‑2 in phosphatidylinositol (PI) and the phosphatidylinositol mono- and diphosphates of ox brain. |
||||||
| Fatty acids | PI | PI monophosphate | PI diphosphate | |||
|---|---|---|---|---|---|---|
| sn-1 | sn-2 | sn-1 | sn-2 | sn-1 | sn-2 | |
| 16:0 | 15 | 9 | 7 | |||
| 18:0 | 74 | 69 | 69 | |||
| 18:1 | 10 | 10 | 20 | 13 | 21 | 10 |
| 18:2 | 1 | 2 | trace | 1 | 1 | 1 |
| 20:3(n-9) | 5 | 10 | 10 | |||
| 20:3(n-6) | 5 | 11 | 12 | |||
| 20:4(n-6) | 67 | 49 | 52 | |||
| 22:3 | 7 | 10 | 7 | |||
| 22:6(n-3) | trace | trace | trace | |||
| Data from Holub, B.J. et al., J. Lipid Res., 11, 558-564 (1970);
DOI. As examples of molecular species data for a range of tissues and animals, see Barneda, D. et al. Biochim. Biophys. Acta, Lipids, 1870, 159640 (2025); DOI. |
||||||
Changes in the concentrations of phosphoinositides or of their fatty acid or molecular species compositions in plasma and other tissues have been reported in various disease states, where they may serve as biomarkers. These include diseases of the liver, cardiovascular system, kidney, lung and brain, as well as cancer, sepsis and diabetes.
Biosynthesis: Phosphatidylinositol is the primary precursor of seven phosphoinositides (PIP), the head groups of which have different structures and charges that impact directly on membrane and metabolic properties. The concentrations of individual phosphoinositides are maintained at steady state levels in membranes by a continuous and sequential series of phosphorylation and dephosphorylation reactions by kinases, phosphatases and phospholipase C enzymes, which are regulated and/or relocated with multiple interdependencies through cell surface receptors for extracellular ligands, i.e., the phosphoinositide cycle. While this has been termed a ‘futile cycle’, which can consume a significant proportion of cellular ATP production, it is only part of a wider pattern of reactions - the phosphatidylinositol cycle (see below).
Controlled synthesis of these different phosphoinositides occurs in different intracellular compartments for characteristic and independently regulated tasks with spacially distinct target enzymes or receptors. In animals, the complexity is such that 18 phosphoinositide inter-conversion reactions have been identified to date, and these are mediated by at least 20 phosphoinositide kinases and 34 phosphoinositide phosphatases that span 8 and 10 classes, respectively, with some yet to be characterized. Some of these are part of large multi-protein megacomplexes of >700 kDa, and PI4K, for example, is encoded by four genes (PI4KA, PI4KB, PI4K2A and PI4K2B) with each isoform operating in different subcellular membrane compartments to produce phosphatidylinositol 4-phosphate for particular signalling purposes; at the plasma membrane PI4KA exists in the form of a complex with two regulatory proteins and two other trans-membrane proteins. Most of these enzymes are conserved across the eukaryota, and each has its own specificities that cannot be replaced by related isoforms.
As a generality, most mono-phosphorylations occur in endomembranes, such as the endosomes and the Golgi network, while second and third phosphorylations occur primarily at the plasma membrane, and this is reflected in the lipid composition of each membrane. While these enzymes work independently and sequentially to produce each product, there remains a possibility that some participate in mega-protein/enzyme complexes to coordinate their efficiency. Transporters, e.g., the 'Nir2' protein, facilitate the exchange of phosphoinositides between membranes. It should be noted that there are links to the metabolism of phosphatidylcholine, which can be hydrolysed by phospholipase D to phosphatidic acid, which interacts with key kinases.
This can be exemplified by phosphatidylinositol 4-phosphate (PI(4)P), which is produced by the action of a phosphatidylinositol 4-kinase (PI4K) in the Golgi, and this is in turn phosphorylated by a phosphatidylinositol phosphate 5-kinase (PIPK I) to form phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) at the plasma membrane, although the latter can also be formed by phosphorylation of phosphatidylinositol 5‑phosphate (PI(5)P) by a 4‑kinase (PIPK II). To further enrich the arachidonic acid content, some limited selectivity in the formation of molecular species or remodelling may occur.
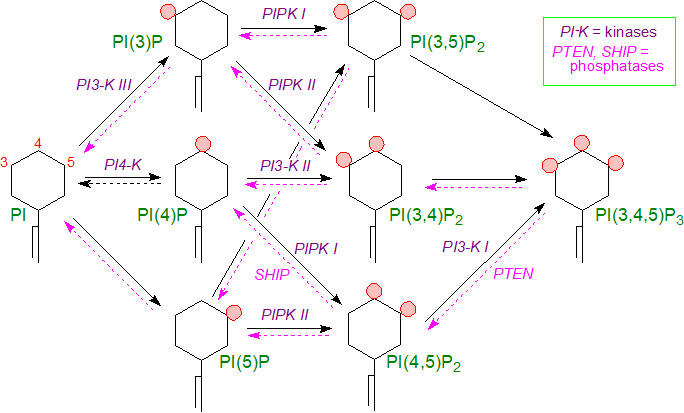 
|
| Figure 4. Polyphosphoinositide metabolism in animal tissues. |
Subsequently, it was discovered that phosphatidylinositol is phosphorylated by a 3-kinase (PI3K III or the VPS 34 complex) to produce phosphatidylinositol 3‑phosphate (PI(3)P) in the early endosomes or the cytosolic leaflet of cellular membranes. Three phosphatidylinositol 3-kinases families (eight isoforms) have been described to date, each with its own substrate specificities. A second phosphoinositide signalling pathway involves activation of two of these 3‑kinases, stimulated by growth factors and hormones, that phosphorylate PI(4,5)P2 (by PI3K I - four isoforms) and PI(4)P (by PI3K II - three isoforms) to produce phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) and phosphatidylinositol 3,4‑bisphosphate (PI(3,4)P2), respectively. While PI(3)P and other 3‑phosphorylated metabolites amount to only about 0.5% of the total phosphoinositides in resting mammalian cells, they are now recognized to have profound importance for cellular metabolism.
In addition to kinases, the amounts of these various metabolites are regulated by phosphoinositide phosphatases, which are highly conserved in eukaryotes and dephosphorylate phosphoinositides at the 3, 4 or 5 positions of the inositol ring. So-called ‘SHIP’ phosphatases convert PI(4,5)P2 back to PI(4)P by hydrolysis of the 5-phosphate group, but 3‑phosphorylated phosphoinositides are only degraded by phosphatases, such as those of the PTEN ('phosphatase and tensin homolog deleted on chromosome ten') family, and they differ from the other phosphoinositides in that they are not hydrolysed by phospholipase C.
 The various organelles
in cells have membranes with their own functions and molecular compositions, but all the phosphatidylinositol precursor is formed at the
endoplasmic reticulum, and the different lipid products must be transported between membrane sites via trafficking processes/proteins.
A family of phosphatidylinositol transfer proteins with five members is known in mammals, three of which are soluble proteins
(PITPα, PITPβ and PITPNC1) and two are membrane-associated proteins containing multiple domains (PITPNM1/Nir2 and PITPNM2/Nir3).
There is selective recruitment of effector proteins to particular membranes by binding only to a single type
of phosphoinositide, and this is followed by interactions between the phosphoinositide-binding proteins and various enzymes
to channel phosphoinositide production to the required metabolic outcomes and to regulate signalling.
Much of the PI(4)P and PI(4,5)P2 for signalling purposes is probably formed at contact sites between the endoplasmic reticulum
and plasma membrane and other organelles where they facilitate transfer of Ca2+, phospholipids, cholesterol and a motor protein.
The various organelles
in cells have membranes with their own functions and molecular compositions, but all the phosphatidylinositol precursor is formed at the
endoplasmic reticulum, and the different lipid products must be transported between membrane sites via trafficking processes/proteins.
A family of phosphatidylinositol transfer proteins with five members is known in mammals, three of which are soluble proteins
(PITPα, PITPβ and PITPNC1) and two are membrane-associated proteins containing multiple domains (PITPNM1/Nir2 and PITPNM2/Nir3).
There is selective recruitment of effector proteins to particular membranes by binding only to a single type
of phosphoinositide, and this is followed by interactions between the phosphoinositide-binding proteins and various enzymes
to channel phosphoinositide production to the required metabolic outcomes and to regulate signalling.
Much of the PI(4)P and PI(4,5)P2 for signalling purposes is probably formed at contact sites between the endoplasmic reticulum
and plasma membrane and other organelles where they facilitate transfer of Ca2+, phospholipids, cholesterol and a motor protein.
A concept has emerged in which each phosphoinositide has its own role, the ‘lipid code hypothesis’, in which defined lipids act as labels for each cellular membrane to organize cells into dynamic and responsive membrane-bound compartments and maintain the orderly flow for membrane trafficking and spatio-temporal signalling reactions. Thus, PI(4)P, PI(4,5)P2, PI(3)P and phosphatidylinositol 3,5‑bisphosphate (PI(3,5)P2) are found mainly on the Golgi, plasma membrane, early endosomes and late endocytic organelles, respectively, where they are sometimes regarded as landmarks for these compartments. PI(4,5)P2 is present throughout the plasma membrane where it is considered to be a general marker, while PI(3,4,5)P3 is a characteristic component of the basolateral region of this membrane in a polarized cell but is absent from the apical part.
As a caveat, it should be noted that this map of phosphoinositides to each organelle is derived from their steady state distributions, but the highly dynamic generation and consumption of different phosphoinositides in response to different stimuli in the various sub-cellular compartments in living cells by the action of kinases and phosphatases together with lipase reactions, may lead to the formation of transient pools of differing molecular forms. There must be a continuous replenishment of the precursors by new synthesis, especially of phosphatidylinositol.
Functions: The characteristic subcellular locations of the different phosphoinositide species, together with the rapid and reversible nature of phosphorylation, gives them a central position to act as molecular switches that control cell signalling cascades, membrane dynamics and remodelling. The precise locations of the phosphoinositides are factors that contribute a characteristic identity to each cellular compartments, including the plasma membrane, endosomes, lysosomes, protein scaffolds and the nucleus, and sometimes even to each face of these, such as the cis and trans faces of the Golgi apparatus, and this enables directional transport of cellular constituents between organelles or membranes. In lysosomes, phosphoinositide switches regulate such critical functions as nutrient sensing, mTORC1 activity and membrane repair to maintain cellular homeostasis, while nuclear phosphoinositide signalling regulates transcription, DNA repair and oncogenic pathways. Dysregulation of phosphoinositide metabolism and signalling is a factor in many diseases, including cancer, and many genetic disorders have been identified. All are precursors for the water-soluble inositol phosphates (see below).
Phosphoinositides can act in signalling directly by binding via their polar head groups to cytosolic domains of certain membrane proteins, thereby triggering downstream signalling cascades, often in conjunction with another acidic phospholipid, such as phosphatidylserine or phosphatidic acid at an adjacent-binding site. The term 'lipidon' has been coined to describe the unique collection of co-located lipids that distinguish nano-environments of membranes and provide the context for PI recognition in vivo. In this way, they can regulate the operation of innumerable proteins integral to membranes by relocating a protein from one area of the cell to another, e.g., from the cytosol to the inner leaflet of the plasma membrane, or they can attract cytoskeletal and signalling components to the membrane. The distinctive phosphoinositide composition of membranes in different organelles adds strength and specificity to the interactions by cooperative binding with other membrane proteins.
Binding is usually through electrostatic interactions with the negative charges of the phosphate groups on the inositol ring with characteristic clusters of basic amino acid residues in proteins to recruit them to intracellular membranes that often leads to conformational changes and thence increased activity of unstructured peptides. At least 70 types of binding sites for phosphoinositides have been identified in proteins, and a region termed the pleckstrin homology (PH) domain, consisting of ~120 amino acids, is the most abundant of these with more than 250 proteins identified in the human proteome. This ensures binding with particular polyphosphoinositides, often simultaneously with other proteins. While the interaction is driven by non-specific electrostatic interactions initially, it is followed by selective binding to increase the membrane residence time. The phox homology (PX) domain family with 49 members in humans is unique in that it can recognize all seven phosphoinositide forms, while proteins with a FYVE domain, which is enriched in cysteine and is stabilized by two zinc atoms, binds only to PI(3)P. Among the proteins that bind to phosphoinositides in this way are phospholipases, protein kinases, regulators of membrane trafficking, and cytoskeletal, scaffold and ion channel proteins. For example, the protein kinase C family have C1 or C2 domains which recognize PI(4,5)P2 and PI(3,4,5)P3 (and sometimes other lipids).
Phosphatidylinositol 3-phosphate and the other phosphatidylinositol monophosphates are present in cells at low levels only, and their concentrations do not fluctuate greatly. The phosphoinositide 3-kinase pathway is a master regulator of cellular growth and division, and plays essential roles in controlling immune cell function, metabolism, chemotaxis and proliferation through generation of the signalling lipid PI(3)P. This is a marker lipid that recruits certain cytosolic proteins to the early endosomes and defines their identity. It is pivotal for the initiation of autophagy, i.e., the controlled internal degradation and turnover of cellular constituents, while PI(3,5)P2 is utilized for the autophagosome-lysosome fusion step and in the subsequent acidification of this organelle. After sorting of the lysosomal contents, components of the internalized cargo are recycled to the plasma membrane and PI(3)P is dephosphorylated to phosphatidylinositol by its own phosphatase, before this is in turn phosphorylated to PI(4)P. Thus, the processes of internalization, sorting and trafficking of membrane proteins depend on the interconversion of phosphoinositides by coordinated phosphorylation-dephosphorylation reactions.
 In general,
PI(3)P controls cellular processes by recruiting effector proteins through low to moderate affinity interactions
with characteristic PI(3)P binding domains.
A protein designated Akt (protein kinase B) is recognized as a direct initiator of the PI3K signalling cascade with receptor tyrosine kinases
as the main upstream activators, and it is now known that every phosphatidylinositol phosphate has a unique set
of effector proteins that are recruited to target membranes or are allosterically regulated by receptors.
Further, PI(3)P regulates the final stage of cell division (cytokinesis), and the lipid is known to accumulate where cells divide.
In the yeast Saccharomyces cerevisiae, PI3P is critical for the targeting of α-synuclein to the plasma membrane via the secretory
pathway.
Mycobacterium tuberculosis can subvert phosphoinositide signalling to arrest phagosome maturation by dephosphorylation of PI(3)P.
In general,
PI(3)P controls cellular processes by recruiting effector proteins through low to moderate affinity interactions
with characteristic PI(3)P binding domains.
A protein designated Akt (protein kinase B) is recognized as a direct initiator of the PI3K signalling cascade with receptor tyrosine kinases
as the main upstream activators, and it is now known that every phosphatidylinositol phosphate has a unique set
of effector proteins that are recruited to target membranes or are allosterically regulated by receptors.
Further, PI(3)P regulates the final stage of cell division (cytokinesis), and the lipid is known to accumulate where cells divide.
In the yeast Saccharomyces cerevisiae, PI3P is critical for the targeting of α-synuclein to the plasma membrane via the secretory
pathway.
Mycobacterium tuberculosis can subvert phosphoinositide signalling to arrest phagosome maturation by dephosphorylation of PI(3)P.
Dysregulation of PI3K pathways has been implicated in the aetiology and maintenance of various diseases and metabolic disorders, which include insulin-independent glucose transport and many of the physiological actions of insulin. This affects cancer, inflammation and autoimmunity, and in relation to lung cancer, RAS proteins, which are signalling switches for the control of proliferation, differentiation and survival of eukaryotic cells, regulate type I phosphatidylinositol 3-kinase (PI3K), which drives tumour initiation and maintenance. Loss of neuronal PI(3)P results in progressive neurodegeneration. In consequence, pharmaceutical companies are pursuing the development of inhibitors of this enzyme.
Phosphatidylinositol 3,4-bisphosphate can be produced by two routes and regulates a variety of cellular processes with relevance to health and disease that include B cell activation and autoantibody production, insulin sensitivity, neuronal dynamics, endocytosis and cell migration. It is known to bind selectively to several proteins, and it acts as a secondary messenger by recruiting the protein kinases Akt (protein kinase B) and so may influence the cell cycle, cell survival, angiogenesis and glucose metabolism. In the endolysosomal system where it controls the maturation of endocytic coated pits, it is produced from PI(4,5)P2 with its synthesis and turnover spatially segregated. In epithelial cells, it is located on the apical membrane, i.e., facing the lumen, as opposed to the basolateral membranes, and it is a determinant of the identity of the apical membrane.
Phosphatidylinositol 3,5-bisphosphate, is present at low levels only in cells (0.04-0.1% of the total phosphatidylinositides), unless synthesis is induced by growth factors, but it is required for membrane and protein trafficking in the late endosomes in eukaryotes, where conversion of PI(3)P to PI(3,5)P2 promotes endosomal maturation and degradative sorting. It mediates signalling in response to stress and hormonal cues and in the control of ion transport in membranes, while genetic studies confirm that it is necessary for healthy embryonic development, especially in the nervous system.
Phosphatidylinositol 3,4,5-trisphosphate, derived from the action of PI3 kinase on PI(4,5)P2, is almost undetectable in quiescent cells, but its intracellular level rises rapidly from synthesis at the plasma membrane in response to agonists such as extracellular growth factors and hormonal stimuli. By recruiting proteins with pleckstrin homology (PH) domains, such as the protein kinases Akt, PDK1 and BTK, to the plasma membrane, it has been implicated in a variety of cellular processes that include growth, cell survival, proliferation, cytoskeletal rearrangement, intracellular vesicle trafficking and cell metabolism, and it is also a component of a signalling pathway in the cell nucleus that helps cells to cope with DNA damage. In epithelial cells, it is located on the basolateral membrane, i.e., facing adjacent cells, where it is regarded as a determinant of the identity of this membrane.
In contrast to PI(3)P, it opposes autophagy by binding to the PH domain of Akt to induce cell proliferation. During feeding, various physiological responses lead to the secretion of insulin, which stimulates the phosphorylation of PI(4,5)P2 to PI(3,4,5)P3 and triggers a signalling cascade that leads to the suppression of autophagy. When this pathway is impaired, it is harmful towards the insulin resistance associated with various metabolic diseases including obesity and diabetes. PI(3,4,5)P3 has been implicated in tumour cell migration and metastasis, and the PTEN phosphatase, which removes the 3-phosphate group, is a known tumour suppressor. In the nucleus and nucleoli of cells, it takes part in RNA processing/splicing, cytokinesis, protein folding and DNA repair. Like phosphatidylserine, it is reportedly transferred to the outer leaflet of the plasma membrane in aged or damaged cells as an 'eat‑me' signal for apoptosis by phagocytes.
The human immune system utilizes highly mobile neutrophils to eliminate pathogens from infected tissue, and the first step is to track and then pursue molecular signals such as cytokines emitted by pathogens. It has been established that two phospholipids operate in sequence to point the neutrophils in the correct direction. The first of these is PI(3,4,5)P3, which binds to a protein DOCK2 and enables it to translocate to the plasma membrane, before phosphatidic acid, generated by the action of phospholipase D on phosphatidylcholine, takes over and directs the DOCK2 to the leading edge of the plasma membrane. This causes polymerization of actin within the cell and reshapes it to point it in the direction from which the pathogen signals are coming.
Phosphatidylinositol 4-phosphate is the precursor for PI(4,5)P2, but it binds to a protein on the cytoskeleton of the cell and has its own characteristic functions. It has been described as the central regulator of Golgi function that influences membrane trafficking, lipid metabolism and signalling to control cargo sorting, glycosylation and organelle architecture through a range of effector proteins. As the most widely distributed of the phosphoinositides, it is present in late endosomes, lysosomes, secretory vesicles and autophagosomes as well as the Golgi and the plasma membrane; as a part of protein-lipid complexes, it takes part in nuclear processes. In yeast, it is employed in anterograde transport from the trans-Golgi and retrograde transport from the Golgi to the endoplasmic reticulum, and it is necessary for the formation of secretory vesicles in the Golgi that are targeted to the plasma membrane. Some PI(4)P in the plasma membrane is exchanged for phosphatidylserine by the action of transport proteins at junctions with the endoplasmic reticulum. PI(4)P facilitates the conversion of ceramide at the Golgi to glucosylceramide, a precursor for complex glycosphingolipids, by binding to the glucosylceramide transfer protein FAPP2 via its PH domain.
PI(4)P has been called the 'fuel' that drives cholesterol transport as its hydrolysis provides the energy that enables the establishment of sterol concentration gradients across membrane-bound compartments with the aid of the oxysterol-binding protein (OSBP)-related protein (ORP family), which is a regulator of cholesterol, oxysterol and PI(4)P concentrations in membranes, as discussed in our web page on cholesterol.
PI(4)P is necessary for the structure and efficient working of the late endosomes, where it is a factor in the recruitment of proteins that control cargo exit and participate in vesicle formation (following hydrolysis of PI(3)P). It accumulates rapidly in damaged lysosomes and is needed for their repair by recruiting multiple members of the OSBP family, which catalyse robust endoplasmic reticulum-to-lysosome transfer of phosphatidylserine and cholesterol to restore lysosomal integrity. PI(3)P initiates autophagy, but then PI(4)P, PI(4,5)P2 and their binding proteins are modulators of the process at later stages.
In the plasma membrane, PI(4)P can support the ion channels, and it contributes to the anchoring of proteins with polybasic domains, although it is not utilized for synthesis of PI(4,5)P2 in this membrane. PI(4)P derived from PI(4,5)P2 in the membrane of primary cilia in the retina is necessary for vision. In contrast, intracellular bacteria such as Salmonella and Legionella manipulate PI(4)P-enriched compartments to evade host defences and promote replication, and this lipid has an influence on the progression of many diseases, including virus replication, cancer and various inflammatory diseases; inhibitors of PI4-kinase are under study for their therapeutic potential.
Phosphatidylinositol 4,5-bisphosphate is found primarily in the inner leaflet of the plasma membrane, where it may define membrane identity in eukaryotic cells, although it is present in endosomes, the endoplasmic reticulum and nucleus in small amounts. It is crucial for the maintenance of epithelial characteristics and provides the cell-cell adhesion that maintains the integrity of multicellular organisms. Because of its large head group and multivalent negative charge, PI(4,5)P2 has been described as an "electrostatic beacon" that interacts in various ways with membrane proteins, other lipids and cellular cations. In consequence and despite its relatively low concentration, it is a regulator of innumerable events at the plasma membrane, including cell adhesion and motility, vesicle endocytosis and Ca2+-dependent vesicle exocytosis, and the operation of ion channels for potassium, calcium and sodium, where it is an obligatory factor by its interactions with appropriate proteins; its hydrolysis by phospholipase C acts in opposition to this. An intriguing proposal is that PI(4,5)P2 is not so much a signalling molecule but rather serves as a selective cofactor for cellular processes at the plasma membrane where it is a ‘master regulator’.
PI(4,5)P2 interacts with cationic residues of hundreds of proteins in concert with cholesterol to form localized membrane domains that differ from the sphingolipid-enriched rafts. Although its absolute concentration is relatively low (if higher than other phosphoinositides), it can magnify its influence by forming clusters in which the headgroups are linked by bridges of multivalent cations such as Ca2+ and Mg2+ and by binding to proteins. Together with its diacylglycerol metabolites, it assists in vesicle formation in membranes, and a major route for internalization of cell surface proteins such as transferrin is the clathrin-coated vesicle pathway in which it binds to the relevant proteins in the membrane, increasing the number of clathrin-coated pits and permitting internalization of proteins. It operates in a similar way in caveolae, where it is concentrated at the rim.
 Through
its attachment to the apical plasma membrane, PI(4,5)P2 has a key role in the development of the actin cytoskeleton and thereby
controls cell shape, motility, division and many other processes.
It binds to effectors such as vinculin, a membrane-cytoskeletal protein that links integrin
adhesion molecules to the actin cytoskeleton, and dysregulation has been implicated in the migration and metastasis of tumour cells.
In yeasts, the presence of stearic acid in position sn‑1 of the molecule is essential for such binding.
In the cell nucleus, as well as being a precursor for further signalling molecules, PI(4,5)P2 assists in
maintaining chromatin, the complex combination of DNA, RNA and protein that makes up chromosomes, in its transcriptionally optimum
conformation, and it has a role in gene transcription and in the modulation of RNA polymerase.
Through
its attachment to the apical plasma membrane, PI(4,5)P2 has a key role in the development of the actin cytoskeleton and thereby
controls cell shape, motility, division and many other processes.
It binds to effectors such as vinculin, a membrane-cytoskeletal protein that links integrin
adhesion molecules to the actin cytoskeleton, and dysregulation has been implicated in the migration and metastasis of tumour cells.
In yeasts, the presence of stearic acid in position sn‑1 of the molecule is essential for such binding.
In the cell nucleus, as well as being a precursor for further signalling molecules, PI(4,5)P2 assists in
maintaining chromatin, the complex combination of DNA, RNA and protein that makes up chromosomes, in its transcriptionally optimum
conformation, and it has a role in gene transcription and in the modulation of RNA polymerase.
PI(4,5)P2 is a cofactor for phospholipase D and so affects the cellular production of phosphatidic acid with its own signalling properties. Like ceramide-1-phosphate, it interacts with the Ca2+-dependent phospholipase A2 to generate the arachidonate for eicosanoid production. One molecule of PI(4,5)P2 is bound to each subunit of the protein in the crystal structure of the mammalian GIRK2 potassium channel, where it enables a conformational change that assists ion transport by the protein. It is also a cofactor for most transient receptor potential (TRP) ion channels. By binding to ceramide kinase, the enzyme responsible for synthesis of ceramide-1-phosphate, it has an influence on sphingolipid metabolism (and vice versa). In neurons, the endocannabinoid 2‑arachidonoylglycerol is derived from PI(4,5)P2 mainly.
Perhaps, the best characterized of the phosphoinositide signalling events from the hydrolysis of phosphatidylinositol phosphates by phospholipase C isoforms is to produce sn-1,2-diacylglycerols and inositol 3,4,5-trisphosphate (see next section), which act as second messengers. Only those polyunsaturated diacylglycerols derived from PI(4,5)P2 are able to activate protein kinase C (α, ε, δ) isoforms both in vitro and in vivo by interaction with a basic patch distal to a Ca2+ binding site that targets them selectively to the plasma membrane.
Via protein binding, the phosphoinositide is a component of the immune response of animal tissues to toxic bacterial lipopolysaccharides, and it is involved in the pathophysiology of the HIV virus via an interaction with the Tat protein secreted by infected cells.
Phosphatidylinositol 5-phosphate, the properties of which have taken longer to unravel because of technical difficulties in the separation of this isomer, is part of the mechanism for osmoregulation both in animals and plants. While it is the least abundant phosphatidylinositol monophosphate, it takes part in signalling at the nucleus and in the cytoplasm, modulating cellular responses to various stresses, hormones and growth factors. It has been linked to such cellular processes as actin remodelling, vesicle trafficking, metabolic homeostasis and apoptosis/cell survival.
3. Water-Soluble Inositol Phosphates (and Diacylglycerols)
As mentioned briefly above, hydrolysis of phosphatidylinositol phosphates by calcium-dependent phospholipase C (or 'phosphoinositidase C') leads to generation of sn‑1,2‑diacylglycerols (see this web page), which act as second messengers in animal cells and are of great metabolic significance. There are many different enzymes of this type, but the phosphoinositide-specific phospholipase C is utilized in the first step in the inositide signalling pathways. The enzyme exists in six families consisting of at least 13 isoenzymes, all of which have conserved regions such as the pleckstrin homology (PH) binding domain, with each one having a characteristic distribution that is linked to its own cellular process. These enzymes are stimulated by signalling molecules such as G‑protein coupled receptors, receptor tyrosine kinases, Ras-like GTPases and calcium ions, thus linking the hydrolysis of phosphatidylinositol phosphates to a wide range of other cellular signals. As phospholipase C is a soluble protein located mainly in the cytosol, translocation to the plasma membrane is a crucial step in signal transduction. Regulation of these isoenzymes, but mainly PLCβ, is vital for health as they are associated both positively and negatively with pathophysiological processes in relation to cancer (form PLCγ1), while aberrant expression of PLCγ2 may be a factor in neurodegenerative diseases.
Phosphatidylinositol 4,5-bisphosphate is usually considered the main precursor for diacylglycerol signalling, but phosphatidylinositol 4‑phosphate in the plasma membrane may be of equal value following interaction with G protein–coupled receptors (phosphoinositides with a phosphate in position 3 are not substrates for phospholipase C).
Phosphatidylinositol-specific phospholipase Cs are highly conserved across mammals, plants and bacteria, each with distinct structural organizations and regulatory mechanisms. In plants, they regulate growth and stress responses via phosphatidic acid and inositol phosphate signalling, while those in bacteria contribute to virulence by targeting the host cell membranes and glycosylphosphatidylinositol-anchored proteins.
Water-soluble inositol phosphates are versatile small molecules, which are the other products of the phospholipase C reaction. Up to 60 different compounds of this type are possible, and at least 37 of these have been found in nature at the last count, all of which are important in cellular metabolism. Under the action of various physiological stimuli in animals, including sphingosine-1-phosphate, and acting via various G‑protein-coupled receptors, PI(4,5)P2 in the plasma membrane is hydrolysed to release inositol 1,4,5‑trisphosphate, a cellular messenger that diffuses into the cytosol and triggers calcium release from an ATP-loaded store in the endoplasmic reticulum via ligand-gated calcium channels. The reaction takes place at endoplasmic reticulum-plasma membrane contact sites, which are specialised domains for the control of Ca2+ dynamics and various Ca2+‑dependent cellular processes. The increase in calcium concentration, together with the altered phosphorylation status, can activate or inhibit many different protein targets to enable cells to respond in an appropriate manner to an extracellular demand. The diacylglycerols produced remain in the membrane to recruit members of the protein kinase C family. In the parasite T. cruzi, inositol polyphosphates are produced by a phospholipase C-independent pathway involving carbohydrate and sphingolipid metabolism.
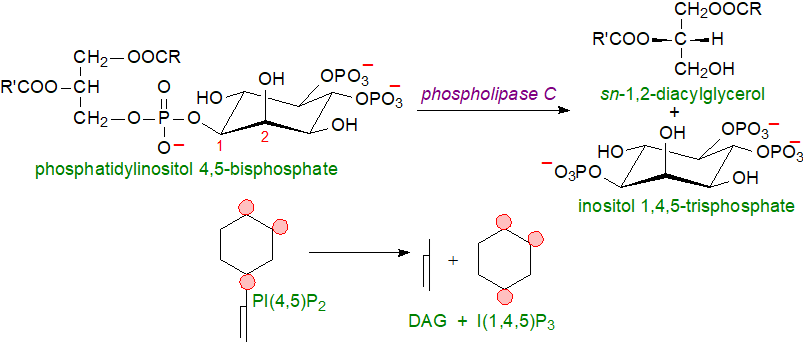 |
| Figure 5. Generation of water-soluble inositol phosphates. |
All the various inositol phosphates assist in the control of cellular events in characteristic ways, but mainly in the organization of signalling pathways, the rearrangement of the actin cytoskeleton and intracellular vesicle trafficking, together with gene transcription, RNA editing, nuclear export and protein phosphorylation. As these metabolites can be rapidly synthesised and degraded in discrete membrane domains or even sub-nuclear structures, they are considered to be ideal regulators of dynamic cellular mechanisms. Structural studies of inositol polyphosphate-binding proteins suggest that such inositides act in part at least by modifying protein function as structural cofactors, ensuring that proteins adopt their optimum conformations. Inositol polyphosphates in the nucleus of the cell are vital for DNA and RNA dynamics, and they may be switches that determine the operation of the nuclear complexes responsible for such processes, with the phosphorylation state of the inositol ring as the essential feature. As different isomers have their own functions at each level of gene expression, extracellular events must coordinate the production of these compounds in a highly synchronous manner.
As examples of other functions, inositol 1,4,5-trisphosphate (IP3) is an important contributor to the regulation of intracellular concentrations of calcium, which is a universal intracellular messenger relevant to innumerable functions that include cell division, apoptosis and the concentrations of other ions. It binds to calcium-permeable IP3 receptors (three in mammals), which are located mainly in the endoplasmic reticulum and release calcium into the cytosol, but have been found at contact sites with mitochondria and couple cellular calcium homeostasis and mitochondrial function. In contrast, inositol 1,4,5,6-tetrakisphosphate (Ins(1,4,5,6)P4) does not regulate calcium homeostasis but influences chloride secretion in intestinal epithelial cells. Phytic acid or IP6 has protective effects in many pathological conditions by ameliorating inflammation and oxidative stress while reducing circulating cholesterol levels
In organisms from plants to mammals, an extra tier of regulatory mechanisms is produced by kinases that generate energetic diphosphate (pyrophosphate)-containing molecules from inositol phosphates. Conversely, these can act as phosphate donors so recovering the original inositol phosphates. These inositol pyrophosphates and the enzymes for their metabolism are likewise utilized in the regulation of cellular processes by interacting with proteins in a variety of ways.
It should be noted that the phospholipase C isoenzymes regulate the concentration of PI(4,5)P2 and related lipids and thence how and where they operate in addition to the generation of new metabolites that may be lipid mediators. For rapid replenishment of the phosphatidylinositides used for this purpose, a cycle of reactions - the phosphatidylinositol cycle - must occur (see below). Some phosphatidic acid is synthesised from the diacylglycerols produced within the plasma membrane through diacylglycerol kinases, and this is transported back to the endoplasmic reticulum and ultimately can be re-utilized for phosphatidylinositol biosynthesis.
4. Phosphatidylinositides in Plants and Lower Organisms
In plants as in animals, there are innumerable requirements for phosphatidylinositol and polyphosphoinositides, which regulate metabolism by acting as ligands that bind to protein targets via lipid-binding domains so altering their enzymatic properties. After the divergence of the animal and plant kingdoms during evolution, polyphosphoinositide metabolism developed in different ways so the details of the processes in each are very different, not least because the subcellular locations of phosphoinositides differ appreciably between plants and animals. As in animals, they are located in membranes with the polar headgroup in the cytosol. Phosphatidylinositol is of course the precursor of the phosphorylated forms and determines their fatty acid compositions. It inhibits apoptosis by acting as the biosynthetic precursor of the sphingolipid ceramide phosphoinositol and so reduces the levels of ceramide.
As in animals, the various phosphoinositides (five in total) are produced and inter-converted rapidly by kinases and phosphatases (in many isoforms) in different cellular membranes in response to environmental or developmental cues. Phosphatidylinositol is generated mainly in the endoplasmic reticulum, while PI 4-kinases (3 isoforms in Arabidopsis) and their product are located in the trans-Golgi network and nucleus, and PI(4)P 5-kinases (11 isoforms in Arabidopsis) and product are present in the plasma membrane. During the biosynthesis of polyphosphoinositides, the first phosphorylation occurs at the hydroxyl groups at positions 3 or 4 of the inositol ring, catalysed by the appropriate kinases, while the second phosphorylation then takes place at position 5; PI 5-phosphate is produced by the action of a phosphatase on PI 3,5‑bisphosphate. Most other metabolites are produced via PI(3)P, and confirmation of reports that some phosphatidylinositol 3,4,5-trisphosphate may be produced from phosphatidylinositol 4,5‑bisphosphate is needed. In contrast to mammalian phosphatidylinositol 3‑kinases, which accept both phosphatidylinositol and its monophosphates as substrates, the plant enzyme acts only on the former.
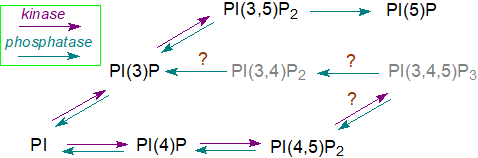 |
| Figure 6. Polyphosphoinositide metabolism in plants. |
The reverse reaction in plants is accomplished by phosphoinositide phosphatases, which can be grouped into three main families with many members, each having differing subcellular locations, substrate specificities and regulatory mechanisms. 4- or 5-Phosphates are hydrolysed by phosphatases of the suppressor of actin (SAC) family and the 5‑phosphatase (5‑Ptase) family, while 3-phosphates by are hydrolysed by the PTEN family.
Although what might be considered normal levels of phosphatidylinositol 4-phosphate are present, the concentrations of phosphatidylinositol 4,5‑bisphosphate and other phosphoinositides are extremely low in plants (10 to 20-fold lower than in mammalian cells), although they are nonetheless required for vital purposes. There are differences between cell types, but in Arabidopsis epidermal root cells, PI(4,5)P2 is present at highest concentration in the plasma membrane (apex region) and nucleus, while PI4P slowly distributes between the plasma membrane and Golgi with the highest concentration in the former. Multivesicular bodies/late endosomes accumulate both PI(3)P and PI(3,5)P2, and the tonoplasts and autophagosomes contain PI(3)P. How the various metabolites are transported between membranes has yet to be determined, but non-vesicular transport may occur at membrane contact sites, and vesicular transport probably occurs also.
Highly polarized distributions of phosphoinositides are found within membranes, in general oriented toward the cytosolic leaflet, and they are organized in nanoclusters together with other lipids and proteins. PI(4)P controls the electrostatic state and necessary for cell division as the only phosphoinositide present at the membrane separating two daughter cells, i.e., the cell plate. PI(4)P may control the location and activities of many membrane proteins, including those used for development, reproduction, immunity, nutrition and signalling, and it may interact with salicylic acid in the plant immune response. Functions are now being discovered for each of the plant phosphoinositides, which are produced rapidly in response to osmotic, salt and heat stress, and it has become evident that a continuous turnover is essential for cell growth and development and for the operation of guard cells. In the nucleus, proteins have been identified that bind to phosphoinositides via the acyl chains, leaving the head group exposed for enzymatic modifications and signal transduction.
 PI(4,5)P2
is present mainly in the plasma, where it is enriched in the detergent-resistant component commonly equated with
'rafts', but occurs in the nucleus and transiently in membrane endocytotic vesicles.
Phosphoinositides are of great importance in microdomains at the tip of growing tissues such as the shoot apical meristem, pollen tubes and
root hairs where PI(4,5)P2 is used for stem cell maintenance and organogenesis.
Although its concentration is low, PI(4,5)P2 has been shown to be a component of signalling mechanisms
by binding to many different target proteins, which have characteristic binding domains.
Together with phosphatidic acid, PI(4,5)P2 regulates a number of actin-binding proteins, and these in turn control
the operation of the actin cytoskeleton and microtubules, which have a role in plant growth, the movement of subcellular organelles,
cell division and differentiation, and plant defence.
In addition, this lipid exerts a control over ion channels, ATPases and phospholipase C-mediated lipid degradation, and the production
of further second messengers.
It is a factor in both clathrin-mediated endocytosis and in exocytosis.
The efficiency of many of the interactions may be dependent on the fatty acid composition of the lipid and the activity of
phosphatidylinositol 4‑phosphate 5-kinase.
PI(4,5)P2
is present mainly in the plasma, where it is enriched in the detergent-resistant component commonly equated with
'rafts', but occurs in the nucleus and transiently in membrane endocytotic vesicles.
Phosphoinositides are of great importance in microdomains at the tip of growing tissues such as the shoot apical meristem, pollen tubes and
root hairs where PI(4,5)P2 is used for stem cell maintenance and organogenesis.
Although its concentration is low, PI(4,5)P2 has been shown to be a component of signalling mechanisms
by binding to many different target proteins, which have characteristic binding domains.
Together with phosphatidic acid, PI(4,5)P2 regulates a number of actin-binding proteins, and these in turn control
the operation of the actin cytoskeleton and microtubules, which have a role in plant growth, the movement of subcellular organelles,
cell division and differentiation, and plant defence.
In addition, this lipid exerts a control over ion channels, ATPases and phospholipase C-mediated lipid degradation, and the production
of further second messengers.
It is a factor in both clathrin-mediated endocytosis and in exocytosis.
The efficiency of many of the interactions may be dependent on the fatty acid composition of the lipid and the activity of
phosphatidylinositol 4‑phosphate 5-kinase.
As in animals, phosphoinositides have a role in endosomal sorting but through the central vacuole, a lytic and storage organelle in plants. Phosphatidylinositol 3,5-bisphosphate is the least abundant of the phosphoinositides, but it is a crucial lipid for membrane trafficking systems. The PI to PI(3)P to PI(3,5)P2 cascade, the second step with a kinase designated FAB1, is required for endosomal sorting events leading to membrane protein degradation or retrieval, vacuolar morphogenesis and autophagy. PI(3,5)P2 has a role in stomatal closure and the growth of root hairs, and it is induced in salt stress.
Although it is not certain whether phosphatidylinositol is itself a substrate in vivo, many different enzymes of the phospholipase C type specific for polyphosphoinositides have been isolated from higher plants; they are activated by Ca2+ and unlike their mammalian counterparts, they are not regulated by G‑proteins. Less is known of the metabolism of the water-soluble inositol phosphates produced in comparison to animals, and plants appear to lack a receptor for inositol 1,4,5-trisphosphate (IP3), although it is the most abundant metabolite of this type and is reported to induce release of calcium ions to trigger stomatal closure. However, there is increasing evidence for lipid signalling mediated by phospholipase C in development and abiotic stress tolerance in plants. There is a general if contested belief that inositol hexakisphosphate (phytic acid or IP6), produced at least in part by sequential phosphorylation of inositol 1,4,5-trisphosphate, is more relevant as a cellular messenger in plants and mobilizes an endomembrane store of calcium ions. Inositol-1,2,4,5,6-pentakisphosphate (IP5) is a structural co-factor of the jasmonic acid receptor coronatine insensitive 1, linking phosphoinositide signalling with phytohormone-controlled pathways.
Phosphoinositides are part of the defence mechanisms against biotic stresses, especially in plant immunity in relation to bacterial, fungal and viral infections. While they may mediate defensive signalling, they can promote pathogen entry into host cells and have been implicated in viral replication.
In plants in contrast to animals as discussed briefly above, diacylglycerols, the other product of phospholipase C hydrolysis of phosphoinositides, and while plants lack protein kinase C and diacylglycerols have not been considered relevant to signal transduction, they do have proteins with related properties that are influenced by these lipids. Instead, diacylglycerols are rapidly converted to phosphatidic acid, with the action of phospholipase D a further source, and this is a well-characterized signalling molecule in plants, especially in defence.
Fungi and protozoa: Yeasts produce only five phosphoinositides (with more phosphatidylinositol 4,5-bisphosphate than plants) but no detectable phosphatidylinositol 3,4‑bisphosphate or phosphatidylinositol 3,4,5-trisphosphate. They are produced rapidly in response to nitrogen starvation, and phosphatidylinositol 3,5-bisphosphate synthesis is induced by osmotic stress. Fungi contain one phospholipase C subtype, which is related to the mammalian PLC-δ and may be a factor in its pathogenicity. In contrast, parasitic protozoa, such as Trypanosoma brucei, have a cycle of phosphoinositide biosynthesis and presumably function that is close to that in animals. The Amoebozoa, such as Dictyostelium discoideum, possess Class I PI3Ks, which produce phosphatidylinositol 3,4,5‑trisphosphate directly at the plasma membrane from 1‑hexadecyl-2-(11Z-octadecenoyl)-sn-glycero-3-phospho-(1'-myo-inositol), i.e., with an ether-linked 16:0 chain at the sn-1 position.
5. The Phosphatidylinositol Cycle
Phosphatidylinositol is at the centre of a cycle of reactions and intermediates that take part in innumerable aspect of cellular signalling in animals (a similar cycle could be described for plants). These are discussed individually at length above, but it is useful to point out how each component forms part of a larger pattern. In brief as illustrated, the various synthetic and hydrolytic reactions for phosphoinositide metabolism can be considered to constitute a phosphatidylinositol cycle with enzymes located both in the endoplasmic reticulum and plasma membrane, so lipids have to be transferred across the cytosol in both directions between the two to complete the cycle, probably via adjacent membrane structures and facilitated by proteins of the phosphatidylinositol transfer protein membrane-associated family (PITPs - PITPNM or nir2), which may channel phosphoinositide production to the necessary outcomes.
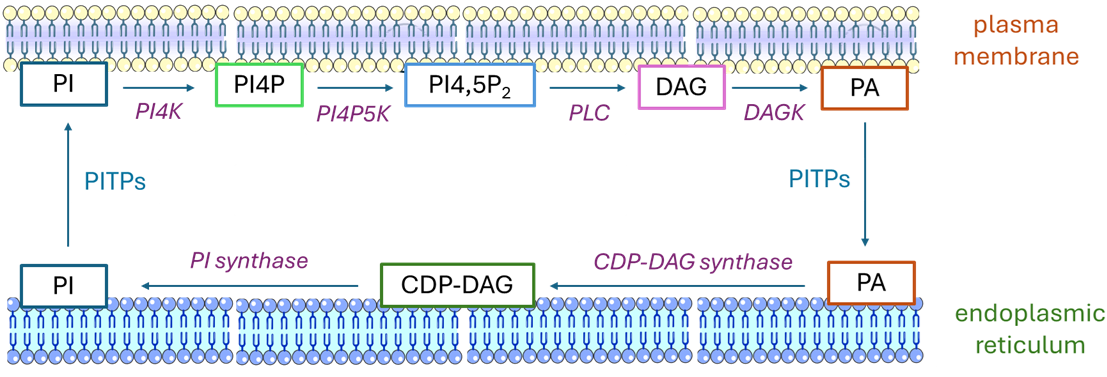 |
| Figure 7. Schematic representation of the phosphatidylinositol cycle. |
Phospholipase C and phosphatidylinositol-4-phosphate 5-kinase (PI4P5K) are located in the plasma membrane, while the cytidine diphosphate-diacylglycerol synthase (CDS2) and phosphatidylinositol synthase are in the endoplasmic reticulum. The epsilon isoform of diacylglycerol kinase (DGKε) is located at contact sites between the endoplasmic reticulum and plasma membrane, but there are nine further isoforms with differing cellular and subcellular locations that may be needed at points in the cycle. Each turn of the cycle uses a great deal of energy and consumes three moles of ATP, together with cytidine triphosphate and inositol. If it is assumed that the pyrophosphate is hydrolysed by endogenous pyrophosphatases to inorganic phosphate, the cycle can proceed in one direction only.
Factors such as membrane curvature must be considered, and the diagram is of necessity a considerable over-simplification. Many of the lipid intermediates can be precursors for other lipids, and for example, diacylglycerols are potential precursors for triacylglycerols while phosphatidic acid is a precursor for phosphatidylcholine and phosphatidylethanolamine. Each lipid intermediate is subject to remodelling of the acyl chains via the Lands cycle, and polyunsaturated fatty acids released can be utilized for eicosanoid production. A further by-product of the cycle is inositol triphosphate, which contributes to the regulation of intracellular calcium levels.
It has been suggested that the unique molecular composition of phosphoinositides (mainly 18:0-20:4) could influence their selective recycling back into phosphatidylinositol as many of the enzymes prefer this substrate, while another suggestion is that the phosphatidylinositol cycle could act to enrich this species through multiple passages around the cycle.
6. Lyso-Phosphoinositides and Other Relevant Lipids
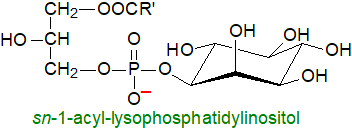 Lysophosphatidylinositols: Lysophosphatidylinositols (LPI),
i.e., with a single fatty acid linked to the glycerol moiety, are formed as intermediates in the remodelling of the fatty acid compositions
of the diacyl-lipids by the action of phospholipase A1 or phospholipase A2 (e.g., cPLA2α),
and when arachidonic acid is released for eicosanoid biosynthesis (see above).
It has become apparent relatively recently that like other lysophospholipids, LPI and the polyphospho analogues may
be signalling molecules, and it has long been known that LPI induces the release of insulin from pancreatic cells,
suggesting that it has a role in glucose homeostasis.
Lysophosphatidylinositols: Lysophosphatidylinositols (LPI),
i.e., with a single fatty acid linked to the glycerol moiety, are formed as intermediates in the remodelling of the fatty acid compositions
of the diacyl-lipids by the action of phospholipase A1 or phospholipase A2 (e.g., cPLA2α),
and when arachidonic acid is released for eicosanoid biosynthesis (see above).
It has become apparent relatively recently that like other lysophospholipids, LPI and the polyphospho analogues may
be signalling molecules, and it has long been known that LPI induces the release of insulin from pancreatic cells,
suggesting that it has a role in glucose homeostasis.
sn‑2-Arachidonoyl-lysophosphatidylinositol, a product of hydrolysis by a phospholipase A1, is an endogenous ligand for a G protein-coupled receptor GPR55 and thereby can induce rapid phosphorylation of certain enzymes, including a protein kinase, which promote cancer cell proliferation, migration and metastasis. Indeed, LPI is a biomarker for poor prognosis in cancer patients (15µM in ascites in ovarian cancer), and its concentration is elevated significantly in highly proliferative cancer cells in vitro. GPR55 is expressed in many regions of the brain, the intestines, endocrine pancreas and islets (where it may trigger insulin release). It has been implicated in macrophage activation and inflammation and in several metabolic diseases (it is present at high levels in obese subjects). Via the action of human glycerophosphodiesterase 3 as a lysophospholipase C, it can be a precursor of the endocannabinoid 2‑arachidonoylglycerol, and in so doing this enzyme suppresses the receptor for LPI and so acts as a switch between GPR55 and endocannabinoid (CB2) signalling.
Glycerophosphoinositol:  Sequential removal of both fatty acids
from phosphatidylinositol by a phospholipase A2 (PLA2IVα), which
is both a phospholipase A2 and a lysophospholipase releases water-soluble glycerophosphoinositol.
While this can be hydrolysed by a glycerophosphodiester phosphodiesterase to inositol 1-phosphate, glycerophosphoinositol per se
has its own functions, as do related compounds derived from the phosphatidylinositol phosphates.
Glycerophosphoinositol is anti-inflammatory in that it inhibits the inflammatory
and thrombotic responses induced by bacterial lipopolysaccharides (endotoxins).
Sequential removal of both fatty acids
from phosphatidylinositol by a phospholipase A2 (PLA2IVα), which
is both a phospholipase A2 and a lysophospholipase releases water-soluble glycerophosphoinositol.
While this can be hydrolysed by a glycerophosphodiester phosphodiesterase to inositol 1-phosphate, glycerophosphoinositol per se
has its own functions, as do related compounds derived from the phosphatidylinositol phosphates.
Glycerophosphoinositol is anti-inflammatory in that it inhibits the inflammatory
and thrombotic responses induced by bacterial lipopolysaccharides (endotoxins).
Diol lipids: Bacteria of the genus Thermomicrobia contain unusual long-chain 1,2-diol-containing phosphoinositides in which the stereochemistry of the diol unit is the same as the corresponding positions in sn-glycerol-3-phosphate. C17 to C23 Straight-and branched-chain saturated fatty acids are linked to position 2 of the diol.

Other lipids: Phosphatidylinositol is known to be the anchor that links a variety of proteins to the external leaflet of the plasma membrane via a glycosyl bridge (glycosyl-phosphatidylinositol(GPI)-anchored proteins), but this was considered to be a topic worthy of its own web page. Lipophosphoglycans (lipoarabinomannans and arabinogalactans) and phosphatidylinositol mannosides are components of the membranes of parasitic protozoa and bacteria, but for convenience they too are discussed with the GPI-anchored proteins.
7. Analysis
Like all acidic phospholipids, phosphatidylinositol is not easy to isolate in a pure state. Care must be taken to ensure that it is fully resolved from phosphatidylserine, but this can be accomplished by adsorption TLC or HPLC with appropriate procedures. The phosphatidylinositol phosphates are a different matter because of their high polarity and low abundance. Acidified solvents must be used to extract them efficiently from tissues and to ensure that they are in a single salt form. For isolation of individual components, TLC methods are usually favoured, although detection can be a problem - one approach being to equilibrate with radioactive phosphorus to facilitate detection and quantification by liquid scintillation counting. HPLC with mass spectrometric detection is now showing great promise, although regiospecific analysis of fatty acid distributions on the glycerol moiety still seems to be fraught with difficulties. Analysis of the lipid-glycoconjugate-protein complexes and of the lipophosphoglycans is a rather specialized task for which modern mass spectrometric and NMR facilities are necessary.
Recommended Reading
- Balla, T. Phosphatidylinositol 4-phosphate; A minor lipid with multiple personalities. Biochim. Biophys. Acta, Lipids, 1870, 159615 (2025); DOI - one of many papers on the theme of ‘The Multiverse of Phosphoinositides’ in this and the next journal volume.
- Barneda, D., Cosulich, S., Stephens, L. and Hawkins, P. How is the acyl chain composition of phosphoinositides created and does it matter? Biochem. Soc. Trans., 47, 1291-1305 (2019); DOI.
- Blunsom, N.J. and Cockcroft, S. Phosphatidylinositol synthesis at the endoplasmic reticulum. Biochim. Biophys. Acta, Lipids, 1865, 158471 (2020); DOI.
- Burke, J.E., Triscott, J., Emerling, B.M. and Hammond, G.R.V. Beyond PI3Ks: targeting phosphoinositide kinases in disease. Nature Rev. Drug Discovery, 22, 357-386 (2023); DOI.
- Calvillo-Robledo, A., Cervantes-Villagrana, R.D., Morales, P. and Marichal-Cancino, B.A. The oncogenic lysophosphatidylinositol (LPI)/GPR55 signaling. Life Sci., 301, 120596 (2022); DOI.
- Cheng, Z.L. and Montgomery, M.K. Physiological roles of phosphoinositides and inositol phosphates: Implications for metabolic dysfunction-associated steatotic liver disease. Clin. Sci., 139, 1073-1122 (2025); DOI.
- Epand, R.M. Features of the phosphatidylinositol cycle and its role in signal transduction. J. Membrane Biol., 250, 353-366 (2017); DOI.
- Heilmann, M. and Heilmann, I. Regulators regulated: Different layers of control for plasma membrane phosphoinositides in plants. Curr. Opinion Plant Biol., 67, 102218 (2022); DOI - see also - DOI1.
- Hou, X.T. and others. Phosphoinositide signalling in cell motility and adhesion. Nature Cell Biol., 27, 736-748 (2025); DOI - see also - DOI1.
- Ishino, Y., Shimanaka, Y., Aoki, J. and Kono, N. Mass spectrometry-based profiling of phosphoinositide: advances, challenges, and future directions. Mol. Omics, in press (2025); DOI.
- Ivanova, A. and Atakpa-Adaji, P. Phosphatidylinositol 4,5-bisphosphate and calcium at ER-PM junctions - Complex interplay of simple messengers. Biochim. Biophys. Acta, Mol. Cell Res., 1870, 119475 (2023); DOI.
- Jensen, J.B. and 10 others. Biophysical physiology of phosphoinositide rapid dynamics and regulation in living cells. J. Gen. Physiol., 154, e202113074 (2022); DOI.
- Kang, J.H., Toita, R., Kawano, T., Murata, M. and Kano, A. Phospholipids and their metabolites as diagnostic biomarkers of human diseases. Prog. Lipid Res., 99, 101340 (2025); DOI.
- Lai, S.Y., Huang, W.B. and He, K.M. Spatial organization of phosphoinositide signaling. FEBS Letts, in press (2025); DOI.
- Lolicato, F., Nickel, W., Haucke, V. and Ebner, M. Phosphoinositide switches in cell physiology - From molecular mechanisms to disease. J. Biol. Chem., 300, 105757 (2024); DOI.
- Macrae, C., Lalovic, D., Bunney, T.D. and Katan, M. Phosphoinositide-specific phospholipase C enzymes: Recent advances in a long journey. Biochim. Biophys. Acta, Lipids, 1870, Biochim. Biophys. Acta, Lipids (2025); DOI.
- Noack, L.C. and Jaillais, Y. Functions of anionic lipids in plants. Annu. Rev. Plant Biol., 71, 71-102 (2020); DOI.
- Overduin, M. and Kervin, T.A. The phosphoinositide code is read by a plethora of protein domains. Exp. Rev. Proteomics, 18, 483-502 (2021); DOI.
- Powis, G., Meuillet, E.J., Indarte, M., Booher, G. and Kirkpatrick, L. Pleckstrin Homology [PH] domain, structure, mechanism, and contribution to human disease. Biomed. Pharmacotherapy, 165, 115024 (2023); DOI.
- Rossignol, F., Lamari, F. and Mitchell, G.A. Phosphoinositide metabolism: biochemistry, physiology and genetic disorders. J. Inher. Metab. Dis., 48, e70008 (2025); DOI.
- Vidalle, M.C. and others. Nuclear phosphoinositides as key determinants of nuclear functions. Biomolecules, 13, 1049 (2023); DOI.
- Wills, R.C. and Hammond, G.R.V. PI(4,5)P2: signaling the plasma membrane. Biochem. J., 479, 2311-2325 (2022); DOI.
- and in relation to the history of the topic -
- Agranoff, B.W. Turtles all the way: reflections on myo-inositol. J. Biol. Chem., 284, 21121-21126 (2009); DOI.
- Irvine, R.F. A short history of inositol lipids. J. Lipid Res., 57, 1987-1994 (2016); DOI.
- Michell, R.H. What was the scientific context in which I wrote my 1975 Inositol phospholipids and cell surface receptor function review? Biochim. Biophys. Acta, Lipids, 1870, 159601 (2025); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
