Fatty Acids: Hydroxy and Other Oxygenated
 The following is an
introduction to the structures, natural compositions and biochemistry of the more important fatty acids with hydroxy, epoxy (furanoid),
methoxy and oxo substituents that are bulk constituents of tissues as opposed to lipid mediators, but it is not intended to be a comprehensive
list.
There is also brief discussion of some other oxygenated fatty acids that do not easily fit into other categories elsewhere in this website.
As with more conventional fatty acids, oxygenated fatty acids exist in nature mainly in lipid-bound form as esters or amides as in
glycero- or sphingolipids, and they can exist in ester linkage to other fatty acids via their hydroxyl groups.
Di-, tri- and polyesters of the last type are usually termed estolides and some examples are listed below,
although others are described on separate web pages, i.e., ceramides,
lipid A and rhamnolipids.
Some hydroxy fatty acids can self-esterify to form lactones, especially when the hydroxyl group is in position 4
or 5 relative to the carboxyl group, and these are usually comparatively volatile and can serve in communication between organisms.
The following is an
introduction to the structures, natural compositions and biochemistry of the more important fatty acids with hydroxy, epoxy (furanoid),
methoxy and oxo substituents that are bulk constituents of tissues as opposed to lipid mediators, but it is not intended to be a comprehensive
list.
There is also brief discussion of some other oxygenated fatty acids that do not easily fit into other categories elsewhere in this website.
As with more conventional fatty acids, oxygenated fatty acids exist in nature mainly in lipid-bound form as esters or amides as in
glycero- or sphingolipids, and they can exist in ester linkage to other fatty acids via their hydroxyl groups.
Di-, tri- and polyesters of the last type are usually termed estolides and some examples are listed below,
although others are described on separate web pages, i.e., ceramides,
lipid A and rhamnolipids.
Some hydroxy fatty acids can self-esterify to form lactones, especially when the hydroxyl group is in position 4
or 5 relative to the carboxyl group, and these are usually comparatively volatile and can serve in communication between organisms.
The prostaglandins and other eicosanoids and the plant oxylipins are in essence fatty acids with oxygen-containing functional groups, but these require their own documents in this website because of their distinctive metabolic and signalling properties. While the intention here is to identify those oxygenated fatty acids that differ from these in their occurrence and possible biological activities, there are times when it can be difficult to determine whether an oxygenated fatty acid is a lipid mediator, and on-going research can change our perceptions; the FAHFA (Fatty Acid ester of Hydroxy Fatty Acid) fall into this category. Inconsistencies are therefore inevitable in this account. Hydroxy, oxo and epoxy fatty acids can arise adventitiously from hydroperoxides, and these are not discussed here. Nor are the many oxygenated fatty acids that are produced by synthetic means for industrial applications.
1. Hydroxy Fatty Acids in Animals
2R-Hydroxy fatty acids are common lipid components, especially of animal sphingolipids, (see our Introduction to sphingolipids). The chain-lengths vary from about C16 to C26, and they are normally saturated or monoenoic, but sphingomyelin containing 2‑hydroxylated polyenoic very-long-chain fatty acids has been found in mammalian testes and spermatozoa.

The hydroxyl group adds to the hydrogen-bonding capacity of sphingolipids, helping to stabilize membrane structures and strengthen the interactions with membrane proteins. It is introduced prior to the biosynthesis of the ceramide component of sphingolipids by a fatty acid 2‑hydroxylase, which is essential for the normal working of the nervous system while regulating differentiation of various cell types. As mutations to the enzyme in humans and mice give rise to a demyelination disorder, it is evident that sphingolipids containing 2‑hydroxy acids have unique functions in membranes that cannot be substituted by non-hydroxy analogues.
2‑Hydroxyoleic acid in particular suppresses the growth and induces autophagy in certain cancer cells by a mechanism that is uncertain but may involve phosphatidylcholine metabolism and not induction of sphingomyelin synthesis as once thought. Intriguingly, it is the unnatural S‑enantiomer that produces this effect. It therefore has appreciable potential as a non-toxic anticancer drug, and the European Medicines Agency has designated it as an orphan drug for the treatment of glioma.
2-Hydroxy fatty acids, including isomers with iso/anteiso-methyl branches, occur in ester linkage in wool wax and Harderian gland secretions, and they have been found in the glycerophospholipids, especially phosphatidylethanolamine, of sponges. 2,3-Dihydroxy-long-chain fatty acids are occasionally reported from the glycosphingolipids of marine invertebrates, and similar fatty acids have been found in the uropygial gland secretions of storks, together with 2- and 3‑mono-hydroxy components.
 2-Hydroxy-phytanic acid
is formed during alpha-oxidation of phytanic acid (see the web page on branched-chain fatty acids)
by liver mitochondria and peroxisomes, but it is detected in tissues only in patients with peroxisomal disorders.
2-Hydroxy-phytanic acid
is formed during alpha-oxidation of phytanic acid (see the web page on branched-chain fatty acids)
by liver mitochondria and peroxisomes, but it is detected in tissues only in patients with peroxisomal disorders.
3-Hydroxy-fatty acids (C4 to C16) are formed during beta-oxidation of fatty acids in mammalian tissues (see our web page on carnitines), and increased concentrations of the free acids or acyl-carnitines in blood and urine are indicative of disorders of fatty acid oxidation. D‑β‑Hydroxybutyrate is synthesised in the liver via the metabolism of fatty acids, including butyrate, and ketogenic amino acids through a series of reactions usually with acetoacetate (a ketone body) as an intermediate. Its concentration in plasma increases during ketosis, and it can be used an energy source by the brain when the blood glucose concentration is low. 3‑Hydroxy-dicarboxylic acids, produced by omega-oxidation of 3-hydroxy fatty acids, are present in urine, while 3‑hydroxy-pristanic acid has been found in the plasma of patients with defects of beta-oxidation. C8 to C12 3-Hydroxy acids are normal components of the wax secretions from the uropygial glands of some birds.
Cow's milk contains small amounts of a range of saturated and monoenoic hydroxy- and keto-fatty acids with the hydroxyl group in positions 5 to 16, but their biosynthetic origin is not known.
A family of enzymes, cytochromes P450s, in liver microsomes oxidize fatty acids with the 12-carbon lauric acid as the primary substrate, although other fatty acids can be utilized, They are unusual in that they hydroxylate with high specificity at the energetically unfavourable terminal (ω) or ω-1 carbons; the products do not accumulate in significant amounts in tissues (discussed in greater detail in our web page on hydroxyeicosatetraenoic acids).
Lactones:  Sebum lipids from animals of the horse family contain appreciable amounts of very-long-chain ω‑hydroxy fatty acids
(C33, C35, C37 mainly) that have cyclized to form lactones;
those from the horse are monoenes and have a methyl branch, while those from the donkey are mainly saturated and unbranched.
Certain frogs from Madagascar possess femoral glands on their hind legs that disseminate volatile compounds
including macrocyclic lactones derived from fatty acids oxidized in the ω-1 position.
Of more practical relevance, 4- and 5-hydroxy fatty acids form γ- and δ‑lactones, respectively, that contribute to the flavour
of cow's milk and dairy products.
Sebum lipids from animals of the horse family contain appreciable amounts of very-long-chain ω‑hydroxy fatty acids
(C33, C35, C37 mainly) that have cyclized to form lactones;
those from the horse are monoenes and have a methyl branch, while those from the donkey are mainly saturated and unbranched.
Certain frogs from Madagascar possess femoral glands on their hind legs that disseminate volatile compounds
including macrocyclic lactones derived from fatty acids oxidized in the ω-1 position.
Of more practical relevance, 4- and 5-hydroxy fatty acids form γ- and δ‑lactones, respectively, that contribute to the flavour
of cow's milk and dairy products.
 Insects:
15-Hydroxy-hexadecanoic and 17-hydroxy-octadecanoic acids are major components of beeswax
(together with small amounts of other homologues and (ω‑2)‑hydroxy-isomers).
Royal jelly, a secretion produced by worker honey bees (Apis mellifera), contains a number of mono- and dihydroxy fatty acids
(C8 to C14), but especially 10‑hydroxy-decanoic and 10‑hydroxy-2E-decenoic acids and homologues
(plus C8 and C10 dicarboxylic acids), which are widely believed to provide a variety of health benefits as
anti-inflammatory agents when consumed by humans.
9‑Hydroxy-2E-decenoic and the oxo-analogue are queen retinue pheromones.
Insects:
15-Hydroxy-hexadecanoic and 17-hydroxy-octadecanoic acids are major components of beeswax
(together with small amounts of other homologues and (ω‑2)‑hydroxy-isomers).
Royal jelly, a secretion produced by worker honey bees (Apis mellifera), contains a number of mono- and dihydroxy fatty acids
(C8 to C14), but especially 10‑hydroxy-decanoic and 10‑hydroxy-2E-decenoic acids and homologues
(plus C8 and C10 dicarboxylic acids), which are widely believed to provide a variety of health benefits as
anti-inflammatory agents when consumed by humans.
9‑Hydroxy-2E-decenoic and the oxo-analogue are queen retinue pheromones.
Bees and many other insects produce lactonized hydroxy fatty acids as pheromones or simply for recognition by their kin. As highly strained structures, 3‑hydroxy fatty acids would not be expected to form lactones, but at least one is known, i.e., vittatalactone, an insect pheromone. Of the many other insect waxes and secretions that contain hydroxy fatty acids, perhaps the best known is the insect Tachardia (Laccifer) lacca, which produces the polymeric material shellac with 9,10,16‑trihydroxy-hexadecanoic (aleuritic), 6‑hydroxy-tetradecanoic (butolic) and other acids as the monomers.
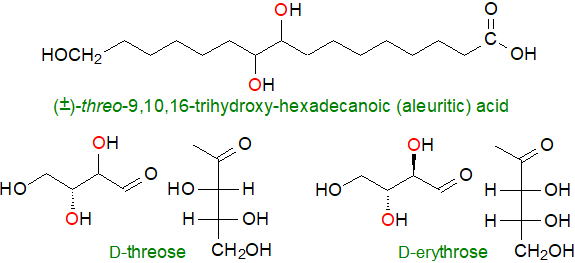 |
| Figure 1. Aleuritic acid and the stereochemistry of 1,2-diols |
Note that the stereochemistry of adjacent hydroxyl groups in the figure is defined from that of the sugars threose and erythrose with threo-dihydroxyls on opposite sides of the alkyl-chain, while erythro groups are on the same side.
2. Estolides (FAHFA) in Animal Tissues
9-Hydroxy-octadecanoic acid is a minor component of animal tissues and induces apoptosis in vitro in cell lines, including cancers. This fatty acid and its isomers with a free carboxyl group and a centrally located hydroxyl group to which a further fatty acid is linked as an estolide or 'FAHFA' (Fatty Acid ester of Hydroxy Fatty Acid), such as the palmitoyl ester of 9‑hydroxy-stearic acid (16:0-(9-O-18:0)) illustrated, have been found in the adipose tissue (white and brown, 100 to 150 ng/g), serum, milk and many other tissues of mice and humans as well as many common foods. In the circulation of healthy humans, the palmitoleic acid ester of 9‑hydroxystearic acid and the oleic acid ester of 9‑hydroxystearic acid are reported to be the main forms. At least 51 families or 300 molecular species of such lipids have been detected in rat adipose tissue, including 8 or more regioisomers of the hydroxy component (5, 7 to 13), and these vary in concentration with age, but unfortunately, doubt has been cast on the reliability of some recent data because of artefact formation (fatty acid dimerization) during mass spectrometry. In many analyses to date, FAHFA chirality has not been determined. Short-chain fatty acids (acetic and propionic) linked to long-chain (>C20) 2‑hydroxy acids have been detected in plasma and tissues, and in the contents of the gastrointestinal tract, where bacteria produce a wide range of diverse structures that differ from those synthesised by the host. As more is learned of their biochemistry and how they can act as lipid mediators, FAHFA are increasingly being classified among the oxylipins.

Relatively little is known of the biosynthesis of these lipids, and oxygenation of a saturated fatty acid to generate the hydroxy component requires a unique reaction. It has been established that they are produced endogenously with defined stereochemistry, i.e., the hydroxyl group has mainly the R‑configuration, and candidate genes for a mono-oxygenation reaction have been proposed. Adipose triacylglycerol lipase is the transacylase responsible for the esterification step. In white adipose tissue, biosynthesis of the precursor hydroxy fatty acids saturated FAHFAs is upregulated during inflammation and is positively regulated by carbohydrate responsive element binding protein (ChREBP), while the transcription factor nuclear factor erythroid-2-related factor 2 (Nrf2), which regulates the expression of antioxidant genes, may contribute to this.
Two atypical integral membrane hydrolases, designated AIG1 and ADTRP (Androgen Dependent TFPI Regulating Protein), and carboxyl ester lipase hydrolyse the estolide bond of FAHFAs, especially isomers with the ester bond at carbon 9, and they are presumed to have a regulatory role. Rather than having catalytic serine residues, these enzymes depend upon threonine nucleophiles.
FAHFA-containing triacylglycerols are present in adipose tissue at concentrations more than 100-fold greater than those of non-esterified FAHFAs higher concentrations in females than males), and as they can be released by lipases, it is suggested that this tissue may be a major reservoir for such compounds. Adipose triglyceride lipase acts in this process and participates in transesterification and remodelling reactions that can change the acyl compositions, but hormone-sensitive lipase is a more potent estolide bond hydrolase for both triacylglycerol-bound and free FAHFAs.
Having entered the circulation, FAHFA act as lipokines, i.e., lipid molecules derived from adipose tissue that can act as hormonal regulators and coordinate a wide array of cellular processes that are beneficial in general. FAHFAs, such as palmitic acid hydroxy stearic acids, have anti-diabetic and anti-inflammatory effects, even when administered orally, and they protect against colitis by regulating gut innate and adaptive immune responses. They help to maintain normal blood sugar levels by improving insulin sensitivity and glucose tolerance in mice by enhancing glucose-stimulated insulin secretion and insulin-stimulated glucose transport, while reducing adipose tissue inflammation. In so doing, they must communicate between adipose tissue and liver, where they act as selective agonists for the GPR40 and GPR120 receptors. Obese human patients were found to have lower FAHFA levels than non-obese controls. Many other FAHFA isomers are present in tissues and their functions are being explored, so for example, 9‑POHSA (palmitoleic acid ester of 9-hydroxy stearic acid) has been shown to attenuate inflammation-related indices induced by bacterial lipopolysaccharides in hepatocytes in rats.
While most of the FAHFAs in animal tissues are not derived from the essential fatty acids, others have been found in adipose tissue with docosahexaenoic acid (DHA) esterified to 9- and 13‑hydroxyoctadecadienoic and 14-hydroxydocosahexaenoic acids; they are present at concentrations like those of the specialized pro-resolving mediators and are potent anti-inflammatory molecules. For example, the estolide of DHA and 12-hydroxystearate is a potent activator of NRF2, which upregulates antioxidant enzymes and may be required for protective purposes in cells.
Estolides - others: Fatty acids with esterified hydroxyl groups in either the 2- or terminal position are present in meibomian secretions and tears of some animals, and more than 70 different molecular species of this type (ω-O-acyl) have been found in humans. Triacylglycerol estolides are present in the paracloacal gland of the brushtail possum with an 18:1 or 18:2 hydroxy fatty acid component.
The skin from animals, but studied especially in pigs and humans, contains O-acyl ceramides in which a long-chain fatty acid component (up to C36) has a terminal hydroxyl group, which may be in the free form or esterified with linoleic acid (see the web page on Ceramides for a detailed discussion of the structures, biosynthesis and properties of these lipids). These ceramides eventually lose the estolide fatty acid and link directly via the terminal hydroxyl group to structural proteins, as vital components of the epidermal permeability barrier. Comparable (O‑acyl)-ω‑hydroxy-fatty acids, i.e., with a terminal rather than a centrally located hydroxyl group, have now been identified in sperm and amniotic fluid. Vernix caseosa, the biofilm that coats the skin of the foetus towards the end of gestation, contains cholesterol esters of ω‑(O‑acyl)-hydroxy fatty acids, i.e., with very-long chain ω-hydroxy acids (e.g., 32:1) linked to more conventional fatty acids (14:0 to 18:1), giving 50 to 52 carbons in total. These also exist in free form together with analogous compounds derived from 2‑hydroxy fatty acids.
The larvae of the European cabbage butterfly, Pieris rapae, secret droplets of an oily liquid consisting of 11R-hydroxy-α-linolenic acid to which straight-chain saturated fatty acids are linked as estolides and termed 'mayolenes', which act as potent chemical deterrents to larval predators such as ants.

Estolides have long been known as components of seed oils and fungi, and some examples of these are described below.
3. Hydroxy Fatty Acids in Higher Plants
 Many unusual fatty acids are produced by certain plant families, and these are found most often in seed oils but only rarely
in membrane lipids.
However, hydroxy fatty acids are key components of plant polymers, such as cutin (see below).
As in animal tissues, 2R‑hydroxy fatty acids, mainly saturated, occur in appreciable amounts in the sphingolipids of plants,
but other 2-hydroxy acids may be encountered in the glycerolipids of seed oils.
For example, 2‑hydroxy-octadeca-9,12,15-trienoate is a minor component of the seed oil of
Thymus vulgaris, 2‑hydroxy-oleic and linoleic acids are found in Salvia nilotica,
and 2‑hydroxy-sterculic acid is occasionally encountered in seed oils of the Malvales.
γ‑Lactones, presumably derived from 4-hydroxy acids (C9 to C12) are present in many fruits to which they impart
pleasant odours.
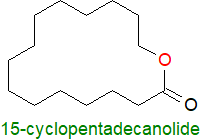
ω‑Hydroxy acids are rarely encountered other than in cutins, but cyclopentadecanolide derived from 15‑hydroxy-pentadecanoic acid
(C15), occurs in small quantities in the essential oil of angelica root and is responsible for a musk-like odour
that gives it commercial value in perfumes.
Many unusual fatty acids are produced by certain plant families, and these are found most often in seed oils but only rarely
in membrane lipids.
However, hydroxy fatty acids are key components of plant polymers, such as cutin (see below).
As in animal tissues, 2R‑hydroxy fatty acids, mainly saturated, occur in appreciable amounts in the sphingolipids of plants,
but other 2-hydroxy acids may be encountered in the glycerolipids of seed oils.
For example, 2‑hydroxy-octadeca-9,12,15-trienoate is a minor component of the seed oil of
Thymus vulgaris, 2‑hydroxy-oleic and linoleic acids are found in Salvia nilotica,
and 2‑hydroxy-sterculic acid is occasionally encountered in seed oils of the Malvales.
γ‑Lactones, presumably derived from 4-hydroxy acids (C9 to C12) are present in many fruits to which they impart
pleasant odours.
ω‑Hydroxy acids are rarely encountered other than in cutins, but cyclopentadecanolide derived from 15‑hydroxy-pentadecanoic acid
(C15), occurs in small quantities in the essential oil of angelica root and is responsible for a musk-like odour
that gives it commercial value in perfumes.
The seed oils of several higher plants contain hydroxy acids, some of which are agricultural commodities, while others are primarily of interest from a scientific standpoint, and some of these are illustrated.
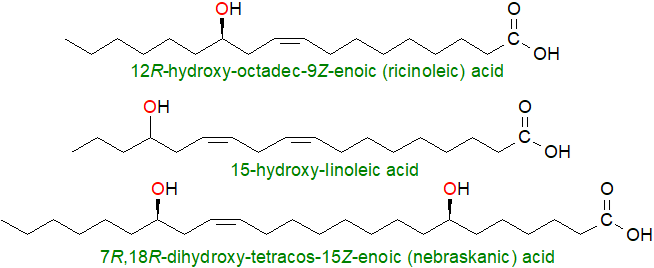 |
| Figure 2. Examples of hydroxy acids from seed oils. |
The best known and most important of these commercially is ricinoleic acid (12R‑hydroxy-octadec-9Z-enoic acid), which comprises up to 90% of castor oil (from Ricinus communis). Biosynthesis in plastids occurs via the action of an oleate 12‑hydroxylase (FAH12), an enzyme closely related structurally to a fatty acyl desaturase. In this instance, the substrate oleate is attached to position sn-2 of phosphatidylcholine and the reaction requires molecular oxygen and NADPH or NADH. Free ricinoleic acid is released into the cytosol after hydrolysis by LPCAT or PLA2/LACS and is transferred to the endoplasmic reticulum for esterification into triacylglycerols and eventually for incorporation into oil bodies.
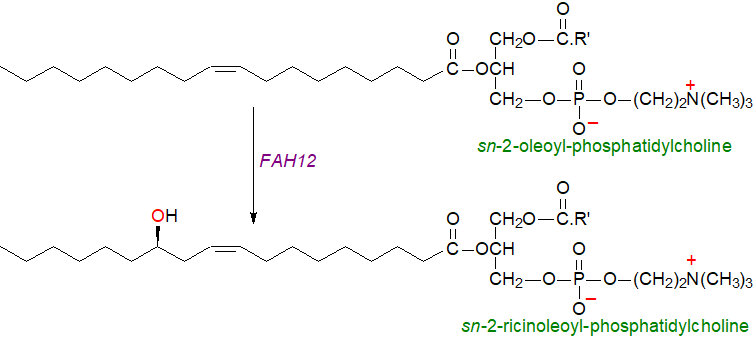 |
| Figure 3. Biosynthesis of ricinoleic acid in R. communis. |
The homologous 14-hydroxy-eicos-11Z-enoic (lesquerolic) acid is a component of seed oils from the genus Lesquerella, while the isomeric 9‑hydroxy-octadeca-12Z-enoic (isoricinoleic) acid is found in the genus Strophanthus. Other non-conjugated dienoic fatty acids, which may be related biosynthetically to these monoenes have been reported in seed oils, and include 12-hydroxy-octadeca-9Z,15Z-dienoic (densipolic) and 14-hydroxy-eicosa-11Z,17Z-dienoic (auricolic) acids. Among many others, 7,18‑dihydroxy-tetracos-15-enoic (nebraskanic) and an isomer with a further double bond in position 21 (wuhanic acid) acid have been characterized from the seed triacylglycerols of the Chinese violet cress (Orychophragmus violaceus). The last are of special interest in that the sequence of chain-length elongation reactions in their biosynthesis is interrupted in a manner usually seen only with bacterial fatty acids. As these fatty acids are present in the oil as oligomeric estolides in addition to the link to glycerol, it may have industrial value as a natural lubricant.
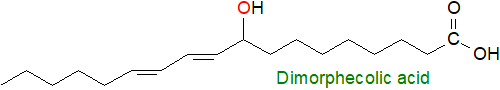
Conjugated dienoic fatty acids with a hydroxyl group and mainly C18 in chain-length are known from certain seed oils, some of which are produced commercially. These include 9-hydroxy-octadeca-10E,12E-dienoic (dimorphecolic) acid from the seed oil of Dimorphotheca sinuata, while the geometrical isomer with a cis/z-double bond in position 12 is present in the seed oil of Calendula officinalis among others. 13‑Hydroxy-octadeca-9Z,11E-dienoic (coriolic) acid has been reported from Coriaria nepalensis seed oil. Further conjugated hydroxy-fatty acids have acetylenic bonds, including ximenynolic (8-hydroxy,9a,11E) and isanolic (8‑hydroxy,9a,11a,17E) acids.
In those plants that contain hydroxy and other substituents in their fatty acids, the unusual fatty acids (~450 are known) are in general only found in the triacylglycerols of seed oils and not in the structural phospholipids. From a study with Physaria (Lesquerella) fendleri, the explanation is that extensive remodelling of triacylglycerols containing the more common range of fatty acids occurs post synthesis by a partial degradation and resynthesis cycle. For this purpose, a unique triacylglycerol lipase and two diacylglycerol acyltransferases (DGAT) are utilized that are selective for different stereochemical and acyl-containing species and may be located together in a remodelling metabolon near the lipid droplet-endoplasmic reticulum junction in seeds.
Similar estolide lipids to the FAHFA found in animal tissues described above have been reported from a variety of plants, but especially in seed oils, with stearic acid linked to hydroxy stearic acid often the most abundant form. One study found 47 FAHFA isomers (5 dominant) in 47 vegetable oil samples (13 types), with concentrations ranging from 0.56 to 1.76 x 104 ng/g. Olive oil is reported to be a good source of source of oleic acid-hydroxy stearic acid, which is anti-inflammatory and significantly improved glucose homeostasis when fed to mice. 15‑Hydroxylinoleate and an estolide of this acid with linoleate are components of the digalactosyldiacylglycerols in the seeds of oats (Avena sativa). Intriguingly, the 15-OH group has the R-configuration in the galactolipid, but the opposite in the oil. After ingestion of oat oil by humans, the free estolide been detected in plasma, and it has been shown to be anti-inflammatory in an animal system in vitro in that it suppresses lipopolysaccharide-stimulated secretion of cytokines and the expression of pro-inflammatory genes. However, the functions of such estolides in the parent plants are not known. Many other lipid-linked estolides have been described from seed oils, including castor oil and the Chinese tallow tree (Sapium sebiferum), which contains 2E,4Z-decadienoic acid in estolide linkage to the allenic 8-hydroxy-5,6-octadienoic acid.
Dihydroxy acids, such as 9,10-dihydroxy-octadecanoic acid and higher homologues, can be minor components of several seed oils, including castor oil, but they occur in significant concentrations in the seed oil of Cardamine impatiens, and trihydroxy (+)-threo-9,10,18-trihydroxyoctadecanoic (phloionolic) and (+)‑threo-9,10,18-trihydroxy-12Z-octadecenoic acids occur in the seed oil of Chamaepeuce afra.
Cutin and suberin: The aerial surfaces of higher plants are covered with a continuous extracellular layer, termed the cuticle, that contains cutin as the major structural component. Suberin is a related material that forms near the plasma membrane in the plant cells of the periderm, i.e., the tissue that surrounds secondary stems in woody plants as part of the bark, and it comprises both aliphatic and aromatic polymers and glycerol; it is also present on root surfaces. With the aid of embedded hydrophobic waxes, these layers act as the primary barrier between the plant and its environment to control the movement of gases, water and solutes and to impart resistance to pathogens or herbivores, while some components impart systemic acquired resistance. Suberin reduces passive Na+ influx and restricts sodium uptake to protect plants from ion toxicity.
Both cutin and suberin contain polyesters with linear and branched chains that consist mainly of mono-, di- and trihydroxy fatty acids together with α,ω‑dicarboxylic fatty acids; the last may be linked via glycerol moieties. In cutin, these fatty acids have chain lengths of 16 and 18 carbons, while suberin is much more complex as it contains phenolic acids, and the range of chain-lengths can go up to C28.
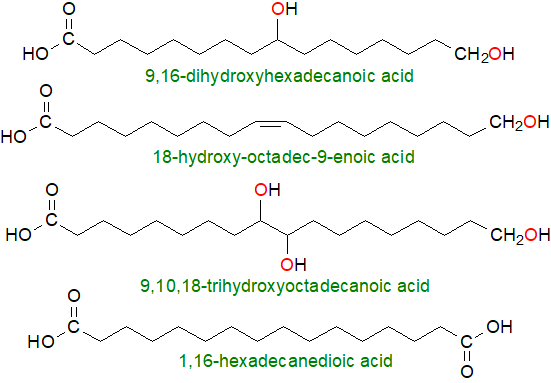 |
| Figure 4. Structures of some typical cutin/suberin fatty acids. |
The fatty acids of cutins include ω-monohydroxy acids (saturated and monoenoic), 9(or 10),16-dihydroxy-hexadecanoic acid (and analogous C18 acids), 9,10,18‑trihydroxy-octadecanoic acid, and occasionally related fatty acids with epoxyl or keto groups in central positions, e.g., 9,10-epoxy,18-hydroxy-octadecanoate. Suberins contain comparable fatty acids but with a wider range of chain-lengths and varying degrees of oxygenation, including phenylpropionic acids, e.g., coumaric and ferulic, and the best known and characterized source is cork from the bark of the cork oak (Quercus suber). In the 'model' plant Arabidopsis thaliana, leaf cutin is enriched in 1,18‑octadec-6,9-dienedioic acid, derived from linoleate, while flower cutin contains predominantly 10,16‑dihydroxypalmitic acid.
The lipid precursors of both cutin and suberin are 16:0, 18:0, 18:1 and 18:2 fatty acids synthesised in plastids and trafficked to the endoplasmic reticulum, the 'hub' for the production of cutin/suberin monomers, where they can undergo chain elongation and reduction to alcohols. For biosynthesis of oxygenated fatty acids, a hydroxyl group is introduced first at the terminal carbon atom of an existing fatty acid by means of cytochrome P450-dependent fatty acid monooxygenases, and this can then be converted to a dicarboxylic acid by a fatty acid oxygenase. Epoxidation by cytochrome P450 enzymes followed by the action of an epoxide hydrolase is required for the introduction of mid-chain hydroxyl groups with for example, oleic acid as the biosynthetic precursor of 18-hydroxy-oleic, 9,10,18‑trihydroxy-octadecanoic, 1,18‑octadec-9-enedioic and 9,10‑dihydroxy-1,18-octadecanedioic acids, together with 9,10-epoxy analogues. Long-chain acyl-coenzyme A synthetase and glycerol-3-phosphate (G3P) acyltransferases are required enzymes. The final step is polymerization by a cutin synthase in the cell wall, although it has been proposed that some cutin formation may occur by non-enzymatic polymerization of aggregates of the precursors in "cutinsomes". While A. thaliana has been widely used for biosynthetic studies of cutin lipids, many appear to regard this species as atypical.
Although much remains to be learned of how the various cutin monomers are transported to the plant surface prior to polymer formation, it is likely that ABCG transporters, exocytosis or lipid transfer proteins are involved possibly via microtubules. The non-specific lipid transfer proteins involved are known to have lipid-specific antimicrobial properties, they carry oxylipins and other signalling molecules essential to systemic acquired resistance at the same time, and they support redox balance by scavenging reactive oxygen species. Some fungal pathogens contain cutinases that can break down the cutin layer and use it as an energy source, but plants respond to this by synthesising oxylipins as a defence mechanism.
Less is known of suberin biosynthesis, but long-chain acyl-coenzyme A synthetases and a family of lipase/esterases and acyltransferases are involved with ABCG transporters and lipid transfer proteins facilitating the transport of suberin monomers to the plant surface layer. 2-Acyl lysophosphatidates are formed at the endoplasmic reticulum and acyl-CoA:glycerol-3-phosphate acyltransferases (GPAT5/7, and GPAT4/8 in Arabidopsis root epidermal cells) are crucial for the formation of the suberin lamellae. Some phosphate groups may be retained to form phosphoester linkages within polymers, although 2-monoacyl-sn-glycerols may be the preferred transport form for the fatty acid monomers in roots at least.
The cutin/suberin layers are distinct from the waxy coating that covers the external surface of plants (see our web page on waxes) and indeed act as a support for it. This wax can contain a range of very-long-chain fatty acids (C20 to C38+), some with hydroxyl or keto groups.
Sporopollenin is the structured outer pollen wall or exine surrounding pollen grains and in spores from flowering plants, and its chemical resilience is evident from its preservation in fossil spores from 450 million years ago that provide the earliest record of plant life on land. This chemical stability has meant that analysis has proved to be technically very difficult as until recently there was no easy way to solubilize and break down such a complex biopolymer for analysis; it is now known to be soluble in ethanolamine, which eases the task. While it seems that there is still much to be learned, it is now evident that it consists of hydroxylated aliphatic and phenylpropanoid units and conventional fatty acids linked to polyhydroxylated alpha-pyrone subunits with extensive cross-linking (probably with the differences between plant species). Through a gene-targeted approach, it has been determined that polyketide and fatty acid synthases, together with cytochrome P450 oxygenases, produce the backbone of polyhydroxylated subunits, with a remarkable conservation of biochemical pathways across the plant kingdom.

4. Hydroxy Fatty Acids in Bacteria and Fungi
Bacteria: Ester- and/or amide-bound 2-hydroxy fatty acids, with both straight and branched chains, are common constituents of bacterial lipids, but in bacterial lipopolysaccharides, the 2-hydroxyl group has the S- not the R-configuration as in animals and plants. As such, they are often found in environmental samples, like soils, biofilms, or sediments. Fatty acids of this kind are usually saturated, in some instances with iso- or anteiso-methyl branches, but monoenes are occasionally detected. Estolides with a fatty acid linked to a 2-hydroxy fatty acid occur in intestinal bacteria.
3(or β)R-Hydroxy long-chain fatty acids, linked by both ester and amide bonds to glucosamine or other amino-sugars, are constituents of lipopolysaccharides and lipid A from Gram-negative bacteria, and usually have a saturated fatty acid attached via an estolide linkage. They are essential to the endotoxin activity, and endotoxin levels in in environmental samples, such as dust, aerosols, soils and sewage, are often determined by analysis of the content of 3‑hydroxy acids. In plants, immune defence responses are induced by the 3‑hydroxy acids derived from lipopolysaccharides rather than by lipid A or the lipopolysaccharide per se. 3‑Hydroxy acids are the main fatty acid constituents of the surfactant rhamnolipids produced by pseudomonads again in part in estolide linkage. Most fatty acids of this type tend to be fully saturated, sometimes with iso- or anteiso-methyl branches. Exophilins are derived from related glycolipids and contain estolide fatty acids and hydroxyl (or acetyl) groups in position 3, while mycolic acids, discussed elsewhere in the pages, contain a hydroxyl group in position 3 and can have keto and methoxyl groups elsewhere in the chain.
3‑Hydroxy acids are produced in yeasts and bacteria by direct hydroxylation, mainly of saturated fatty acids, but as the ubiquitous beta-oxidation system can also be involved in their synthesis, fatty acid of many different kinds can be 3-hydroxylated.
Many bacteria (and some algae) produce fatty acid lactones as signalling molecules, and Streptomycetes sp. use butenolides, a family of α,β-unsaturated γ-lactones with further hydroxyl, keto or methyl groups in the aliphatic chain, to regulate their production of secondary metabolites; these have antibacterial and anti-inflammatory properties in host animals. Many different ten-membered lactones have been characterized from fungi and a few bacteria, and they are produced by polyketide synthases. Macrolactins have a 24-membered lactone ring derived from an ω-1 hydroxy fatty acid with three separate diene structural elements and two hydroxyl groups, and they have been detected in several marine bacteria.

The short-chain hydroxy acid, β-hydroxybutyrate, occurs in the form of a polyester in intracellular inclusions (carbonosomes) in many bacteria, where it is a reserve of carbon and of energy, often in the form of copolymers with 3‑hydroxy-valerate or other C5 to C16 3‑hydroxyalkanoate units. In their synthesis, 3-keto fatty acids are produced as intermediates or by-products in the biosynthesis of conventional fatty acids, or as β-oxidation products, before as CoA esters, these are reduced to the 3R‑hydroxyacyl forms; a polyhydroxyalkanoate synthase generates the polymeric structure. Currently, these polyhydroxyalkanoates are of interest to industry as a source of biodegradable polymers, with the potential to substitute for petroleum-derived plastics in innumerable applications.

10-Hydroxyoctadecanoate and 10-hydroxyhexadecanoate, together with the corresponding oxo fatty acids, are major components of adipocere, the waxy material produced by microbial decay in corpses by many bacteria (including lactic acid bacteria), and the first of these is a major component of the lipids of the protozoon Cryptosporidium parvum. They are synthesised from oleic acid by a bacterial oleate hydratase, which requires FAD as a cofactor and catalyses the addition of water to the double bond to produce 10R-hydroxystearic acid with high stereospecificity, a possible mechanism to remove potentially toxic unsaturated fatty acids in their environment. As they have potential industrial value, microbial enzymes that produce saturated and unsaturated hydroxy and keto acids are attracting special interest.
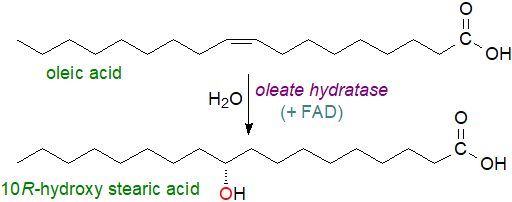 |
| Figure 5. Biosynthesis of hydroxy acids by bacterial fatty acid hydratases. |
Some bacteria are capable of hydroxylating fatty acids close to the terminal part of the molecule, and preparations from
Bacillus megaterium can convert palmitic acid to 14‑hydroxypalmitate mainly with some 15- and 13-hydroxy-isomers.
27‑Hydroxyoctacosanoic acid is a characteristic component of the lipopolysaccharide Lipid A from all soil bacteria
of the family Rhizobiaceae.
 Hydroxy acids
are produced by bacteria such as Lactobacillus plantarum in the gut and affect the host metabolism.
10‑Hydroxy-12Z-octadecenoic acid is synthesised by the action of a hydratase produced by this organism on dietary
linoleate, and this has anti-inflammatory protective effects on the intestinal cell walls, and in mice, it is beneficial
towards obesity induced by high fat diets.
Oxo-metabolites of linoleate, conjugated linoleate and linolenate, which can be incorporated into host tissues, are formed in the same way
and can affect adipose tissue metabolism and the response of macrophages by activating various receptors.
Hydroxy acids
are produced by bacteria such as Lactobacillus plantarum in the gut and affect the host metabolism.
10‑Hydroxy-12Z-octadecenoic acid is synthesised by the action of a hydratase produced by this organism on dietary
linoleate, and this has anti-inflammatory protective effects on the intestinal cell walls, and in mice, it is beneficial
towards obesity induced by high fat diets.
Oxo-metabolites of linoleate, conjugated linoleate and linolenate, which can be incorporated into host tissues, are formed in the same way
and can affect adipose tissue metabolism and the response of macrophages by activating various receptors.
Fungi: In yeasts and fungi, 2-hydroxy fatty acids (amide linked) are major constituents of sphingolipids as in plants and animals, some with a trans/E double bond in position 3, while 2,3-dihydroxy fatty acids have been reported from yeasts. Unusual fatty acids with hydroxyl groups in central positions, together with methyl groups and double bonds, occur in fungi, lichens and slime moulds, and 7‑hydroxy-8,14-dimethyl-9-hexadecenoic acid, and structurally related fatty acids, were found in fungi belonging to the Ascomycetes and Basidiomycetes. 9,10,13- and 9,12,13‑Trihydroxyoctadecenoic acids found in beer are derived from microbial fermentation of linoleic acid. Candida albicans produces 3‑hydroxy-14:2 as a signalling molecule, and it can synthesise a 3-hydroxy oxylipin from arachidonic acid in cells of animal hosts.
Ricinoleic acid, the main component of castor oil, is a major component (44%) of the triacylglycerols of the fungus ergot (Claviceps purpurea), but there are no free hydroxyl groups as these are acylated to form tetra, penta- and hexa-acid molecules capped by normal long-chain fatty acids, i.e., as estolides. The biosynthetic pathway differs from that in the castor oil plant in that linoleic acid is the substrate, and hydroxylation occurs under anaerobic conditions with the hydroxyl group coming from water and not molecular oxygen. Some yeasts produce extra-cellular glycosides containing ω‑1 hydroxy fatty acids (C16 and C18), while certain filamentous fungi of the genus, Ustilago, contain ustilagic acids, 15,16-dihydroxy- and 2,15,16-trihydroxyhexadecanoic acids linked to β‑cellobiose. By means of polyketide synthases, many fungi produce ten-membered lactones with a wide spectrum of biological properties as phytotoxic, cytotoxic, antifungal and antibacterial agents.
One of the great challenges in organic chemistry is to be able to insert an oxygen atom onto an unactivated carbon atom. Fungi seem to do it relatively easily and can use peroxygenases to place hydroxyl groups in the alkyl chains of fatty acids with both positional and stereochemical specificity. The enzymes have no cofactor requirements and simply need a source of H2O2; each enzyme molecule can accomplish the reaction over 100,000 times before its potency declines. The main challenge for biotechnological applications is to express the enzymes, which are produced by complex post-translational mechanisms, in sufficient quantity in more amenable host organisms.
5. Epoxy Fatty Acids in Animals and Plants
cis-9,10-Epoxy-octadecanoic acid has been detected and quantified in plasma of humans where it may be formed by epoxidation of the double bond of oleic acid by a cytochrome P450 enzyme, although its physiological function and fate are not known. Similar mono-epoxy fatty acids are formed in lung and other tissues from linoleate and are termed leukotoxins (the term includes a range of diverse compounds), because they highly toxic towards leukocytes. Together with dihydroxy metabolites formed on ring opening, they have unpleasant cardiovascular effects and influence acute respiratory distress syndrome, especially in burn patients. As epoxidized polyunsaturated fatty acids are bioactive oxylipins, it is more appropriate to consider these here in the context of eicosanoid metabolism.
F.D. Gunstone found and characterized the first natural epoxy fatty acid, (+)-vernolic acid or cis-12,13-epoxy-octadec-9Z-enoic acid, from the seed oil of Vernonia anthelmintica in 1954. In this instance, the stereochemistry of the epoxyl group was subsequently shown to be 12S,13R, but the optical antipode, (‑)‑vernolic acid, has been isolated from certain seed oils of the Malvaceae.

Subsequently, the isomeric cis-9,10-epoxy-octadec-12Z-enoic ('coronaric') acid, cis-15,16-epoxy-octadeca-9Z,12Z-dienoic acid, and cis‑9,10‑epoxy-octadecanoic were found in seed oils. Although epoxy acids are usually minor components, vernolic acid can amount to 60% of the total fatty acids in the seed oil of Vernonia galamensis. Biosynthesis of vernolic and coronaric acids involves epoxygenase enzymes, closely related in structure to desaturases, on linoleic acid, and on prolonged storage, small amounts of epoxy (or hydroxy) acids of defined stereochemistry are generated by enzymic processes in many different seed oils. A fatty acid epoxygenase (CYP77B1) and epoxide hydrolases have been characterized from A. thaliana. Saturated epoxy fatty acids, such as 9,10‑epoxy-octadecanoic and 9,10-epoxy,18-hydroxy-octadecanoate acids, are found in plant cutins (see above).
6. Furanoid Fatty Acids from Natural Sources
Furanoid fatty acids are heterocyclic lipids with the general structural formula illustrated, and they are minor but widespread components of algae and plant lipids. The first natural acid of this type (m = 6 and n = 7 and with no methyl substituents) was found in the seed oil of Exocarpus cupressiformis, although there has been a suggestion that it may have been an artefact of the isolation method. Interest in these compounds was stimulated by the discovery that they are present at low levels in fish, especially in their reproductive tissues, although they are presumed to originate in the diet (other marine sources include sponges and soft corals). Subsequently, they were found in other animals, especially dairy products, where it is assumed that they come from the diet of the herbivores, in human plasma and erythrocytes, and in some bacteria, where they are present mainly esterified to phospholipids. They have distinctive mass spectra and are easily characterized by GC-MS. A short-hand naming system has been recommended by Vetter and Wendlinger (2013, DOI ).
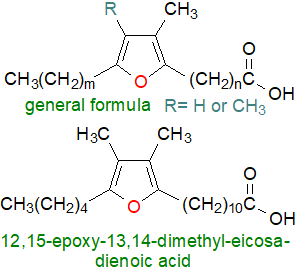 |
| Figure 6. Representative furanoid fatty acids. |
It is much more usual to encounter furanoid fatty acids with a methyl substituent in position 3 of the ring (R = H), or with two methyl substituents in the ring, with the latter more common in fish and possibly the only forms in microalgae. Most natural fatty acids of this type known to date have 16 to 22 carbon atoms, and they occur in series with mostly terminal propyl (m = 2) or pentyl (m = 4) groups. At least 23 different furanoid fatty acids have now been detected in fish, including most fish oils, where they can amount to 0.1 to 1% of the total fatty acids, and the most abundant is often 12,15‑epoxy-13,14-dimethyleicosa-12,14-dienoic acid, together with its homologues and smaller relative proportions of monomethyl acids, such as 12,15-epoxy-13-methyleicosa-12,14-dienoic acid. Trace amounts have been detected of some forms with one or two further double bonds in positions outwith the ring, often with a trans/E configuration e.g., 9E-(3-methyl-5-pentylfuran-2-yl)non-8-enoic acid. In fish, furanoid fatty acids occur in triacylglycerols and cholesterol esters, especially the latter where they can sometimes occur in higher concentrations than the conventional fatty acids.
Fatty acids of this kind have been found in a variety of plant sources and in fungi. In the latex of the rubber tree Hevea brasiliensis, 10,13‑epoxy-11-methyloctadeca-10,12-dienoic acid has been identified as the main component of the lipid fraction, and this and other furanoid fatty acids have been detected as minor components in numerous other plants (seeds, leaves and fruit) in amounts comparable to the tocopherols, i.e., 15 to 2650 µg/100 g fresh weight), as well as in yeasts, algae and marine bacteria. Indeed, it is suggested that plants, algae and bacteria are the main source of furanoid fatty acids in animals and fish via the food chain, especially for the pentyl-substituted fatty acids and those with double bonds in the terminal part of the chain. Linoleic acid is reported to be the biosynthetic precursor of the more abundant furanoid fatty acids, but this has been questioned. While the origin of the propyl-substituted compounds is less debated, they may be derived from metabolism of 9,12-hexadecadienoic acid in algae such as Phaeodactylum tricornutum.
In plants and algae, there is limited information only on the mechanism of biosynthesis, but there is evidence that it proceeds via a hydroperoxide intermediate from the action of a lipoxygenase followed by cylization and then methylation of the furan ring with S‑adenosylmethionine (SAM) as the methyl donor.
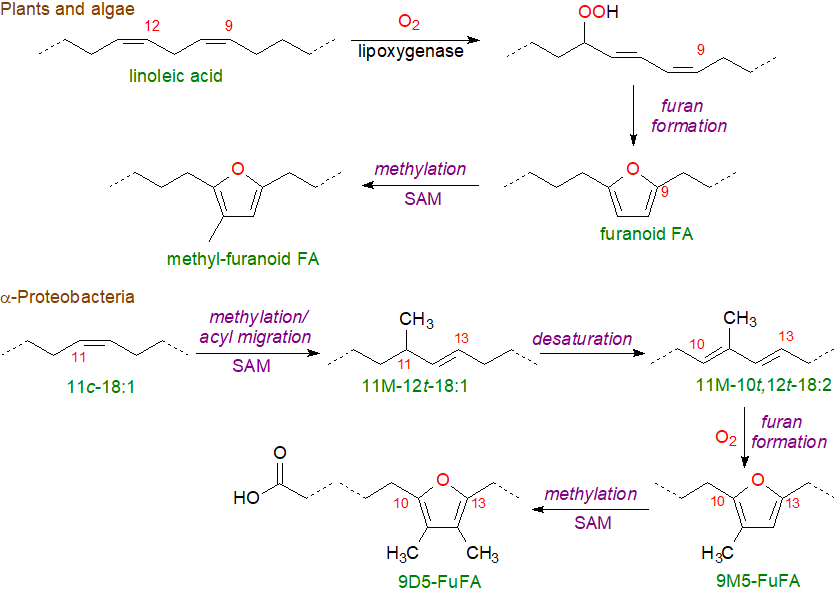 |
| Figure 7. Biosynthesis of furanoid fatty acids in plants, algae and proteobacteria. |
Recently, furanoid fatty acids were detected in bacteria for the first time (from Dehalococcoides sp.); 9‑(5‑pentyl-2-furyl)-nonanoate, 9‑(5‑butyl-2-furyl)-nonanoate and 8-(5-pentyl-2-furyl)-octanoate are present in the phospholipids. The biosynthetic mechanism is very different from that in plants and has been studied in two photosynthetic α‑proteobacteria (Rhodobacter sphaeroides and Rhodopseudomonas palustris) in which 11Z‑octadecenoic acid is the primary precursor with all steps occurring on pre-existing phospholipid fatty acid chains. The first step in biosynthesis in these species is now known to involve methylation of carbon 11 with S‑adenosylmethionine (SAM), and this is accompanied by migration of the double bond with a change in conformation to produce 11M‑12E‑18:1. This is followed by a desaturation step to produce 11M‑10E,12E‑18:2, and then oxygenation by a novel monooxygenase and cyclization to form the furan ring (9M5-FuFA) with molecular oxygen (O2) as the source of the oxygen atom. A further methylation reaction can introduce a second methyl into the ring to form 9D5-FuFA.
Furanoid fatty acids can arise spontaneously from autoxidation of fatty acids with conjugated double bonds. They are scavengers of hydroxyl and hydroperoxyl radicals, and this may be why natural furanoid fatty acids are present in membranes, i.e., as antioxidants and anti-inflammatory agents. This evolutionary convergence in the nature of the products from plants and bacteria suggests a common role, probably as a defence mechanism to protect membranes by mitigating the effect of reactive oxygen species. Similarly, it has been suggested that furanoid fatty acids may play a part in the cardio-protective effects of dietary fish oils, although it would not be surprising if such distinctive oxylipins were found to have other signalling functions in vivo.
Metabolites: Several short-chain dibasic furanoid fatty acids have been isolated from human blood and plasma and have been termed urofuranic acids. They are presumably beta-oxidation metabolites of the longer-chain furanoid fatty acids from fish in the diet and are reported to have immuno-metabolic properties. When kidney metabolism is impaired, 3‑carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF) can accumulate, and it may be a significant uremic toxin. The concentration of this metabolite increases in plasma of patients who progress from pre-diabetes to type 2 diabetes, and it may be a marker for this disease as it reportedly increases oxidative stress and impairs insulin secretion. It can be used to estimate fish oil consumption in humans.

Others: A fatty acid containing a tetrahydrofuran ring, (+)-(2S,3S,5R)-tetrahydro-3-hydroxy-5-[(1R)-1-hydroxyhexyl]-2-furanoctanoic acid, is a secreted pheromone that controls the migratory behaviour of a fish, the sea lamprey. A tetrahydropyran ring-containing fatty acid, 2,3‑dihydroxy-9,13-oxy-7E-octadecenoic acid, occurs linked to taurine in the protozoon Tetrahymena thermophila. In animal tissues, isofurans with a tetrahydrofuran ring are formed adventitiously together with isoprostanes by autoxidative processes .
7. Methoxy Fatty Acids
The mycolic acids, described in our web page on branched-chain fatty acids, are unusual in many ways not least in that they contain methoxy substituents. Otherwise, methoxy fatty acids are not common in nature, although 2-methoxy substituted fatty acids, all with the R‑configuration at the chiral centre, have been isolated from sponges, especially those of Caribbean origin. They include saturated, monoenoic and very-long chain dienoic acids. Some mid-chain methoxylated fatty acids such as lyngbic acid have been isolated from marine cyanobacteria, especially Lyngbya sp., where they exist as N‑substituted amides termed ‘malyngamides’, while other methoxy fatty acids are occasionally reported from algae, fungi and bacteria. Methoxy fatty acids are currently of some pharmaceutical interest as they display antibacterial, antifungal, anticancer and antiviral activities.

Unfortunately, methoxyl groups can be introduced to fatty acyl chains artefactually during analysis if cyclopropane or brominated fatty acids are methylated by inappropriate methods.
8. Keto (Oxo) Fatty Acids
Keto fatty acids are often reported as artefacts of oxidation of fatty acids but relatively rarely as natural fatty acids, although analogues of some of the common plant hydroxy fatty acids are occasionally found. For example, 13-oxo-9E,11E-octadecadienoic acid, an analogue of coriolic acid, is a major component of Monnina emarginata seed oil, where it is accompanied by a small amount of 13-oxo-9E-octadecenoic acid, while 9‑oxo-10E,12E-octadecadienoic acid is present with dimorphecolic acid in Dimorphotheca seed oils. Oxo-monoenoic fatty acids have been found in a few seed oils including Cuspidaria pterocarpa and Mappia foetida. The keto acid, licanic or 4-oxo-9Z,11E,13E-octadecatrienoic or 4-keto-α-eleostearic acid, amounts to 60% of the seed oil of Licania rigida, while a 4‑keto analogue of α‑parinaric acid was found in Chrysobalanus icacao. The traumatin family of plant oxylipins contains keto- or terminal aldehyde-moieties.

5-Oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-Oxo-ETE) is an oxylipin that is best discussed in that context. 3-Keto fatty acids found as minor components of animal tissues are generally intermediates in β-oxidation, but the origin of a range of minor keto fatty acids, differing in chain length and the positions of double bonds and oxo groups, in cow's milk is not known (51 isomers characterized). Small amounts of saturated oxo-fatty acids have recently been detected in human plasma and may be beneficial to health. In the royal jelly produced by queen honeybees, 9-oxo,2E-decenoic acid is a pheromone that control the actions of workers bees.
Recommended Reading
There appear to be no general reviews on this topic currently available, but the following are useful guides to various aspects of the chemistry, biochemistry and analysis of naturally occurring oxygenated fatty acids.
- Badami, R.C. and Patil, K.B. Structure and occurrence of unusual fatty acids in minor seed oils. Prog. Lipid Res., 19, 119-153 (1981); DOI
- Bates, P.D. and Shockey, J. Towards rational control of seed oil composition: dissecting cellular organization and flux control of lipid metabolism. Plant Physiol., 197, kiae658 (2025); DOI.
- Beltran-Nogal, A., Sanchez-Moreno, I., Mendez-Sanchez, D., de Santos, P.G., Hollmann, F. and Alcalde, M. Surfing the wave of oxyfunctionalization chemistry by engineering fungal unspecific peroxygenases. Curr. Opinion Structural Biol., 73, 102342 (2022); DOI.
- Brechany, E.Y. and Christie, W.W. Identification of the saturated oxo fatty acids in cheese. J. Dairy Res., 59, 57-64 (1992); DOI - also unsaturated at - DOI.
- Cahoon, E.B. and Li-Beisson, Y. Plant unusual fatty acids: learning from the less common. Curr. Opinion Plant Biol., 55, 66-73 (2020); DOI.
- Carballeira, N.M. New advances in the chemistry of methoxylated lipids. Prog. Lipid Res., 41, 437-456 (2002); DOI.
- Chang, F., Rowart, P., Salvatore, S.R., Rom, O., Mascal, M. and Schopfer, F.J. The emerging significance of furan fatty acids in food, nutrition, and potential therapeutic use. Food Chem, 479, 143759 (2025); DOI.
- Chen, X. and others. Polymeric phenylpropanoid derivatives crosslinked by hydroxyl fatty acids form the core structure of rape sporopollenin. Nature Plants, 10, 1790-1800 (2024); DOI.
- Dalton, B., Bhagabati, P., De Micco, J., Padamati, R.B. and O'Connor, K. A review on biological synthesis of the biodegradable polymers polyhydroxyalkanoates and the development of multiple applications. Catalysts, 12, 319 (2022); DOI.
- Heng, Y.C., Wong, G.W.J. and Kittelmann, S. Expanding the biosynthesis spectrum of hydroxy fatty acids: unleashing the potential of novel bacterial fatty acid hydratases. Biotechnol. Biofuels Bioprod., 17, 131 (2024); DOI.
- Khanal, S., Ngo, W., Nichols, K.K., Wilson, L., Barnes, S. and Nichols, J.J. Human meibum and tear film derived (O-acyl)-omega-hydroxy fatty acids in meibomian gland dysfunction. Ocular Surface, 21, 118-128 (2021); DOI.
- Kosma, D.K., Graça, J. and Molina, I. Update on the structure and regulated biosynthesis of the apoplastic polymers cutin and suberin. Plant Physiol., 197, kiae653 (2025); DOI.
- Lemke, R.A.S. and others. A bacterial biosynthetic pathway for methylated furan fatty acids. J. Biol. Chem., 295, 9786-9801 (2020); DOI.
- Merheb, C., Gerbal-Chaloin, S., Casas, F., Diab-Assaf, M., Daujat-Chavanieu, M. and Feillet-Coudray, C. Omega-3 fatty acids, furan fatty acids, and hydroxy fatty acid esters: dietary bioactive lipids with potential benefits for MAFLD and liver health. Nutrients, 17, 1031 (2025); DOI.
- Parchuri, P., Bhandari, S., Azeez, A., Chen, G., Johnson, K., Shockey, J., Smertenko, A. and Bates, P.D. Identification of triacylglycerol remodeling mechanism to synthesize unusual fatty acid containing oils. Nature Commun., 15, 3547 (2024); DOI.
- Revol-Cavalier, J., Quaranta, A., Newman, J.W., Brash, A.R., Hamberg, M. and Wheelock, C.E. The octadecanoids: synthesis and bioactivity of 18‑carbon oxygenated fatty acids in mammals, bacteria, and fungi. Chem. Rev., 125, 1-90 (2025); DOI.
- Schulz, S. and Hötling, S. The use of the lactone motif in chemical communication. Nat. Prod. Rep., 32, 1042-1066 (2015); DOI.
- Tan, D. and Saghatelian, A. The measurement, regulation and biological activity of FAHFAs. Nature Chem. Biol., 21, 796-806 (2025); DOI.
- Zhang, W.X., Hu, W., Zhu, Q.F., Niu, M.T., An, N., Feng, Y.Q., Kawamura, K. and Fu, P.Q. Hydroxy fatty acids in the surface Earth system. Sci. Total Environ., 906, 167358 (2024); DOI.
For tutorials on mass spectral analysis of oxygenated fatty acids of the kind discussed here - see our mass spectrometry pages.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
