Prostanoids: Prostaglandins, Prostacyclins
and Thromboxanes
The prostanoids are part of the oxylipin family of lipid mediators that are derived from the action of cyclooxygenases or prostaglandin synthases upon the twenty-carbon essential fatty acids or eicosanoids, primarily arachidonic acid. They can be further subdivided by structure into two main groups, the prostacyclopentanes, comprising the prostaglandins and prostacyclins, and the thromboxanes with a 6‑membered ether-containing ring, each of which is concerned with some aspect of signalling and especially of the inflammatory response. In general, prostanoids occur at very low levels in tissues, of the order of nanomolar concentrations, but they have profound actions as short-lived autocrine and paracrine signalling molecules. While most studies have been concerned with their occurrence and function in mammals, they have been detected in birds, ray-finned fishes, marine invertebrates, trypanosomes, blood flukes, and perhaps surprisingly some algae and yeasts.
Prostaglandin research began in the 1930s with studies of seminal fluid, and they were named for the prostate gland, thought to be their source, but it was the 1960s before the structures were fully elucidated, and the biosynthetic relationships to essential fatty acids were described. The Nobel Prize for Medicine for 1982 was awarded to Professors Bengt Samuelsson, John Vane and Sune Bergström for their discoveries in this field.
1. Nomenclature and Structures of Prostanoids
In defining the various structures, prostanoids are best considered as derivatives of a C20 saturated fatty acid, prostanoic acid, although this does not occur naturally. A key feature of prostaglandins is a five-membered ring encompassing carbons 8 to 12, as illustrated below, while the thromboxanes are similar but have heterocyclic oxane structures. They are all synthesised by enzyme systems, which confer stereospecificity and chirality to every structural feature, and they are thus distinct from the isoprostanes, which are produced by non-enzymic means with greatly limited stereospecificity (and have their own web page).
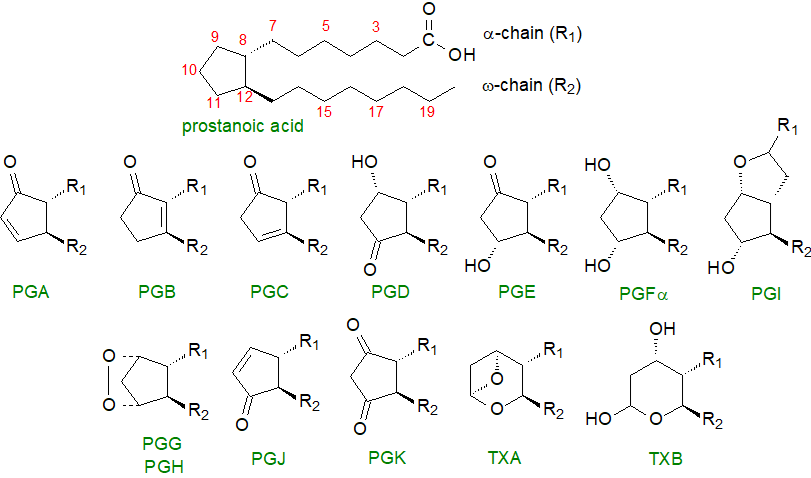 |
| Figure 1. Structures of the prostanoids: prostaglandins (PG), prostacyclin (PI), thromboxanes (TX). |
In the approved nomenclature, each prostaglandin is named using the prefix 'PG' followed by a letter A to K depending on the nature and position of the substituents on the ring. Constant features of all prostaglandins are a hydroxyl group of the S‑configuration on carbon 15 and a trans-double bond at carbon 13 of the alkyl substituent (R2), while the side chains (R1 and R2)are trans to each other on the cyclopentane ring, i.e., they are spatially opposed. PGA to PGE and PGJ are distinguished by a keto group in various positions on the ring, together with the presence or absence of double bonds or hydroxyl groups in various positions in the ring, while PGF has two hydroxyl groups, and PGK has two keto substituents on the ring. PGG and PGH are bicyclic endoperoxides, while an oxygen bridge between carbons 6 and 9 distinguishes prostacyclin (PGI). Thromboxane A (TXA) contains an unstable bicyclic oxygenated ring structure, but thromboxane B (TXB) has a stable oxane ring.
To define prostaglandins further, a numerical subscript (1 to 3) is used to denote the total number of double bonds in the alkyl substituents, which is determined by the nature of the fatty acid precursor, and a Greek subscript (α or β) is used with prostaglandins of the PGF series to describe the stereochemistry of the hydroxyl group on carbon 9. This is illustrated for prostaglandins PGE and PGFα of the 1, 2 and 3 series below.
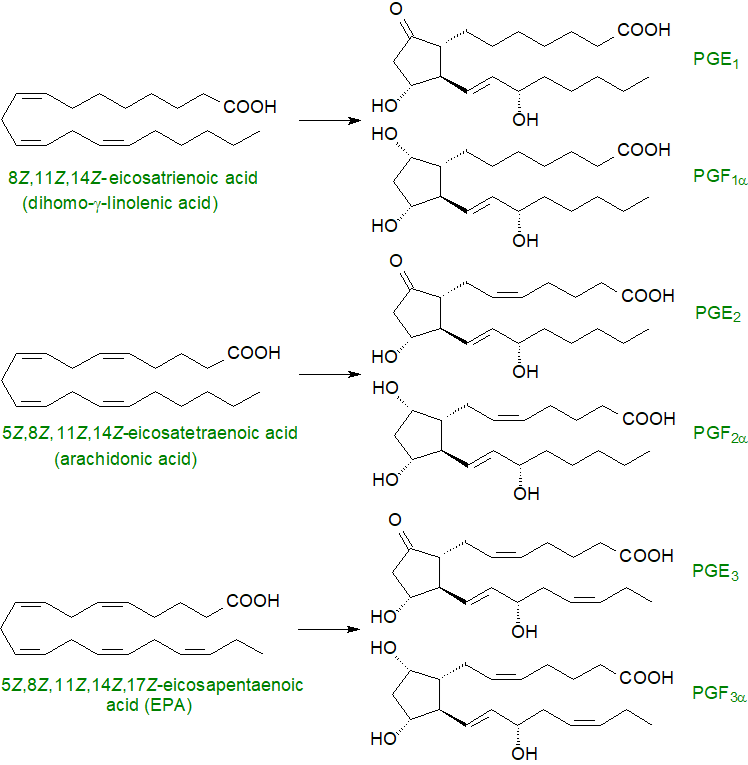 |
| Figure 2. Prostaglandin precursors and products. |
Prostaglandins PGE1, PGE2 and PGE3 are derived from 8c,11c,14c-eicosatrienoic (dihomo-γ-linolenic), 5c,8c,11c,14c-eicosatetraenoic (arachidonic) and 5c,8c,11c,14c,17c-eicosapentaenoic acids, respectively. Of these, PGE2 is detected most often, and it participates in innumerable physiological processes. Dihomo-prostaglandins derived from adrenic acid (22:4(n-6) have been found in cell preparations, but no such metabolites are produced from docosahexaenoic acid (DHA).
Prostanoids are sometimes grouped somewhat simplistically according to their main physiological actions in cells, i.e., prostaglandins with an involvement in pro-inflammatory processes mainly, prostacyclins, which reduce inflammation, and thromboxanes with the related but often opposing roles to the latter of platelet aggregation and vasoconstriction.
2. Biosynthesis of Prostaglandins
Cyclooxygenases: Eicosanoids, including the prostanoids, are not in general stored within cells but are synthesised as required in response to hormonal stimuli. The prostaglandins PGE2 and PGF2α were first isolated and fully characterized from human seminal fluid in 1963 by Samuelsson, but prostaglandins and other eicosanoids are now known to be produced in a highly selective manner by most cell types, depending on the activation state and the physiological condition of the tissues in which they occur. As the first step in their synthesis, a substrate fatty acid such as arachidonic acid is released from the cellular phospholipids by the action of the enzyme phospholipase A2 discussed in the Introductory document to this series.
Next, the free acids are acted upon by one of two related enzymes, cyclooxygenase-1 (COX‑1) and cyclooxygenase-2 (COX-2), more correctly termed prostaglandin endoperoxide H synthases-1 and 2 (PGHS-1 and PGHS-2), respectively (or as the prostaglandin G/H synthases, PTGS1 and PTGS2). These are heme-containing enzymes that are both oxygenases and peroxidases and catalyse the first committed steps in the synthesis of prostanoids from fatty acid precursors. COX-1 is always present in tissues, while COX-2 is induced by appropriate physiological stimuli, such as hormones, cytokines, tumour promoters or growth factors. The two iso-enzymes have approximately 90% sequence identity, and they are similar in structure, but an important difference is that COX-2 has a larger pocket at the catalytic site because of an isoleucine to valine substitution. Although arachidonic acid is the preferred substrate for both enzymes, COX-2 is more permissive in that it can utilize fatty acids such as dihomo-γ-linolenic and eicosapentaenoic acids (and even linoleic and α-linolenic acids in vitro at least).
In humans, COX-1 and COX-2 are homodimers of 576 and 581 amino acids, respectively, and each has three highly conserved mannose-containing oligosaccharides N-linked to it at asparagine residues, one of which facilitates protein folding. A fourth oligosaccharide is found only in COX-2 and regulates its degradation. Although the enzymes are sequence homodimers, they are conformational heterodimers because one monomer is the catalyst while the other is an allosteric regulator; this quaternary structure is necessary for enzymatic activity. Each subunit of the dimer consists of three domains: an epidermal growth factor-like (EGF) domain, a membrane binding domain and a catalytic domain. The EGF-like domain is located at the interface of the dimer, and it may facilitate dimerization and perhaps some membrane binding, while the membrane binding domain has four amphipathic α‑helices that insert into one face of the bilayer. The substantial catalytic domain contains separate oxygenase and peroxidase sites on opposite sides of the heme prosthetic group. These enzymes are integral membrane proteins of the endoplasmic reticulum, where they are located on the lumenal side only of the bilayer, and of the inner and outer nuclear membranes (COX‑2 is located on the Golgi in cancer cell lines).
Both enzymes catalyse the same two reactions with each carrying out a cyclooxygenase reaction in which two molecules of oxygen are added to arachidonic acid to form a bicyclic endoperoxide and then a further hydroperoxy group in position 15, i.e., to form prostaglandin PGG2. The first reaction occurs at a hydrophobic channel in the centre of the enzyme to form a hydroperoxide intermediate, which is transferred across to the heme-containing site for reduction by the peroxidase to form prostaglandin PGH2.
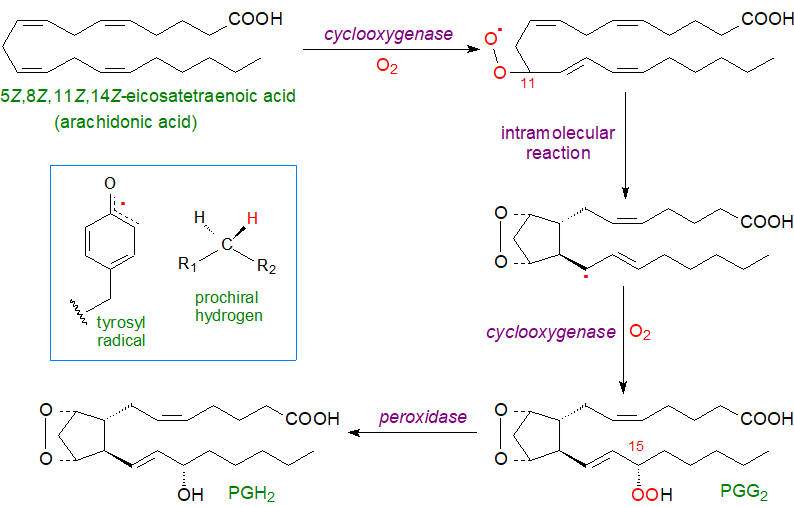 |
| Figure 3. Dual function of prostaglandin H synthases 1 and 2 (or COX1 and 2) in prostaglandin biosynthesis. |
Although the reactions occur at different sites, they work cooperatively, and with both enzymes, the combined reactions are initiated by the oxidation of the heme group involved in the peroxidase reaction by traces of endogenous hydroperoxides with formation of a tyrosyl radical. COX-1 abstracts the 13‑pro‑S hydrogen from arachidonic acid and initiates the cyclooxygenase reaction by formation of a carbon-centred radical at C-11; attack of molecular oxygen at this position leads to an intramolecular rearrangement with formation of a bicyclic endoperoxide and a further carbon-centred radical at C-15. This radical reacts with another molecule of oxygen to form a hydroperoxide, PGG2, and thence PGH2 is produced via the peroxidase action of each enzyme by comparable mechanisms. The tyrosyl radical is regenerated so that COX can carry out multiple turnovers without a need to repeat the activation step. As the catalytic tyrosyl radical can be transferred to an adjacent tyrosyl residue and become inactive after about 300 turnovers, the enzymes must be re‑expressed constantly to generate metabolites. The PGH2 formed is unstable and is the starting point for the biosynthesis of most other prostanoids. The reaction is illustrated here for arachidonic acid, but other precursor polyunsaturated fatty acids interact with the enzymes in analogous ways.
 Despite the structural homology, separate genes encode COX-1 (on chromosome 9 in humans) and COX-2 (on chromosome 1), and they are regulated
independently by different systems.
The enzymes differ in their subcellular localization, substrate specificity and how they are coupled to upstream and downstream enzymes, while
the catalytic domains differ in structure so that the susceptibilities to some inhibitors are not the same.
Despite the structural homology, separate genes encode COX-1 (on chromosome 9 in humans) and COX-2 (on chromosome 1), and they are regulated
independently by different systems.
The enzymes differ in their subcellular localization, substrate specificity and how they are coupled to upstream and downstream enzymes, while
the catalytic domains differ in structure so that the susceptibilities to some inhibitors are not the same.
The requirement for two distinct cyclooxygenases is not fully understood, but it is apparent that they have different roles in tissues. Although it is almost certainly an over-simplification, it is usually suggested that COX-1 is used for ‘housekeeping’ (homeostatic) purposes, responding rapidly to circulating hormones, which require constant monitoring and regulation. It is a constitutive enzyme that produces prostaglandins in the endoplasmic reticulum, which exit cells and signal through G-protein-linked receptors at the cell surface, although there are suggestions that it operates only at relatively high concentrations of arachidonic acid, as during platelet aggregation, cell injury or acute inflammation. In those tissues where prostaglandins act as specialized signalling molecules, such as kidney, stomach, vascular endothelium and blood platelets, COX-1 is expressed at higher concentrations, i.e., where the enzyme provides precursors for thromboxane synthesis.
In contrast, COX-2 is an inducible enzyme that is not present in unstimulated tissues other than lung, kidney and brain, and in the last, COX‑2 is constitutive in neurons and radial glia but not in other cell types. It is expressed under the control of the pro-inflammatory transcription factor NF‑κB in response to a wide range of extracellular and intracellular stimuli, such as cytokines, growth factors and tumour promoters, and it produces prostanoids that are primarily pathophysiological or that operate during defined stages of cellular development. It can utilize much lower concentrations of arachidonic acid and substrates other than the free acid. As COX-2 expression is inducible by bacterial lipopolysaccharides, it is most important in cells that are concerned with inflammation, such as macrophages and monocytes, and it is the form of the enzyme that has the main responsibility for the synthesis of those prostanoids associated with the most severe inflammatory states, including cancer, rheumatoid arthritis, Alzheimer's disease and respiratory disorders, although COX-1 is relevant in this context as well. On the other hand, COX-2 provides the substrate for synthesis of prostacyclin, which opposes the actions of thromboxanes (see below). COX-2 is activated by hydroperoxide concentrations that are approximately tenfold lower than those for COX-1, raising the possibility that under limiting concentrations of peroxide, COX-2 may be fully active while COX-1 is not. Induction of COX-2 expression is regulated by sphingosine-1-phosphate, a crucial sphingolipid.
Other prostaglandin synthases: As research has expanded on these oxylipins, many more enzymes have been identified, and their naming has changed over time, but I tend to use the older more common names here, as opposed to those that may now be considered more correct from a strict academic standpoint. PGH2 produced by the COX enzymes is an unstable intermediate from which all other prostanoids are derived by a variety of different enzymic reactions, some of which are illustrated next for arachidonate as the primary precursor. The nature and proportions of the various enzymes and of the prostanoids produced vary according to cell type, and forms of some of the enzymes must exist that differ in amino acid sequence, structure and co-factor requirements. Before they can act, newly synthesised prostanoids must be transported from the cytosol and across various membranes by means of transporter systems including cellular export and import.
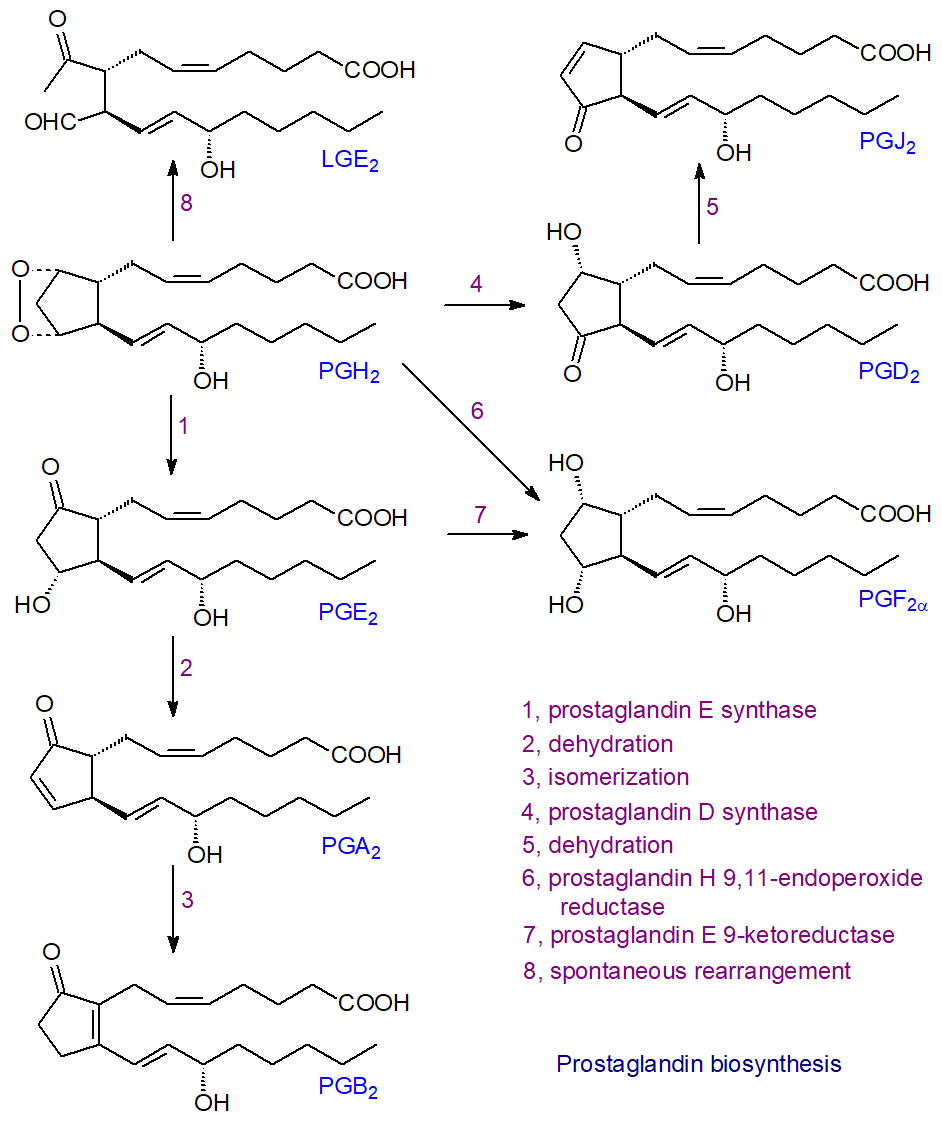 |
| Figure 4. Biosynthesis of prostaglandins - the synthases. |
Thus, PGE2 is produced from PGH2 by prostaglandin E synthases, of which at least three forms exist that are structurally distinct; two microsomal forms (mPGES-1/2) require glutathione, while a cytosolic form is glutathione independent. The most important of these is the cytosolic enzyme (cPGES), which together with mPGES-2 is expressed constitutively in many different types of cell. The other microsomal PGE synthase (mPGES‑1) is induced by inflammatory stimuli and works in concert with the inducible COX-2; it is over-expressed in various tumours. The membrane-bound mPGES-2 is a bifunctional enzyme that may be a factor in the pathogenesis of liver diseases. PGE2 is the major product of prostaglandin biosynthesis pathways induced by pro-inflammatory substances and with its metabolite PGF2α, it takes part in a positive feedback loop to regulate COX-2 expression.
PGD2 is formed in an analogous manner through an intramolecular rearrangement from PGH2 by the action of prostaglandin D synthases, which exist in two forms that are evolutionarily distinct but convergent in the nature of the products formed; one is located in the central nervous system, testes and heart (glutathione independent) and the other in inflammatory and immune cells (glutathione dependent). In rat peritoneal macrophages, PGD synthase and COX‑1 work cooperatively.
Prostaglandin F2α (PGF2α), the most common stereochemical form, is synthesised by two main routes. PGE2 is the precursor for a cytosolic enzyme prostaglandin E 9-ketoreductase (carbonyl reductase 1), which is an NADPH-dependent oxidoreductase with a wide range of substrate specificities and tissue expression, but PGF2α can be produced directly from PGH2 by the action of prostaglandin H‑endoperoxide reductase, requiring NADPH. Interestingly, this enzyme can utilize PGD2 as a substrate for the synthesis of another of the four stereochemical forms of PGF2α, i.e., 9α,11β-PGF2α, in the uterus to promote uterine contractions.
The cyclopentenone prostaglandins A and J, with reactive α,β-unsaturated keto groups, are produced by spontaneous dehydration reactions (non-enzymatic) from PGE and PGD, respectively, i.e., removal of two hydrogen atoms and one oxygen atom across the 9‑hydroxyl-carbon 10 region to form a cyclopentenone ring, and further modifications can then occur. PGA2 isomerizes to form the highly unstable PGC2, which rapidly undergoes a secondary isomerization to produce PGB2. In vitro, in the presence of human serum albumin, it has been demonstrated that PGD2 is transformed into three dehydration products, i.e., 15‑deoxy-PGD2, Δ12-PGJ2 and 15‑deoxy-Δ12,14-PGJ2 (the last two via the intermediate PGJ2).
 Levuglandins, such as LGE2
(sometimes termed ‘isoketals’), are formed from PGH2 by a non-enzymic rearrangement.
They have a very short half-life and react more rapidly than most lipid oxidation products
with the free primary amine groups of proteins and phosphatidylethanolamine to form covalent adducts (see below).
Indeed, the reaction is so rapid that the free levuglandins have never been isolated and are only known in adduct form.
Levuglandins, such as LGE2
(sometimes termed ‘isoketals’), are formed from PGH2 by a non-enzymic rearrangement.
They have a very short half-life and react more rapidly than most lipid oxidation products
with the free primary amine groups of proteins and phosphatidylethanolamine to form covalent adducts (see below).
Indeed, the reaction is so rapid that the free levuglandins have never been isolated and are only known in adduct form.
In a minor side reaction, COX-2 operating as a dioxygenase can act as a lipoxygenase to introduce one dioxygen molecule without endoperoxide formation. After reduction, this can lead to the formation of 11R‑hydroxy-eicosatetraenoic acid (11R‑HETE), 15R-HETE, and 15S‑HETE, from which it is able to produce diHETE metabolites (and from 5‑HETE, i.e., 5S,15R‑diHETE).
Aspirin: Both COX iso-enzymes and thence prostaglandin synthesis are inhibited by non-steroidal anti-inflammatory drugs ('NSAIDs'), such as aspirin (acetylsalicylic acid - a ubiquitous plant hormone and one of the earliest known and most widely used of all pharmaceuticals) and ibuprofen. Aspirin exerts this inhibition by binding to the cyclooxygenase site and transferring its acetyl group irreversibly to a particular serine residue (Ser-530), which then protrudes into the catalytic site and prevents the first step of creating the tyrosyl radical that starts the cyclooxygenase reaction. Because of differences in the structures of the binding sites, COX-1 is completely inhibited by this means, but aspirin acetylation of COX-2 causes a shift in the reaction to convert the enzyme from a cyclooxygenase to a lipoxygenase to result in the generation of 15R‑hydroxy-5,8,11,13-eicosatetraenoic acid (15R‑HETE), i.e., with the opposite chirality to that produced in the lipoxygenase reaction. A small amount of PGD2, but not PGE2, may be formed - again with the 15R‑configuration. In human mast cells, both enantiomers of 15-HETE are produced by COX-1, but the 15S-isomer is selectively depleted by reaction with the catabolic enzyme 15‑hydroxyprostaglandin dehydrogenase (see below).
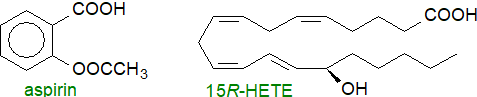 |
| Figure 5. Aspirin and the product of is interaction with COX-2. |
The selective inhibition of cyclooxygenases by aspirin is the reason for its well-known analgesic, anti-pyretic and anti-inflammatory properties as a pharmaceutical. As it inhibits thromboxane synthesis and thence platelet aggregation, it is recommended for cardiovascular therapy. However, this does not fully explain aspirin's anti-inflammatory behaviour, and through an action with COX-2, it is now reported to take part in the generation of oxygenated lipid mediators such as the ‘aspirin-triggered’ protectins (resolvins) and the epi‑lipoxins, as well as eicosanoids with an R‑configuration, all of which are potent anti-inflammatory agents, especially in neuro-inflammation. In contrast, ibuprofen and all other drugs of this type bind reversibly and compete with arachidonic acid for the catalytic sites.
Synthesis of COX-2 is inhibited by certain steroidal anti-inflammatory drugs at the level of transcription. As the catalytic site of COX-2 is smaller than that of COX‑1, it has proved possible to develop several other drugs that inhibit the action of COX-2 only, and as well as having analgesic and anti-inflammatory actions, these are used clinically to prevent cancer of the colon. Unfortunately, some of the latter have been associated with an increased risk of cardiovascular disease and have been withdrawn from the market.
Endocannabinoid metabolism: There is a significant difference in the substrate requirements of the two COX iso-enzymes. While both utilize unesterified arachidonic acid as substrate, COX-2 can metabolize dihomo-γ-linolenic and eicosapentaenoic acids. When triggered by Kdo2-lipid A, COX-2 can react with endocannabinoids, and with 2‑arachidonoylglycerol, it produces esterified 2‑prostanoylglycerol derivatives, i.e., hydroxy endoperoxides analogous to PGH2, which can be further metabolized by downstream prostaglandin synthases, such as mPGES-1. Although these can be hydrolysed by esterases such as the lysophospholipid lipase LYPLA2 present in blood and some tissues to release the prostaglandin in free form, there is evidence that 2-prostanoylglycerol affects calcium mobilization through novel receptors as well as via the PPARδ receptor. COX‑1, in contrast, can only utilize free fatty acids as substrates.
Similarly, with anandamide (arachidonoylethanolamine) and arachidonoylglycine, COX-2 produces ‘prostamides’, though with lower efficiency. While these may simply serve as precursors of free prostanoids through hydrolysis, there is increasing evidence that they are new classes of lipid mediators in their own right. These amide derivatives are relatively long-lived in plasma, and amides of PGF2α are available as drugs to lower ocular pressure and treat glaucoma.
Other esterified forms: Although they are generated in an unesterified form, PGE2 and PGD2 synthesised from PGH2 in platelets by the action of COX-1 are rapidly esterified to position sn-2 of 1‑lysophosphatidylethanolamines. COX-2 can utilize 2-arachidonoyl-lysophospholipids to generate the oxylipin-containing lysophospholipids, including PGE2- and 11-HETE-lysophosphatidylcholine, with potential biological activity (see our web pages on HETE and oxidized phospholipids). Prostaglandyl-(15-4')-myo-inositol (1':2'-cyclic)-phosphate (cyclic PIP) is synthesised in rat liver from prostaglandin E and the novel inositol phosphate, guanosine diphospho-4-myo-inositol 1:2-cyclic phosphate; it is a natural agonist for cyclic AMP.
 COX-1 in
thrombin-activated human platelets generates a diepoxy oxylipin from unesterified arachidonic acid in nanogram
amounts that has been identified as 8,9‑11,12‑diepoxy-13-hydroxyeicosadienoic acid (8,9‑11,12-DiEp-13-HEDE or DiEpHEDE), which
stimulates and primes the expression of human neutrophil integrin while having a role in innate immunity and acute
inflammation (it was first erroneously identified as 8‑hydroxy-9,10-dioxolane A3 (DXA3)).
After synthesis, it is rapidly esterified to position sn-2 of phosphatidylethanolamine in which position sn‑1
is occupied by a 16:0, 18:0 or 18:1 vinyl ether or an 18:0 fatty acid, and although the intact phospholipid remains in the membrane,
it is as effective as the free eicosanoid in its actions.
Related endoperoxides may be formed in tissues via the co-occurrence of LOX and cytochrome P450 or peroxygenase enzymes in tissues.
COX-1 in
thrombin-activated human platelets generates a diepoxy oxylipin from unesterified arachidonic acid in nanogram
amounts that has been identified as 8,9‑11,12‑diepoxy-13-hydroxyeicosadienoic acid (8,9‑11,12-DiEp-13-HEDE or DiEpHEDE), which
stimulates and primes the expression of human neutrophil integrin while having a role in innate immunity and acute
inflammation (it was first erroneously identified as 8‑hydroxy-9,10-dioxolane A3 (DXA3)).
After synthesis, it is rapidly esterified to position sn-2 of phosphatidylethanolamine in which position sn‑1
is occupied by a 16:0, 18:0 or 18:1 vinyl ether or an 18:0 fatty acid, and although the intact phospholipid remains in the membrane,
it is as effective as the free eicosanoid in its actions.
Related endoperoxides may be formed in tissues via the co-occurrence of LOX and cytochrome P450 or peroxygenase enzymes in tissues.
Related enzymes: The nematode Caenorhabditis elegans lacks cyclooxygenase enzymes, but it can produce a molecule that is identical to prostaglandin F2α by some as yet unknown mechanism. Certain pathogenic fungi and yeasts produce 3‑hydroxy-eicosanoids from host arachidonic acid, and they can hijack the host’s COX-2 enzymes to produce 3‑hydroxy-prostaglandins from these that have similar properties to the normal compounds. Other fungi produce enzymes with significant homologies to mammalian cyclooxygenases COX-1 and COX-2 and termed Ppo proteins that synthesise PGH2. While the yeast Candida albicans and related pathogenic fungi produce PGE2 and other prostanoids in vitro from exogenous arachidonate, the relevant enzymes have not yet been characterized. Indeed, whole genome sequencing has revealed that fungi have no homologues for the mammalian enzymes, suggesting that they have evolved alternative mechanisms for the synthesis of eicosanoids. A prostaglandin H synthase isolated from the red alga Gracilaria vertniculophylla is very different in structure from its animal counterparts, but it may work in the same way, although it is not inhibited by non-steroidal anti-inflammatory drugs. Some pathogenic protozoa including the Chaga's disease agent Trypanosoma cruzi produce COX-like proteins that are distant evolutionarily from mammalian COX; thromboxane A2 is the main prostanoid found with a little PGF2α. There is further discussion below in relation to 'exotic' prostanoids.
Insects: The phospholipids in most insects tend to contain very little arachidonic acid, so the starting point in the biosynthesis of prostaglandins is the release of linoleic acid by the action of phospholipase A2. When required, linoleic acid is elongated and desaturated to form arachidonate, and this is acted upon by a peroxidase termed peroxinectin (Pxt), and not by cyclooxygenases, to produce PGH2, which is then converted to PGE2 and PGD2 by PGE2 and PGD2 synthases, respectively. Mosquitos are an exception and require arachidonic acid from animal blood as a nutrient. Insects have differing catabolic enzymes for prostanoids also.
3. Prostacyclin and Thromboxane Biosynthesis
Prostacyclin is synthesised by a prostacyclin synthase (CYP8A1), constitutively expressed in endothelial cells and in neurons and glial cells, that converts PGH2 (synthesised by COX-2) directly to PGI2 (half-life 42 seconds), while a thromboxane A2 synthase (CYP5A1) catalyses the production of thromboxane TXA2 from PGH2 (synthesised by COX-1 to which the thromboxane A synthase may be located in close physical proximity in rat peritoneal macrophages). These enzymes are related to the cytochrome P450 super-family of proteins and are located on the cytosolic face of the endoplasmic reticulum, so the precursor PGH must cross the membrane. PGI is the main prostanoid formed in endothelial and smooth muscle cells and TXA is most abundant in platelets and lung. In addition, PGI2 and some other prostanoids can be produced by cell-cell interactions by using enzymes in adjacent cells, i.e., PGH2 of platelet origin is converted to PGI2 in the vascular epithelium, while prostacyclin production by erythrocytes is at least in part dependent on PGH2 from lymphocytes. Subsequently, PGI2 can be released by endothelial cells to induce a signalling cascade with G-protein coupled receptors on nearby platelets. Although platelets can synthesise thromboxane TXA2 from endothelial PGH2 in vitro, this may not be a major pathway in vivo.
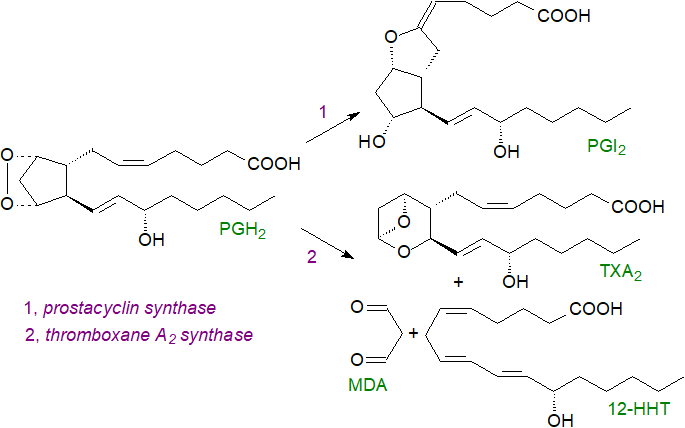 |
| Figure 6. Biosynthesis of prostacyclin and thromboxane. |
The thromboxane A2 synthase produces a further metabolite 12S-hydroxy-5Z,8E,10E-heptadecatrienoic acid (12-HHT) from PGH2 by a rearrangement of the cyclopentane-endoperoxide structure with malondialdehyde (MDA) as a by-product in epithelial cells in various tissues but mainly the intestine and skin; relatively large amounts are produced in activated platelets during skin injury and may contribute to wound healing. This oxylipin is of relevance to leukotriene metabolism, as it is an endogenous agonist for the leukotriene receptor BLT2. It can be oxidized by 15‑hydroxyprostaglandin dehydrogenase to the 12-keto metabolite (12‑KHT) with some functions that oppose those of thromboxane A2.
4. Prostanoid Catabolism
Prostanoids work close to the site of synthesis, and they are often subjected to partial catabolism before they are exported into the circulation as inert metabolites, which are often more easily analysed than are their precursors and can serve as markers for these. Some, such as PGI and TXA, are deactivated spontaneously, but the key enzyme systems react with the 15S‑hydroxyl group as discussed in the Introductory web page. Prostanoids are first imported from the extracellular fluid across the plasma membrane to the cytoplasm by the prostaglandin transport protein (PGT), a member of the OATP family, where, for example, prostaglandin PGE2 is oxidized by 15‑hydroxyprostaglandin dehydrogenase to 15‑keto-PGE2. Although this metabolite was long thought to be inert, it is now recognized that catabolism of PGE2 is a vital step in halting tumour cell proliferation, and that 15‑keto-PGE2 is an electrophilic molecule that operates in association with PPARγ (see below) and other proteins for this purpose. 13,14‑Dihydro-15-keto-prostaglandin-E2 is indeed inert.
Further oxidation of prostanoids eventually yields metabolites such as 11α‑hydroxy-9,15-dioxo-2,3,4,5-tetranor-prostane-1,20-dioic acid or PGE-M, which is the main metabolite of PGE2 found in urine. Many more catabolic products can be formed, and when PGD2 is injected into humans, 25 metabolites have been identified. Some PGE2 and PGF2α excreted in human urine is from the kidney rather than the general circulation.
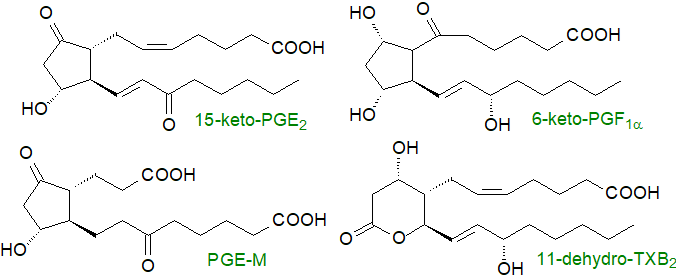 |
| Figure 7. Some products of the catabolism of prostanoids and thromboxanes. |
As the vinyl ether moiety in prostacyclin is unstable below pH 8.0, it is rapidly deactivated by a non-enzymatic hydrolysis reaction to form 6‑keto-PGF1α. Similarly, TXA2 contains an unstable ether linkage with a half-life of 30 seconds, and it is hydrolysed non-enzymatically to open the bicyclic oxygenated ring and form inert thromboxane B2 (TXB2) with hydroxyl groups in positions 9 and 11, a significant portion of which then undergoes dehydrogenation at C‑11 by an 11‑dehydroxythromboxane B2 dehydrogenase to form 11‑dehydro-TXB2, a metabolite found in human blood plasma and urine that can be monitored to assess COX‑1 activity and its responses to drug treatments.
5. The Functions of Prostanoids
Prostanoids are ubiquitous lipids in animal tissues that coordinate a multitude of physiological and pathological processes at concentrations down to 10‑9g per g of tissue, either within the cells in which they are formed in response to stimuli (autocrine) or in closely adjacent cells (paracrine). Under normal physiological conditions, they are cytoprotective in gastric mucosa, renal physiology, gestation and parturition, but in contrast, they have been implicated in many pathological conditions, such as inflammation, cardiovascular disease and cancer. Different prostanoids can be complementary or opposing depending on tissue and physiological conditions, and the correct balance between them can often be crucial. Such is the complexity of these interactions that an outline only of some of the more important can be presented here.
Receptors:  Prostanoids are sometimes described as local hormones that
act mainly in an autocrine fashion close to the site of their synthesis to coordinate other hormones in the circulation,
although some can undergo facilitated transport from the cell via transporters, mainly members of the ABC transporter superfamily,
to exert paracrine actions (they are rendered inert too readily to be transported far).
In this mode, they each interact with cell-surface G‑protein-linked receptors (GPCRs),
which comprise a large protein family with seven trans-membrane domains that sense molecules outside the cell
and induce signal transduction pathways inside the cell and thence the cellular responses.
When a ligand binds to a GPCR, it causes a conformational change, which allows it to act as a guanine nucleotide exchange factor
for an associated G‑protein.
Prostanoids are sometimes described as local hormones that
act mainly in an autocrine fashion close to the site of their synthesis to coordinate other hormones in the circulation,
although some can undergo facilitated transport from the cell via transporters, mainly members of the ABC transporter superfamily,
to exert paracrine actions (they are rendered inert too readily to be transported far).
In this mode, they each interact with cell-surface G‑protein-linked receptors (GPCRs),
which comprise a large protein family with seven trans-membrane domains that sense molecules outside the cell
and induce signal transduction pathways inside the cell and thence the cellular responses.
When a ligand binds to a GPCR, it causes a conformational change, which allows it to act as a guanine nucleotide exchange factor
for an associated G‑protein.
Five classes (and several sub-classes) of GPCR have been identified in mice and man that interact with prostanoids, and these are specific for PGE2 (designated EP or four subclasses EP1 to EP4), PGD2 (DP or two subclasses DP1 and DP2), PGF2α (FP), PGI2 (IP) and TXA2 (TP or two isoforms - TPα and TPβ), most of which belong to the α‑branch of Class A GPCRs, excepting DP2 (CRTH2) in the γ-branch of Class A GPCRs. The immediate result of binding to these receptors is an increase or decrease in the rate of generation of cytosolic second messengers (cAMP or Ca2+), a change in membrane potential, or activation of a protein kinase. The different receptors characterized from diverse cell types tend to have high, but not absolute, specificity for particular prostanoids and characteristic requirements in each cell, a phenomenon that can be explained by interactions of receptors with other G-proteins, which alter their mode of action. Thus, the EP4 receptor can couple with the Gαs‑protein to trigger adenylyl cyclase to form cAMP when stimulated by PGE2, or it can couple with the Gαi-protein to induce kinases that include phosphatidylinositol 3-kinase. This can mean the difference between being pro- and anti-inflammatory or pro- and anti-cancer.
Certain of the cyclopentanone prostanoids (PGA and PGJ series) interact at the cell nucleus with peroxisome proliferator-activated receptors (PPARs) of which there are three, but in this context PPARγ is most relevant. This is a nuclear hormone receptor or transcription factor, which regulates the expression of genes for adipogenesis, glucose homeostasis and lipid metabolism. All PPARs heterodimerize with the retinoid X receptor (RXR), which must itself be activated by binding to 9‑cis-retinoic acid, and bind to characteristic regions on the DNA of target genes.
The picture of prostanoid actions is complicated by the fact that a given prostanoid can have many different effects, sometimes opposing, according to the cell type, the nature of the stimulatory response and the type of receptor. Depending on its interactions with one of four receptors in different cell types, PGE2 can be either pro- or anti-inflammatory, while the relationship between the two iso-enzymes COX-1 and COX-2 determines how their products act in any given circumstance.
Inflammation and immune responses: The best known of the functions of prostaglandins and thromboxanes in cells is that they modify the inflammatory response to affect such symptoms as pain, fever and swelling, although it should be recognized that inflammation is an intrinsically beneficial event that leads to removal of offending molecules and restoration of tissue structure and metabolism. The main cause for concern is when acute inflammation fails to resolve and causes tissue damage as in sepsis, in which a prolonged and excessive immune response is followed by an immunosuppressive stage that can lead to high mortality rates. In the early days of prostaglandin research, it was evident that prostaglandins injected into tissues could induce all the symptoms of inflammation, but it is now recognized that the interactions are complex, and prostanoids can act both in a pro- and anti-inflammatory manner according to the nature of the inflammatory stimulus and the prostanoid produced, together with the profile of prostanoid receptors in each type of cell. For example, EP3 receptors are involved in the development of fever, while EP2 and EP4 are concerned with allergy and bone resorption. Receptor-specific actions of prostaglandins heighten neuronal excitability and so generate and transmit pain signals.
 Under normal conditions,
prostanoid levels in cells are low, but during inflammation both the nature and concentration of prostanoids can change dramatically.
Macrophages produce both PGE2 and TXA2, but the ratio changes to an excess of PGE2 with an inflammatory
stimulus by enhancement of the release cascade of pro-inflammatory cytokines such as interleukin (IL)-1, IL‑2 and tumour necrosis
factor‑α (TNF‑α).
In this context, prostanoids are best viewed as part of complex regulatory networks that modulate the actions of immune cells.
Under normal conditions,
prostanoid levels in cells are low, but during inflammation both the nature and concentration of prostanoids can change dramatically.
Macrophages produce both PGE2 and TXA2, but the ratio changes to an excess of PGE2 with an inflammatory
stimulus by enhancement of the release cascade of pro-inflammatory cytokines such as interleukin (IL)-1, IL‑2 and tumour necrosis
factor‑α (TNF‑α).
In this context, prostanoids are best viewed as part of complex regulatory networks that modulate the actions of immune cells.
PGE2 is a potent pro-inflammatory agent and participates in all the processes leading to the classic signs of inflammation, including the induction of fever and enhancing pain, and it can cause the transition to chronic inflammation by acting as a cytokine amplifier. Signalling by PGE2 through its EP2 receptor in mice promotes an energy-deficient state in the brain that drives pro-inflammatory responses that lead to cognitive decline, which can be reversed by inhibition of myeloid EP2 signalling. Its concentration in brain is significantly elevated in autism spectrum disorders. Selective silencing of the EP2 receptor in Schwann cells in mice substantially abrogates persistent pain elicited by inflammatory stimuli without affecting protective inflammation, suggesting new treatment possibilities. There is a particular interest in findings that in its pro-inflammatory role, PGE2 promotes the growth of colorectal tumours (see below), and it is a factor in the pathology of rheumatoid arthritis and in respiratory diseases; with the last, it can have both positive and negative effects depending upon circumstances.
On the other hand, PGE2 has anti-inflammatory properties in some circumstances, such as suppressing lymphocyte proliferation and inhibiting the production of certain interleukins and other cytokines. It is preventative towards 5-lipoxygenase action, which is crucial for the synthesis of pro-inflammatory leukotrienes. In this manner, PGE2 has a role in initiating the inflammatory response and in its eventual resolution. By enhancing the production of endothelial progenitor cells, PGE2 promotes wound healing and tissue regeneration, and while acting via the receptor EP4, it stimulates the migration of dendritic cells into the skin to trigger the immune system. The use of inhibitors of the catabolic enzyme 15-hydroxyprostaglandin dehydrogenase may have therapeutic potential in this instance. While 15-keto-PGE2 can sometimes promote the resolution of inflammation, PGD2 opposes this, and thromboxanes are important in this context (see below). Prostaglandins with 15R‑stereochemistry are anti-inflammatory.
Prostaglandin PGF2α exacerbates chronic inflammatory diseases such as rheumatoid arthritis. In contrast to its protective role in cardiovascular disease (see below), PGI2 is a mediator of the oedema and pain that accompany acute inflammation, and it is produced rapidly following tissue injury or inflammation. It is the most abundant prostanoid in synovial fluid in human arthritic knee joints, and it may contribute to neuropathic pain. PGI synthase is expressed in inflammatory cells including macrophages, where PGI can be either pro- or anti-inflammatory.
The high levels of prostanoids found in inflammation are presumed to be due to the recruitment of leukocytes and the induction of COX-2 (COX-1 may have a minor role only), which then produces the pro-inflammatory prostanoids mainly in various tissues. This explains the interest in COX-2 inhibitors for treating arthritis and other chronic inflammatory diseases and the role of non-steroidal drugs, such as aspirin, in reducing the symptoms of fever. Simplistically, COX-2 may be pro-inflammatory in the early stages of inflammation, but it is beneficial at later stages by generating anti-inflammatory prostanoids, while prostanoids derived from COX-1 may sustain the inflammatory response. In the brain, COX-2 in neurons has been implicated in the progression of Alzheimer's disease.
Immune responses are initiated and coordinated by T lymphocytes, which are influenced by prostanoids in a variety of ways, including modification of their development and maturation. Thus, PGE2 inhibits lymphocyte activation and proliferation, while TXA2 acts in opposition to this. PGE2 is beneficial to the innate and adaptive immune systems by regulating immunity and host defence against viral, fungal and bacterial pathogens, but prostanoids induced by such organisms can prolong infections. Again, the actions of COX-2 (and COX-1) may be factors in triggering antigen-specific inflammation.
Although PGD2 is pro-inflammatory in allergic responses when released from mast cells (eosinophils) and in brain in the perception of pain, in other circumstances, it is recognized as an anti-inflammatory prostanoid that may participate in the resolution of inflammation. It is the principal ligand for two receptors, DP1 and DP2, and it is an agonist of the thromboxane receptor, TP. PGD2 is a significant anti-inflammatory agent in experimental colitis, and its synthesis is elevated rapidly in response to tissue injury, including ischemic damage, to counter the pro-inflammatory PGE2 and other chemotaxins; PGD2 decreases food intake, while PGE2 increases it. It has been suggested that because of the similarities in structure of these two prostanoids, they may act as partial agonists of each other's cognate receptors.
 PGJ2
with Δ12-PGJ2 and the short-lived 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2),
the cyclopentenone-containing J‑series of prostaglandins produced by non-enzymatic dehydration of PGD2, are now well established
as anti-inflammatory regulators, which are ligands for PPARγ as discussed briefly above, although they bind to the DP2 receptor
as well; PGA2 produced enzymatically must be considered in this context.
15d-PGJ2 enters cells and binds to PPARγ, causing it to form heterodimers with retinoid X receptor alpha (RXRα),
which bind to particular DNA sequences and thereby express genes of concern to lipid metabolism, inflammatory responses and immunity.
Fatty acid binding proteins (FABP3 and FABP5) may modulate how it signals in the brain.
The J‑series of prostaglandins influence the immune response as they are produced in antigen-presenting cells such as
T lymphocytes, and 15d‑PGJ2 affects the resolution of the inflammatory response by inducing apoptotic cell death of
macrophages.
PGJ2
with Δ12-PGJ2 and the short-lived 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2),
the cyclopentenone-containing J‑series of prostaglandins produced by non-enzymatic dehydration of PGD2, are now well established
as anti-inflammatory regulators, which are ligands for PPARγ as discussed briefly above, although they bind to the DP2 receptor
as well; PGA2 produced enzymatically must be considered in this context.
15d-PGJ2 enters cells and binds to PPARγ, causing it to form heterodimers with retinoid X receptor alpha (RXRα),
which bind to particular DNA sequences and thereby express genes of concern to lipid metabolism, inflammatory responses and immunity.
Fatty acid binding proteins (FABP3 and FABP5) may modulate how it signals in the brain.
The J‑series of prostaglandins influence the immune response as they are produced in antigen-presenting cells such as
T lymphocytes, and 15d‑PGJ2 affects the resolution of the inflammatory response by inducing apoptotic cell death of
macrophages.
PGA2 and PGJ2 promote the resolution of chronic inflammation associated with bacterial, parasitic and viral infections, as for example, with the last, they repress viral protein synthesis, alter viral protein glycosylation, and inhibit virus transmission.
As it contains an electrophilic α,β-unsaturated ketone moiety in its cyclopentenone ring, 15d-PGJ2 can act as an endogenous electrophile, which can undergo Michael addition with cellular nucleophiles such as the free cysteine residues of proteins and of glutathione and cysteine per se. Covalent modifications of this type may be one mechanism by which it induces many of its responses in tissues, and for example, cyclopentanone prostanoids are redox regulators of actin and can bind covalently on Cys374 of actin to induce morphological changes to the cytoskeleton of leukocyte, endothelial and muscle cells. It inhibits tumorigenesis in cancer as discussed below.
Polyunsaturated fatty acids of the omega-3 family are known to be anti-inflammatory, possibly by inhibiting the release of arachidonate from membrane phospholipids for eicosanoid production, or alternatively, by competing with arachidonate for the same enzymes of eicosanoid biosynthesis such as production of PGE3 from eicosapentaenoic acid (EPA). In general, the 3-series prostanoids derived from EPA operate in very different ways from those of the 2-series and tend to be much less inflammatory. Prostaglandins derived from dihomo-γ-linolenic acid (20:3(n‑6)), i.e., 1-series prostanoids, differ from 2-series, and PGE1 has been shown to suppress inflammation and promote vasodilation. The protectins, resolvins and maresins ('specialized pro-resolving mediators') produced from omega-3 precursors are relevant to this topic, as they bring about the resolution of inflammation.
Cardiovascular effects: Two prostanoids have opposing functions in the maintenance of vascular homeostasis, i.e., thromboxane TXA2 and prostacyclin PGI2, although other prostaglandins, including PGE2 and PGD2 are relevant. TXA2 is synthesised mainly in platelets (which express only COX-1), production being enhanced during platelet activation, and acting via the thromboxane receptors TPα and TPβ, it induces an increase in intracellular calcium and protein kinase C and thereby promotes platelet aggregation, vasoconstriction and smooth muscle proliferation, even though it has a half-life of only 20-30 seconds. This is part of a repair mechanism for wound healing, including damaged vessel walls, and via TP receptor signalling, it is responsible for timely tissue regeneration. On the other hand, when the damage is too great, blood clots can form with the potential to cause strokes or heart attacks. TXA2 induces angiogenesis and/or lymphangiogenesis through greater production of growth factors and cytokines, while the thromboxane metabolite 12‑HHT is required for skin regeneration.
In contrast, PGI2 is the main product of macro-vascular endothelial cells via COX-2. It is produced as required, with a half-life of only about 42 seconds, and it is a potent vasodilator locally through the IP receptor mainly but also through the cytosolic nuclear receptor PPARβ. By inhibiting platelet aggregation and smooth muscle cell proliferation, it contributes substantially to myocardial protection. Both TXA2 and PGI2 are therefore crucial mediators of pathological vascular events that include thrombosis and atherogenesis, and it is evident that the correct balance between the two is necessary for good cardiovascular health. The ratio of TXA2:PGI2 seems to be a better indicator than the absolute amounts of these mediators produced in vivo.
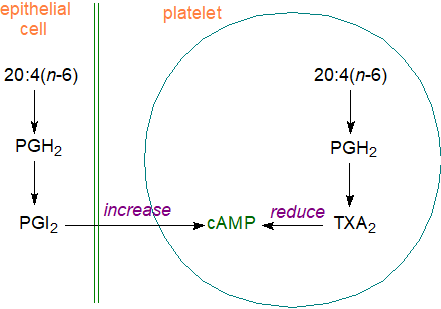 |
| Figure 8. Opposing effects of thromboxane and prostacyclin. |
In platelets and certain other cells, PGI2 activates adenyl cyclase and elevates cAMP concentrations by stimulating the IP receptor, but TXA2 has the opposite effect. While prostacyclin has an acute impact that is evident rapidly after adding prostacyclin to a system, it can operate in the longer term by directing gene transcription. Further relevant factors are increased expression of the TP receptor (for TXA2) in atherosclerotic lesions, which can directly accelerate atherogenesis and plaque growth.
The cardio-protection afforded by a low dose of aspirin (81 mg/d) that has been established by clinical trials is exerted by the irreversible long-term inhibition of platelet COX-1 and thence of TXA2 biosynthesis for the lifetime of a platelet in the circulation (aspirin does not affect PGI synthesis). Indeed, aspirin appears to be the only COX inhibitor proven to be cardio-protective and demonstrates the causality of eicosanoids in the development of cardiovascular pathophysiologies. It may be relevant that 15R-PGD2, produced by aspirin-treated COX-2, inhibits aggregation of human platelets. In contrast, there is some concern that some COX-2 inhibitors may be pro-thrombotic by inhibiting prostacyclin synthesis relative to that of thromboxanes. In clinical practice, such potential adverse properties of these drugs must be balanced against positive results in other tissues since only 1-2% of patients are believed to be at risk. Again, polyunsaturated fatty acids of the omega-3 family are reported to be beneficial via the action of specialized pro-resolving mediators. As inflammation promotes atherogenesis and the associated thrombotic events, there is concern that inflammatory prostaglandins may be harmful, but PGE2 has been shown to be of benefit against the progression of atheromous plaques.
Lung: PGE2 produced by COX-2 in lung epithelial cells is a robust anti-inflammatory and anti-asthmatic agent by binding to the EP2 receptor. PGI2 signalling through the IP receptor inhibits allergic airway inflammation, and PGI2 analogues are used to treat pulmonary arterial hypertension. Although the roles of PGD2 and thromboxane A2 are more complex, they are mainly pro-inflammatory in relation to asthma, and antagonists for the DP2 receptor are in phase III clinical trials for the treatment of this disease.
Gastrointestinal system: COX-1 is always present throughout the human gastrointestinal tract and produces PGI2 and PGE2, which are protective towards the gastrointestinal mucosa. Both prostanoids reduce acid secretion from parietal cells, while increasing blood flow and stimulating the secretion of mucus. In this instance, the non-steroidal anti-inflammatory drugs, such as aspirin, have a negative impact, while the COX-2 inhibitors can be beneficial. On the other hand, these findings are challenged by studies showing that COX-2 is expressed in the intestinal mucosa and is induced in ulceration whereby large amounts of prostaglandins are produced that assist in healing. PGD2 is beneficial, as discussed above.
Kidney: Prostaglandins generated by both COX-1 and COX-2, especially PGE2, assist in the regulation of kidney function by maintaining vascular tone, blood flow, and salt and water excretion. PGE2 is required for the regulation of sodium re-absorption, while PGI2 (and possibly PGE2) increases potassium secretion. Vasodilatory PGI2 increases the flow of blood and other fluids through the kidney, actions again mediated via its receptors.
Reproductive system: Prostaglandins produced both by COX-1 and COX-2 take part in many aspects of reproduction in females, from ovulation and fertilization through to labour, with synthesis in the foetus and in the placenta as well as in other reproductive tissues. In women, the synthesis of PGE2 and PGF2α is increased appreciably during labour, and these prostanoids can be used as drugs to induce labour, while PGF2α is used to induce abortions in women in mid-trimester and to initiate ovulation in cows. Increased levels of placental thromboxanes are produced in patients with pre-eclampsia, a disease state during pregnancy that results in high blood pressure and often kidney failure, symptoms that can be ameliorated by the administration of aspirin. In males, seminal prostaglandins PGE2 and PGE1 are required for sperm concentration and motility, with significantly reduced levels seen in infertile men, while PGF2α facilitates sperm transport within the female reproductive tract by inducing peristaltic contractions.
Adipose tissue: Prostaglandins have diverse and opposing roles in adipose tissue metabolism to affect the regulation of adipogenesis and various metabolic disorders, such as obesity and dyslipidemia. PGE2 and PGF2α act together to inhibit the differentiation of pre-adipocytes, while PGD2 promotes adipogenesis by acting as a ligand for PPARγ and suppressing lipolysis via its receptor DP2. On the other hand, PGE2 stimulates thermogenesis in beige and brown adipocytes and so influences energy balance. The PGJ2 series trigger PPARγ to up-regulate lipid accumulation in adipocytes, while PGI2 influences the regulation of the life cycle of adipocytes more broadly and impacts upon terminal differentiation.
Bone: PGE2 is central to pain symptoms in the bone disease osteoarthritis, but it is of benefit to bone repair and regeneration during bone remodelling as the disease progresses. Nonsteroidal anti-inflammatory drugs (NSAIDs) used to relieve pain may not help the regeneration process.
 Cancer: COX-2 is over-expressed in many cancers, including those of the breast, colon and prostate.
In particular, PGE2 produced by this enzyme together with the PGE synthase mPGES-1 occurs at much higher concentrations in tumour
than in normal tissues, while urinary concentrations of its metabolite PGE-M are considered to be biomarkers for predicting the risk and
prognosis for some cancer types.
PGE2 has been shown to promote intestinal tumour initiation and growth by silencing certain tumour suppressor and DNA repair genes via
DNA methylation, and via the immune system and inflammation, it inhibits the destruction of tumours.
It promotes survival of tumour cells by inhibiting apoptosis and inducing proliferation and by increasing cell motility and migration.
In addition, the receptors EP2 and EP4 are often upregulated in cancer and support cell proliferation, migration, invasion and metastasis
through induction of multiple signalling pathways, while EP1 is involved in chemically induced colon cancer.
A metabolite of PGE2, PGE-M, is elevated in the urine of patients with gastric and colon cancers and is associated with a poor
outcome.
Cancer: COX-2 is over-expressed in many cancers, including those of the breast, colon and prostate.
In particular, PGE2 produced by this enzyme together with the PGE synthase mPGES-1 occurs at much higher concentrations in tumour
than in normal tissues, while urinary concentrations of its metabolite PGE-M are considered to be biomarkers for predicting the risk and
prognosis for some cancer types.
PGE2 has been shown to promote intestinal tumour initiation and growth by silencing certain tumour suppressor and DNA repair genes via
DNA methylation, and via the immune system and inflammation, it inhibits the destruction of tumours.
It promotes survival of tumour cells by inhibiting apoptosis and inducing proliferation and by increasing cell motility and migration.
In addition, the receptors EP2 and EP4 are often upregulated in cancer and support cell proliferation, migration, invasion and metastasis
through induction of multiple signalling pathways, while EP1 is involved in chemically induced colon cancer.
A metabolite of PGE2, PGE-M, is elevated in the urine of patients with gastric and colon cancers and is associated with a poor
outcome.
In consequence both the non-steroidal anti-inflammatory drugs, such as aspirin, and COX-2 inhibitors have been found to be beneficial towards some types of cancer with aspirin reducing the risk of gastrointestinal, breast, bowel and prostate cancers, although side effects remain a problem. Drugs that are better targetted towards PGE2 are under development, and clinical trials of EP2 and EP4 antagonists are underway.
In contrast, PGD2 can be either pro- or anti-tumorigenic depending on the experimental model. PGE3, derived from the n-3 eicosapentaenoic acid (EPA), is anti-proliferative in various cancers, possibly by interfering with PGE2. PGF2α promotes greater tumour progression and aggressiveness, while thromboxane TXA4 is a pro-carcinogenic mediator that affects several tumour cell survival pathways, including cell proliferation, apoptosis and metastasis, but again balanced by the opposing influence of prostacyclin PGI2 and aspirin.
15-Deoxy-Δ12,14-PGJ2 (15d-PGJ2) is produced by a variety of cells and is a potent anti-inflammatory regulator via its interaction with PPARγ to regulate adipogenesis and tumorigenesis. A transport system may carry it to the cells where it is required, and thence it is transported into the nucleus, where it stimulates gene transcription. Unlike PGE2, 15d-PGJ2 is a potent anti-tumour agent, inhibiting tumour growth both in vitro and in vivo in many tissues. It acts in various ways, for example to inhibit proliferation and induce apoptosis directly, while indirectly, it can interact to inhibit migration of tumour cells. It can affect surrounding cells to reduce the expression of some receptors, although some experimental conditions have been identified in which it works in a contrary manner. In general, PGE2 and 15d‑PGJ2 have affect tumorigenesis in profoundly different ways, and the prostaglandin synthases that are responsible for their biosynthesis are targets for the development of anticancer drugs. This may not be a simple solution, as COX-2 catalyses the production of pro-resolving as well as anti-inflammatory oxylipins.
Stem cells: Signalling by Wnt proteins, a family of proteolipids containing covalently esterified palmitoleic acid, controls the self-renewal of hematopoietic stem cells and bone marrow repopulation for which PGE2 is required, and it has been suggested that the PGE2/Wnt interaction is a master regulator of vertebrate regeneration and recovery in stem cells and other organ systems.
Protein metabolism: γ-Keto aldehydes such as the levuglandins (see above) and isolevuglandins, the latter produced in an analogous manner to the isoprostanes, have a remarkable reactivity towards proteins to form adducts with greatly modified functions. Thus, these di-aldehydes react with lysyl residues on proteins to form first Schiff base adducts and thence pyrrole derivatives, which can form intra- and intermolecular protein-protein cross-links. Pyrrole adducts are in turn sensitive to oxygen and are further oxidized in vivo to stable lactam and hydroxylactam products. Protein adducts of this type are not at all easy to analyse, but those in brain have been correlated with the severity of Alzheimer’s disease. Indeed, levuglandins and isolevuglandins are among the most potent neurotoxic products of lipid oxidation.
Levuglandins react with phosphatidylethanolamine to form products that are readily oxidized to hydroxy-lactam derivatives, and from a practical standpoint, these may be better markers of oxidative injury than protein-conjugates as they are more easily analysed.
Insects: Prostaglandins are signalling molecules in insects as in vertebrates, including hormone actions in the fat body and affects upon reproduction, fluid secretion and the immune response, although little appears to be known of their receptors. As a means of increasing their virulence, some insect-borne pathogens target phospholipase A2 to reduce prostaglandin biosynthesis and bring about immunosuppression.
Parasitic infections: It has been established that several parasitic organisms produce prostaglandins by comparable enzymic mechanisms to their mammalian hosts, and these may play a part in the pathogenesis of parasitic diseases.
6. Some Exotic Prostanoids
Marine invertebrates, including sponges, corals and molluscs, contain a wide range of prostaglandins, many of which are of the conventional type such as PGE2, PGF2 and so forth. They are presumed to have a similar biochemistry and metabolism as in mammals, although they occur largely in esterified form or as lactones rather than as the free acids, and they are involved in the regulation of oogenesis and spermatogenesis, in ion transport, and perhaps as defence compounds. Remarkably, one species of coral (Plexaura homomalla) contains up to 8% of its dry mass as prostanoid esters, but these have the 15-hydroxyl group in the R- rather than the S‑configuration so can have very different biological properties from the conventional prostanoids in that they are potent anti-inflammatory agents.
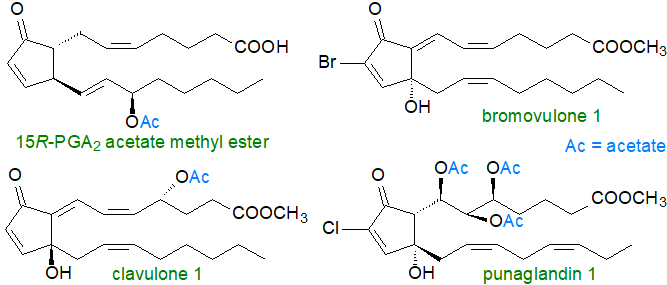 |
| Figure 9. Some exotic marine prostanoids |
Some examples of the many novel prostanoids of corals are illustrated above, and these differ in stereochemistry from the typical prostanoids, or contain acetyl groups, or are substituted with halogen atoms, such as chlorine or bromine. Little is known of the biochemistry of these clavulones, bromovulones or punaglandins in marine organisms, but there is increasing interest in them because of they may be anti-tumour agents.
It is perhaps more surprising that some red algae (seaweeds) such as Gracilaria species contain prostaglandins (PGE2, PGF2α and others) and are known to have a cyclooxygenase gene. In Gracilaria vermiculophylla, PGG2 is first synthesised from arachidonate by a cyclooxygenase, and this is converted to 15‑hydroperoxy-PGE2, which can then react either enzymatically or non-enzymatically to generate PGE2 or 15-keto-PGE2; a similar but rather minor pathway has since been discovered in animals. Why these organisms, which produce toxins for lethal food poisonings that have occurred in Japan, need such oxylipins is not known, but they be defensive. The brown alga Ectocarpus siliculosus synthesises prostanoid-like compounds (ectocarpins) from α-linolenic acid and EPA by means of a hydroperoxide bicyclase, while prostaglandins have been found in phytoplanktonic microalgae (diatoms) and dinoflagellates that may be defensive against copepod predators.
7. Prostanoid Analysis
Analysis of prostanoids is not a simple task because they occur at such low levels in tissues and because of their inherent chemical instability. Extraction must be carried out under mild conditions as rapidly as possible, and solid-phase extraction methods are now available that set the standard for isolation as a class, before HPLC linked to mass spectrometry is usually necessary for detailed analysis, which is now possible to the 0.1-1 pg level. Many primary standards, including deuterated forms, are now available commercially for comparison, calibration or validation purposes. Chiral chromatography can be used to distinguish between prostaglandins and isoprostanes. Immunoassays are available that may be suitable for some clinical applications, but they are not sensitive to minor differences in prostanoid structure.
Suggested Reading
- Badimon, L., Vilahur, G., Rocca, B. and Patrono, C. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc. Res., 117, 2001-2015 (2022); DOI.
- Biringer, R.G. The enzymology of the human prostanoid pathway. Mol. Biol. Rep., 47, 4569-4586 (2020); DOI.
- Biringer, R.G. A review of prostanoid receptors: expression, characterization, regulation, and mechanism of action. J. Cell Commun. Signal., 15, 155-184 (2021); DOI.
- Braune, S., Küpper, J.H. and Jung, F. Effect of prostanoids on human platelet function: an overview. Int. J. Mol. Sci., 21, 9020 (2020); DOI.
- Di Costanzo, F. Di Dato, V., Ianora, A. and Romano, G. Prostaglandins in marine organisms: a review. Marine Drugs, 17, 428 (2019); DOI.
- Fujimori, K. Prostaglandin D2 and F2α as regulators of adipogenesis and obesity. Biol. Pharm. Bull., 45, 985-991 (2022); DOI.
- Fujino, H. The biased activities of prostanoids and their receptors: review and beyond. Biol. Pharm. Bull., 45, 684-690 (2022); DOI.
- Hajeyah, A.A., Griffiths, W.J., Wang, Y., Finch, A.J. and O’Donnell, V.B. The biosynthesis of enzymatically oxidized lipids. Front. Endocrinol., 11, 591819 (2020); DOI.
- Idborg, H. and Pawelzik, S.C. Prostanoid metabolites as biomarkers in human disease. Metabolites, 12, 721 (2022); DOI.
- Jin, K.P., Qian, C., Lin, J.T. and Liu, B. Cyclooxygenase-2-prostaglandin E2 pathway: A key player in tumor-associated immune cells. Front. Oncol., 13, 1099811 (2023); DOI.
- Kim, Y. and Stanley, D. Eicosanoid signaling in insect immunology: new genes and unresolved issues. Genes, 12, 211 (2021); DOI.
- Lagarde, M. and Nicolaou, A. (Editors) Oxygenated metabolism of PUFA: analysis and biological relevance. Biochim. Biophys. Acta, Lipids (Volume 1851, Issue 4, Pages 307-518) (2015) - special issue.
- Lee, B.R., Paing, M.H. and Sharma-Walia, N. Cyclopentenone prostaglandins: biologically active lipid mediators targeting inflammation. Front. Physiol., 12, 640374 (2021); DOI.
- Li, J.J., Guo, C.Y. and Wu, J.Y. 15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), an endogenous ligand of PPAR-γ: function and mechanism. PPAR Res., 7242030 (2019); DOI.
- Maseda, D., Ricciotti, E. and Crofford, L.J. Prostaglandin regulation of T cell biology. Pharmacol. Res., 149, 104456 (2019); DOI
- Niu, M.Y. and Keller, N.P. Co-opting oxylipin signals in microbial disease. Cell. Microbiol., 21, e13025 (2019); DOI.
- Parchem, K. and others. Oxylipin profiling for clinical research: Current status and future perspectives. Prog. Lipid Res., 95, 101276 (2024); DOI.
- Peebles, R.S. Prostaglandins in asthma and allergic diseases. Pharmacol. Therapeut., 193, 1-19 (2019); DOI.
- Schebb, N.H. and others. Technical recommendations for analyzing oxylipins by liquid chromatography-mass spectrometry. Sci. Signal., 18, 678 (2025); DOI.
- Smith, M.L. and Murphy, R.C. The eicosanoids: cyclooxygenase, lipoxygenase and epoxygenase pathways. In: Biochemistry of Lipids, Lipoproteins and Membranes (6th Edition). pp. 260-296 (Edited by N.D. Ridgeway and R.S. McLeod, Elsevier, Amsterdam) (2016) - see Science Direct.
- Zhang, M., Li, W. and Li, T. Generation and detection of levuglandins and isolevuglandins in vitro and in vivo. Molecules, 16, 5333-5348 (2011); DOI.
- and from a historical standpoint on the topic -
- Patrono, C. Fifty years with aspirin and platelets. Brit. J. Pharm., 180, 25-43 (2023); DOI.
- Samuelsson, B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J. Biol. Chem., 287, 10070-10080 (2012); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: November 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
