Fatty Acids: Polyunsaturated with Other than
Methylene-Interrupted Double Bonds
The structures, occurrence and biochemistry of two types of unsaturated fatty acids are considered here - those with conjugated double bond systems and those in which the double bonds are separated by two or more methylene groups. Related fatty acids from plant systems may have acetylenic bonds or oxygenated groups, and these are discussed on separate web pages. Similarly, the eicosanoids and hydroperoxides, which can have conjugated double bond systems, are discussed in other documents. Mainstream polyunsaturated fatty acids with methylene-interrupted double bonds of course have their own web page.
1. Conjugated Fatty Acids Derived from Bacteria in Animal Tissues
Fatty acids with conjugated diene systems are found in small but significant amounts in tissues of ruminant animals, where they are formed as intermediates or by-products in the biohydrogenation of linoleic and linolenic acids by microorganisms in the rumen, and thence they make their way into meat and dairy products and eventually into human tissues (together with vaccenic and stearic acids). The main conjugated isomer, 9Z,11E‑octadecadienoic acid, amounts to 1-2% of the fatty acids of milk fat, and it may be accompanied by a small proportion of positional and geometrical isomers with conjugated double bonds (6,8- to 12,14-18:2) or less often with two or more methylene groups between double bonds, while an analogous fatty acid derived from α-linolenic acid, 9Z,11E,15Z-octadecatrienoic acid is usually detected at trace levels only. One consequence of the biohydrogenation process is that the levels of the precursor essential fatty acids in ruminant tissues are very low in comparison with monogastric animals, such as horses on a similar grass-based diet. Although most of the conjugated fatty acids in tissues of other animals are probably obtained from the diet, some may be produced by linoleate isomerases in intestinal microorganisms even in humans.
Several routes to the biosynthesis of the conjugated linoleate isomer have been suggested for various bacterial species, and for example, linoleate isomerases have been identified from Butyrivibrio fibrisolvens and Propionibacterium sp. In Lactobacillus plantarum the process occurs in several steps by a multi-enzyme complex, and in the first, a linoleate 10‑hydratase produces the intermediate 10-hydroxy-octadec-12Z-enoic acid, which is converted to a ketone and three steps later to 9Z,11E-octadecadienoic acid. Other bacterial species produce the 10E,12Z-isomer (or both).
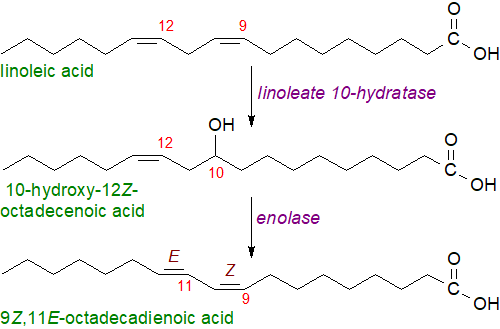 |
| Figure 1. Biosynthesis of 9Z,11E-octadecadienoic acid in Lactobacillus plantarum. |
There is considerable interest in conjugated linoleic acid ('CLA') at present because of reports that it has beneficial medical properties, including as an anti-cancer agent. Depending on the specific isomer, it is claimed to have anti-atherosclerosis effects, to help the immune system, and to affect energy metabolism by promoting protein deposition as opposed to fat. The material sold as a nutritional supplement, and approved by the FDA, is produced commercially by alkaline isomerization of linoleate-rich oils and consists of equimolar amounts of 9Z,11E- and 10E,12Z-18:2, together with variable amounts of positional and geometrical isomers. This mixture does not necessarily have the same benefits as the individual isomers. As described in our web pages for methylene-interrupted polyunsaturated fatty acids, CLA isomers can undergo chain elongation and desaturation to eventually form an analogue of arachidonic acid with a conjugated double bond system, i.e., 5Z,8Z,12Z,14E-20:4. Although the rate of this reaction is low, it is possible that such fatty acids could have biological activity if utilized for eicosanoid biosynthesis. Shorter-chain metabolites of CLA are found in tissues as a result of β‑oxidation.
It is now recognized that conjugated linoleate produces nitro fatty acids with great facility in presence of reactive nitrogen species in animal tissues in vitro, and it may be the primary endogenous substrate for fatty acid nitration in vivo; nitro-CLA isomers have been detected in plasma and urine of healthy humans.
2. Conjugated Fatty Acids Produced in Animals
Some synthesis of conjugated fatty acids from dietary precursors occurs within organs such as the liver of animals. Thus, 7E,9Z-octadecadienoate is synthesised endogenously from 7E-18:1 by means of a Δ9-desaturase in dairy cows. While 9Z,11E-18:2 is synthesised by a comparable route in human tissues by desaturation of 11E-octadecenoate (vaccenic acid, primarily of microbial origin) from the diet, rodent tissues, including lactating mammary gland, can produce the 11E,13Z isomer from the same precursor by means of the FADS3 desaturase, i.e., a unique methyl-end Δ13‑desaturation in animals.
 Fatty
acids, which are all-trans-polyenes, are the yellow pigment in budgerigar feathers, and unusually for an animal product,
they are produced by a polyketide synthase rather than a fatty acid synthase.
In addition, the nematode Caenorhabditis elegans utilizes a polyketide synthase to produce a C18 fatty acid with 3/4 double bonds
in conjugation and other functional groups linked to tetrapeptides (nemamides A and B).
A short-chain conjugated fatty acid, 2E,4E-hexadienoic (sorbic) acid, is a characteristic component of triacylglycerols
in the surface wax of aphids, where it is found exclusively in position sn-2 (this acid was first described from unripe berries of the
Rowan tree (Sorbus aucuparia)).
Fatty
acids, which are all-trans-polyenes, are the yellow pigment in budgerigar feathers, and unusually for an animal product,
they are produced by a polyketide synthase rather than a fatty acid synthase.
In addition, the nematode Caenorhabditis elegans utilizes a polyketide synthase to produce a C18 fatty acid with 3/4 double bonds
in conjugation and other functional groups linked to tetrapeptides (nemamides A and B).
A short-chain conjugated fatty acid, 2E,4E-hexadienoic (sorbic) acid, is a characteristic component of triacylglycerols
in the surface wax of aphids, where it is found exclusively in position sn-2 (this acid was first described from unripe berries of the
Rowan tree (Sorbus aucuparia)).
3. Conjugated Fatty Acids from Plants
Seed oils are important sources of conjugated fatty acids, though conjugated dienes are only rarely encountered. 10E,12E-Octadecadienoic acid comprises about 10% of the seed oil of Chilopsis linearis and may be the only long-chain conjugated dienoic fatty acid from a plant source, although the short-chain 2E,4Z-decadienoic acid is present in estolide linkage to an allenic hydroxy acid in Stillingia oil. Those conjugated dienoic fatty acids found in refined vegetable oils are mainly artefacts of processing.
In contrast, fatty acids with conjugated triene systems have been found in many different plant species. Of these, 9Z,11E,13E-octadecatrienoic (α‑eleostearic) acid is the most widespread and best known, and tung oil is the main commercial source.

Other geometrical isomers of this fatty acid are found in various seed oils, and these include 9Z,11E,13Z- and 9E,11E,13Z-octadecatrienoic acids ('punicic' and 'catalpic' acids, respectively), the first of which is found in the seed oil from pomegranate and related species and the second from Catalpa ovata. Two 8,10,12-18:3 isomers are known, i.e., 8E,10E,12Z-18:3 ('calendic' acid) from marigold seeds (Calendula officinalis), and 8Z,10E,12Z-18:3 from Jacaranda seeds.
 A conjugated
tetraenoic acid, 9Z,11E,13E,15Z-octadecatetraenoic or 'α-parinaric' acid, is a major
constituent of seed oils from Parinarium and Impatiens species, and unusual fatty acids with four conjugated double bonds
as well as methylene-interrupted double bonds are produced by some marine algae
(e.g., 4Z,7Z,9E,11E,13Z,16Z,19Z-docosaheptaenoic acid).
A conjugated
tetraenoic acid, 9Z,11E,13E,15Z-octadecatetraenoic or 'α-parinaric' acid, is a major
constituent of seed oils from Parinarium and Impatiens species, and unusual fatty acids with four conjugated double bonds
as well as methylene-interrupted double bonds are produced by some marine algae
(e.g., 4Z,7Z,9E,11E,13Z,16Z,19Z-docosaheptaenoic acid).
All conjugated tri- and tetraenoic acids isomerize readily to the all-trans or 'beta'-forms, which are more stable thermodynamically, especially in the presence of alkali or on heating.
Calendic acid (8E,10E,12Z-18:3) is synthesised in Calendula officinalis from linoleate (9Z,12Z-18:2) by an unusual Δ12‑oleate desaturase (a FAD2 variant) that converts the Z-double bond in position 9 to an 8E,10E-conjugated double bond system. As a result of a small change in the position of the iron oxidant at the catalytic centre of the enzyme relative to the substrate, the site of the initial hydrogen abstraction is at C-11 rather than C-12, as is more usual. Punicic acid is synthesised in plants from linoleate by a related desaturase that converts the Z-double bond in position 12 to an 11E,13Z-conjugated double bond system.
 |
| Figure 2. Biosynthesis of calendic acid. |
There is increasing interest in the potential health-giving properties of natural conjugated trienoic fatty acids for which studies in vitro and in vivo have demonstrated anti-inflammatory, anti-obesity, immuno-modulatory and anti-carcinogenic activities, as well as improvements to cardiovascular health. For example, punicic acid is reported to decrease oxidative damage and inflammation by increasing the expression of peroxisome proliferator-activated receptors (PPARs) and to reduce the symptoms of neurological disorders. However, further systematic clinical studies are needed with subjects of various ages to standardise dosing and formulations to determine optimal intake levels for health benefits (commercial supplements vary widely in concentration and purity). There is also a need for studies of the bioavailability and metabolic fate of such fatty acids in humans as the FDA has yet to approve their use as nutraceuticals.
Aside from seed oils, Rhizobium bacteria interact with leguminous plants in a host-specific manner to form nitrogen-fixing root nodules, and an unusual C18 tetraenoic fatty acid with three double bonds in conjugation, 2E,4E,6E,11Zoctadecatetraenoic acid, is a key component of the signalling mechanism that initiates the process.
4. Bis- and Polymethylene-Interrupted Unsaturated Fatty Acids from Marine Invertebrates
The primitive animals the sponges (family - Spongillidae) and some other marine invertebrates are known to contain a wide range of distinctive fatty acids, the demospongic acids, with bis-methylene-interrupted cis-double bonds and ranging in chain-length from C16 to C34. These fatty acids have a cis,cis-dienoic system of two types, either with the double bonds in positions 5 and 9 or derived from 5,9‑16:2 by chain-elongation. The second type is usually a relatively minor component of sponges and was first reported from the cellular slime mould Dictyostelium discoideum.

Homologous fatty acids from 5,9-16:2 to 5,9-34:2, both odd and even-numbered, can be found in a single species of sponge, while 5,9‑16:2, 7,11-18:2, 9,13-20:2 and so forth to 23,27-34:2, which are presumably formed by sequential chain-elongation of 5,9-16:2, have been found in another species. Related fatty acids occur with a methyl branch, often in the iso- or anteiso-position but also located more centrally (e.g., 22‑methyl-5,9-28:2), and multi-branched (isoprenoid) demospongic fatty acids have been found in at least one fresh-water species. Comparable fatty acids have been identified in many other marine invertebrates, including nudibranchs, limpets, sea anemones and gorgonians. 5,11- and 5,13-20:2 and their homologues 7,13- and 7,15-22:2 are typical components of marine Echinoderms.
One trienoic fatty acid with two bis-methylene-interrupted double bond systems in effect, i.e., 5,9,13-20:3, has been found in sponges and other marine invertebrates, but most trienoic demospongic acids have one bis-methylene-interrupted double bond system with the third double bond in the n-7 or n-9 positions (e.g., 5,9,25-32:3). Some tetraenoic demospongic acids have two bis-methylene-interrupted double bond systems that are widely separated (e.g., 5,9,21,25-32:4). The most unusual is (all Z)-34S-methylhexatriaconta-5,9,12,15,18,21-hexaenoic acid, which is present in syriacin, a strange, sulfated ceramide glycoside from the freshwater sponge Ephydatia syriaca.
Although the double bonds are normally of the cis (Z) configuration, a series of E,E-5,9-dienoic fatty acids was isolated from the sponge Plakortis halichondroides, while 5Z,9Z,19E-26:3 constituted 10% of the fatty acids from a fresh-water sponge, and related fatty acids have been found in the brittle star fish.
More than a hundred different fatty acids can be found in a single species of sponge. Although many of these are derived from the microflora and fauna that make up the food chain, the demospongic acids are certainly synthesised de novo by sponges. Thus, in the sponge Microciona prolifera, it has been demonstrated that 16:0 is elongated to the 26:0 fatty acid, which is then desaturated by Δ5 and Δ9 desaturases to form 5-26:1 and 9-26:1, respectively. These are both further desaturated to 5,9-26:2 by the same enzymes, i.e., unlike other animal systems, a double bond can be inserted before or after an existing double bond. 5,9,19-26:3 is formed in an analogous manner by elongation of 9-16:1 to 19-26:1, followed by desaturation in positions 5 and 9.
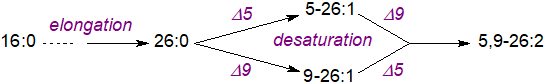 |
| Figure 3. Biosynthesis of 5,9-unsaturated fatty acids in sponges. |
Dienoic fatty acids in which more than two methylene groups separate the double bonds are found in many marine invertebrates, but especially in molluscs and cephalopods, and the most common of these are 5,11- and 5,13-20:2, and 7,13- and 7,15-22:2. 6,11-18:2 and 6,11-20:2 Fatty acids have been found in a marine sponge, while invertebrates from deep-sea hydrothermal vent ecosystems contain a number of unusual non-methylene-interrupted polyenes, including 5,13,16-20:3, 5,14,17-20:3 and 5,13,16,19-20:4. In bivalves, 7,13-22:2 and 7,15-22:2 are synthesised de novo by chain elongation, followed by Δ5 desaturation and further elongation of 9-18:1 and 9-16:1, respectively. The increasing availability of genetic information has enabled identification and characterization of both elongases and desaturases from such organisms. As anti-cancer activities have been reported, the pharmacological properties of some of these fatty acids are under investigation.
Incidentally, low levels of polymethylene-interrupted fatty acids have been detected in vernix caseosa, the waxy layer covering human newborns, and in some human cancer cell lines.
5. Bis- and Polymethylene-Interrupted Unsaturated Fatty Acids from Plants
Δ5-Unsaturated polymethylene-interrupted fatty acids with cis-double bonds only occur in appreciable amounts in seeds, leaves and other tissues of the relatively primitive plants, the gymnosperms (conifers), and of these, the best known is probably 5Z,9Z,12Z-octadecatrienoic ('pinolenic') acid, which is widespread in species of pine trees (Pinus genus).

Several such fatty acids have been identified, some of which are characteristic of certain families of gymnosperms, and they include 5,11-18:2, 5,11-20:2, 5,9,12,15-18:4 (coniferonic), 5,11,14-20:3 (sciadonic) and 5,11,14,17-20:4 (juniperonic). Related branched-chain fatty acids, anteiso-methyl-5,9-18:2 and anteiso-methyl-5,9,12-18:3 acids have been found in pine wood extracts. 7,11-20:2 and 7,11,14-20:3 occur in some species, and they are presumably derived from chain elongation of the Δ5-C18 precursors. Most have been given trivial names, although they are only likely to be of interest to specialists. Incidentally, sciadonic acid has been detected in vivo in hormone-positive breast cancer tissue, where it presumably results from a defect in the biosynthesis of arachidonic acid.
5Z,13Z-Docosadienoic acid (16% of the total fatty acids) occurs in the seed oil of a plant from a quite different family Limnanthes alba (meadowfoam). Trace levels of dienoic fatty acids with cis-double bonds in positions 5 and 9 have been found in a few other plant species, while 9,15-octadecadienoate occurs in mango pulp. An analogue of pinolenic acid with a trans double bond in position 5 (5E,9Z,12Z-18:3) is the main fatty acid constituent of the seed oil of Aquilegia vulgaris (columbine), while a positional isomer of this, i.e., 3E,9Z,12Z-octadecatrienoate (accompanied sometimes by 3E,9Z-18:2 and 3E,9Z,12Z,15Z-18:4), is a common constituent of seed oils from the Compositae together on occasion with the all-Z isomer.
Pinolenic acid or 5,9,12-18:3 is synthesised in gymnosperms by the action of a Δ5 desaturase on linoleate, and presumably the other unusual acids in such plants are synthesised in the same way from appropriate precursors. For example, in the gymnosperm, Torreya grandis, analysis of the genome has demonstrated that sciadonic acid is synthesised by elongation of linoleate by a C18 Δ9-elongase followed by the action of a C20 Δ5-desaturase.
 |
| Figure 4. Biosynthesis of sciadonic acid in gymnosperms. |
Two distinctive front-end Δ5 desaturases have been characterized from developing seeds of Anemone leveillei, one having a broad specificity for fatty acid substrates, while the other prefers C20 fatty acids for the biosynthesis of 5,11,14-20:3 especially. There are claims of health benefits towards weight reduction, lipid-lowering, diabetes and inflammatory disorders when pinolenic acid is included in animal diets, and it is reported to suppress cell invasion and motility in cancer.
6. Protozoa, Fungi and Bacteria
 The
opportunistic protozoan pathogen Acanthamoeba castellanii contains 4,21,24‑triacontatrienoic acid
(not 5,21,24‑30:3 as originally reported) as components of phosphatidylethanolamine and phosphatidylserine.
Slime moulds contain fatty acids with bis- and poly-methylene-interrupted double bond systems in the 5,9- and 5,11-positions.
Amanita pantherina, a poisonous mushroom, contains long-chain fatty acids with a 2,4-conjugated double bond system and
termed pantheric acids.
Mycobacteria such as Mycobacterium phlei produce a series of polyunsaturated fatty acids with multiple bis-methylene-interrupted
double bonds and up to C40 in chain-length; in the main isomer m= 15 and n=5.
In Mycobacterium abscessus, they are present as trehalose polyphleates.
The
opportunistic protozoan pathogen Acanthamoeba castellanii contains 4,21,24‑triacontatrienoic acid
(not 5,21,24‑30:3 as originally reported) as components of phosphatidylethanolamine and phosphatidylserine.
Slime moulds contain fatty acids with bis- and poly-methylene-interrupted double bond systems in the 5,9- and 5,11-positions.
Amanita pantherina, a poisonous mushroom, contains long-chain fatty acids with a 2,4-conjugated double bond system and
termed pantheric acids.
Mycobacteria such as Mycobacterium phlei produce a series of polyunsaturated fatty acids with multiple bis-methylene-interrupted
double bonds and up to C40 in chain-length; in the main isomer m= 15 and n=5.
In Mycobacterium abscessus, they are present as trehalose polyphleates.
Recommended Reading
- Almoraie, M., Spencer, J. and Wagstaff, C. Punicic acid: a potential nutraceutical compound in pomegranate seed oil and its cardiovascular benefits. Foods, 14, 2412 (2025); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Dembitsky, V.M., Rezanka, T. and Srebnik, M. Lipid compounds of freshwater sponges: family Spongillidae class Demospongiae. Chem. Phys. Lipids, 123, 117-155 (2003); DOI.
- Du, M., Gong, M., Wu, G., Jin, J., Wang, X. and Jin, Q. Conjugated linolenic acid (CLnA) vs conjugated linoleic acid (CLA): a comprehensive review of potential advantages in molecular characteristics, health benefits, and production techniques.. J. Agric. Food Chem., 72, 5503–5525 (2024); DOI.
- Gunstone, F.D., Harwood, J.L. and and Dijkstra, A.J. (Editors). The Lipid Handbook (3nd Edition). Chapman & Hall, London) (2007) - see CRC Press.
- Kornprobst, J.-M. and Barnathan, G. Demospongic acids revisited. Marine Drugs, 8, 2569-2577 (2010); DOI.
- Lou, H. and others. The Torreya grandis genome illuminates the origin and evolution of gymnosperm-specific sciadonic acid biosynthesis. Nat. Commun., 14, 1315 (2023); DOI.
- Monroig, Ó., Shu-Chien, A.C., Kabey, N., Tocher, D.R. and Castro, L.F.C. Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: From genes to functions. Prog. Lipid Res., 86, 101157 (2022); DOI
- Takala, R., Ramji, D.P. and Choy, E. The beneficial effects of pine nuts and its major fatty acid, pinolenic acid, on inflammation and metabolic perturbations in inflammatory disorders. Int. J. Mol. Sci., 24, 1171 (2023); DOI.
- Wu, C., Chen, H., Mei, Y., Yang, B., Zhao, J., Stanton, C. and Chen, W. Advances in research on microbial conjugated linoleic acid bioconversion. Prog. Lipid Res., 93, 10125 (2024); DOI.
- Yang, B., Gao, H., Stanton, C., Ross, R.P., Zhang, H., Chen, Y.Q., Chen, H. and Chen, W. Bacterial conjugated linoleic acid production and their applications. Prog. Lipid Res., 68, 26-36 (2017); DOI.
For tutorials on mass spectral analysis of these fatty acids - see our mass spectrometry pages.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: October 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
