Phosphatidic Acid, Lysophosphatidic Acid and Related Lipids
Phosphatidic acid or 1,2-diacyl-sn-glycero-3-phosphate is an intermediate in the biosynthesis both of other glycerophospholipids and of triacylglycerols. It is structurally one of the simplest of the phospholipids and was long thought to be important only as a precursor of other lipids, where it is indeed a key molecule, but it is now known to have many other functions in animals, plants and other organisms by its influence on membrane structure and dynamics and by its interactions with various proteins. As a lipid mediator, it modulates various signalling and cellular processes, such as membrane tethering, conformational changes and enzymatic activities of proteins, and vesicular trafficking. Moreover, its 1‑acyl metabolite lysophosphatidic acid is recognized as a signalling molecule that influences many metabolic pathways through its own receptors.
1. Phosphatidic Acid – Occurrence and Biosynthesis
Phosphatidic acid is not an abundant lipid constituent of any living organism, seldom greater than picomolar concentrations in cells, but it is essential both as an intermediate in the biosynthesis of other glycerophospholipids and triacylglycerols and as a signalling molecule or precursor of signalling molecules. Indeed, it is often over-estimated in tissues as it can arise by inadvertent enzymatic hydrolysis during inappropriate storage or extraction conditions during analysis. It is the simplest diacyl-glycerophospholipid, the only one with a phosphomonoester as the head group, and as such it is acidic and carries a negative charge, i.e., it is an anionic lipid.
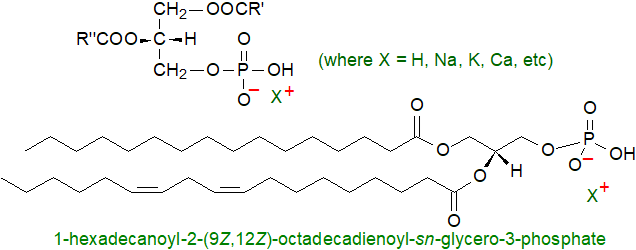 |
| Figure 1. Structural representations of phosphatidic acid. |
sn‑Glycerol-3-phosphate pathway: There are at least four biosynthetic pathways for phosphatidic acid biosynthesis in different organelles under various conditions, and these may result in the formation of molecular species with differing fatty acid compositions. A universal route in prokaryotes, plants, yeast and animals consists of sequential acylation of sn‑glycerol-3-phosphate, derived from catabolism of glucose, by coenzyme A derivatives of fatty acids (acyl-CoA) as illustrated (the web page on biosynthesis of triacylglycerols has further discussion). First, one type of acyltransferase catalyses the acylation of position sn-1 to form lysophosphatidic acid (1‑acyl-sn-glycerol-3-phosphate), and then a second acyltransferase catalyses the acylation of position sn‑2 to yield phosphatidic acid.
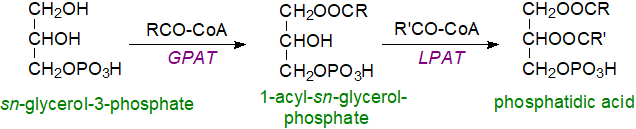 |
| Figure 2. Biosynthesis of phosphatidic acid via glycerol-3-phosphate. |
In mammals, the glycerol-3-phosphate acyltransferase that catalyses the first step exists in four isoforms, two in the mitochondrial outer membrane (designated GPAT1 and 2) and two in the endoplasmic reticulum, which are evolutionarily conserved and predominate in most tissues (GPAT3 and 4); all are membrane-bound enzymes, which span the membranes. GPAT1 is highly expressed in the liver and adipose tissue, where it is responsive to changes in feeding status via the sterol regulatory element binding protein-1 (SREBP-1), a master transcriptional regulator of lipogenic enzymes that is essential to direct fatty acyl-CoA esters towards glycerolipid synthesis as opposed to β-oxidation. GPAT3 and 4 are required for triacylglycerol storage, with calcineurin B homologous protein 1 (CHP1) as a critical regulator that is essential for their stability, enzymatic activity and location in lipid droplets. GPAT4 is the main contributor to lysophosphatidic acid synthesis in liver and brown adipose tissue.
For the second step in phosphatidic acid biosynthesis, five mammalian acyl-CoA:lysophosphatidic acid acyltransferases are known of which three are in the endoplasmic reticulum (LPLAT1, 2 and 3), with a further two (LPLAT4 and 5) on the outer mitochondrial membrane (the nomenclature of these and related enzymes in the literature has changed and can be confusing; LPLAT is now preferred over LPAT, MBOAT or AGPAT). While LPLAT1 and 2 have strict specificity for lysophosphatidic acid as acyl acceptor, other isoforms can esterify other lysophospholipids. Human LPLAT1 shows higher activity with 14:0-, 16:0- and 18:2‑CoAs, while LPLAT2 prefers 20:4-CoA, and LPLAT3 produces phosphatidic acid containing docosahexaenoic acid (22:6(n-3)), especially in retina and testes. LPLAT4 and 5 have a preference for oleoyl-CoA and polyunsaturated acyl-CoAs as the acyl donor, suggesting a dual role in glycerolipid synthesis and remodelling (cf., the Lands' cycle). Biosynthesis in the endoplasmic reticulum predominates in adipose tissue, but the mitochondrial enzymes are responsible for half the production in liver. As there is traffic of phosphatidic acid between the mitochondria and endoplasmic reticulum for remodelling or for synthesis of other lipids, the relative contributions of the two can be difficult to assess.
In plants, the sn-glycerol-3-phosphate pathway exists both in plastids and at the endoplasmic reticulum with multiple isoforms of the two types of acyltransferase as well as differences in the acyl substrates. This is the main pathway towards structural lipids, and it is discussed in some detail in our web page on galactosyldiacylglycerols. In brief, most plant lipid biosynthesis begins with fatty acid biosynthesis in the chloroplasts, before the acyltransferase ATS1 transfers an 18:1 acyl group from acyl-acyl carrier protein (acyl-ACP) to position sn-1 of glycerol 3-phosphate, and ATS2 transfers a palmitoyl group from ACP to position sn-2 to produce phosphatidic acid at the inner leaflet of the chloroplast inner envelope membrane (IEM). Fatty acids intended for the endoplasmic reticulum are released from ACP in the chloroplast stroma by IEM-associated thioesterases, exported and then activated by acyl-CoA synthases of the outer envelope membrane to produce species with C18 fatty acids in both positions. Thus, those fatty acids used for phosphatidic acid biosynthesis in the endoplasmic reticulum are markedly different in composition from those in the plastids. Subsequently, phosphatidic acid in the plastids is utilized for biosynthesis of galactosyldiacylglycerols, while that in the endoplasmic reticulum is used for synthesis of triacylglycerols and phospholipids.
In bacteria, two families of enzymes are responsible for acylation of position sn-1 of glycerol-3-phosphate. One present in Escherichia coli utilizes the acyl-acyl carrier protein (acyl-ACP) products of fatty acid synthesis as acyl donors as well as acyl-CoA derived from exogenous fatty acids. In a second wider group of bacteria, including cyanobacteria, there are enzymes (PlsX and PlsY) that make use of unique acyl donors, acyl-phosphates derived in part from acyl-ACP, to acylate position sn-1. Acylation of position sn-2 in this instance is performed by a further family of enzymes (PlsC) that uses acyl-ACP as the acyl donor, although some bacteria may use acyl-CoA. Mycobacterium tuberculosis is unique in having two enzymes that can acylate either glycerol-3-phosphate or lysophosphatidic acid in each of the two positions.
Dihydroxyacetone phosphate pathway: In animals and yeast, a second biosynthetic pathway utilizes dihydroxyacetone phosphate (DHAP) as the primary precursor for the peroxisomal enzyme, DHAP acyltransferase, which produces acyl-DHAP. This intermediate is converted to lysophosphatidic acid by reduction of the keto group in position sn-2 in a NADPH-dependent reaction catalysed by acyl-DHAP reductase, and this is in turn acylated to form phosphatidic acid by the same LPLAT as in the first mechanism. This pathway is required for the biosynthesis of ether lipids.
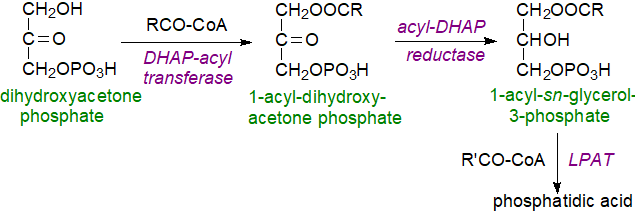 |
| Figure 3. Biosynthesis of phosphatidic acid via dihydroxyacetone phosphate. |
Other pathways: A third route to phosphatidic acid is via hydrolysis of other phospholipids, but mainly phosphatidylcholine, by the enzyme phospholipase D (or by a family or related enzymes of this kind), which utilizes water as the nucleophile to catalyse the hydrolysis of phosphodiester bonds in phospholipids and is dependent on and regulated by neurotransmitters, hormones, small monomeric GTPases and lipids. The various forms are phosphoproteins, which are regulated by kinases and phosphatases and by binding to phosphatidylinositol 4,5‑bisphosphate. Phospholipase D is readily available for study in plants, where the special functions of phosphatidic acid have long been known (see below), but it is now recognized that it is present in bacteria, yeasts and most animal cells. In the last, it exists in two main isoforms with differing substrate preferences and cellular locations; PLD1 is found mainly in the Golgi-lysosome continuum and is induced by extracellular signals from small G proteins (ARF, Rho, and Rac) and protein kinase C, while PLD2 is present mainly in the plasma membrane and has high basal activity. In mitochondria, a distinctive enzyme of this type utilizes cardiolipin as substrate.
 |
| Figure 4. Generation of phosphatidic acid by the action of phospholipase D. |
In addition to generating phosphatidic acid for signalling purposes and for the maintenance of membrane composition, phospholipase D influences intracellular protein trafficking, cytoskeletal dynamics, cell migration and cell proliferation, partly through protein-protein interactions. It is a factor in inflammation and in cancer growth and metastasis as a downstream transcriptional target of proteins involved in the pathophysiology of these diseases. By a transphosphatidylation reaction with ethanol, it generates phosphatidylethanol, a useful biomarker for ethanol consumption in humans. Perhaps surprisingly, it is also a guanine nucleotide exchange factor.
Phosphatidic acid can be generated by the action of diacylglycerol kinases on 1,2-diacyl-sn-glycerols, such as those produced from other phospholipids by the action of phospholipase C (see our web page on diacylglycerols). Diacylglycerol kinases, of which at least ten isoforms (DGKα to DGKκ) exist in five subtypes based on functional domains with different sub-cellular locations and functions in animals, use ATP as the phosphate donor to generate phosphatidic acid. While the epsilon isoform (DGKε) utilizes the 1‑stearoyl-2-arachidonoyl species of diacyl-sn-glycerols preferentially to produce phosphatidic acid for the biosynthesis of phosphatidylinositol, the other isoenzymes are selective for saturated-monosaturated diacylglycerol combinations. Aside from producing phosphatidic acid for phospholipid production or signalling, these enzymes may attenuate signalling by diacylglycerols, and they can contribute to cellular asymmetry and control the polarity of cells by regulating the gradients in diacylglycerol and phosphatidic acid concentrations. Dysregulation of DGK activity is associated with several diseases, including cancer and metabolic disorders.
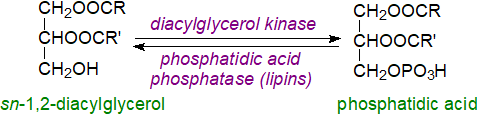 |
| Figure 5. Biosynthesis of phosphatidic acid by diacylglycerol kinases and the reverse reaction. |
A further possible route to phosphatidic acid production for signalling purposes is via acylation of lysophosphatidic acid, which can be produced independently as a lipid mediator as discussed below. This pathway may be most relevant in membranes, where the protein endophilin is an LPLAT and generates phosphatidic acid from lysophosphatidic acid to alter the curvature of the membrane bilayer.
Catabolism: The reverse reaction, i.e., hydrolysis of phosphatidic acid by lipins (phosphatidic acid phosphohydrolases), is discussed in our web page on triacylglycerols and briefly below. These enzymes regulate the local concentrations of phosphatidic acid and thence its availability for lipid synthesis or for signalling.
2. Phosphatidic Acid as a Lipid Precursor
Phosphatidic acid generated via 1-acyl-sn-glycerol-3-phosphate is a primary precursor for biosynthesis of other glycerolipids, although alternative pathways may be more important for generating this lipid for signalling purposes. On the other hand, whether separate pools of phosphatidic acid for specific purposes really exist is not certain, as dynamic changes of intracellular distribution can occur under various cellular conditions that are attributed to inter-organelle transfer via vesicular transport or at membrane contact sites by lipid transfer proteins. Control of its concentration in membranes, such as the endoplasmic reticulum, is therefore necessary, and a transcriptional repressor 'Opi1', which binds only to phosphatidic acid in membranes, is a regulatory factor, although many other phosphatidic acid-binding proteins have been identified that influence how it is used either as a biosynthetic precursor or for signalling purposes. The mechanisms for phosphatidic acid homeostasis vary among animals, plants, yeasts and bacteria in response to differing requirements in these organisms. One difficulty in studying the problem is that the steady-state concentration of phosphatidic acid in cells can be too low for accurate measurement.
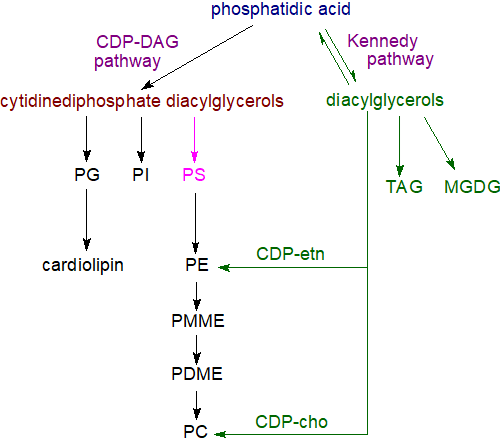 |
| Figure 6. Biosynthesis of complex lipids with phosphatidic acid as precursor. |
In addition to dietary, hormonal and tissue factors in animals, the extent to which fatty acids are channelling either into triacylglycerol synthesis for storage in lipid droplets and secretion in lipoproteins or into glycerophospholipids for membrane formation depends to a large extent upon the enzymes of glycerol-3-phosphate pathway, their isoform expression, activities and locations. Phosphatidic acid is not only a biosynthetic precursor of other lipids but also a regulatory molecule in the transcriptional control of the genes for glycerolipid synthesis, and control of its concentration in cells for this purpose is essential. For example, the local concentration of phosphatidic acid in the endoplasmic reticulum is a factor in the biogenesis of lipid droplets.
The subsequent steps in the utilization of phosphatidic acid in the biosynthesis of triacylglycerols and of the various glycerophospholipids are described in separate documents of this website. Thus, hydrolysis of phosphatidic acid by phosphatidate phosphatase enzymes (including lipins 1, 2 and 3) is the source of sn‑1,2‑diacylglycerols (DG), which are the precursors for the biosynthesis of the most abundant glycerolipids, i.e. triacylglycerols (TAG), phosphatidylcholine (PC), phosphatidylethanolamine (PE) and in plants monogalactosyldiacylglycerols (MGDG) via the so-called Kennedy pathway.
Via reaction with cytidine triphosphate, phosphatidic acid is the precursor of cytidine diphosphate diacylglycerol, which is the key intermediate in the synthesis of phosphatidylinositol (PI), phosphatidylglycerol (PG) and thence of cardiolipin (CL), and in prokaryotes and yeast but not animals, phosphatidylserine (PS). Depending on the organism and other factors, phosphatidylserine can be a precursor for phosphatidylethanolamine, while the latter can give rise to phosphatidylcholine by way of mono- and dimethyl-phosphatidylethanolamine intermediates. The cytidine diphosphate diacylglycerol synthase is another enzyme that consumes phosphatidic acid and modulates its concentration in cells so regulating processes mediated by this lipid.
While the fatty acid composition of phosphatidic acid can resemble that of the eventual products, the latter are generally much altered by re-modelling after synthesis via deacylation-reacylation reactions (the Lands' cycle - see the web page on phosphatidylcholine).
3. Phosphatidic Acid as a Lipid Mediator in Animals
As well as being an intermediate in lipid biosynthesis, phosphatidic acid and especially that generated by the action of phospholipase D and by diacylglycerol kinases may be a signalling molecule as a second messenger, although it is not certain whether every action suggested by studies in vitro operates in vivo. Nonetheless, phosphatidic acid has been implicated in many aspects of animal cell biochemistry and physiology.
Some of the observations may be explained simply by the physical properties of phosphatidic acid, which has a propensity to form a hexagonal II phase in the presence of calcium ions. Thus, hydrolysis of phosphatidylcholine, a cylindrical non-fusogenic lipid, converts it into cone-shaped phosphatidic acid, which promotes negative membrane curvature and fusion of membranes because its small anionic phosphomonoester head group lies relatively close to the hydrophobic interior of the lipid bilayer. In model systems, phosphatidic acid can bring about membrane fusion, probably because of its ability to form non-bilayer phases, and phosphatidic acid biosynthesis favours intraluminal budding of endosomal membranes with the formation of exosomes. Vesicle trafficking, secretion and endocytosis in many cell types may require phosphatidic acid derived from the action of phospholipase D.
Its overall negative charge is relevant in this context, and it is not always clear whether some of the observed effects are characteristic for phosphatidic acid or simply to negatively charged phospholipids in general. In contrast to phosphoinositide-interacting proteins, which have defined structural folds, the binding motifs of proteins with phosphatidic acid are not highly conserved. Nonetheless, it has been demonstrated that the positively charged lysine and arginine residues of proteins can bind with some specificity to phosphatidic acid through hydrogen bonding with the phosphate group, thus distinguishing it from other phospholipids. An ‘electrostatic-hydrogen bond switch model’ has been proposed in which the head group of phosphatidic acid forms a hydrogen bond to an amino acid residue, leading to de-protonation of the head group, increasing its negative charge from -1 to -2 and thus enabling stronger interactions with basic residues and tight docking with the membrane interacting protein. In this way, phosphatidic acid can tether certain proteins to membranes, and it can simultaneously induce conformational changes, hinder ligand binding and/or oligomerize proteins to alter their catalytic activity, stability and interactions with other molecules. It is a cellular pH sensor in that binding to proteins is dependent on intracellular pH and the protonation state of its phosphate headgroup.
The nature of the fatty acid components is relevant; phosphatidic acid linked to 16:0 strongly inhibits insulin aggregation in vitro to lower cell toxicity but that linked to 18:0 has the opposite effect, while an unsaturated component (18:1) changes the physical form of insulin in yet another manner.
 One target
of the lipid is mTOR, a serine/threonine protein kinase with a signalling cascade that regulates cell growth, proliferation, motility and
survival, together with protein synthesis and transcription by integrating both nutrient and growth factor signals.
This forms two distinct complexes of accessory proteins that regulate downstream targets.
Of these, mTORC1 interacts directly with phosphatidic acid, and this interaction allosterically induces the enzyme complex
to regulate protein synthesis, mitochondrial metabolism and the transcription of enzymes for lipid biosynthesis.
In contrast, phosphatidic acid appears to inhibit mTORC2 in relation to insulin signalling.
One target
of the lipid is mTOR, a serine/threonine protein kinase with a signalling cascade that regulates cell growth, proliferation, motility and
survival, together with protein synthesis and transcription by integrating both nutrient and growth factor signals.
This forms two distinct complexes of accessory proteins that regulate downstream targets.
Of these, mTORC1 interacts directly with phosphatidic acid, and this interaction allosterically induces the enzyme complex
to regulate protein synthesis, mitochondrial metabolism and the transcription of enzymes for lipid biosynthesis.
In contrast, phosphatidic acid appears to inhibit mTORC2 in relation to insulin signalling.
Phosphatidic acid regulates membrane trafficking events, and that produced by phospholipase D has been shown to contribute to exocytosis of secretory vesicles in many cell types, as well as vesicular transport within cells. By binding to targeted proteins, including protein kinases, protein phosphatases and G-proteins, it can either increase or inhibit them, and for example, it activates the enzyme NADPH oxidase, which operates as part of the defence mechanism against infection and tissue damage during inflammation. Impacts upon gene transcription have been observed that are linked to inhibition of peroxisome proliferator-activated receptor (PPAR). In yeast, phosphatidic acid in the endoplasmic reticulum binds directly to a transcriptional repressor to keep it inert outside the nucleus; when the lipid precursor inositol is added, this phosphatidic acid is rapidly depleted, releasing the transcriptional factor so that it can be translocated to the nucleus where it is able to repress target genes. This may be a mechanism to control phospholipid synthesis.
Phosphatidic acid regulates many aspects of phosphoinositide metabolism, and the murine phosphatidylinositol 4‑phosphate 5-kinase, the main enzyme generating the lipid second messenger phosphatidylinositol 4,5-bisphosphate, does not appear to operate unless phosphatidic acid is bound to it. Following generation by the action of phospholipase D, this lipid recruits the kinase to the membrane and induces a conformational change that is regulatory. Similarly, it may promote phospholipase A2, a key enzyme in eicosanoid production from phosphoinositide precursors.
In relation to signalling, it should be noted that phosphatidic acid can be metabolized to sn-1,2-diacylglycerols or to lysophosphatidic acid (see below), which each have their own signalling pathways. Both lipid mediators can be rendered inert by conversion back to phosphatidic acid.
Phospholipase D isoforms and phosphatidic acid have been implicated in a variety of pathologies, including neurodegenerative diseases, blood disorders, late-onset Alzheimer's disease and cancer, leading to attempts to develop inhibitors of the enzyme for therapeutic purposes. The expression of LPLAT isoforms can enhance the proliferation and chemoresistance of some cancer cells, while diacylglycerol kinase alpha (DGKα) is highly expressed in several refractory cancer cells, where it attenuates apoptosis and promotes proliferation. DGKα is also highly abundant in T cells and induces a nonresponsive state, which enables advanced cancers to escape immune action, and inhibition of this enzyme is seen as a promising treatment strategy.
4. Phosphatidic Acid as a Lipid Mediator in Plants
Phosphatidic acid is present at higher levels in roots of plants in comparison to leaves and is necessary for root architecture, but while its concentration is elevated in flowers and reproductive tissues, the significance of this is not known. As well as being one of the central molecules in lipid biosynthesis, it facilitates the transport of lipids across plant membranes, and it is the main plant lipid second messenger, which is rapidly and transiently generated in response to many different biotic and abiotic stresses. In contrast to animal metabolism, the diacylglycerol signalling pathway is relatively insignificant in plants. One further difference from animal metabolism is that diacylglycerol pyrophosphate can be synthesised from phosphatidic acid in plants (see below).
The main source of phosphatidic acid for signalling purposes is the action of phospholipase D (PLD) on membrane phospholipids, such as phosphatidylcholine and phosphatidylethanolamine, although diacylglycerol kinase 5, which is regulated by phosphorylation, is relevant in Arabidopsis under stress. Numerous related enzymes of the phospholipase D type occur in plants, 12 in the 'model' plant Arabidopsis and 17 in rice, in comparison with two in humans and one in yeast, and individual iso-enzymes can be characteristic of certain tissues and may elicit specific responses. In the former, the isoforms are grouped into six classes, based on the genic architecture, sequence similarities, domain structures and biochemical properties, and to operate optimally, these depend mainly on their lipid-binding domains, with some homologous to the human and yeast enzymes and with most containing a characteristic ‘C2’ (calcium- and lipid-binding) domain. The most widespread of these is PLDα, which does not require binding to phosphatidylinositol 4,5-bisphosphate, in contrast to other PLD isoforms and the mammalian enzyme, but millimolar levels of Ca2+ are necessary. The composition of the molecular species formed depends on the nature of the stimulus and that of the phospholipase D isoform. Studies with fluorescent biosensors suggest that phosphatidic acid accumulates in the subapical region of the cytosolic leaflet of the plasma membrane.
 Many phosphatidic
acid-binding proteins have been identified that are necessary for plant signal transduction, although there does not appear to be a
characteristic recognition motif.
It is involved in many different cell responses induced by hormones, stress and developmental processes, and it often acts in concert with
phosphatidylinositol 4,5-bisphosphate by binding to certain proteins rather than acting via a receptor.
As in mammalian cells, it can both activate or inhibit enzymes, and targets for such signalling include protein kinases and phosphatases
in addition to proteins that take part in membrane trafficking and the organization of the cytoskeleton.
If the target protein is soluble, binding to phosphatidic acid can cause the protein to be sequestered
into a membrane to influence downstream targets.
Phosphatidic acid is required to bind and allosterically activate the
monogalactosyldiacylglycerol synthase (MGDG1), located in the inner envelope membrane of the chloroplast,
and so it may be a regulator of the biosynthesis of thylakoid membranes.
Many phosphatidic
acid-binding proteins have been identified that are necessary for plant signal transduction, although there does not appear to be a
characteristic recognition motif.
It is involved in many different cell responses induced by hormones, stress and developmental processes, and it often acts in concert with
phosphatidylinositol 4,5-bisphosphate by binding to certain proteins rather than acting via a receptor.
As in mammalian cells, it can both activate or inhibit enzymes, and targets for such signalling include protein kinases and phosphatases
in addition to proteins that take part in membrane trafficking and the organization of the cytoskeleton.
If the target protein is soluble, binding to phosphatidic acid can cause the protein to be sequestered
into a membrane to influence downstream targets.
Phosphatidic acid is required to bind and allosterically activate the
monogalactosyldiacylglycerol synthase (MGDG1), located in the inner envelope membrane of the chloroplast,
and so it may be a regulator of the biosynthesis of thylakoid membranes.
Phospholipase D and the phosphatidic acid produced have long been recognized as of importance during seed germination and leaf senescence, and they are required for the response to stress damage and pathogen attack, both in higher plants and in green algae. A high content of phosphatidic acid induced by phospholipase D action during wounding or senescence brings about a loss of the membrane bilayer phase, because of the conical shape of this negatively charged phospholipid in comparison to the cylindrical shape of structural phospholipids, with crucial effects upon lipid-protein interactions, "the electrostatic-hydrogen bond switch model" described above. By promoting negative curvature at the plasma membrane and binding to clathrin proteins, it facilitates the process of endocytosis. Comparable phenomena may explain why phosphatidic acid influences the response to other forms of stress, including osmotic stress (salinity or drought), cold and oxidation, and for the interactions of plants with pathogens, nitrogen-fixing bacteria and arbuscular mycorrhizal fungi.
Although much remains to be learned of mechanistic aspects, phosphatidic acid promotes the response to the plant hormone abscisic acid, and it may interact with salicylic acid to mediate defence responses. It promotes the growth of pollen-tubes and root hairs, decreasing peroxide-induced cell death, and mediating the signalling processes that lead to responses to ethylene and again to the hormone abscisic acid. Thus, in Arabidopsis, phosphatidic acid interacts with a protein phosphatase to signal the closure of stomata promoted by abscisic acid and with a further enzyme to mediate the inhibition of stomatal opening by abscisic acid. Together these reactions constitute a signalling pathway that regulates water loss from plants.
It is noteworthy that phosphatidic acid production can be initiated by opposing stress factors, such as cold and heat, as well as by hormones that can be antagonistic, such as abscisic acid and salicylic acid. There is evidence that phosphatidic acid molecules synthesised by the two main pathways differ in composition and cellular distributions and so may produce different responses. Certainly, during low temperature stress, phosphatidic acid is generated by the action of diacylglycerol kinase, and it seems likely that the cellular environment controls where the lipid is produced, together with the availability of target proteins or other molecules with which it can act synergistically. Genes encoding enzymes for phosphatidic acid metabolism have been manipulated to explore their potential application for crop improvements in plant growth, development and stress responses.
As in animals, phosphatidic acid is catabolized and its signalling is terminated by lipid phosphate phosphatases (including glycerophosphodiester phosphodiesterases) and phosphatidic acid hydrolases, and by acyl-hydrolases and lipoxygenases with the production of fatty acids and other small molecules, which are subsequently absorbed and recycled.
5. Lysophosphatidic Acid
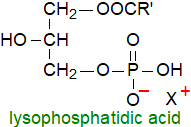 Lysophosphatidic acid (LPA) or
1-acyl-sn-glycero-3-phosphate (and its sn-2 isomer) differs structurally from phosphatidic acid in having only one mole of fatty
acid per mole of lipid, and as such, it is one of the simplest possible glycerophospholipids.
It exists esterified to many different fatty acids (16:0 to 22:6) or instead with a 1‑O‑alkyl/alkenyl-ether linkage, and there
is evidence that saturated and polyunsaturated species (18:0- versus 20:4-LPA) may differ in their biological properties, especially in
the regulation of signalling pathways.
Lacking one fatty acid in comparison to phosphatidic acid, it is a much more hydrophilic molecule, while the additional hydroxyl group
strengthens hydrogen bonding within membranes, properties that may be required in cells.
As the sn‑1-acylated form is more stable thermodynamically, facile isomerization ensures that this isomer tends to predominate,
although both regio-isomers may have their own characteristic interactions with certain proteins.
Lysophosphatidic acid (LPA) or
1-acyl-sn-glycero-3-phosphate (and its sn-2 isomer) differs structurally from phosphatidic acid in having only one mole of fatty
acid per mole of lipid, and as such, it is one of the simplest possible glycerophospholipids.
It exists esterified to many different fatty acids (16:0 to 22:6) or instead with a 1‑O‑alkyl/alkenyl-ether linkage, and there
is evidence that saturated and polyunsaturated species (18:0- versus 20:4-LPA) may differ in their biological properties, especially in
the regulation of signalling pathways.
Lacking one fatty acid in comparison to phosphatidic acid, it is a much more hydrophilic molecule, while the additional hydroxyl group
strengthens hydrogen bonding within membranes, properties that may be required in cells.
As the sn‑1-acylated form is more stable thermodynamically, facile isomerization ensures that this isomer tends to predominate,
although both regio-isomers may have their own characteristic interactions with certain proteins.
Although lysophosphatidic acid is present at very low levels only in animal tissues, it is utilized in innumerable biochemical processes. It is a biosynthetic precursor of phosphatidic acid, but there is particular interest in its role as a lipid mediator with growth factor-like properties that acts via its own receptors. Indeed, it is now recognized that it is a central regulator and pleiotropic signalling molecule both in normal physiology and disease that controls such diverse cellular processes such as proliferation, survival, migration, immune modulation and tissue remodelling. As a consequence of its role in disease, it is a target for the development of drugs, some of which have reached the stage of formal clinical trials.
Biosynthesis: In the circulation, the main source of lysophosphatidic acid is a lysophospholipase D known as ‘autotaxin’, which reacts with lysophosphatidylcholine (200 μM in plasma) to yield LPA in an albumin-bound form mainly, although it is relatively soluble in aqueous media because of its polarity and small size. This lipid is more abundant in serum (1 to 5 μM) than in plasma (100 nM), because of the release of its main precursor, lysophosphatidylcholine, from activated platelets during coagulation. Autotaxin is a member of the nucleotide pyrophosphatase-phosphodiesterase family, which is secreted by many different cell types but mainly platelets and adipocytes into the extracellular space, although it is also present in cerebrospinal and seminal fluids and many other tissues, including cancer cell lines from which it was first isolated and characterized. Indeed, the name derives from the finding that it promotes chemotaxis on melanoma cells in an autocrine fashion. Lysophosphatidic acid binds to target cells via integrin and heparan sulfate proteoglycans, and this may assist delivery to its receptors. Both genetic deletion and overexpression of the enzyme in mice result in aberrant vascular and neuronal development and soon lead to death of the embryos.
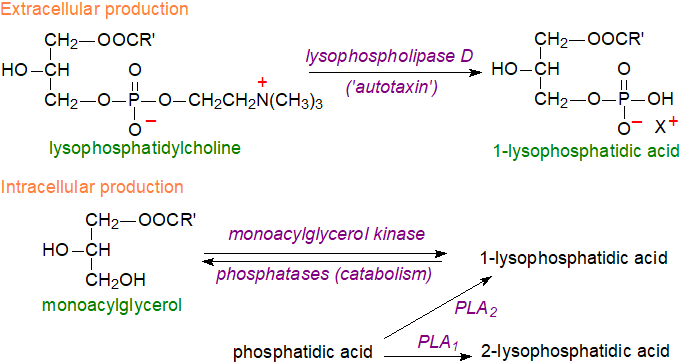 |
| Figure 7. Biosynthesis of lysophosphatidic acid. |
While autotaxin is the primary source of extracellular lysophosphatidic acid, it is now established that it is produced intracellularly in a wide variety of cell types by various mechanisms often with phosphatidic acid, derived from other phospholipids by the action of phospholipase D, as the immediate precursor. Hydrolysis of phosphatidic acid by a phospholipase A2 (PLA2) is the main mechanism in platelets, but other cellular enzymes that are relevant include a phosphatidic acid-selective phospholipase A1 (PLA1) producing sn‑2‑acyl-lysophosphatidic acid, a monoacylglycerol kinase (utilizing monoacylglycerols produced by the action of lipid phosphate phosphatases), and glycerol-3-phosphate acyltransferase (the first step in phosphatidic acid biosynthesis). Upon stimulation by pro-inflammatory cytokines, secretory PLA2-IIA (sPLA2‑IIA) can induce the release of LPA from phosphatidic acid exposed on the surface of extracellular vesicles derived from platelets and Ca2+‑loaded erythrocytes.
General function: Although lysophospholipids are relatively small molecules, they carry a high content of information through the nature of the phosphate head group, the positional distribution of the fatty acids on the glycerol moiety, the presence of ether or ester linkages to the glycerol backbone, and the chain-length and degree and position of unsaturation of the fatty acyl chains. Lysophosphatidic acid acts upon nearly all cell types, often as a proliferative and pro-survival signal, inducing cellular invasion, migration and differentiation, while stimulating smooth muscle and fibroblast contraction, cytoskeletal rearrangement, secretion of cytokines/chemokines and numerous other effects. The 1-O-alkyl- and alkenyl-ether forms, which can be derived from platelet-activating factor and other phospholipids, act in much the same way. On the other hand, it is possible that much of the lysophosphatidic acid produced intracellularly is used for synthesis of other phospholipids rather than for signalling purposes.
Receptors: The informational content of the lysophosphatidic acid molecule leads to selectivity in the relationship with cell receptors. At least six G protein-coupled receptors that are specific for lysophosphatidic acid have now been identified in vertebrates and designated LPAR1 to LPAR6, each found in particular organs and coupled to at least one or more of the heterotrimeric Gα to induce cellular responses; LPAR1 couples to Gαi, Gαq, and Gα13, for example, depending upon tissue and context. These receptors vary appreciably in amino acid sequences but are classified into two subgroups, the endothelial differentiation gene (EDG) family (LPAR1-3) and the non-EDG (or P2Y) family (LPAR4-6), with differing tissue distributions and with most cell types expressing them in different combinations, although LPAR1 is virtually ubiquitous in tissues. Lysophosphatidic acid with an acyl chain at the sn-2 position (2‑acyl-LPA) is preferred to 1-acyl-LPA as ligand for LPAR3 and LPAR6. Among other receptors, there is some interaction with transient receptor potential cation channel V1 (TRPV1), epidermal growth factor receptor (EGFR), and the transcription factors to integrate its signalling with broader cellular networks. Plasma lysophosphatidic acid interacts with its receptors while it is bound to albumin.
 Characterization
of the cloned lysophosphatidic acid receptors in combination with strategies of molecular genetics has allowed determination of signalling
and other effects that are dependent on receptor mechanisms, and it is evident that a range of downstream signalling cascades are mediated
by lysophosphatidic acid through the LPAR receptors.
These include activation of adenylyl cyclase, cAMP production, intracellular Ca2+ and K+ production (via ion
channels), protein kinases, phospholipase C, phosphatidylinositol 3-kinase, small GTPases (Ras, Rho, Rac), release of arachidonic acid,
and much more.
In this way, lysophosphatidic acid regulates cell survival, proliferation, cytoskeleton re-arrangement, motility, cytokine secretion,
cell differentiation and many other vital cellular processes - too many to describe in detail here.
Sometimes, lysophosphatidic acid appears to work in contradictory ways, and there is evidence that it is a factor in cell survival
in some circumstances and in programmed cell death in others.
Characterization
of the cloned lysophosphatidic acid receptors in combination with strategies of molecular genetics has allowed determination of signalling
and other effects that are dependent on receptor mechanisms, and it is evident that a range of downstream signalling cascades are mediated
by lysophosphatidic acid through the LPAR receptors.
These include activation of adenylyl cyclase, cAMP production, intracellular Ca2+ and K+ production (via ion
channels), protein kinases, phospholipase C, phosphatidylinositol 3-kinase, small GTPases (Ras, Rho, Rac), release of arachidonic acid,
and much more.
In this way, lysophosphatidic acid regulates cell survival, proliferation, cytoskeleton re-arrangement, motility, cytokine secretion,
cell differentiation and many other vital cellular processes - too many to describe in detail here.
Sometimes, lysophosphatidic acid appears to work in contradictory ways, and there is evidence that it is a factor in cell survival
in some circumstances and in programmed cell death in others.
Normal physiology: Signalling by lysophosphatidic acid regulates innumerable basic physiological functions from embryogenesis to tissue repair and immune modulation, although much research is now concerned with its influence upon various disease states. Signalling by lysophosphatidic acid is a regulatory factor in the mammalian reproductive system by facilitating oocyte maturation and embryo implantation in females and spermatogenesis in males through the action of the receptors LPAR1 to LPAR3. During early gestation, it regulates vascular remodelling at the maternal-foetal interface, and it is involved in embryonic brain development through its activity in neural progenitor cells, neurons and glia and in vascular remodelling. In the central nervous system, LPAR1 plays a central role in both triggering and maintaining neuropathic pain by mechanisms that may include demyelination of damaged nerves.
Lysophosphatidic acid has been found in saliva in significant amounts, and it has been suggested that it is involved in wound healing in the upper digestive organs such as the mouth, pharynx and oesophagus. When applied topically to skin wounds, it has similar effects by stimulating proliferation of new cells to seal the wound. After bone injury, it facilitates intercellular remodelling and enhances osteoclastogenesis and bone resorption via LPAR1/3. Receptor LPAR6 together with the phospholipase A1 is required for the regulation of the endothelial blood-brain barrier and for the development of hair follicles. The proliferation and survival of stem cells and their progenitors is regulated by lysophosphatidic acid signalling, and while acting via LPAR1 in bone cells, lysophosphatidic acid is required for bone mineralization and repair. LPAR6 in lung and LPAR1 in kidney and the skin are employed in multiple steps in the progression of fibrosis.
Cancer: There is great interest in the influence of lysophosphatidic acid on various disease states and cancer especially, as increased expression of autotaxin and subsequent increased levels of lysophosphatidic acid have been reported in several primary tumours. One important finding is that lysophosphatidic acid is markedly elevated in the plasma and peritoneal fluid (ascites) of ovarian cancer patients compared to healthy controls, and elevated plasma levels in patients in the first stage of the disease suggest that it may be a useful marker for the early detection (the 18:0 and 20:4 species are selectively associated with shorter relapse-free survival). The secretory form of phospholipase A2 acts preferentially on lipids from damaged membranes or microvesicles, such as those produced by malignant cells, and this eventually results in increased levels of lysophosphatidic acid. In turn, this induces the expression of genes for many different enzymes that lead to the proliferation of ovarian and other cancer cells and may induce cell migration via receptors LPAR1 to LPAR3 and possibly LPAR6, while LPAR4 and LPAR5 have opposing effects. Autotaxin and LPARs have been implicated in resistance to chemotherapy and radiation treatment during cancer therapy.
As lysophosphatidic acid has growth-factor-like properties that induce cell proliferation and migration in many cell types, changes in cellular shape and increases of endothelial permeability, it is perhaps not surprising that it is relevant to tumour biology. Treatment of various cancer cell types with lysophosphatidic acid promotes the expression and release of interleukin 8 (IL-8), which is a potent angiogenic factor, i.e. it is critical for embryonic blood vessel formation and for the growth and spread of cancers by enhancing the availability of nutrients and oxygen. There is evidence that signalling by lysophosphatidic acid is causally linked to hyperactive lipogenesis in cancer. For example, it stimulates the sterol regulatory element-binding protein (SREBP), together with the fatty acid synthase and AMP-activated protein kinase–ACC lipogenic cascades that lead to elevated synthesis of new lipids. Increased autotaxin expression has been demonstrated in many different cancer cell lines, and the expression of many of the surface receptors for lysophosphatidic acid in cancer cells is aberrant. Cancer cells must evade the immune system during metastasis, and lysophosphatidic acid facilitates this process by inhibiting the operation of T cells. Therefore, lysophosphatidic acid metabolism is a target of the pharmaceutical industry in the search for new drugs for cancer therapy, aided by a knowledge of the crystal structures of three of the receptors. At least one inhibitor of autotaxin is under evaluation in a phase III clinical trial.
Inflammation: Signalling by lysophosphatidic acid has been implicated in many aspects of chronic inflammation, which it promotes by affecting the endothelium in several ways, such as enhancing endothelial cell migration and the secretion of chemokines-cytokines, and by regulation of the integrity of the endothelial barrier. Problems with lysophosphatidic acid signalling together with changes in autotaxin expression are factors in such metabolic and inflammatory disorders as obesity, insulin resistance, non-alcoholic fatty liver disease, rheumatoid arthritis, multiple sclerosis and cardiovascular disease. Further, there is evidence that it contributes to many neurological disorders, such as Alzheimer's disease and neuropathic pain, and to asthma and bone malfunction. Drugs that interact with the lysophosphatidic acid receptors are reported to attenuate symptoms of several diseases in animal models, and three have passed phase I and II clinical trials for idiopathic pulmonary fibrosis and systemic sclerosis in human patients. Drugs that target autotaxin production and catabolism of lysophosphatidic acid are in development, and the steroidal anti-inflammatory agent, dexamethasone, appears to be of great value.
 Cardiovascular disease: Under certain conditions, lysophosphatidic acid can become athero- and thrombogenic and
can aggravate cardiovascular disease.
As oxidized low-density lipoproteins promote the production of lysophosphatidic acid,
its content in atherosclerotic plaques is high, suggesting that it might serve as a biomarker for cardiovascular disease.
Indeed, lysophosphatidic acid promotes pro-inflammatory events that lead to the development of atheroma
as well encouraging progression of the disease.
By mediating platelet aggregation, it could lead to arterial thrombus formation.
Dysregulation of lysophosphatidic acid metabolism causes changes that affect heart disease with impacts upon obesity cardiomyopathy, and cardiac
mitochondrial dysfunction and injury due to myocardial infarction/ischemia-reperfusion.
Cardiovascular disease: Under certain conditions, lysophosphatidic acid can become athero- and thrombogenic and
can aggravate cardiovascular disease.
As oxidized low-density lipoproteins promote the production of lysophosphatidic acid,
its content in atherosclerotic plaques is high, suggesting that it might serve as a biomarker for cardiovascular disease.
Indeed, lysophosphatidic acid promotes pro-inflammatory events that lead to the development of atheroma
as well encouraging progression of the disease.
By mediating platelet aggregation, it could lead to arterial thrombus formation.
Dysregulation of lysophosphatidic acid metabolism causes changes that affect heart disease with impacts upon obesity cardiomyopathy, and cardiac
mitochondrial dysfunction and injury due to myocardial infarction/ischemia-reperfusion.
Catabolism: Deactivation of lysophosphatidic acid is accomplished by dephosphorylation to produce monoacylglycerols by a family of three lipid phosphate phosphatases (LPP1, 2 and 3), which also dephosphorylate sphingosine-1-phosphate, phosphatidic acid and ceramide 1-phosphate. These are integral membrane proteins with the catalytic site in the plasma membrane facing the extracellular environment, enabling them to access and hydrolyse extracellular lysophosphatidic acid and other phospholipids. Mice with a constitutive LPP3 deficiency are not viable, but this is not true for LPP1 and LPP2 knockout mice.
Lysophosphatidic acid can be converted back to phosphatidic acid, and so rendered inert, by a membrane-bound O-acyltransferase (MBOAT2), which has a preference for lysophosphatidic acid (and lysophosphatidylethanolamine) with oleoyl-CoA as the optimum substrate.
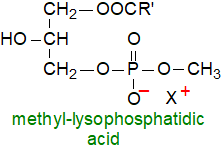 Related lipids: Acute leukaemia cells produce
methyl-lysophosphatidic acids in which the polar head-group is methylated (they have also been found in
hibernating hamsters).
As these act as antigens to which one group of human T cells (CD1c) react strongly, it is possible that they might be a target for the
immunotherapy of haematological malignancies.
Methyl-phosphatidic acids are reportedly formed as artefacts during extraction of bacterial lipids with chloroform-methanol.
Related lipids: Acute leukaemia cells produce
methyl-lysophosphatidic acids in which the polar head-group is methylated (they have also been found in
hibernating hamsters).
As these act as antigens to which one group of human T cells (CD1c) react strongly, it is possible that they might be a target for the
immunotherapy of haematological malignancies.
Methyl-phosphatidic acids are reportedly formed as artefacts during extraction of bacterial lipids with chloroform-methanol.
Other lysophospholipids are known to have biological functions other than as lipid precursors (see separate web pages). The sphingolipid analogue, sphingosine-1-phosphate, resembles lysophosphatidic acid in how it operate in cells, and the two lipids are often discussed together in the same contexts, although they may sometimes have opposing effects. One significant difference is that much (but not all) of the sphingosine-1-phosphate is produced intracellularly, while in the main, lysophosphatidic acid is produced extracellularly for signalling purposes.
6. Cyclic Phosphatidic Acid
 Cyclic phosphatidic acid
(sometimes termed ‘cyclic lysophosphatidic acid or cPA’) was isolated originally from a slime mould, but it has since been detected
in a wide range of organisms including humans in the brain and bound to albumin in serum
(at a concentration of 10-7M, or a tenth that of lysophosphatidic acid), and it is most abundant in tissues subject to injury.
It has a cyclic phosphate at the sn-2 and sn-3 positions of the glycerol carbons,
and this structure is necessary for its activity.
In human serum, the main molecular species contains palmitic acid together with smaller amounts of 18:0, 18:1 and 18:2 fatty acids.
An unusual plasmenylcyclic phosphatidic acid has been isolated from the intestinal bacterium Bifidobacterium longum subs. infantis.
Cyclic phosphatidic acid
(sometimes termed ‘cyclic lysophosphatidic acid or cPA’) was isolated originally from a slime mould, but it has since been detected
in a wide range of organisms including humans in the brain and bound to albumin in serum
(at a concentration of 10-7M, or a tenth that of lysophosphatidic acid), and it is most abundant in tissues subject to injury.
It has a cyclic phosphate at the sn-2 and sn-3 positions of the glycerol carbons,
and this structure is necessary for its activity.
In human serum, the main molecular species contains palmitic acid together with smaller amounts of 18:0, 18:1 and 18:2 fatty acids.
An unusual plasmenylcyclic phosphatidic acid has been isolated from the intestinal bacterium Bifidobacterium longum subs. infantis.
In humans, it has been demonstrated that glycerophosphodiesterase 7 (GDE7), a Ca2+-dependent lysophospholipase D located on the luminal side of the endoplasmic reticulum generates cyclic phosphatidic acid in living cells as well as in a cell-free system, while a study with foetal bovine serum suggest that it can be produced by the enzyme autotaxin, the serum lysophospholipase D that produces lysophosphatidic acid (see above). Bacterial, fungal and spider enzymes akin to sphingomyelinases D catalyse reactions with lysophosphatidycholine and sphingomyelin to generate cyclic phosphates instead of the expected monoester phosphates in serum by intramolecular transphosphatidylation as opposed to hydrolysis. However, cyclic phosphatidic acid can be formed artefactually by the addition of strong acid to serum.
While cyclic phosphatidic acid may signal in an analogous manner to lysophosphatidic acid per se in that it binds to some of the same receptors, it has some quite distinct functions in animal tissues, and for example, it is known to be an inhibitor of DNA polymerase alpha, and it inhibits the platelet aggregation induced by lysophosphatidic acid, possibly by inhibiting autotaxin. It is a high-affinity ligand for the nuclear receptor PPARγ, which is involved in the regulation of adipogenesis, glucose homoeostasis and processes related to type 2 diabetes. Cyclic phosphatidic acid is an inhibitor of cancer cell invasion and metastasis, a finding that is currently attracting great pharmacological interest, and derivatives of cPA, in which the sn-2 or sn‑3 oxygen of the glycerol backbone is replaced by a methylene group ('2- and 3-carba-cPA'), are stable analogues that are being tested for this purpose in clinical trials. In the central nervous system, it can enhance cell survival and neurite extension in neurons, while it is neuroprotective against apoptosis. It is beneficial in an animal model of multiple sclerosis, it attenuates neuropathic pain, and it relieves the symptoms of osteoarthritis. In skin, it improves hydration by stimulating the synthesis of hyaluronic acid, a major component of the extracellular matrix.
7. Pyrophosphatidic Acid
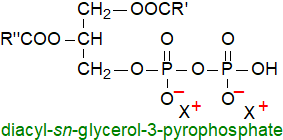 Pyrophosphatidic acid or sn-1,2-diacylglycero-3-pyrophosphate is an unusual and little-known
phospholipid, which was first identified as a minor component in yeasts and is now known to be present in mushrooms and higher plants as a
product of the enzyme phosphatidic acid kinase, which is present in all plant tissues but mainly the plasma membrane.
Pyrophosphatidic acid or sn-1,2-diacylglycero-3-pyrophosphate is an unusual and little-known
phospholipid, which was first identified as a minor component in yeasts and is now known to be present in mushrooms and higher plants as a
product of the enzyme phosphatidic acid kinase, which is present in all plant tissues but mainly the plasma membrane.
It is rapidly metabolized back to phosphatidic acid by a phosphatase and thence to diacylglycerols, and it may be part of the phospholipase D signalling cascade in plants, perhaps by attenuating the effects of phosphatidic acid. Pyrophosphatidic acid is barely detectable in plant cells under normal conditions, but its concentration increases very rapidly in response to stress situations, including osmotic stress and attack by pathogens. Such findings add to the belief that it is a signalling molecule in plants under stress, often in relation to abscisic acid responses. In yeasts, it may have a role in the regulation of the synthesis and metabolism of phospholipids such as phosphatidylserine.
8. Analysis
Phosphatidic acid and related lipids are not the easiest to analyse. On adsorption chromatography, retention times tend to be variable and may be dependent to some extent on the nature of the cations associated with the acidic lipids, but two-dimensional TLC can give good results. Phosphatidic acid, bis(monoacyl)glycerophosphate and pyrophosphatidic acid have never been easy to distinguish, but modern liquid chromatography-mass spectrometric methods appear to be the answer.
Recommended Reading
- Athenstaedt, K. Phosphatidic acid biosynthesis in the model organism yeast Saccharomyces cerevisiae - a survey. Biochim. Biophys. Acta, Lipids, 1866, 158907 (2021); DOI.
- Jiang, S.F., Yang, H.L. and Li, M.Q. Emerging roles of lysophosphatidic acid in macrophages and inflammatory diseases. Int. J. Mol. Res., 24, 12524 (2023); DOI.
- Jose, A. and Kienesberger, P.C. Autotaxin-LPA-LPP3 axis in energy metabolism and metabolic disease. Int. J. Mol. Sci., 22, 9575 (2021); DOI.
- Jose, A., Fernando, J.J. and Kienesberger, P.C. Lysophosphatidic acid metabolism and signaling in heart disease. Can. J. Physiol. Pharmacol., 102, 685-696 (2024); DOI.
- Karalis, T. and Poulogiannis, G. The emerging role of LPA as an oncometabolite. Cells, 13, 629 (2024); DOI.
- Kim, S.-C. and Wang, X. Phosphatidic acid: an emerging versatile class of cellular mediators. Essays Biochem., 64, 533-546 (2020); DOI.
- Kitakaze, K. and others. GDE7 produces cyclic phosphatidic acid in the ER lumen functioning as a lysophospholipid mediator. Communications Biol., 6, 524 (2023); DOI.
- Kolesnikov, Y., Kretynin, S., Bukhonska, Y., Pokotylo, I., Ruelland, E., Martinec, J. and Kravets, V. Phosphatidic acid in plant hormonal signaling: from target proteins to membrane conformations. Int. J. Mol. Sci., 23, 3227 (2022); DOI.
- Lee, J. and Ridgway, N.D. Substrate channeling in the glycerol-3-phosphate pathway regulates the synthesis, storage and secretion of glycerolipids. Biochim. Biophys. Acta, Lipids, 1865, 158438 (2020); DOI - and other articles in this journal issue.
- Liu, Y., Yuan, S. and Wang, X. Phosphatidic acid-mediated signaling. In: Lipid-Mediated Protein Signaling (Adv. Exp. Med. Biol., Vol. 991), pp. 159-176 (edited by D.G.S. Capelluto, Springer Science+Business Media, Dordrecht) (2013); DOI.
- Martin-Salgado, M., Ochoa-Echeverría, A. and Mérida, I. Diacylglycerol kinases: A look into the future of immunotherapy. Adv. Biol. Reg., 91, 1009994 (2024); DOI.
- McDermott, M.I., Wang, Y., Wakelam, M.J.O. and Bankaitis, V.A. Mammalian phospholipase D: Function, and therapeutics. Prog. Lipid Res., 78, 101018 (2020); DOI.
- Nadhan, R., Nath, K., Basu, S., Isidoro, C., Song, Y.S. and Dhanasekaran, D.N. Decoding lysophosphatidic acid signaling in physiology and disease: mapping the multimodal and multinodal signaling networks. Signal Transd. Targ. Therapy, 10, 337 (2025); DOI.
- Ridgway, N.D. and McLeod, R.S. (Editors) Biochemistry of Lipids, Lipoproteins and Membranes (6th Edition). (Elsevier, Amsterdam) (2016) - see Science Direct.
- Strawn, L., Babb, A., Testerink, C. and Kooijman, E.E. The physical chemistry of the enigmatic phospholipid diacylglycerol pyrophosphate. Front. Plant Sci., 3, 40 (2012); DOI.
- Yao, J.W. and Rock, C.O. Exogenous fatty acid metabolism in bacteria. Biochimie, 141, 30-39 (2017); DOI.
- Yao, S.B., Yang, B., Li, J.W., Tang, S., Tang, S.H., Kim, S.C. and Wang, X.M. Phosphatidic acid signaling in modulating plant reproduction and architecture. Plant Commun., 6, 101234 (2025); DOI.
- Yu, J., Loh, K., Song, Z.Y., Yang, H.Q., Zhang, Y. and Lin, S. Update on glycerol-3-phosphate acyltransferases: the roles in the development of insulin resistance. Nutr. Diabetes, 8, 34 (2018); DOI.
- Zhou, H.J., Huo, Y.W., Yang, N. and Wei, T.T. Phosphatidic acid: from biophysical properties to diverse functions. FEBS J., 291, 1870-1885 (2024); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: September 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
