Sterols: 5. Bile Acids and Alcohols
 Bile acids (mainly C24 but also C27) are end products of
cholesterol catabolism in animals, and their best-known function is to act as powerful detergents or
emulsifying agents in the intestines to aid the digestion and absorption of fatty acids, monoacylglycerols and other fatty products, and to
prevent the precipitation of cholesterol in bile.
In addition, it is now recognized that they are hormones with signalling properties in the regulation of multiple metabolic processes,
especially in intestinal epithelial cells, the interface between the body and the luminal contents (including the microbiome), where they
are primary effectors of a host of responses via interactions with receptors.
In addition, they are ligands for receptors in liver cells and other extrahepatic tissues to influence vital metabolic processes, including glucose
and energy metabolism, and by interaction with nuclear receptors, they regulate their own synthesis in the liver.
Bile acids (mainly C24 but also C27) are end products of
cholesterol catabolism in animals, and their best-known function is to act as powerful detergents or
emulsifying agents in the intestines to aid the digestion and absorption of fatty acids, monoacylglycerols and other fatty products, and to
prevent the precipitation of cholesterol in bile.
In addition, it is now recognized that they are hormones with signalling properties in the regulation of multiple metabolic processes,
especially in intestinal epithelial cells, the interface between the body and the luminal contents (including the microbiome), where they
are primary effectors of a host of responses via interactions with receptors.
In addition, they are ligands for receptors in liver cells and other extrahepatic tissues to influence vital metabolic processes, including glucose
and energy metabolism, and by interaction with nuclear receptors, they regulate their own synthesis in the liver.
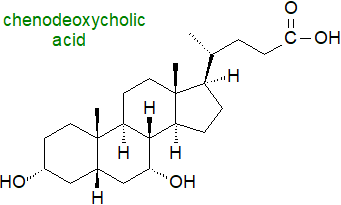
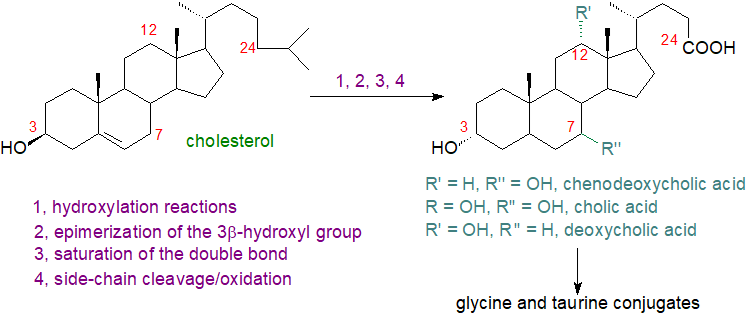
Chenodeoxycholic acid (illustrated) is considered to have the root structure for C24 bile acids in vertebrates, but innumerable different bile acids and alcohols occur in animals, often dependent on species, presumably because multiple enzymatic pathways have evolved to convert cholesterol into these highly water-soluble, amphipathic molecules in the liver. When further modifications by the intestinal microbiome are taken into account, it has become apparent that there are hundreds of known modifications to bile acids and their conjugates and thousands of bile acid-associated genes.
The nomenclature is complex for historical reasons. Many were given trivial names in the 19th century long before their structures were determined, and these continue to be used for reasons of practical convenience; for example, nitrogen-free cholic acid was isolated as long ago as 1838. Although Heinrich Wieland obtained the 1927 Nobel Prize in Chemistry for his work on bile acids, the correct structure of the steroid nucleus was not determined until 1932 by means of the X‑ray diffraction studies of John Desmond Bernal, incidentally the first application of this technique to a biological problem.
1. Structures and Occurrence
Bile acids are a family of steroids with a core structure of seventeen carbon atoms arranged in four fused rings, i.e., three cyclohexane rings (rings A-C) and one cyclopentane ring (ring D), together with a five or eight carbon side chain terminating in a carboxylic acid group (or hydroxyl in the bile alcohols). They contain hydroxyl groups at positions C3, C7 and often C12 and hydrophobic methyl groups at positions C18 and C19. In contrast to most sterols, the junction of the A and B rings of bile acids has a cis or chair configuration. They are sometimes termed 'cholanoids' or 'cholestanoids' and are usually subdivided into three main classification groups, i.e., C27 bile alcohols, C27 bile acids and C24 bile acids, with C27 bile alcohols and acids containing the C8 side chain of cholesterol, while the C24 bile acids have a truncated C5 side chain. Early in this century, 85 unconjugated bile acids (45 C27 and 40 C24) and 25 bile alcohols had been characterized in vertebrates, but novel structures continue to be reported, thanks in part to advances in methodology, and now the numbers are in the hundreds. The trivial names in common use are derived from cholic acid (with an additional hydroxyl on C-12), the first bile acid to be fully characterized, with an element added that is derived from either the chemical structure or the primary animal source.
The three classes of bile acids and alcohols can be considered in terms of ‘default’ structures with hydroxylation of the tetracyclic ring nucleus, i.e., with hydroxyl groups at C-3 (epimerized from the 3β-hydroxyl group of cholesterol) and C-7, together with either a primary alcohol or a carboxyl group at the terminal carbon atom of the side chain. Further substituents can then be added to the default structures, either on a ring or the side chain or on both.
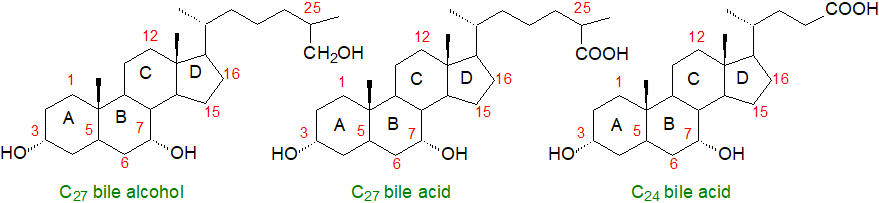 |
| Figure 1. Core structures of bile acids and alcohols. |
In mammals, C24 bile acids predominate, and they are major components of bile amounting to about 12% of the contents (with roughly 4% phospholipids and 1% cholesterol). In non-mammalian vertebrates, such as fish and reptiles, bile alcohols (non-acidic) are formed, while invertebrates do not produce bile acids or alcohols. The main components in human bile are the C24 compounds chenodeoxycholic (45%), deoxycholic and cholic acids (31%) with hydroxyl groups of the 3α,7α-, 3α,12α- and 3α,7α,12α-configurations, respectively. In contrast, bile in mice contains mainly the more hydrophilic muricholic (hydroxylated at the 6β position) and cholic acids, so experimental data from laboratory animals cannot always be extrapolated to humans. Apart from other primates, only hamster bile is similar in composition to that of humans.
Bile alcohols and acids exhibit great structural diversity, with variation occurring in the stereochemistry of the A/B ring juncture of the steroid nucleus, in the sites of hydroxyl or keto groups, and in the orientation of hydroxyl groups, i.e., whether they are α or β to the ring. The length of the side chain can vary, while hydroxyl groups of variable orientation and double bonds can be present or absent, together with differences in the stereochemistry of the C‑25 carbon atom and the site of the carboxyl group. In that there appears to have been a progressive molecular development from C27 alcohols to C27 acids to C24 acids, these structural patterns can be interpreted in terms of relationships between species and families during vertebrate evolution. A 7β-hydroxyl group is present in ursodeoxycholic acid (discussed and illustrated below) found primarily in bears, i.e., it is an epimer of chenodeoxycholic acid derived by flipping the 7α‑hydroxyl group from chenodeoxycholic acid to the β-face to result in very different physicochemical and biological properties, while hyocholic acid (with 3α,6α,7α-hydroxyls) is a major component of pig bile. "Iso-" epimers have the 3‑hydroxyl group in the β-configuration.
The transformation to a cis-fused configuration at the A/B ring junction as illustrated accentuates the change in polarity of the molecule, creating hydrophilic (α) and hydrophobic (β) faces with the hydrophilic hydroxyl groups oriented towards the α-face (concave or lower side), while the hydrophobic methyl groups are oriented towards the β-face (convex or upper side). Bile acids are thus amphipathic molecules and are powerful detergents.
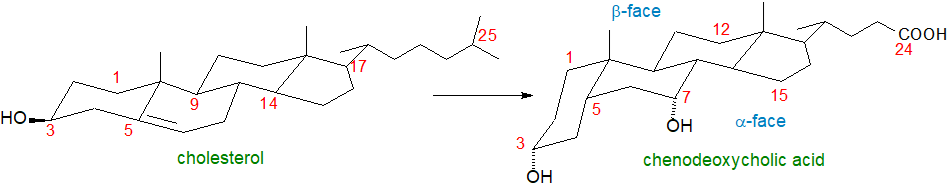 |
| Figure 2. Biosynthesis of bile acids - conformational changes. |
Allo-bile acids in lower vertebrates (fish and reptiles) and a few African mammals are flat because of change in the configuration of the A and B rings (A/B trans-fusion or 5α‑stereochemistry). A few planar bile acids retain the cis-conformation at the A/B ring junction, either because of an alpha arrangement of the C5 hydrogen atom as opposed to the usual beta arrangement or there is a double bond at carbon 5. The most common of these are allo-cholic, 7α,12α-dihydroxy-3-oxochol-4-en-24-oic, 7α-hydroxy-3-oxochol-4-en-24-oic and 12α-hydroxy-3-oxochol-4,6-dien-24-oic acids. Other than certain disease states, these are found only in the healthy human foetus and new-born, pregnant women and some centenarians, but not otherwise in healthy adults. Many further modifications to bile acids occur due to microbial action in the intestines that change the physical and biological properties, often to blur the distinction between the faces.
Bile acid conjugates: A further complication is that much of the bile acids are secreted into bile in the form of conjugates with the carboxyl group and the amino acids taurine and to a lesser extent glycine. There are substantial species differences in the nature of the conjugation, but taurine conjugation is the rule in fish, amphibians, reptiles and birds, and in some mammals such rodents, rats and dogs; C27 bile acids are conjugated exclusively with taurine. Glycine conjugation is typical in herbivores and primates, including humans, although other forms of conjugation occur to a lesser extent, e.g., with glucose or by sulfation, or with N‑acetylglucosamine at C7. In humans, the ratio of glycine to taurine conjugation is about 3 to 1, while in rodents >95% of bile acids are conjugated with taurine. Although the amount of sulfated bile acids in serum and bile tends to be small in humans, they constitute a high proportion (~70%) of those in urine, and this is an important route for detoxification and elimination from the body; bile alcohols conjugated with sulfate have a pKa value like that of taurine conjugates. Formation of 3β-sulfoxy-cholest-5-en-26-oic acid has been demonstrated in hepatocytes in vitro, and this may act as a secretory regulator of lipid metabolism and inflammatory responses. Again, the gut microbiome can alter (and expand) the nature of the conjugation. 3β-Hydroxy-bile acid fatty acid esters have been detected in faecal samples.
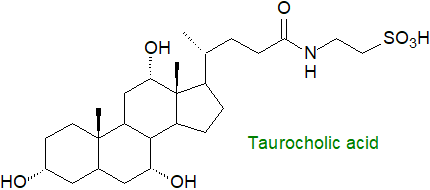
The physical properties of bile acid conjugates are obviously fundamental to their function as this lowers the pKa. Relative to most sterols, the additions increase substantially the acidity of the molecules and their solubility in water. At the physiological pH values in the intestines, bile conjugates ionize and exist in salt form, and they cannot enter the epithelial cells of the biliary tract and small intestines. Their amphipathic nature enables them to form mixed micelles with phosphatidylcholine in solution, and these micelles are relatively stable in the presence of calcium ions.
2. Biosynthesis and Metabolism of Bile Acids
Although there are several different biosynthetic routes to bile acids from cholesterol, there are four main steps, and the liver is the only organ concerned in the production of the primary bile acids. In fact, there are at least 16 enzymes that catalyse up to 17 reactions to convert insoluble cholesterol into a highly soluble conjugated bile salt. At least one transporter and multiple cellular compartments, which includes the cytosol, endoplasmic reticulum, mitochondria and peroxisomes, are involved. What has been termed the ‘classical or neutral’ pathways to the biosynthesis of the ‘root’ bile acid, chenodeoxycholic acid, is the main mechanism in humans under normal physiological conditions and occurs exclusively in hepatocytes. These produce the primary bile acids, while secondary bile acids are formed by microbial action in the intestines as discussed in the next section.
In brief, the first step in the classical pathway is rate limiting and involves the synthesis of 7α-hydroxy-cholesterol by a cholesterol 7α‑hydroxylase (CYP7A1) in the endoplasmic reticulum, as described in our web page on oxysterols. In the next step, epimerization of the 3β‑hydroxyl group is accomplished by a specific oxidoreductase, with 7α-hydroxy-4-cholesten-3-one as an intermediate, before the double bond is hydrogenated by one of two reductases in step 3. These reactions change the configuration of the junction between the steroid rings A and B, resulting in a bend in the molecule. In the last series of reactions, the side chain is oxidized in mitochondria and the resulting 3α,7α‑dihydroxy-5β-cholestanoic acid is converted to the CoA ester in the endoplasmic reticulum for transport into the peroxisomes where the side chain is cleaved by the same enzyme that produces 27-hydroxycholesterol, i.e., sterol-27-hydroxylase (CYP27) (see our web page on oxidized sterols), to remove the three terminal carbons and eventually produce chenodeoxycholic acid.
 |
| Figure 3. Biosynthesis of bile acids by the classical pathway. |
 Synthesis of chenodeoxycholic and cholic acids occurs by a similar route up to a branch point where a sterol
12α‑hydroxylase (CYP8B1 - another of the cytochrome P450 family) introduces a 12‑hydroxyl group into the steroidal side chain
on the way to the latter.
The eventual result is that cholic and chenodeoxycholic acids are produced in approximately equal amounts.
In mice, the formation of muricholates with a 6α- or 6β‑hydroxyl group is catalysed by CYP2c70.
Synthesis of chenodeoxycholic and cholic acids occurs by a similar route up to a branch point where a sterol
12α‑hydroxylase (CYP8B1 - another of the cytochrome P450 family) introduces a 12‑hydroxyl group into the steroidal side chain
on the way to the latter.
The eventual result is that cholic and chenodeoxycholic acids are produced in approximately equal amounts.
In mice, the formation of muricholates with a 6α- or 6β‑hydroxyl group is catalysed by CYP2c70.
An alternative lesser pathway for the synthesis of bile acids is now known to exist that is initiated by 27-, 25- and 24‑sterol hydroxylases and utilizes other oxysterols as the precursors (see our web page on oxysterols). It is often termed the 'acidic pathway' as acidic intermediates are formed when the oxidation of the side chain of cholesterol precedes the modification of the steroid ring. Oxysterol 7α‑hydroxylases are the main enzymes in this second pathway, illustrated for 27‑hydroxycholesterol. In this instance, bile acid synthesis starts in the inner membrane of mitochondria and is catalysed by sterol 27-hydroxylase, but the rate limiting step may be cholesterol transport into the mitochondria of hepatocytes, but also at some extrahepatic sites, including macrophages and endothelial cells. The process continues in the endoplasmic reticulum and the cytoplasm to produce chenodeoxycholic acid. At each step (in both mechanisms), specific transport mechanisms are required that are not fully understood. This biosynthetic pathway produces less than 30% of the bile acids in humans, but the various intermediates are signalling molecules. It has even been suggested that this is their primary role in cells, and that their use in bile may have been a bonus during evolution.
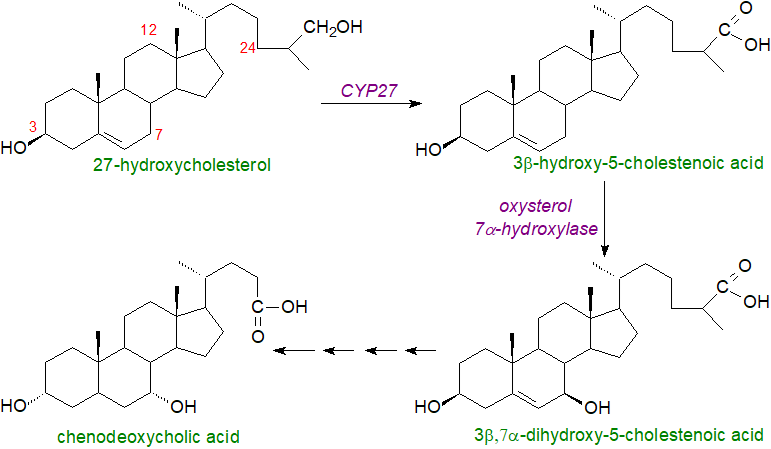 |
| Figure 4. Biosynthesis of bile acids by acidic pathway. |
The presence of planar bile acids in the human foetus and infant may be reflective of less evolved biosynthetic pathways. In patients with the genetic disorder Smith-Lemli-Opitz syndrome, a defect in cholesterol biosynthesis leads to elevated levels of 7-dehydrocholesterol in plasma and thence to a third pathway for bile acid synthesis that proceeds through 7-oxo and 7β-hydroxy intermediates, so avoiding cholesterol, and results in the formation of unusual Δ5‑unsaturated bile acids. This pathway is present in healthy humans to a minor extent, but there is concern for pregnant patients with this syndrome, as some of the intermediates have been detected in amniotic fluid where they have the potential to interfere with hedgehog proteins, which are critical to developmental processes in the infant.
Finally, before secretion into bile, a high proportion of the bile acids produced by both routes is converted in the peroxisomes to conjugates with the amino acids taurine, a sulfonic acid-containing compound derived from cysteine (see our web page on sulfonolipids), or glycine by N‑acylamidation by means of a bile acid:CoA synthase and a bile acid:amino acid transferase.
Regulation of bile acid synthesis involves complex processes, which are linked to the metabolism of cholesterol, retinoids and fatty acids. However, the main control is exerted via the rate-limiting enzyme cholesterol 7α-hydroxylase, the activity of which can be modified by several different pathways that include the action of bile acids and cholesterol on gene transcription via specific receptors (see below).
Enterohepatic circulation and metabolism: Bile acids are stored in the gallbladder and are cycled between the intestines and liver via the enterohepatic circulation, a highly integrated process that enables conservation and recycling of bile acids to maintain a pool for efficient nutrient absorption and for stability in the intestinal microbiota. First, glycine and taurine conjugated bile acids are secreted into the canalicular space between hepatocytes bound to a specific binding protein, and they cross the canalicular membrane in an ATP-dependent fashion by means of a bile salt export pump to enter the bile in the gall bladder (together with phospholipids and cholesterol), where the concentration of bile acids in bile is 100 to 1000 times higher than that in the hepatocytes; this transport against the concentration gradient controls the overall rate of bile acid production. Sulfate and glucuronate conjugated bile acids are secreted at the same time by Multidrug Resistance-Associated Protein 2. Thence after ingestion of a meal and in response to the gut hormone cholecystokinin, the gallbladder is stimulated to contract with relaxation of the sphincter of Oddi and results in the expulsion of bile through bile ducts into the duodenum of the small intestine, where they assist the emulsification, hydrolysis and absorption of the partially hydrolysed lipids from the diet (see our web page on triacylglycerol metabolism).
 At low concentrations,
bile acids are present in a monomeric form, but as their concentration increases, they reach a
'critical micellar concentration', and together with phospholipids they can solubilize triacylglycerols and other non-polar lipids
within in the hydrophobic core of micelles.
These have a diameter up to 30 Å, increasing the surface area and enabling interaction of their lipid contents
with lipases to enhance absorption at the brush border membrane.
By facilitating the binding of pancreatic lipase with its co-lipase, bile acids stimulate lipolysis of triacylglycerols directly.
They may control the growth, digestive capacity and metabolism of the microbial biome in the small intestine, ultimately
with consequences for the physiology and biochemistry of the host, including the metabolic syndrome, obesity and heart failure.
At low concentrations,
bile acids are present in a monomeric form, but as their concentration increases, they reach a
'critical micellar concentration', and together with phospholipids they can solubilize triacylglycerols and other non-polar lipids
within in the hydrophobic core of micelles.
These have a diameter up to 30 Å, increasing the surface area and enabling interaction of their lipid contents
with lipases to enhance absorption at the brush border membrane.
By facilitating the binding of pancreatic lipase with its co-lipase, bile acids stimulate lipolysis of triacylglycerols directly.
They may control the growth, digestive capacity and metabolism of the microbial biome in the small intestine, ultimately
with consequences for the physiology and biochemistry of the host, including the metabolic syndrome, obesity and heart failure.
Microflora in the small intestine de-conjugate a proportion of the bile acids by means of a bile salt hydrolase (BSH), which acts upon a wide range of bile acid conjugates including the six main components of human bile and catalyses the hydrolysis of amide bonds to form the free bile (and amino) acids, a process that continues to near completion in the large bowel. One advantage of this process is that bile acids in free form have enhanced their antimicrobial properties, restricting the growth of Clostridium difficile. Some of these bile acids can be absorbed in the intestines, but some is excreted because deconjugation of bile salts increases their pKa to ~5, making them less soluble and less efficiently reabsorbed.
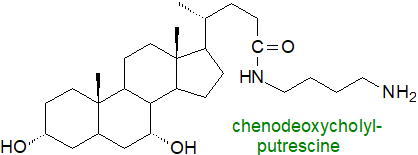 On the other hand, intestinal microorganisms in humans can form conjugates of cholic acid with phenylalanine,
leucine, tyrosine, and other amino acids or amines (e.g. putrescine, cadaverine), which are unlike from those formed within tissues.
These are linked to the carboxylic acid group of the bile acid to form amides (bile acid amidates) in reactions catalysed by
the bile salt hydrolase.
They may have novel involvements in metabolism, including as ligands for receptors, assistance in T cell differentiation, and as
antimicrobial agents.
On the other hand, intestinal microorganisms in humans can form conjugates of cholic acid with phenylalanine,
leucine, tyrosine, and other amino acids or amines (e.g. putrescine, cadaverine), which are unlike from those formed within tissues.
These are linked to the carboxylic acid group of the bile acid to form amides (bile acid amidates) in reactions catalysed by
the bile salt hydrolase.
They may have novel involvements in metabolism, including as ligands for receptors, assistance in T cell differentiation, and as
antimicrobial agents.
Once their main task in assisting the digestion of dietary lipids is completed, much of the non-conjugated bile acids (~95%) are reabsorbed passively throughout the small and large intestines. The more abundant conjugated bile acids require an apical sodium-dependent bile acid transporter in the terminal ileum to cross the brush border membrane of the enterocytes, before they are assisted across the enterocyte by a heterodimer of two proteins, termed organic solute transporters (OSTα and OSTβ), which are responsible for driving bile acids through the basolateral membranes and into the venous blood. They are returned to the liver bound to albumin in the portal blood stream, where they are absorbed by the sodium/taurocholate co‑transporting polypeptide to complete the cycle. The process is continued by the apical bile salt export pump, which transports the bile salts out of the hepatocyte into primary bile against a steep concentration gradient to merge with newly synthesised bile acids in the gallbladder.
In humans, the average pool of bile acids is roughly 2 g, and a conjugated bile salt may complete this cycle from two to six times each day so hepatic secretion into the duodenum is about 12g/day. While only a little of the secondary bile acids is absorbed into tissues, these can accumulate slowly as the human liver is unable to convert deoxycholic acid back to cholic acid. The small proportion of bile acids that avoids hepatic extraction and enters the systemic and portal circulation is dynamic both in amount and molecular species composition, and it follows a meal-dependent and circadian rhythm, which has an appreciable influence upon activation of bile acid receptors.
Efficient enterohepatic cycling ensures that a relatively constant supply of bile acids is available to facilitate lipid digestion and absorption whenever food is ingested. Each stage of the process, from synthesis in the liver to intestinal reabsorption to re-uptake by hepatocytes, is closely regulated by complex signalling pathways between the liver and intestines, with bile acids per se having a key function through their actions on the farnesoid X receptor (FXR) expressed in hepatocytes and epithelial cells lining the intestinal lumen (see below).
In adult humans, roughly 0.5g of cholesterol is utilized for bile acid production each day. It has become evident that the 5% of bile acids that is lost into the faeces represents a significant element of the turnover of cholesterol. Indeed, this is the major pathway for the removal of cholesterol from the body, and it is necessary for the maintenance of cholesterol homeostasis both from quantitative and regulatory standpoints.
Secondary bile acids: 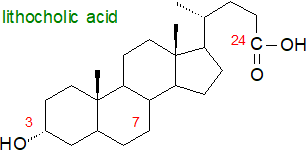 As well as deconjugation, further microbial enzymes (eukaryotic, bacterial
and archaeal) can modify the steroidal structures of bile acids by dehydroxylation, oxidation of hydroxyl groups in positions 3, 7 or 12 to oxo
groups, and epimerization to generate secondary bile acids, of which more than a hundred different forms have been detected in human faeces, all
of which have the potential to interact with the bile acid receptors and influence host metabolism.
It is now recognized that bile acid modification is one of the major bidirectional modes by which the microbiome communicates with its host,
and conversely how the host influences the composition of its symbionts.
As well as deconjugation, further microbial enzymes (eukaryotic, bacterial
and archaeal) can modify the steroidal structures of bile acids by dehydroxylation, oxidation of hydroxyl groups in positions 3, 7 or 12 to oxo
groups, and epimerization to generate secondary bile acids, of which more than a hundred different forms have been detected in human faeces, all
of which have the potential to interact with the bile acid receptors and influence host metabolism.
It is now recognized that bile acid modification is one of the major bidirectional modes by which the microbiome communicates with its host,
and conversely how the host influences the composition of its symbionts.
Clostridium spp. especially act upon the deconjugated bile acids to produce less polar lithocholic from chenodeoxycholic acid and deoxycholic from cholic acid by removing the 7-hydroxyl group (i.e., resulting in 7-deoxy bile acids) as part of a multi-step, bifurcating mechanism that has been termed the Hylemon-Björkhem pathway. Most of these metabolites are absorbed in the colon via passive diffusion, and although some are cytotoxic, they can be detoxified in humans by sulfation and/or glucuronidation, or by oxido-reduction, epimerization and side chain desaturation, to produce modified (or tertiary) bile acids including hyodeoxycholic and ursodeoxycholic acids, with the latter produced by epimerization of chenodeoxycholic acid (and illustrated below). Although epimerization at the C3 hydroxyl group during transit through the intestine can be reversed upon reabsorption and recirculation to the liver, some isodeoxycholic and isolithocholic acids (3β forms) are excreted in faeces as esters of long-chain fatty acids. A balanced pool of primary and secondary bile acids and their conjugates is essential for efficient absorption of lipids from the gut.
Intriguingly, centenarians may have a distinctive gut microbiome that is enriched in microorganisms that can generate unique secondary bile acids, including various isoforms of lithocholic acid (LCA), i.e., iso-, 3-oxo-, allo-, 3-oxoallo- and isoallo-lithocholic acid. These have anti-microbial properties, and of them isoalloLCA is highly potent against Gram-positive microorganisms, including multidrug-resistant pathogens. Lithocholic acid is reported to extend the lifespan of nematodes and fruit flies, but there is no evidence for a similar effect in higher animals, where indeed there can be toxicity problems.
3. The Functions of Bile Acids
Digestion: As discussed above and in our web page on triacylglycerol metabolism, bile acids act as powerful detergents or emulsifying agents in the intestines to aid the hydrolysis of dietary triacylglycerols and other lipids and the subsequent absorption of unesterified fatty acids, cholesterol, monoacylglycerols, fat-soluble vitamins and other nutrients by the intestinal epithelium. However, they participate in many other ways in tissue metabolism, together with modified bile acids produced by bacteria in the gut, which are absorbed and distributed in the bloodstream to other tissues.
Signalling: Bile acids act as signalling molecules that orchestrate blood glucose, lipid and energy metabolism, and in common with most lipid mediators, subtle structural differences have dramatic effects upon their metabolic roles. They are nutrient signalling hormones in the liver and intestines mainly by activating several receptors in the nucleus especially the farnesoid X receptor (FXRα), which forms a heterodimer with the retinoid X receptor alpha (RXRα), and they interact with G‑protein coupled receptors in the plasma membrane, such as the transmembrane G‑protein coupled receptor 5 (TGR5 or GPBAR1). The pregnane-x-receptor (PXR), a xenobiotic sensor, and the vitamin D receptor (VDR) are other nuclear receptors. There are further promiscuous receptors that accommodate bile acids in a non-exclusive manner, including the pregnane X-receptor (PXR), the sphingosine-1-phosphate receptor 2, the muscarinic receptor, and the leukaemia inhibitory factor receptor (LIFR). It is noteworthy that bile acid receptor activation can result in reduced expression of the human NF‑κB-dependent signalling pathway. Through these signalling mechanisms, bile acids regulate the expression of many genes associated with sterol, triacylglycerol and carbohydrate metabolism.
These receptors have selective affinities for different bile acids, for example chenodeoxycholic acid is the most potent stimulator of FXRα, and they exhibit different patterns of expression corresponding to different signalling requirements in tissues. FXR expression is most prevalent in hepatocytes and in the ileum, though it has been found at lower levels in many other tissues including the brain, and bile acids can cross the blood-brain barrier from the circulation to influence brain metabolism with the potential to affect neurological diseases. Both FXR/RXR and the G-protein-coupled bile acid receptors are located at the interface of the host immune system with gut microorganisms, and they are important components of the innate immune system, such as intestinal and liver macrophages, dendritic cells and natural killer T cells, where bile acids may be beneficial.
 Acting via FXRs
(hepatic and intestinal) and various signalling pathways, most bile acids exert a negative feedback regulation
on their own synthesis and enterohepatic circulation, mainly through inhibition of both the activity and expression of the key enzyme CYP7A1.
In collaboration with insulin, they have an influence on the metabolism of lipids and of glucose, and they are involved in the regulation of
triacylglycerol biosynthesis and the production of very-low-density lipoproteins (VLDL) in the liver, thereby lowering the levels of plasma
triacylglycerols.
The FXR/RXRα heterodimer in the distal ileum regulates production of fibroblast growth factor 15/FGF19, a hormone traveling via the
enterohepatic circulation to activate a complex that includes the hepatic FGF receptor 4.
Bile acid signalling via FXR/RXR is a regulator of glucose metabolism, promoting glucose tolerance and insulin sensitivity,
and there are suggestions that modification of bile acid metabolism may be a useful pharmacological approach to the treatment of the metabolic
syndrome, type 2 diabetes and perhaps of atherosclerosis, as the FXR/RXR receptor has a major influence on cholesterol metabolism.
The receptor TGR5 is expressed in many different tissues in addition to the intestines, and the discovery that it was activated by bile acids
led to the realization that they stimulate the production of cAMP and phosphorylation of enzymes relevant to glucose homeostasis and immune
cell regulation with potential impacts upon the control of energy expenditure and on the development of obesity.
Conjugated bile acids activate the sphingosine-1-phosphate receptor-2, which in turn triggers various kinases that impact upon
the regulation of glucose metabolism.
Acting via FXRs
(hepatic and intestinal) and various signalling pathways, most bile acids exert a negative feedback regulation
on their own synthesis and enterohepatic circulation, mainly through inhibition of both the activity and expression of the key enzyme CYP7A1.
In collaboration with insulin, they have an influence on the metabolism of lipids and of glucose, and they are involved in the regulation of
triacylglycerol biosynthesis and the production of very-low-density lipoproteins (VLDL) in the liver, thereby lowering the levels of plasma
triacylglycerols.
The FXR/RXRα heterodimer in the distal ileum regulates production of fibroblast growth factor 15/FGF19, a hormone traveling via the
enterohepatic circulation to activate a complex that includes the hepatic FGF receptor 4.
Bile acid signalling via FXR/RXR is a regulator of glucose metabolism, promoting glucose tolerance and insulin sensitivity,
and there are suggestions that modification of bile acid metabolism may be a useful pharmacological approach to the treatment of the metabolic
syndrome, type 2 diabetes and perhaps of atherosclerosis, as the FXR/RXR receptor has a major influence on cholesterol metabolism.
The receptor TGR5 is expressed in many different tissues in addition to the intestines, and the discovery that it was activated by bile acids
led to the realization that they stimulate the production of cAMP and phosphorylation of enzymes relevant to glucose homeostasis and immune
cell regulation with potential impacts upon the control of energy expenditure and on the development of obesity.
Conjugated bile acids activate the sphingosine-1-phosphate receptor-2, which in turn triggers various kinases that impact upon
the regulation of glucose metabolism.
Bile acids participate in the processes of apoptosis and cell survival, and they influence calcium mobilization, cyclic AMP synthesis and protein kinase C activation via their interactions with receptors. Intestinal cells undergo a continuous process of apoptosis and regeneration, and in general, hydrophobic bile acids, such as lithocholic, are considered to be proapoptotic, while the relatively hydrophilic ursodeoxycholic acid is anti-apoptotic. As a xenobiotic sensor, PXR promotes the transcription of detoxifying enzymes, and it is activated by high levels of bile acids to assist FXR to regulate bile acid synthesis, possibly to prevent liver damage, while activation of the vitamin D receptor in the intestines induces the cytochrome P450 enzyme CYP3A4, which detoxifies excess bile acids.
Bile acids stimulate the proliferation and differentiation of osteoblasts by activating FXR and TGR5, which modulate signal pathways and gene expression in bone cells, ultimately leading to an increase in bone formation. By inhibiting osteoclasts and reducing articular cartilage degradation, they facilitate bone repair.
Other functions: Most of the enzymes for bile acid synthesis play multiple roles in intermediary metabolism. Some are employed in the production of oxysterols, others act on intermediates in hormone biosynthesis, some metabolize very-long-chain fatty acids such as dietary pristanic acid, and another is utilized in vitamin D synthesis. Bile acids bind with high affinity in a hydrophobic pocket in the phosphodiesterase (phospholipase D) responsible for the hydrolysis of N-acyl phosphatidylethanolamine to generate the endocannabinoid anandamide. They stabilize the enzyme to enhance dimer assembly and enable catalysis.
Bile acids and disease: A specific composition of bile acids, including primary versus secondary, conjugated versus unconjugated, and hydroxylated versus non-hydroxylated, may be required to maintain an optimum physiological balance and modulate the pathogenesis of disease. Inefficient biosynthesis and metabolism of bile acids can cause health problems from the neonatal period to adulthood with diverse clinical symptoms that range from cholestatic liver disease to metabolic disorders, intestinal inflammation, and neuropsychiatric symptoms, which include spastic paraplegias. Via receptor-mediated pathways, they influence the progression of metabolic diseases by regulating glucose and lipid metabolism, immune function and energy expenditure. At high concentrations, bile acids are proinflammatory and cytotoxic, and their presence is relevant to the pathogenesis of cancer of the liver, biliary tract and colon and to inflammatory bowel disease. It should be noted that the bile acid profile may be more important than the total concentration, and faecal concentrations of the secondary bile acids 7-deoxycholic and lithocholic acids are elevated in colon cancer and have been correlated with the severity of the disease. The planar bile acids tend to be present in higher concentrations during liver injury and diseases such as cirrhosis. When intestinal bile acid absorption is disrupted, the result is bile acid accumulation in the colon that leads to watery diarrhoea and bile acid loss in the stool (bile acid diarrhoea syndrome or BAD).
Twenty different bile acids have been detected in brain having crossed the blood-brain barrier, where they affect the neurotransmitter receptors, such as the muscarinic acetylcholine and γ-aminobutyric acid receptors, and they influence neurological disorders, such as Alzheimer's, Parkinson's and Huntington's diseases and cognitive disfunction, often in a beneficial manner although this may not always be true for those produced by gut bacteria.
In contrast, the hydrophilic secondary bile acid ursodeoxycholic acid (3α,7β-dihydroxy-5β-cholan-24-oic acid) and its taurine conjugate are used therapeutically for cholesterol gallstone dissolution and in the treatment of primary biliary cirrhosis by stimulating bile flow from the liver. They regulate cholesterol levels by breaking up micelles containing cholesterol in the intestine so reducing the rate of absorption. As this bile acid inhibits apoptosis in epithelial cells, it is being studied for potential benefits in many disease states where apoptosis is deregulated. Ursodeoxycholic acid has been found to be beneficial in improving peripheral blood flow in chronic heart failure patients and in protecting the heart against reperfusion injury, and it is chemo-preventative against cancer by inducing inhibition of proliferation and apoptotic and/or autophagic death of cancer cells (the opposite of its effects in epithelial cells).
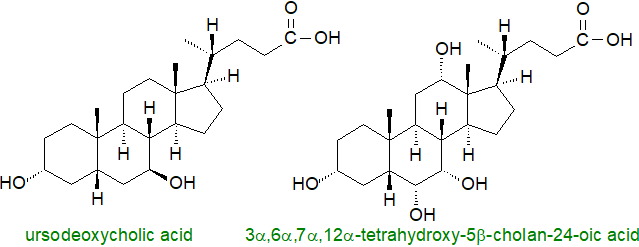
Mice deficient in the Bile Salt Export Pump (Bsep), the primary bile acid transporter in liver cells, produce high levels of tetrahydroxy-bile acids such as 3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid from muricholic acid and thus avoid severe liver damage. Bile acids of this type may have potential in the treatment of cholestatic disease in humans. However, secondary bile acids such as deoxycholate and lithocholate can be cytotoxic to cause oxidative stress, enterohepatic disorders, membrane damage and colonic carcinogenesis in the host.
4. Analysis
For many years, gas chromatography linked to mass spectrometry was the method of choice for the analysis of de-conjugated bile acids, and it is still of value because of the structural information that can be obtained by electron-impact ionization. High-performance liquid chromatography linked to mass spectrometry with electrospray ionization now affords much greater sensitivity when this is required, and this technique is suitable for the analysis of both conjugated and non-conjugated bile acids.
Recommended Reading
- Baiocchi, L. and others. Dual role of bile acids on the biliary epithelium: friend or foe? Int. J. Mol. Sci., 20, 1869 (2019); DOI.
- Choudhuri, S. and Klaassen, C.D. Molecular regulation of bile acid homeostasis. Drug Metab. Disposition, 50, 425-455 (2022); DOI.
- Donkers, J.M., Abbing, R.L.P.R. and van de Graaf, S.F.J. Developments in bile salt based therapies: A critical overview. Biochem. Pharm., 161, 1-13 (2019); DOI.
- Dutta, M., Cai, J.W., Gui, W. and Patterson, A.D. A review of analytical platforms for accurate bile acid measurement. Anal. Bioanal. Chem., 411, 4541-4549 (2019); DOI.
- Evangelakos, I., Heeren, J., Verkade, E. and Kuipers, F. Role of bile acids in inflammatory liver diseases. Seminars Immunopathol., 43, 577-590 (2021); DOI.
- Fan, M.J., Yang, Z.Y., Jin, L.H. and Huang, W.D. Differential roles of individual bile acid in physiology and disease. Pharmacol. Res., 218, 107845 (2025); DOI.
- Fiorucci, S., Distrutti, E., Carino, A., Zampella, A. and Biagioli, M. Bile acids and their receptors in metabolic disorders. Prog. Lipid Res., 82, 101094 (2021); DOI - and also - DOI.
- Fleishman, J.S. and Kumar, S. Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets. Sig. Transduct. Target Ther., 9, 97 (2024); DOI.
- Hanafi, N.I., Mohamed, A.S., Kadir, S.H.S.A. and Othman, M.H.D. Overview of bile acids signaling and perspective on the signal of ursodeoxycholic acid, the most hydrophilic bile acid, in the heart. Biomolecules, 8, 159 (2018); DOI.
- Hurley, M.J., Bates, R., Macnaughtan, J. and Schapira, A.H.V. Bile acids and neurological disease. Pharmacol. Therapeut., 240, 108311 (2022); DOI.
- Kiriyama, Y., Tokumaru, H., Sadamoto, H. and Nochi, H. Biological actions of bile acids via cell surface receptors. Int. J. Mol. Sci., 26, 5004 (2025); DOI.
- Li, J.N. and Dawson, P.A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta, Mol. Basis. Dis., 1865, 895-911 (2019); DOI.
- Macierzanka, A., Torcello-Gómez, A., Jungnickel, C. and Maldonado-Valderrama, J. Bile salts in digestion and transport of lipids. Adv. Colloid Interface Sci., 274, 102045 (2019); DOI.
- Mohanty, I. and others. The underappreciated diversity of bile acid modifications. Cell, 187, 1801-1818.e20 (2024); DOI1 - see also - DOI2.
- Monte, M.J., Fàbrega, L., Romero, M.R., Temprano, A.G., Kaplowitz, N., Garcia-Ruiz, C., Marin, J.J.G. and Fernandez-Checa, J.C. Bile acids in liver and gastrointestinal cancer. Seminars Cancer Biol., 116, 45-58 (2025); DOI.
- Ridlon, J.M. and Gaskins, H.R. Another renaissance for bile acid gastrointestinal microbiology. Nat. Rev. Gastroenterol. Hepatol., 21, 348–364 (2024); DOI.
- Wang, B.B., Han, D., Hu, X.Y., Chen, J., Liu, Y.W. and Wu, J. Exploring the role of a novel postbiotic bile acid: Interplay with gut microbiota, modulation of the farnesoid X receptor, and prospects for clinical translation. Microbiol. Res., 287, 127865 (2024); DOI.
- Xie, C., Huang, W.K., Young, R.L., Jones, K.L., Horowitz, M., Rayner, C.K. and Wu, T.Z. Role of bile acids in the regulation of food intake, and their dysregulation in metabolic disease. Nutrients, 13, 1104 (2021); DOI.
- and in relation to the history of the topic -
- Hofmann, A.F. and Hagey, L.R. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J. Lipid Res., 55, 1553-1595 (2014); DOI.
- Ridlon, J.M., Daniel, S.L. and Gaskins, H.R. The Hylemon-Björkhem pathway of bile acid 7-dehydroxylation: history, biochemistry, and microbiology. J. Lipid Res., 64, 100392 (2023); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: January 2026 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
