Long-Chain (Sphingoid) Bases
Long-chain/sphingoid bases are the characteristic and defining structural units of the sphingolipids, which are important membrane constituents and signalling lipids of animals and plants and of a few bacteria (see our Introduction to the topic). These are long-chain aliphatic amines, containing two or three hydroxyl groups and often a characteristic trans-double bond in position 4. To be more precise, they are 2‑amino-1,3-dihydroxy-alkanes or alkenes with (2S,3R)‑erythro stereochemistry, often with various further substituents in the alkyl chain. They are necessary for the physical and biological properties of all complex sphingolipids, but free sphingoid bases are also bioactive and interact with receptors and other target molecules. As discussed below, the mechanisms for biosynthesis of sphingoid bases and of the simple N‑acylated form (ceramides) are intimately linked.
1. Structures and Occurrence
In animal tissues, the most common or abundant of the sphingoid bases is sphingosine ((2S,3R,4E)-2-amino-4-octadecene-1,3-diol) or sphing-4E-enine, i.e., with a C18 aliphatic chain, hydroxyl groups in positions 1 and 3 and an amine group in position 2; the double bond in position 4 has the trans (or E) configuration. This was first characterized in 1947 by Professor Herbert Carter, who was the first to propose the term "sphingolipides” for those lipids containing sphingosine. It is usually accompanied by the saturated analogue dihydrosphingosine (or sphinganine) as a minor component.
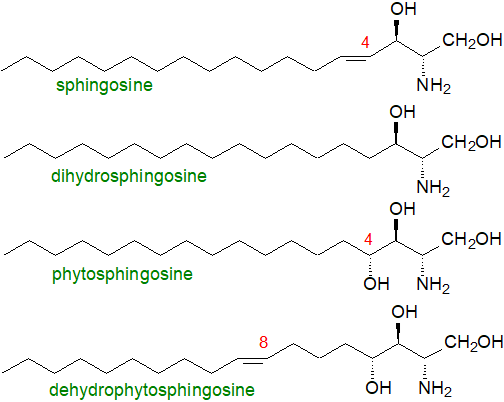 |
| Figure 1. Structures of some sphingoid bases from animals and plants. |
For shorthand purposes, a nomenclature like that for fatty acids can be used as recommended by IUPAC-IUB; the chain length and number of double bonds are denoted in the same manner with the prefix 'd' or 't' to designate di- and trihydroxy bases, respectively. Thus, sphingosine is denoted as d18:1 and phytosphingosine is t18:0. The position of the double bond as in 4-sphingenine may be indicated by a superscript, i.e., as d18:1Δ4t, or by 4E‑d18:1 or d18:1(4E). A modified abbreviation system for use in lipidomic analyses is recommended by Lipid Maps, but other alternative nomenclatures occasionally seen in publications should be avoided.
 The number of
different long-chain bases that has been found in animals, plants and microorganisms now amounts to over one hundred, and many of these may
occur in a single tissue or organism but almost always as part of a complex lipid with an N‑acyl-linked fatty acid and often
phosphate or carbohydrate groups, as opposed to in the free form.
The aliphatic chains can contain from 14 to as many as 28 carbon atoms, and most often they are saturated,
monounsaturated or diunsaturated, with double bonds of either the cis or trans configuration.
In human plasma, the main dienoic long-chain base (sphingadiene) is
D‑erythro-1,3-dihydroxy-2-amino-4E,14Z-octadecadiene (illustrated below), and this is abundant in kidney
with more in women than in men, but it is not present in zebra fish, whcih are used as a model in research.
The number of
different long-chain bases that has been found in animals, plants and microorganisms now amounts to over one hundred, and many of these may
occur in a single tissue or organism but almost always as part of a complex lipid with an N‑acyl-linked fatty acid and often
phosphate or carbohydrate groups, as opposed to in the free form.
The aliphatic chains can contain from 14 to as many as 28 carbon atoms, and most often they are saturated,
monounsaturated or diunsaturated, with double bonds of either the cis or trans configuration.
In human plasma, the main dienoic long-chain base (sphingadiene) is
D‑erythro-1,3-dihydroxy-2-amino-4E,14Z-octadecadiene (illustrated below), and this is abundant in kidney
with more in women than in men, but it is not present in zebra fish, whcih are used as a model in research.
Forms with three double bonds, such as sphinga-4E,8E,10E-trienine, sometimes with a methyl group in position 9, have been found the sphingolipids of some marine invertebrates and in a dinoflagellate. A cytotoxic base isolated from a sponge Haliclona sp., i.e., halisphingosine A, has been characterized as (2R,3R,8R,9Z)-2-amino-octadec-9-ene-1,3,8-triol. In addition, long-chain bases can have branched chains with methyl substituents in the omega‑1 (iso), omega‑2 (anteiso) or other positions, hydroxyl groups in positions 4, 5 or 6, ethoxy groups in position 3, and even a cyclopropane ring in the aliphatic chain in some organisms. N‑Methyl, N,N-dimethyl and N,N,N-trimethyl derivatives of sphingoid bases have been detected in mouse brain, and 1-O-methyl bases can be formed in rat liver homogenates.
The compositions of long-chain bases of sphingomyelins of some animal tissues are listed in Table 2 of our web page on sphingomyelins, where the main C18 components are accompanied by small amounts of C16 to C19 dihydroxy bases, although the latter attain higher proportions in tissues of ruminant animals. Human skin contains a wide range of isomers, including saturated, monoenoic and 6-hydroxy bases and phytosphingosines from C16 to C28 in chain-length, while in gangliosides from human brain and intestinal tissues, eicosasphingosine (2S,3R,4E‑d20:1) occurs in appreciable concentrations with variable amounts in different regions and membranes. Shorter-chain bases are found in many insects, and in the fruit fly, Drosophila melanogaster, which is widely used as a model in genetic and metabolic experiments, the main components are C14 bases. In contrast to higher animals, the nematode Caenorhabditis elegans produces C17 iso-methyl-branched sphingoid bases, which are essential for normal sphingolipid metabolism in the organism.
Long-chain base compositions of individual lipids can vary markedly within a single organelle as well as between species, tissues and organelles. The data in Table 1 is perhaps from an extreme example, but it illustrates that remarkable differences that can exist among lipids in one cellular component, rat liver mitochondria in this instance. Although only part of the data from the paper cited is listed, it illustrates that 3‑keto-sphinganine, produced in the first step of sphingosine biosynthesis and normally a minor component of sphingolipids (often not detectable), can vary from 28 to 100% of the sphingoid bases depending on the lipid class and membrane within the organelle.
Table 1. Long chain base composition of some lipid components of mitochondria from rat liver. |
||||
| Base (%) | ||||
|---|---|---|---|---|
| d18:1 | d18:0-3keto | t21:1 (phyto) | Unidentified | |
| Ceramidesa | 18 | 28 | 53 | - |
| Glucosylceramidesa | 3 | 95 | - | 3 |
| Lactosylceramidesb | 100 | |||
| a whole mitochondria; b mitochondrial inner membrane Data from Ardail, D. et al. FEBS Letts, 488, 160-164 (2001); DOI. |
||||
Phytosphingosine or 4D-hydroxy-sphinganine ((2S,3R,4R)-2-amino-octadecanetriol), i.e., with an additional hydroxyl group in position 4, is a common long-chain base of mainly plant origin. It is a saturated C18-trihydroxy compound, although unsaturated analogues with a trans (or occasionally a cis (Z)) double bond in position 8, i.e., dehydrophytosphingosine or 4D‑hydroxy-8-sphingenine, tend to be much more abundant. In many plant, there are lipid class preferences, and dihydroxy long-chain bases tend to be more enriched in glucosylceramides than in glycosylinositolphosphoceramides, as in the model plant Arabidopsis thaliana, where the data listed for whole tissue is probably representative largely of the latter lipid (Table 2).
Table 2. Sphingolipid long-chain base composition of whole tissue and glucosylceramides from Arabidopsis thaliana. |
||||||
| Base (%) | ||||||
|---|---|---|---|---|---|---|
| t18:1 (8Z) | t18:1 (8E) | t18:0 | d18:1 (8Z) | d18:1 (8E) | d18:0 | |
| Whole tissue | 12 | 70 | 13 | 4 | 1 | |
| Glucosylceramides | 44 | 22 | 5 | 28 | 2 | |
| Data from Sperling, P. et al. Plant Physiol. Biochem., 43, 1031-1038 (2005);
DOI. See Table 2 of our web page on ceramide monohexosides for data on plant species other than Arabidopsis. |
||||||
Other plant long-chain bases have double bonds in position 4, which can be of either the cis or trans configuration, although trans-isomers are by far the more common, while the base d18:2Δ4E,8Z/E is relatively abundant in most plants. In A. thaliana and its relatives Δ4 long-chain bases are found mainly in the flowers and pollen and then exclusively as components of the glucosylceramides. In general, outwith the Brassica family, the composition is dependent on species, but typically it is composed of up to eight different C18‑sphingoid bases, with variable geometry of the double bond in position 8, i.e., (E/Z)-sphing-8-enine (d18:1Δ8), (4E,8E/Z)-sphinga-4,8-dienine (d18:2Δ4,8) and (8E/Z)-4-hydroxy-8-sphingenine (t18:1Δ8); d18:1Δ4, d18:0 and t18:0 tend to be present in small amounts only. In the primitive picoalga Ostreococcus tauri, cis-Δ8-unsaturation only is found.
Phytosphingosine is not restricted to plants but is found in significant amounts in the urinary bladder (with the C20 analogue), intestinal cells and skin of animals, with much smaller relative proportions in kidney. Although non-mammalian sphingoid bases in general tend to be poorly absorbed from the intestines, a small proportion of the phytosphingosine and related sphingoid bases found in animal tissues may enter via the food chain.
Yeasts and fungi tend to have differing and characteristic long-chain base compositions. Filamentous fungi have 9-methyl-4E,8E-sphingadienine as the main sphingoid base in the glucosylceramides but not in the ceramide phosphoinositol glycosides, while yeasts contain mainly the saturated C18 bases sphinganine and phytosphingosine, although some trans-4/8-unsaturated forms are often present. The yeast Wickerhamomyces ciferrii produces appreciable amounts of tetra-acetylphytosphingosine. Only a few bacteria synthesise sphingolipids, but the family Bacteroidetes, which is abundant in the human gut, is an exception, and these usually contain saturated (and iso-methyl-branched) long-chain bases. Other pathogenic bacteria may utilize sphingolipids and sphingoid bases obtained from their hosts.

Sphingoid bases are surface-active amphiphiles with critical micellar concentrations of about 20 μM in aqueous solutions, and they probably exist in the gel phase at physiological temperatures. In that they bear a small positive charge at neutral pH, they are unusual amongst lipids. Although their pKa (9.1) is lower than in simple amines because of intra-molecular hydrogen bonding, together with their relatively high solubility (> 1μM), this enables them to cross membranes or move between membranes with relative ease. In so doing, they increase the permeability of membranes to small solutes. In esterified form in complex lipids, they participate in the formation of ordered lipid domains in membranes such as rafts.
The complex sphingolipids are discussed elsewhere in these web pages, but in most the sphingoid base is first linked via the amine group to a fatty acid, including very-long-chain saturated or monoenoic and 2-hydroxy components to form ceramides, which can then be attached a polar head group, such as phosphate or a carbohydrate, via the primary hydroxyl moiety. Sphingosine-1-phosphate is one exception that is not acylated and is a key signalling molecules in cells, often compared to lysophospholipids (cf., lysophosphatidic acid).
2. Biosynthesis and Metabolism
Biosynthesis of sphingolipids in eukaryotes commences with the biosynthesis of the sphingoid base sphinganine, but subsequent steps, including insertion of double bonds, can occur after they are esterified by fatty acids to form ceramides. Palmitoyl-coenzyme A is the primary substrate, but other fatty acids can be utilized, and these can result in changes in the sphingoid bases produced and in downstream sphingolipid products.
Sphinganine biosynthesis: The basic mechanism for the biosynthesis of sphinganine involves condensation of palmitoyl-CoA with L‑serine, catalysed by the membrane-bound enzyme serine palmitoyltransferase, requiring pyridoxal 5’-phosphate as a cofactor, which binds to a characteristic lysine residue on the enzyme. The reaction occurs on the cytosolic side of the endoplasmic reticulum in animal, plant and yeast cells with formation of 3‑keto-sphinganine. This is the key regulatory or rate-limiting step in sphingolipid biosynthesis that is conserved in all organisms studied to date, and elimination of this enzyme is embryonically fatal in mammals and fruit flies. In mammals, serine palmitoyltransferase is heteromeric and is composed of two main subunits, designated SPTLC1 with either SPTLC2 or SPTLC3 (sometimes termed SPTLC2a and SPTLC2b, respectively). SPTLC1 is necessary for activity, and it is ubiquitously expressed as is SPTLC2, which contains the binding site for pyridoxal 5-phosphate, while SPTLC3 is present in a relatively limited range of tissues and is most abundant in skin and placenta. There are two smaller subunits ssSPTA and ssSPTB in humans, which differ in a single amino acid residue and may be regulatory (multiple forms exist in different organisms); the catalytic site is at the interface between the two main subunits. During development and haematopoiesis, ssSPTA is required by the serine palmitoyltransferase.
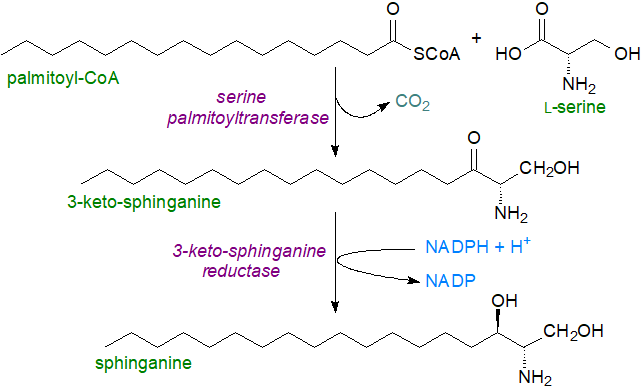 |
| Figure 2. Biosynthesis of sphinganine. |
Addition of either of the two small subunits to the enzyme complexes changes the substrate preferences substantially and enables the synthesis of the wide range of homologues found in nature. In mammals, the SPTLC1-SPTLC2 complex forms C18 sphingoid bases (with some C19 and C20), while the combination of SPTLC1 and SPTLC3 gives a broader product spectrum, including an anteiso-methylbranched-C18 isomer (from anteiso-methyl-palmitate as the precursor). Although such branched bases are synthesised to a limited extent in human skin, they are the main forms in lower invertebrates such as C. elegans. Another requirement is the availability of serine.
The activity of the serine palmitoyltransferase is governed by negative feedback by binding of the central sphingolipid metabolite ceramide to orosomucoid proteins (ORM-like or ORMDL), three in mammals (ORMDL1 to 3) and two in yeast (Orm1/2), which are ubiquitously expressed trans-membrane proteins that are components of a conserved holoenzyme complex ('SPOTs'). Together with the influence of SPTLC1-SPTLC2, this is a mechanism for homeostatic regulation of sphingolipid biosynthesis; longer-term regulation involves ORMDL removal from the complex for trafficking and degradation. In yeast, these proteins are regulated by phosphorylation of serine residues in the amino terminus of Orm, but in animals there is a different regulatory mechanism, and ceramide bound to the SPT/ORMDL complex may trigger inhibition. In animals, the 'sphingomyelin synthase-related protein' is a further regulator of serine palmitoyltransferase.
In plants, serine palmitoyltransferase is a heterodimer composed of LCB1 and LCB2 subunits with some homology to the mammalian enzymes, while in the yeast Saccharomyces cerevisiae, there are three subunits: Lcb1, Lcb2 and Tsc3. The enzyme in the apicomplexan parasite Toxoplasma gondii is a homodimer in contrast to other eukaryotes, but it is located in the endoplasmic reticulum. Serine palmitoyltransferase is inhibited by binding of ceramides to the ORM proteins, and in effect, this is a sensor for intracellular ceramide concentrations that regulates sphingolipid homeostasis. In those bacteria that produce sphingolipids, this enzyme is also a homodimer but is located in the cytoplasm in the gram-negative bacterium Sphingomonas paucimobilis.
 The
second step in sphinganine biosynthesis is reduction of the 3-keto group to a hydroxyl in an NADPH-dependent manner
by a 3‑ketodihydrosphingosine reductase ('3KDSR'), also on the cytosolic side of the endoplasmic reticulum,
a step that must occur rapidly as the intermediate is rarely encountered in tissues.
The enzymes are presumed to be in similar subcellular locations in plant cells.
The
second step in sphinganine biosynthesis is reduction of the 3-keto group to a hydroxyl in an NADPH-dependent manner
by a 3‑ketodihydrosphingosine reductase ('3KDSR'), also on the cytosolic side of the endoplasmic reticulum,
a step that must occur rapidly as the intermediate is rarely encountered in tissues.
The enzymes are presumed to be in similar subcellular locations in plant cells.
Sphingosine biosynthesis via ceramide: Free sphinganine formed in this way is rapidly N‑acylated by acyl-coA to form dihydroceramides by dihydroceramide synthases, which in animals are located primarily on the endoplasmic reticulum, presumably on the cytoplasmic surface. Animals, yeasts and plants have multiple isoforms of this enzyme, for which the abbreviated term ‘ceramide synthase’ is now widely applied as they utilize most other sphingoid bases as substrates, including those produced by hydrolysis of sphingolipids. They are unique gene products with each located on a different chromosome and with considerable variation in the expression of the enzymes in different cell types within tissues, but all have six membrane spanning regions with the only substantial differences in an 11-residue sequence in a loop between the last two putative transmembrane domains. Each isoenzyme has specificities for the chain-length of the fatty acyl-CoA moieties but to a limited extent only for the base, suggesting that ceramides containing different fatty acids have differing roles in cellular physiology. As ceramides are central to all elements of sphingolipid biochemistry, there is much more discussion of their occurrence, biosynthesis and metabolism in a web page dedicated to this lipid.
Humans and mice have six ceramide synthases, which utilize subsets of acyl-CoAs and thus produce ceramides with defined acyl chain lengths. Of these, ceramide synthase 2 is most abundant in lung, liver and kidney and is specific for coA esters of very-long-chain fatty acids (C20 to C26). Ceramide synthase 1 utilizes 18:0 and is located mainly in brain with lower levels in skeletal muscle and testes. Ceramide synthase 3 is responsible for the unusual ceramides of skin and testes and uses C26-CoA and higher including polyunsaturated-CoAs with the latter tissue, while ceramide synthase 4 (skin, liver, heart, adipose tissue and leukocytes) uses C18 to C22-CoAs. Ceramide synthases 5 (lung epithelia and brain grey and white matter) generates C16 (mainly) and C18 ceramides, and ceramide synthase 6 (intestine, kidney and lymph nodes) produces C14 and C16 ceramides. As hydroxylation and the presence or otherwise of double bonds in the acyl-CoAs do not influence the selectivity of the ceramide synthases, and the expression of mRNA expression for ceramide synthases does not always correlate with the fatty acid composition of sphingolipids in a particular tissue, it is evident that other factors determine which molecular species are formed. One such is acyl-coenzyme A-binding protein (ACBP), which facilitates the synthesis of ceramides containing very-long fatty acids and stimulates ceramide synthases 2 and 3. Regulation of the ceramide synthases is complex and involves gene transcription, epigenetic modifications, post-translational modification and phosphorylation.
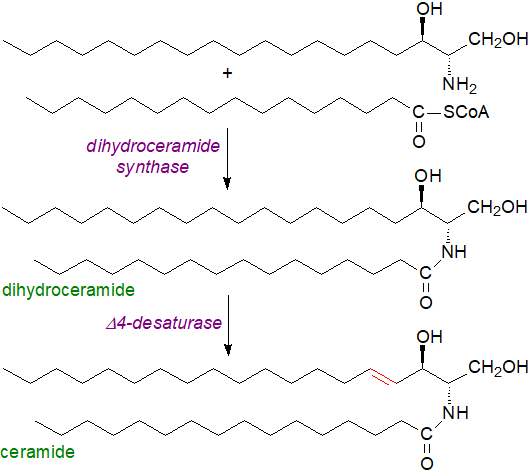 |
| Figure 3. Introduction of double bond into long-chain bases via ceramide. |
Insertion of the trans-double bond in position 4 to produce sphingosine occurs only after the sphinganine has been esterified in this way to form a ceramide as illustrated above, with desaturation occurring at the cytosolic surface of the endoplasmic reticulum. The desaturases were first characterized in plants, and this subsequently simplified the isolation of the appropriate enzymes in humans and other organisms. Two dihydroceramide desaturases have now been identified in animals and designated 'DEGS1' and 'DEGS2', and both insert trans double bonds in position 4, but DEGS2 is a dual function enzyme that acts as a hydroxylase to generate phytoceramides, i.e., to add a hydroxyl group on position 4. Distribution of the enzymes in tissues is very different, with DEGS1 expressed ubiquitously but highest in liver, brain, Harderian gland, kidney and lung, while DEGS2 expression is largely restricted to skin, intestine and kidney, where phytoceramides are more abundant. An early study by W. Stoffel and colleagues demonstrated that Δ4‑desaturation starts with syn-removal of the C(4)- HR and then the C(5)-HS hydrogens, the first evidence that desaturases in general operate in this stepwise fashion.
The enzyme responsible for the insertion of the cis-14 double bond to produce sphinga-4-trans,14-cis-dienine is the fatty acid desaturase 3 (FADS3), which utilizes ceramides containing sphingosine as the precursor and not free spingoid bases. In this case, FADS3 exhibits specificity with respect to the chain length and branching of the sphingoid base, but not that of the fatty acid moiety of the ceramide, which is eventually directed to sphingomyelin synthesis mainly. This enzyme is also known to insert a cis-double bond in position 13 of the CoA ester of vaccenic acid (11E-18:1) to produce the conjugated diene 11E,13Z-18:2.

Synthesis of sphingoid bases de novo is essential in most eukaryotes, and inhibition of the biosynthetic pathways affects growth and viability. In mice, this can depend upon tissue, as deletion of the liver SPTLC2, was found to have no effect on liver metabolism, while a comparable deletion of adipocyte SPTLC1 caused major tissue defects. Presumably, the latter tissue is unable to take up enough sphingolipid from the circulation to remedy the problem. Deficiencies in SPTLC3 are related to dermal pathologies, and genetic variants of SPTLC3 are associated with dyslipidemia and atherosclerosis. In the nervous system, deficiencies in DEGS1 can lead to an accumulation of dihydroceramides and neuronal dysfunction, while a further metabolite is produced by insertion of a cis-double bond in position 14 of sphingosine by FADS3 to produce d18:1(14Z) (or 18:1(14Z);O2), regarded as a biomarker for DEGS1-related hypomyelinating leukodystrophy.
Phytosphingosine and plant sphingoid bases: Phytosphingosine or 4D-hydroxy-sphinganine is formed from sphinganine, produced as above, by hydroxylation in position 4, possibly via the free base in plants, although in yeasts, it can be formed both from sphinganine and a ceramide substrate. While a single sphinganine C4‑hydroxylase is present in yeast, Arabidopsis has two such enzymes (SBH1 and 2), which are critical for growth and viability. Much remains to be learned of these processes, but it is known that the enzyme responsible is closely related to a Δ4 desaturase, and it has been demonstrated that there are bifunctional Δ4‑desaturase/Δ4-hydroxylases in Candida albicans and mammals, especially in keratinocytes (DEGS2 discussed above) with which either 4‑hydroxylation or Δ4‑desaturation is initiated by removal of the proR C-4 hydrogen. In mice and humans, dihydroceramide:sphinganine C4‑hydroxylase is expressed exclusively in urothelial cells of the urinary bladder, and sphinganine linked to ceramide is the substrate for 4‑hydroxylations.
In the leaves of Arabidopsis thaliana, 90% of the sphingoid bases are phytosphingosine with a Δ8E‑double bond. In plants in general, in addition to Δ4‑desaturation, two further types (20 gene products) of sphingoid Δ8-desaturase have been characterized that catalyse the introduction of a double bond at position 8,9 of phytosphingosine. These are evolutionarily distinct from the Δ4‑desaturases, and one type produces the trans (E)-8 isomer mainly and the other mostly the cis (Z)-8 isomer, with overall the trans-isomer tending to predominate but dependent upon plant species. The trans isomer is formed when the hydrogen on carbon 8 is removed first, and the cis when carbon 9 is the point of attack. While the main group of Δ8‑desaturases requires a 4‑hydroxysphinganine moiety as substrate, the second does not.
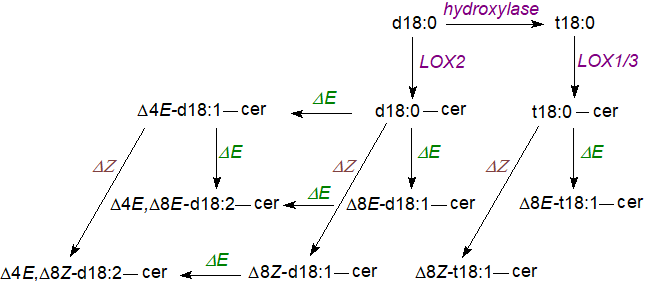 |
| Figure 4. Ceramide formation and desaturation of sphingoid bases in plants (some of the desaturases are species specific). |
Three different isoforms of ceramide synthase have been identified in Arabidopsis and denoted LOH1, LOH2 and LOH3. Phytosphingosine is used efficiently by LOH1 and LOH3 (class II synthases), but only LOH2 (class I synthase) uses sphinganine efficiently; LOH2 and 3 prefer unsaturated long-chain bases. Marked fatty acid specificity is observed with LOH2 using only palmitoyl-CoA and dihydroxy bases, while LOH1 shows greatest activity for 24:0- and 26:0-CoAs and trihydroxy bases; none utilize unsaturated acyl-CoA esters efficiently. In plants, fatty acid desaturases and hydroxylases are closely related, and sphingolipid fatty acid α‑hydroxylation occurs on the ceramide, as opposed to the free acyl chain (two hydroxylases, FAH1/2 occur in rice) . The Δ8‑desaturase utilizes ceramide as the substrate and the channels the products selectively into the synthesis of highly complex sphingolipids, while Δ4‑desaturation channels ceramides for synthesis of glucosylceramide. The free ceramide pool of leaves has a very different sphingoid base composition in comparison with complex sphingolipids and consists of roughly equimolar amounts of t18:1Δ8E and t18:0.
It has been established that long-chain bases with 4-hydroxyl groups are necessary for the viability of the filamentous fungus Aspergillus nidulans and for growth in plants such as A. thaliana. The presence of an 8E double bond confers aluminium tolerance to yeasts and plants, and it is important for chilling resistance in tomatoes, but a trans-4 double bond in the sphingoid base is not needed for growth and development in Arabidopsis.
Fungal sphingoid bases: Fungi produce trans Δ8-isomers only, but Δ4- and Δ8-desaturases do not occur in the widely studied yeast S. cerevisiae. In the biosynthesis of sphingoid bases in fungi, the double bonds in positions 4 and 8 and the characteristic methyl group in position 9 are inserted sequentially into the sphinganine portion of a ceramide, the last by means of a methyltransferase similar to plant and bacterial cyclopropane fatty acid synthases.
In S. cerevisiae the ceramide synthase is a heteromeric protein complex, containing three subunits, Lag1, Lac1 and Lip1, of which the first two are homologous proteins that feature eight transmembrane domains. In the yeast Pichia pastoris, there is a distinct ceramide synthase, which utilizes dihydroxy sphingoid bases and C16/C18 acyl-coenzyme A as substrates to produce ceramides. The long-chain-base components of the ceramide are then desaturated in situ by a Δ4‑desaturase and the fatty acid components are hydroxylated in position 2. Further desaturation of the long-chain base component by a Δ8-(trans)-desaturase occurs before the methyl group in position 9 is introduced by an S-adenosylmethionine-dependent sphingolipid C-9 methyltransferase. As a final step, a trans-double bond may be introduced into position 3 of the fatty acid component. These ceramides are used exclusively for the production of glucosylceramides, and a separate ceramide synthase encoded by a different gene produces the ceramide precursors for ceramide phosphorylinositol mannosides.
Bacterial sphingoid bases: Genomic and biochemical studies have identified six proteins in the complete pathway for ceramide synthesis in bacteria found in the human microbiome, starting with the serine palmitoyltransferase and production of 3‑ketodihydrosphinganine, which is esterified by a long-chain fatty acid as such before the keto group is reduced to form the dihydroceramide. Serine palmitoyltransferase and ceramide synthase are in the cytoplasm, while the ceramide reductase is in the periplasmic space. The order of the reactions differs from that in eukaryotes, and phylogenetic analyses suggest that the bacterial and eukaryotic ceramide pathways evolved independently.
Viral sphingoid bases: The genome of a marine virus (EhV) encodes for a novel serine palmitoyltransferase, which hijacks the metabolism of algal hosts to produce unusual hydroxylated C17 sphingoid bases; these accumulate in lytic cells of infected algae such as the bloom-forming Emiliania huxleyi. While this may seem a rather esoteric topic, viruses constitute a high proportion of the marine biome, and their control of the growth of algal blooms has global consequences.
Unesterified sphingosine: A cycle of reactions occurs in tissues by which sphingoid bases are incorporated via ceramide intermediates into complex sphingophospho- and sphingoglycolipids, which are utilized for innumerable purposes before eventually being broken down to their component parts. It is worth noting that all the free sphingosine in tissues must arise from ceramides by this route, in particular by the action of ceramidases, five of which are known with differing pH optima and varying subcellular locations. The levels of free sphingoids and their capacities to act as lipid mediators (see below) are controlled mainly by enzymic re‑acylation to form ceramides, although some is acted upon by sphingosine kinases to produce sphingosine-1-phosphate.
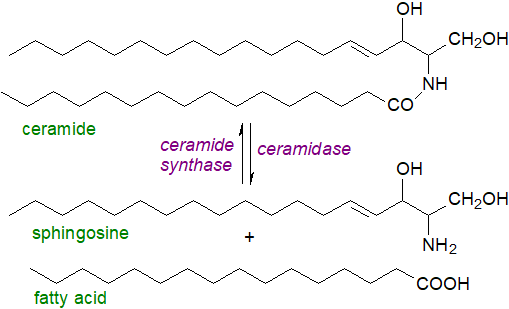 |
| Figure 5. Free sphingosine production from ceramide (and resynthesis). |
Free sphingoid bases are absorbed by enterocytes following digestion of dietary sphingolipids in animals (including some from gut microorganisms), and while some of this is converted to complex sphingolipids, much is catabolized with eventual formation of palmitic acid.
Catabolism of sphingosine and other long-chain bases occurs after conversion to sphingosine-1-phosphate and analogues as discussed in our web page on this metabolite. In yeasts, an alternative means of detoxification has been reported in which an excess of phytosphingosine is first acetylated and then converted to a vinyl ether prior to export from the cells.
3. Functions of Unesterified Sphingoid Bases
Primarily, sphingoid bases serve as basic components of ceramides and complex sphingolipids, where variations in their compositions can influence the physical and biological properties of these lipids. Independently of this in their free (unesterified) form, they are mediators of many cellular events even though they are present at low levels only in tissues (typically 25 and 50 nM in plasma), with intracellular levels determined by hydrolysis by ceramidases or by the action of sphingosine kinases (sphingosine-1-phosphate production). In animal cells, they inhibit protein kinase C indirectly, possibly by a mechanism involving interference with the binding of activators of the enzyme, such as diacylglycerols or phorbol esters. They are involved in the regulation of lipid metabolism and cell growth and survival by their impact upon such enzymes as phosphatidate phosphohydrolase-1 (PAP-1 or lipin), diacylglycerol kinase (DAGK) and phospholipase D (PLD).
In the process of apoptosis, they take part in a manner distinct from that of ceramides by by modulating the phosphorylation of some transcription factors, and sphingosine has been shown to stimulate a sphingosine-activated protein kinase in cultured rat hippocampal neurons and astrocytes to trigger apoptosis. Sphingoid bases are known to be potent inhibitors of cell growth, although they induce cell proliferation and DNA synthesis. By regulating ion channels in the plasma membrane, such as the transient receptor potential melastatin channel, sphingosine influences sensory perception and other cellular processes. While sphingosine does not participate in raft formation in membranes, it may rigidify pre-existing gel domains in mixed bilayers, although any such effects will be dependent on local concentrations and pH. It should be noted that some experimental observations may be due to conversion to sphingosine-1-phosphate.
 Some human
neurodegenerative and neurodevelopmental disorders due to defective serine palmitoyltransferase are known.
Free sphingosine has been implicated in various pathological conditions, and for example, plasma sphingosine levels are increased
in hyperthyroidism and in patients with type 2 diabetes.
Lysosomal storage of the lipid is an initiating factor in Niemann Pick type C disease, a neurodegenerative disorder,
where it causes a change in calcium release leading to a build-up of cholesterol and sphingolipids.
In the human adrenal cortex, sphingosine produced in situ by the acid ceramidase
is required for steroid production by serving at the cell nucleus as a ligand for steroidogenic factor 1,
which controls the transcription of genes for the conversion of cholesterol to steroid hormones.
Some human
neurodegenerative and neurodevelopmental disorders due to defective serine palmitoyltransferase are known.
Free sphingosine has been implicated in various pathological conditions, and for example, plasma sphingosine levels are increased
in hyperthyroidism and in patients with type 2 diabetes.
Lysosomal storage of the lipid is an initiating factor in Niemann Pick type C disease, a neurodegenerative disorder,
where it causes a change in calcium release leading to a build-up of cholesterol and sphingolipids.
In the human adrenal cortex, sphingosine produced in situ by the acid ceramidase
is required for steroid production by serving at the cell nucleus as a ligand for steroidogenic factor 1,
which controls the transcription of genes for the conversion of cholesterol to steroid hormones.
Unesterified sphingoid bases be protective against cancer of the colon in humans. Thus, N,N‑dimethylsphingosine and dihydrosphingosine, like the deoxysphingoid bases, are known to induce cell death in various types of malignant cells, and there is evidence that sphingadienes of plant and animal origin inhibit colorectal cancer in mouse models by reducing sphingosine-1-phosphate levels. In consequence, synthetic analogues of long-chain bases are being tested for their potential as pharmaceuticals. N,N‑Dimethylsphingosine is of further interest in that it inhibits protein kinase C, sphingosine kinase and many other enzyme systems, while it induces mechanical hypersensitivity to neuropathic pain.
With some bacterial pathogens, sphingosine is reported to be bactericidal due to binding of the protonated NH2 group to the negatively-charged lipid cardiolipin in bacterial plasma membranes so promoting rapid permeabilization of the membrane and thence bacterial cell death. It is also effective against some fungal, e.g., Candida albicans and viral infections, e.g., herpes simplex and COVID-19. In skin, free sphingoid bases derived from ceramide have antimicrobial properties and are mediators of host epithelial defence against bacterial invasion, and they inhibit the effects of bacterial pathogens in pulmonary infections. Free phytosphingosine is anti-proliferative and anti-inflammatory and is important for the maintenance of skin homeostasis. Sphinganine may have functions that differ from those of sphingosine in various disease states that include cardiovascular, liver and kidney diseases, while impaired 3-ketosphinganine metabolism also has implications for human pathology.
In plants, free sphingosine has a signalling role by controlling pH gradients across membranes, and synthesis of sphingoid bases is stimulated by infection with bacterial pathogens as part of the immune response; they are secreted in root exudates where they inhibit pathogenic fungi in soils. In Arabidopsis, the plant pathogenic oomycete Phytophthora infestans triggers a defence response when its 9-methyl-branched sphingoid base is recognized by a plasma membrane lectin receptor-like kinase, RDA2, after hydrolysis of its complex sphingolipids by a plant ceramidase. Free sphingoid bases (and the correct balance with the 1‑phosphate derivatives) are crucial for the regulation of apoptosis in plants.
4. 1-Deoxy-Sphingoid Bases
A few rare genetic disorders have been found in which there is a defect in one of the subunits of the serine palmitoyltransferase or one of the regulatory proteins (ORM), or of ceramide synthesis, and the best known of these results in the formation of sphingoid base analogues that lack the hydroxyl group on carbon 1. In animal cells, 1-deoxysphinganine (2-amino,3-hydroxy-octadecane) can be formed at low levels by condensation of palmitoyl-CoA with L‑alanine catalysed by the serine palmitoyltransferase, especially when cells are depleted in L‑serine. Any unsaturated 1-deoxysphingenine produced has a cis-double bond in position 14 introduced by the FADS3 desaturase as in sphingadiene (see above) and not a trans-double bond in position 4. In the same way, reaction with glycine produces 1‑desoxymethylsphinganine ((2R)‑1‑aminoheptadecan-2-ol or M17:0). Both types of deoxy-base are N-acylated subsequently by ceramide synthases to form ceramide analogues, which can eventually be hydrolysed by ceramidases. Ceramides formed from deoxysphinganine are present in normal cell tissues at low levels, e.g., liver, and in many foods, but they are not usually noticed because they are swamped by the much larger amounts of conventional ceramides. They have also been detected in anaerobic bacteria from the Black Sea.
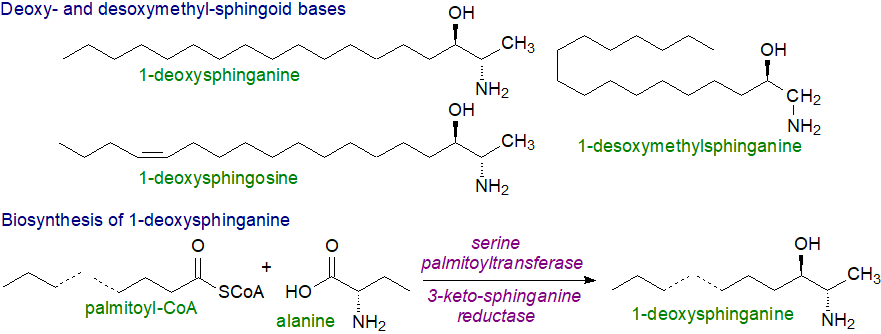 |
| Figure 6. Structures of deoxy- and desoxymethyl-sphingoid bases and biosynthesis of the former. |
In yeasts, one subunit of the serine palmitoyltransferase promotes alanine utilization for deoxy-base formation, while commensal bacteria from the Bacteroidetes phylum produce some deoxy-sphingolipids, which can be absorbed by intestinal cells.
As they lack a terminal hydroxyl group, deoxy-bases cannot form complex ceramide derivatives such as the glycosphingolipids or sphingomyelin. For the same reason, they cannot be catabolized by the normal pathway via sphingosine 1-phosphate, although degradation can be accomplished slowly by the action of cytochrome P4504F enzymes involving hydroxylation and desaturation reactions that produce a number of intermediate metabolites.
There is no known requirement for deoxy-sphingoid bases, and they can be harmful (cytotoxicity) in animals, presumably depending on their degradative capacity; 1‑deoxysphingosine is less toxic than the saturated analogue. In a rare genetic disorder, hereditary sensory and autonomic neuropathy type 1 (HSAN1), such lipids are formed in a reaction with alanine and glycine because of defects in the sub-units of the serine palmitoyltransferase, and they accumulate in vitro in cells treated with the fungal toxin fumonisin B1 (see below). Elevated concentrations have been reported in human serum and visceral adipose tissue, in various tissues of aged mice, and in patients with type 2 diabetes, and plasma levels are prospective biomarkers for the development of this disease; they interfere with the survival of pancreatic β-cells and insulin production. While deoxysphingoid bases are neurotoxic in that they cause mitochondrial fragmentation and dysfunction, promising preliminary results have been obtained with dietary supplements of serine to "compete out” these deleterious effects.
 In
contrast, they may be beneficial as modulators of the activity of NR2F1 and 2, which are orphan nuclear hormone receptors that are
critical for the development of the nervous system, heart, veins and lymphatic vessels.
1‑Deoxy bases inhibit the growth of cancer cells by binding to sphingosine kinase I, and a synthetic phytosphinganoid analogue termed
‘enigmol’ ((2S,3S,5S)-2-amino-3,5-dihydroxyoctadecane), which can be administered orally, has shown promise
against colon and prostate cancer cells in culture.
This and various other sphingoid mimics are under evaluation in phase I clinical trials in cancer patients.
In
contrast, they may be beneficial as modulators of the activity of NR2F1 and 2, which are orphan nuclear hormone receptors that are
critical for the development of the nervous system, heart, veins and lymphatic vessels.
1‑Deoxy bases inhibit the growth of cancer cells by binding to sphingosine kinase I, and a synthetic phytosphinganoid analogue termed
‘enigmol’ ((2S,3S,5S)-2-amino-3,5-dihydroxyoctadecane), which can be administered orally, has shown promise
against colon and prostate cancer cells in culture.
This and various other sphingoid mimics are under evaluation in phase I clinical trials in cancer patients.
5. Unusual Marine Sphingoid Bases and Fungal Toxins
Some plants and animals, especially marine organisms, synthesise long-chain bases lacking the hydroxyl group in position 1 or 2, i.e., they are 1‑deoxy-sphingoid bases, and indeed, 1‑deoxysphinganine per se was first found in a marine organism where it was given the trivial name 'spisulosine'. Among the more unusual of these deoxy bases are the C28 α,ω- or two-headed-sphingoid base-like compounds from sponges, such as calyxinin and oceanin and their β‑glucosides, i.e., calyxoside and oceanapiside, respectively. Some bacteria of the family Bacteroidetes produce sulfonolipids (capnoids), which have structural similarities to the deoxy bases.
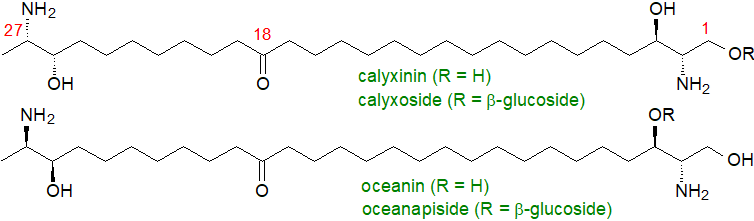 |
| Figure 7. Structures of some unusual sphingoid bases of marine origin. |
Myriocin or 2-amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxo-eicos-6-enoic acid from the thermophilic fungus Isaria sinclairii is a potent inhibitor of serine palmitoyltransferase, the first step in sphingosine biosynthesis, and it is a powerful immunosuppressant. A family of six related molecules from Aspergillus fumigatus and termed sphingofungins has analogous properties. In this species, both sphingoid bases and the sphingofungins are synthesised by some of the same enzymes in the endoplasmic reticulum, ER-derived vesicles and the cytosol, so some mechanism must exist to escape self-poisoning. They differ in the final step, in which there is condensation of a C18 precursor with the uncommon substrate aminomalonate. Via a programme of structural modification, a drug termed ‘fingolimod’ has been developed from myriocin for the treatment of multiple sclerosis, as is discussed further in our web page on sphingosine-1-phosphate.
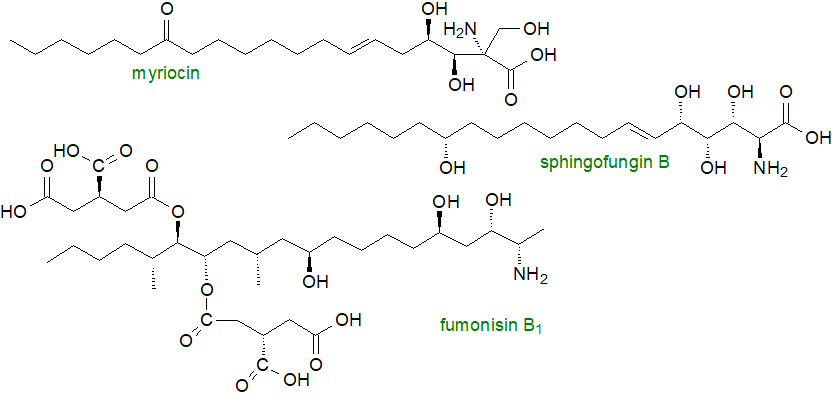 |
| Figure 8. Fungal toxins related to sphingoid bases in structure. |
Fungal toxins that have structural similarities to deoxysphingoid bases, e.g., fumonisin B1 with propane-1,2,3-tricarboxylic acid groups esterified at the C14 and C15 positions (illustrated), are found in maize and other crop plants where they are a cause of disease and can cause cell death. By inhibiting the dihydroceramide synthases, they disturb the synthesis of both the structural and signalling sphingolipids, such that there is reduced formation of dihydroceramides, ceramides and complex sphingolipids, but elevated levels of sphinganine, sphingosine and their 1-phosphates, together with formation of novel 1-deoxy-sphingoid bases. When ingested by humans and other animals, fumonisin can exacerbate or cause several disease states, including oesophageal cancer, non-alcoholic steatohepatitis and diabetes.
6. Analysis
The first step in the analysis of the sphingoid bases of sphingolipids is hydrolysis of any glycosidic linkage or phosphate bonds as well as the amide bond to the fatty acyl group. This should be accomplished by a procedure in which the minimum degradation or rearrangement of the bases occurs, such as artefactual dehydration, rearrangements and O- or N‑methylation. While many analysts claim that base-catalysed hydrolysis causes least disruption, rapid acid-catalysed methods are often preferred for speed and convenience.
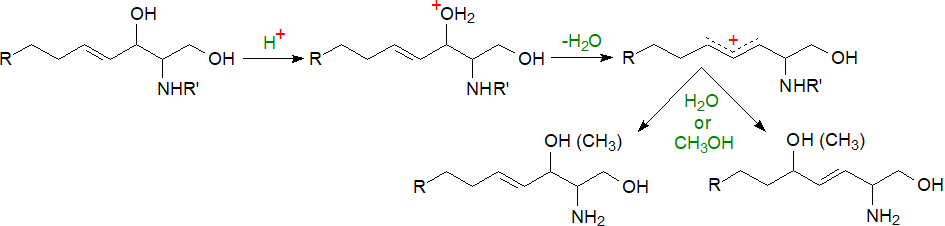 |
| Figure 9. Artefacts formed from sphingoid bases under acid hydrolysis conditions. |
Subsequently, the bases are best analysed by gas chromatography after derivatization to reduce their polarity, although analysis of long-chain bases while in intact sphingolipids by liquid chromatography-mass spectrometry methods is a valuable alternative.
Suggested Reading
- AL Mughram, M.H., Kellogg, GE. and Wattenberg, B.W. Three kingdoms and one ceramide to rule them all. A comparison of the structural basis of ceramide-dependent regulation of sphingolipid biosynthesis in animals, plants, and fungi. Adv. Biol. Reg., 91, 101010 (2024); DOI.
- Carreira, A.C., Santos, T.C., Lone, M.A., Zupančič, E., Lloyd-Evans, E., Almeida, R.F.M., Hornemann, T. and Silva, L.C. Mammalian sphingoid bases: Biophysical, physiological and pathological properties. Prog. Lipid Res., 75, 100988 (2019); DOI.
- Chen, J., Li, Z.M., Cheng, Y., Gao, C.S., Guo, L.T., Wang, T.H. and Xu, J.P. Sphinganine-analog mycotoxins (SAMs): chemical structures, bioactivities, and genetic controls. J. Fungi, 6, 312 (2020); DOI.
- Futerman, A.H. Sphingolipids. In: Biochemistry of Lipids, Lipoproteins and Membranes, 6th Edition, pp. 297-326 (ed. N.D. Ridgeway and R.S. McLeod, Elsevier, Amsterdam) (2016) - see Science Direct - there is now a 7th edition..
- Harrison, P.J., Dunn, T.M. and Campopiano, D.J. Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep., 35, 921-954 (2018); DOI.
- Haslam, T.M. and Feussner, I. Diversity in sphingolipid metabolism across land plants. J. Exp. Botany, 73, 2785-2798 (2022); DOI.
- Hornemann, T. Sphingoid base diversity. Atherosclerosis, 401, 119091 (2025); DOI.
- Jamjoum, R., Majumder, S., Issleny, B. and Stiban, J. Mysterious sphingolipids: metabolic interrelationships at the center of pathophysiology. Front. Physiol., 14, 1229108 (2024); DOI.
- Jojima, K. and Kihara, A. Metabolism of sphingadiene and characterization of the sphingadiene-producing enzyme FADS3. Biochim. Biophys. Acta, Lipids, 1868, 159335 (2023); DOI.
- Kuo, A. and Hla, T. Regulation of cellular and systemic sphingolipid homeostasis. Nature Rev. Mol. Cell Biol., 25, 802–821 (2024); DOI.
- Lone, M.A., Hülsmeier, A.J., Saied, E.M., Karsai, G., Arenz, C., von Eckardstein, A. and Hornemann, T. Subunit composition of the mammalian serine-palmitoyltransferase defines the spectrum of straight and methyl-branched long-chain bases. Proc. Natl. Acad. Sci. USA, 117, 15591-15598 (2020); DOI.
- Lone, M.A., Santos, T., Alecu, I., Silva, L.C. and Hornemann, T. 1-Deoxysphingolipids. Biochim. Biophys. Acta, Lipids, 1864, 512-521 (2019); DOI.
- Mahawar, U. and Wattenberg, B. Intricate regulation of sphingolipid biosynthesis: an in-depth look into ORMDL-mediated regulation of serine palmitoyltransferase. Bioessays, 47, e70036 (2025); DOI.
- Marquês, J.T., Marinho, H.S. and de Almeida, R.F.M. Sphingolipid hydroxylation in mammals, yeast and plants - an integrated view. Prog. Lipid Res., 71, 18-42 (2018); DOI.
- Merrill, A.H. Don't be surprised when these surprise you: some infrequently studied sphingoid bases, metabolites, and factors that should be kept in mind during sphingolipidomic studies. Int. J. Mol. Sci., 26, 650 (2025); DOI.
- Pruett, S.T., Bushnev, A., Hagedorn, K., Adiga, M., Haynes, C.A., Sullards, M.C., Liotta, D.C. and Merrill, A.H. Biodiversity of sphingoid bases (‘sphingosines’) and related amino alcohols. J. Lipid Res., 49, 1621-1639 (2008); DOI.
- Rodriguez-Cuenca, S., Barbarroja, N. and Vidal-Puig, A. Dihydroceramide desaturase 1, the gatekeeper of ceramide induced lipotoxicity. Biochim. Biophys. Acta, Lipids, 1851, 40-50 (2015); DOI.
- Stankeviciute, G. and others. Convergent evolution of bacterial ceramide synthesis. Nat. Chem. Biol., 18, 305-312 (2022); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: November 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
