Monoacylglycerols
Monoacylglycerols (or "monoglycerides") are esters of the trihydric alcohol glycerol in which only one of the hydroxyl groups is esterified with a long-chain fatty acid. They can exist in three stereochemical forms as illustrated. Our web page on triacylglycerols describes the stereospecific numbering system.
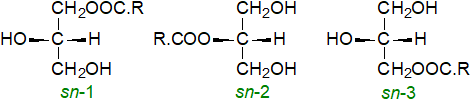 |
| Figure 1. Structures of monoacyl-sn-glycerols. |
Using most chromatographic separation systems, the 1/3-isomers are not distinguished from each other and are termed 'α‑monoacylglycerols', while the 2‑isomers are 'β‑monoacylglycerols'. They tend to be minor components only of most plant and animal tissues, and indeed would not be expected to accumulate because, as strong detergents, they would have a disruptive effect on membranes. From a technological standpoint, synthetic monoacylglycerols can be constituents of commercial detergents, while monoolein has become one of the most widely used lipids in the fields of drug delivery, emulsion stabilization and protein crystallization, where its physical properties as an amphiphile in aqueous emulsions are so distinctive that it has been termed a ‘magic lipid’. Here, the biological properties only of monoacylglycerols are discussed.
They are the main lipid components of adipose tissue macrophages where their function is uncertain. One particular monoacylglycerol, i.e., 2‑arachidonoylglycerol, is of special importance as an endocannabinoid and is more appropriately discussed under that heading in this website, but other monoacylglycerols have their own functions in cells as discussed below. Many marine organisms produce unusual monoacylglycerols, for example with oxylipin or mono- and diterpenoid acyl constituents.
Formation and function: 2-Monoacylglycerols are a major end product of the intestinal digestion of dietary fats by the enzyme pancreatic lipase in animals. They are taken up directly by the intestinal cells and converted to triacylglycerols via the monoacylglycerol pathway before being transported in lymph to the liver (see our web pages on triacylglycerols for further details). Within tissues, monoacylglycerols are produced by lipolysis, as has been studied extensively in relation to triacylglycerols in adipose tissue, with first a reaction with adipose triacylglycerol lipase to generate 2‑monoacylglycerols which can then be further hydrolysed by a monoacylglycerol lipase. Although it is known that 2-monoacylglycerols can spontaneously isomerize to the 1/3‑isomers, which are thermodynamically more stable, the precise conditions under which this occurs in vivo are not clear.
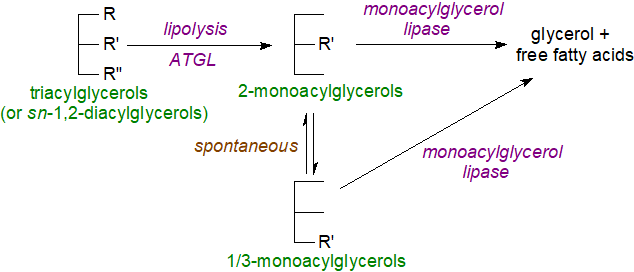 |
| Figure 2. Monoacylglycerol formation and catabolism in adipose tissue. |
Monoacylglycerols are produced in bacteria, fungi, plants and animals from diacylglycerols, but not from triacylglycerols, by the action of diacylglycerol lipases. 1,2‑Diacyl‑sn-glycerols released by the action of phospholipase C on membrane phospholipids in brain and nervous tissue are used to generate 2‑arachidonoylglycerol by this means. In plants such as Arabidopsis, 2-monoacyl-sn-glycerols are generated for synthesis of cutin polymers via an intermediate 2-lysophosphatidic acid produced by glycerol-3-phosphate acyltransferases (GPATs). While the bacterial diacylglycerol lipases can hydrolyse diacylglycerols to glycerol and free fatty acids via monoacylglycerol intermediates, the analogous mammalian enzyme has little monoacylglycerol lipase activity.
It is now recognized that 2-monoacylglycerols and 2-oleoylglycerol in particular are signalling mediators in the intestines by activating a G protein coupled receptor GPR119, sometimes termed the ‘fat sensor’, which may be the only receptor responsible for fat-induced release of the gut hormones glucagon-like peptide-1 (GLP-1), peptide tyrosine-tyrosine (PYY), and neurotensin. When stimulated, this receptor causes a reduction in food intake and body weight gain in rats and regulates glucose-stimulated insulin secretion. The receptor was at first thought to be located at the apical membrane facing the intestinal lumen, but recent evidence suggests that it may be located at the basolateral membrane. Oleoylethanolamine and 1-oleoyl-lysophosphatidylcholine act in the same manner, but 2‑oleoylglycerol is the most abundant of the potential agonists. Whether these 2‑monoacylglycerols have signalling functions in other tissues has yet to be determined.

1-Butyryl-glycerol (monobutyrin) is an angiogenesis factor (the growth of blood vessels from the existing vasculature), which is secreted by differentiating adipocytes. Glycerol monolaurate in human milk is reported to have antimicrobial and anti-inflammatory properties towards several species of Bacillus and Staphylococcus, and it is sometimes added to foods for this purpose; this and other monoacylglycerols are also antifungal agents. 1-Monoacylglycerol species containing long-chain saturated fatty acids bind to the C1‑domain of a protein Munc13-1, which interacts with SNARE proteins to facilitate the priming of insulin granules and consequently to stimulate insulin secretion in pancreatic β-cells. In addition, 1‑monoacylglycerols may play a role in brown adipose metabolism and energy expenditure by activating the peroxisome proliferator-activated receptors PPARα and PPARγ. Acylglycerol kinases that can phosphorylate both 1- and 2‑monoacyl-sn-glycerols to form the important signalling molecule lysophosphatidic acid have been described from brain and other tissues.
 In the fruit fly Drosophila melanogaster, 2-linoleoyl-glycerol is a signalling mediator akin to the endocannabinoids in higher animals.
Monoacylglycerols containing 3,8‑dihydroxy fatty acids (C18 to C24), many of which are further acylated with acetate
residues, occur in poplar leaves and buds (propolis) and have been shown to be strongly antiproliferative against human
gastrointestinal cancers in vitro.
In the fruit fly Drosophila melanogaster, 2-linoleoyl-glycerol is a signalling mediator akin to the endocannabinoids in higher animals.
Monoacylglycerols containing 3,8‑dihydroxy fatty acids (C18 to C24), many of which are further acylated with acetate
residues, occur in poplar leaves and buds (propolis) and have been shown to be strongly antiproliferative against human
gastrointestinal cancers in vitro.
Catabolism: In animal cells, monoacylglycerols are catabolized mainly by the action of a monoacylglycerol lipase with formation of free fatty acids and glycerol, although a ubiquitously expressed serine hydrolase α/β-hydrolase domain 6 (ABHD6) may regulate signalling by monoacylglycerols.
The monoacylglycerol lipase is currently of considerable interest as it is highly expressed in aggressive human cancer cells and primary tumours. The resulting high lipolytic activity increases free fatty acid levels in cancer cells, and these feed into a diverse network of pro-tumorigenic signalling lipids, which promote migration, survival and tumour growth in vivo to support malignancy. In consequence, a considerable effort is going into development of inhibitors of the enzyme. Inhibition of monoacylglycerol lipase has the potential to benefit several other disease states, including neurological and neurodegenerative diseases, and inhibition of the hydrolysis of 2-arachidonoylglycerol might reduce the availability of arachidonic acid for synthesis of pro-inflammatory prostaglandins.
Analysis: During extraction of tissues for analysis, monoacylglycerols undergo acyl migration very rapidly to form a mixture that contains more than 80% of the 1/3‑form. They can be stabilized and purified by chromatography on adsorbents impregnated with boric acid, provided that great care is taken to avoid polar solvents and elevated temperatures. By following appropriate derivatization procedures, stereoisomers of monoacylglycerols can be resolved by chiral chromatography (see our webpage on stereospecific analysis of triacyl-sn-glycerols), but it is more usual to analyse them as a total monoacylglycerol fraction isolated by TLC, for example for gas chromatography of the methyl ester derivatives of the fatty acid components.
References
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Gruden, E. and others. Tumor microenvironment-derived monoacylglycerol lipase provokes tumor-specific immune responses and lipid profiles. Prostaglandins, Leukotrienes Essential Fatty Acids, 196, 102585 (2023); DOI.
- Hansen, H.S. and Vana, V. Non-endocannabinoid N-acylethanolamines and 2-monoacylglycerols in the intestine. Brit. J. Pharm., 176, 1443-1454 (2019); DOI.
- Ianni, F., Carotti, A., Protti, M., Favilli, A., Gerli, S., Furlanetto, S., Mercolini, L. and Sardella, R. Chiral high-performance liquid chromatography analysis of mono-, di-, and triacylglycerols with amylose- and cellulose-phenylcarbamate-based stationary phases. J. Pharm. Biomed. Anal., 236, 115720 (2023); DOI.
- Kulkarni, C.V., Wachter, W., Iglesias-Salto, G., Engelskirchen, S. and Ahualli, S. Monoolein: a magic lipid? Phys. Chem. Chem. Phys., 13, 3004-3021 (2011); DOI.
- Poursharifi, P., Madiraju, S.R.M. and Prentki, M. Monoacylglycerol signalling and ABHD6 in health and disease. Diabetes Obesity Metabolism, 19, 76-89 (2017); DOI.
- Yuan, D., Wu, Z. and Wang, Y. Evolution of the diacylglycerol lipases. Prog. Lipid Res., 64, 85-97 (2016); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
