Sterols: 4. Hopanoids and Related Lipids
Bacteria and other prokaryotic organisms such as blue-green algae do not in general contain any of the conventional sterols found in plants and animals (there are always exceptions), but instead, many species have related molecules, i.e., pentacyclic triterpenoids based on a hopane skeleton with four cyclohexane rings and a cyclopentane E-ring and termed ‘hopanoids’ (from the plant genus Hopea, from which hydroxyhopanone was first isolated as a component of the resin in 1955). They were discovered in living prokaryotes in 1971 in the bacteria Methylococcus capsulatus and Alicyclobacillus acidocaldarius, but it is now known that 10% of bacteria, but especially the Proteobacteria, have the appropriate genes to produce hopanoids; 23% of Alphaproteobacteria have the genetic capacity to produce bacteriohopanepolyol derivatives. In nature, many different structures are now known to occur from simple hopanoids to elongated compounds with polyfunctional side chains, and they extend from living organisms (bacteria, lichens, bryophytes, ferns, tropical trees and fungi) to 'fossil molecules' in geological sediments at least 2.7 billion years back in time.
Perhaps surprisingly, phylogenetic profiling suggests that biosynthesis of hopanoids may be ancestral in the Bacterial domain of life, and it has been proposed that eukaryotic genes for sterol biosynthesis may have been assembled from ancestral bacterial contributions. None of the relevant enzymes, such as triterpene cyclases, are present in Archaea.
1. Hopanoids
The hopanoids are highly diverse in structure, and they are conveniently grouped into two classes, with the first comprising simple hopanoids with a C30 quasi-planar polycyclic hopane skeleton, of which the simplest is diploptene or hop-22(29)-ene, usually found with diplopterol or hopan-22-ol. The second class consists of hopanoids with a polyfunctionalized side chain attached by a carbon-carbon bond, usually derived from ribose, to generate C35 molecules. In general, the molecules are amphiphilic, and in that they modulate the fluidity and the permeability of phospholipid membranes, they have been called ‘sterol surrogates’.
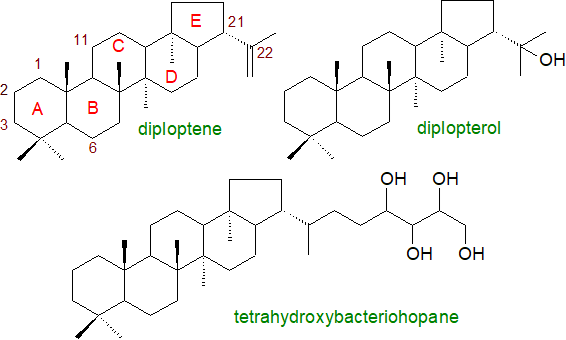 |
| Figure 1. Structures of some representative hopanoids. |
Perhaps the most abundant hopanoid in living organisms is tetrahydroxybacteriohopane (bacteriohopane-32,33,34,35-tetrol), i.e., with a distinctive five-carbon terminal side chain linked by a carbon-carbon bond to the isopropyl group of the hopane framework and with four hydroxyls. The polar nature of this moiety changes the physical properties and thence the potential functions in membranes substantially. Many forms of this are known in which the terminal hydroxyl (on C35) group is substituted, for example with a glycosidic or ether linkage to glucosamine, adenosine or ribose. In some species, the polyhydroxy side chain can be O- or N-acylated, and Frankia species contain the phenylacetate monoester of tetrahydroxybacteriohopane. Further hydroxylated forms are known, and penta- and hexaols and amino-polyols occur in some genera with 63 different structures identified in a recent analytical study of α-proteobacteria.
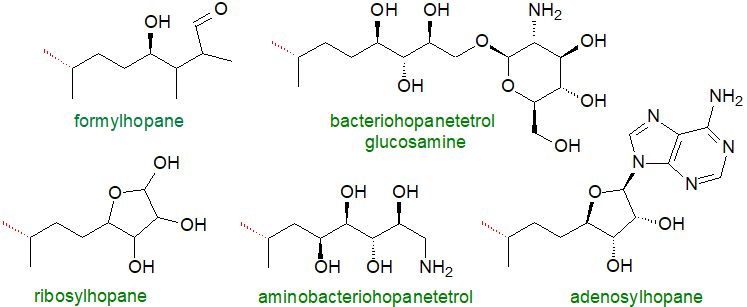 |
| Figure 2. Partial structures of some complex hopanoids (linked to the E ring). |
Some of these have additional methyl groups (C2β, C2α or C3β) in the A ring, with stereochemical isomers adding to the variability, and of these, 2‑methylbacteriohopanepolyols are common in cyanobacteria and α-proteobacteria, especially those of marine species, but are rarely encountered in other phyla. Hopanoids with double bonds in the ring at C6 and/or C11 have also been characterized. The more complex of these are sometimes referred to as ‘composite structures’, and they are seen as useful taxonomic markers for some bacterial genera. As some hopanoids are so tightly bound within organisms that they are not easily extracted for structural analysis, it is likely that many further types will eventually be characterized.
Hopanoids are most abundant in aerobic bacteria (methanotrophs, heterotrophs and cyanobacteria), but they also in some anaerobic bacteria but not in Archaea and rarely in eukaryotes. In particular, they occur in many species of nitrifying (i.e., ammonia- and nitrite-oxidizing) bacteria of marine and terrestrial origin but not in all. Most species in the genus Azotobacter produce hopanoids, but A. chroococcum does not. In most instances, the hopanoid content of prokaryote cells is comparable to that of sterols in eukaryotic cells.
The rings in hopanoids have chair-chair-chair-chair-chair conformations (see Figure 4) in comparison to four-ringed sterols, which have chair-boat-chair-boat-open conformations. The resulting ring structures are planar, rigid and hydrophobic with a length corresponding to half the thickness of a membrane bilayer. Bacteriohopanepolyols are located in the inner and outer membranes of Gram-negative bacteria, as well as in the cytoplasmic membrane of Gram-positive bacteria, in part acylated to lipopolysaccharides. In one symbiotic Bradyrhizobium strain (slow-growing rhizobia), the lipid A spanning the whole of the outer membrane has at least one molecule of carboxyl-bacteriohopanediol or its 2‑methyl derivative linked covalently to the (ω-1)-hydroxyl group of one of its very-long-chain fatty acid components; B. japonicum has two such substituents. This serves to reinforce the stability and rigidity of the outer membrane of the organism to protect against environmental stress, while the inner leaflet contains free hopanoids that permit a higher ordering and strengthening of the cell envelope.
A few higher plants and some ferns, mosses and fungi contain hopanoids, although these lack the complex side chains and often have an oxygen atom at C3. The rare plant metabolite 22-hydroxyhopan-3-one from dammar resin has the diplopterol structure but with an additional ketone group on C3, and although their function in such organisms is not known, it is presumed to be analogous to that of sterols in membranes. Diploptene has been detected in the epicuticular waxes of collembola (springtails), tiny hexapod arthropods, but those hopanoids reported from sponges probably originate in symbiotic or ingested microorganisms.
2. Biochemistry and Function
Biosynthesis: As with sterols, squalene is a primary precursor for the biosynthesis of the hopane skeleton. Biosynthesis of isopentenyl pyrophosphate and dimethylallyl pyrophosphate, the intermediates in squalene biosynthesis, is either via the mevalonate pathway or via the ‘non-mevalonate’ or ‘2C-methyl-D-erythritol-4-phosphate (MEP)’ pathway. Indeed, it was anomalies in the results of biosynthetic studies with hopanoids that lead to the elucidation of the MEP pathway (see our web pages dealing with biosynthesis of cholesterol and plant sterols, respectively, for details).
The mechanism for squalene biosynthesis differs in bacteria from that in eukaryotes. In bacteria, an enzyme HpnD converts two molecules of farnesyl diphosphate to presqualene diphosphate, which is converted to hydroxysqualene in a hydrolytic rearrangement catalysed by HpnC, before undergoing reduction to squalene catalysed by HpnE, a member of the amine oxidoreductase family. In Eukaryotes, a single enzyme catalyses the three steps from farnesyl diphosphate to squalene (step 3 in our web page on cholesterol), but in bacteria, three separate enzymes are involved. These enzymes were probably formed from a common ancestor, but the amino acid sequences have diverged appreciably.
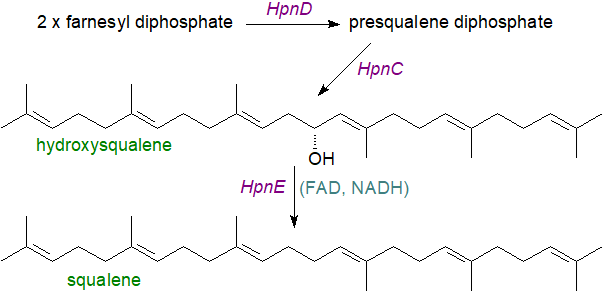 |
| Figure 3. Biosynthesis of squalene in bacteria. |
A further important distinction is that hopanoid biosynthesis does not proceed via 2,3‑epoxysqualene, but rather squalene per se undergoes cyclization without migration of the methyl groups to produce C30 diploptene or diplopterol in a one-step oxygen-independent process (cf., the nineteen-enzymatic step conversion of squalene into 24-ethylsterols in higher plants that requires 12 molecules of dioxygen). In consequence, bacterial hopanoids lack the 3β‑hydroxyl group of sterols.
Synthesis occurs in a single concerted reaction by a squalene-hopene cyclase that is quite different from the squalene epoxide cyclase. From studies of the crystal structure of the former, it is suggested that the active site is located in a large central cavity of a size and shape to bind squalene in the necessary conformation. The cavity is surrounded by loops containing aromatic residues, which may stabilize the putative ionic intermediates. Cyclization probably begins with a reaction in which a carbon-carbon double bond is protonated via an aspartate residue at the top of the cavity. Then, rings A and probably B are formed in a concerted manner before rings C and D are fashioned in ring closure reactions. Finally, the ring E is formed and the carbocation at C-22 is deprotonated to form hopene (or reacts with the elements of water to form diplopterol).
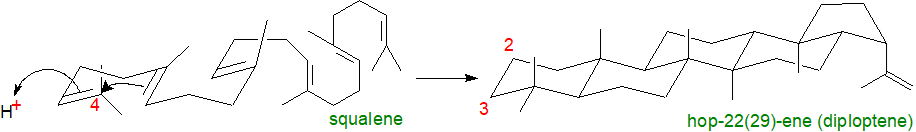 |
| Figure 4. Biosynthesis of hopanoids - cyclization step. |
Like sterol biosynthesis, it is one of the most complex single-step processes known to biochemistry, with the formation of five ring structures, modification of thirteen covalent bonds and the generation of nine new stereochemical centres, all under precise enzymatic control.
Adenosylhopane (see partial structure above) is the first intermediate produced during the biosynthesis of the side chains of bacteriohopanepolyols by an enzyme designated HpnH, a radical S-adenosylmethionine (SAM) enzyme, usually encoded in the hopanoid biosynthetic gene cluster that converts diploptene into adenosylhopane in the presence of SAM, flavodoxin, flavodoxin reductase and NADPH. This is in turn converted to phosphoribosylhopane by a purine nucleoside phosphorylase HpnG. Subsequently, adenosylhopane is utilized to produce the wide range of products formed in microorganisms by down-stream processing. For example, it can lose the adenyl group to produce ribosylhopane, and the D-ribose moiety can in turn be opened to produce the five-carbon side chain bacteriohopanetetrol either directly or via formylhopane. In cyanobacteria and Acidobacteria, addition of methyl groups to form 2β- and 3β-methyl hopanoids by hopanoid methylases (HpnP and HpnR) occurs after synthesis of the hopanoid rings, with SAM as the methyl donor. The enzymology of these reactions is now being revealed.
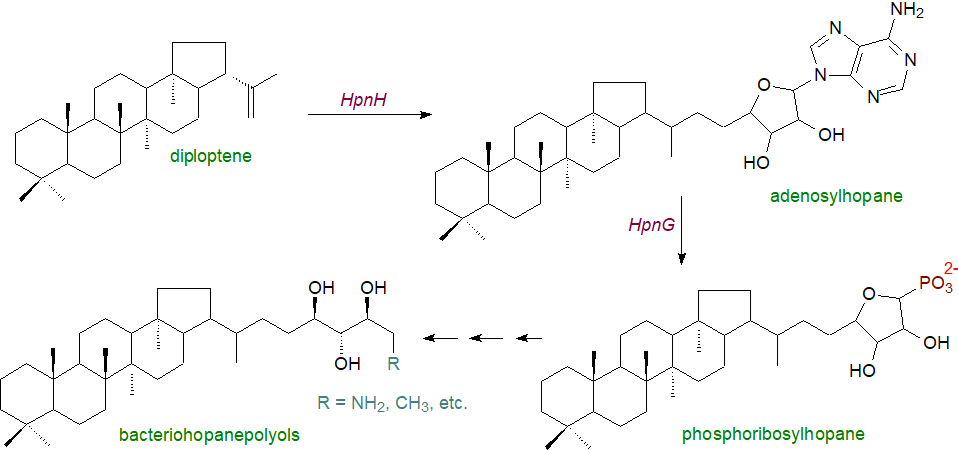 |
| Figure 5. Biosynthesis of complex bacteriohopanepolyols |
The presence of the 3-oxygen atom in some plant hopanoids, e.g., moretenol and mollugogenol, suggests that they are formed via a 2,3‑epoxysqualene intermediate, although in other plant species there may have been horizontal transfer of the genes for the squalene-hopene cyclase from bacteria.
Function: Hopanoids have very similar functions to those of sterols in the membranes of animals and plants in that they can intercalate into phospholipid bilayers and modulate the fluidity of membranes by interacting with their complex lipid components. Physical chemical studies with diplopterol in model membranes have shown that it induces phase segregation and increases lipid compaction, while retaining membrane fluidity and compressibility in the same way as sterols such as cholesterol, stigmasterol and ergosterol. By decreasing membrane permeability, hopanoids limit the diffusion of oxygen, and this may facilitate aerobic nitrogen fixation in the ocean by protecting the extremely O2-sensitive nitrogenase enzyme in cyanobacteria.
 They enable
bacteria to adapt to stress caused by extreme environmental conditions, including high temperatures, low pH, detergents and antibiotics,
and while hopanoids usually comprise around 1 to 5% of the total lipids in cells, the proportion can rise appreciably in response to stress
with the thermophile Bacillus acidocaldarius synthesising roughly seven times more hopanoids at 65°C than at 60°C,
presumably to counteract the increased fluidity of the lipid portion of the membrane and thereby reinforce it.
Resting cells (akinetes) of cyanobacteria produce ten times as much hopanoids as vegetative cells, especially when they are stressed by
desiccation or exposure to prolonged cold.
They enable
bacteria to adapt to stress caused by extreme environmental conditions, including high temperatures, low pH, detergents and antibiotics,
and while hopanoids usually comprise around 1 to 5% of the total lipids in cells, the proportion can rise appreciably in response to stress
with the thermophile Bacillus acidocaldarius synthesising roughly seven times more hopanoids at 65°C than at 60°C,
presumably to counteract the increased fluidity of the lipid portion of the membrane and thereby reinforce it.
Resting cells (akinetes) of cyanobacteria produce ten times as much hopanoids as vegetative cells, especially when they are stressed by
desiccation or exposure to prolonged cold.
Molecular dynamics simulations suggest that diploptene is located between the two leaflets of a membrane bilayer, partitioning to the midplane, where it may decrease membrane permeability by providing an added layer of protection to the cell. In contrast, the extended hopanoid bacteriohopanetetrol in membranes probably adopts an upright orientation inside the lipid bilayer, where it can interact with other membrane lipids to condense the membrane. By increasing the Van der Waals forces between lipids, they limit the penetration of small molecules, and in Frankia species, most of the lipids in the membrane barrier that prevents diffusion of oxygen into the nitrogen-fixing cells are hopanoids; bacteriohopanetetrol phenylacetate monoester is specific to the vesicle envelope. Hopanoids have been located in the plasma membrane and outer membranes of Gram-negative bacteria and in the outer and thylakoid membranes of cyanobacteria, where they interact with glycolipids in bacterial outer membranes to form a highly ordered bilayer in a manner analogous to the interaction of sterols with sphingolipids in forming raft-like microdomains in eukaryotic plasma membranes. As hopanoids have a key role in the structure and operation of pentameric ligand-gated ion channels in membranes in prokaryotes, it has been suggested that these ion channels and sterols may have co-evolved in eukaryotes.
With cultures of single species, bacteriohopanepolyols have only been detected in cyanobacteria capable of nitrogen fixation, implicating them as markers for diazotrophy in the oceans. Similarly in plants such as soybean, the hopanoids of Bradyrhizobium spp. are involved in legume–rhizobia root nodule symbioses, although there is no known mechanistic link between hopanoid production and nitrogen fixation. The fission yeast Schizosaccharomyces japonicus has acquired a squalene-hopene cyclase through horizontal gene transfer, and production of hopanoids enables this species to thrive in anoxia, where sterol biosynthesis is not possible. Under aerobic conditions, both sterols and hopanoids are formed to support membrane properties.
It is apparent that hopanoids are essential for growth in many if not all hopanoid-producing organisms, as inhibition of hopanoid biosynthesis can limit their growth markedly and selectively in comparison with other bacteria. While some species may grow when hopanoid synthesis is inhibited, they are much less resistant to environmental challenges, and hopanoid biosynthesis is seen as a potential target to fight multidrug resistance in pathogenic bacteria.
3. Geohopanoids
As the pentacyclic ring structure of hopanoids is very stable and not readily degraded, geochemists tend to look upon them as molecular fossils ('geohopanoids' or 'homohopanoids') that serve as markers for families of organisms in geological formations from recent sediments to petroleum deposits and rocks (and potentially one day for extra-terrestrial life on Mars). Hopanoids are sometimes claimed to be the most abundant lipids on earth, and in one estimate, their mass in sedimentary rocks and oil reservoirs is said to be ~109 tons, an amount comparable to the total mass of carbon in all living organisms!
Although they are highly resistant, hopanoids are not immune to change in the geological environment over millions of years, and stereomutation, reduction and defunctionalization occur to produce hopanols and hopanoic acids, commonly with 28 to 35 carbon atoms; these processes are termed 'diagenesis'. For example, the 17β,21β(H) configuration of hopanoids in living organisms isomerizes to the thermally more stable 'geological' 17ɑ,21β(H) and 17β,21ɑ(H) configurations.
 |
| Figure 6. Changes to hopanoids with geological time. |
Different bacterial genera possess characteristic hopanoid distributions, which may point to the origins of the organisms producing the deposits. The concentration of 25,28-bisnorgammacerane relative to steranes in geological deposits is treated as a biomarker to trace the change from predominantly eukaryotic life forms to bacterial and back again during the epoch known as the "snowball earth". However, 2α‑methylhopanetetrols that were thought to be unique constituents of photosynthetic cyanobacteria and found in 2.7-billion-year-old-shales in Australia, i.e., from a period long before the atmosphere was oxidizing, are now known to be produced by proteobacteria and acidobacteria also. On the other hand, their primary origin may indeed be in cyanobacteria, as it is believed that the relevant genes appeared later in evolution in Alphaproteobacteria. For related reasons, it is uncertain whether 3‑methyl hopanoids can still be considered as exclusive biomarkers for aerobic methanotrophs. As the pattern of the various products can have indicative value in oil exploration, their analysis is of great practical importance.
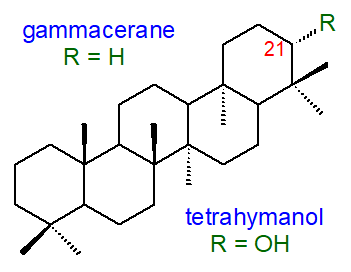
4. Tetrahymanol and Related Lipids
 Several pentacyclic compounds related to the hopanoids are known that are derived from the gammacerane skeleton in which
the E-ring is six-membered (5-six-membered rings in total).
Of these, the most abundant is tetrahymanol (gammaceran-21α-ol), which was first isolated from the ciliate protozoan
Tetrahymena pyriformis.
It was subsequently detected in several other eukaryotic organisms that inhabit oxygen-poor environments,
including fungi, protozoa and even ferns, before it was found in other ciliates and increasingly in anaerobic bacteria,
such as the purple non-sulfur bacterium Rhodopseudomonas palustris.
Several pentacyclic compounds related to the hopanoids are known that are derived from the gammacerane skeleton in which
the E-ring is six-membered (5-six-membered rings in total).
Of these, the most abundant is tetrahymanol (gammaceran-21α-ol), which was first isolated from the ciliate protozoan
Tetrahymena pyriformis.
It was subsequently detected in several other eukaryotic organisms that inhabit oxygen-poor environments,
including fungi, protozoa and even ferns, before it was found in other ciliates and increasingly in anaerobic bacteria,
such as the purple non-sulfur bacterium Rhodopseudomonas palustris.
Various structural variants have been found including 20α-methyltetrahymanol, 2β‑methyltetrahymanol and 2β,20α‑dimethyltetrahymanol, which occur with tetrahymanol per se and various hopanoids in the nitrogen-fixing, symbiotic root-nodule forming bacterium Bradyrhizobium japonicum.
Bacteria produce tetrahymanol by cyclization of squalene to a hopene molecule by squalene-hopene cyclase followed by a ring expansion involving a tetrahymanol synthase. In eukaryotes, tetrahymanol is formed by a squalene-tetrahymanol cyclase with the nature of the E-ring depending on the orientation of the terminal methyl groups during the final cyclization step. When sterols are added to cultures of Tetrahymena pyriformis, synthesis of tetrahymanol is inhibited completely, suggesting that sterols and tetrahymanol have similar functions in this organism, i.e., like the hopanoids, tetrahymanol is a sterol surrogate.
Gammacerane-derived structures have been found in sediments and other geological formations, together with the geohopanoids, where they were initially believed to be useful geochemical markers for ciliate protozoa, although it is now recognized that some could originate in bacteria.
Sporulenes are C35-terpenoid hydrocarbons with an unusual pentacyclic structure produced by Bacillus subtilis during sporulation that increase their resistance to reactive oxygen species (akin to bacteriohopanetetrol phenylacetate monoester in Frankia species). Squalene is not the biosynthetic precursor, but instead they originate from a linear heptaprenyl unit with all head-to-tail isoprene linkages via an enzyme related to the squalene synthase.
5. Analysis
Hopanoids require special extraction methods because of their high polarity. Indeed, some are so tightly bound to membranes that their presence in many organisms may have gone undetected. At one time, the molecular structures were simplified by removal of part of the side chain by chemical means to facilitate analysis, so much information was lost. This may no longer be necessary, and HPLC allied to modern mass spectrometric methods involving atmospheric-pressure chemical ionization or electrospray ionization is be the way forward.
Suggested Reading
- Banta, A.B., Wei, J.H. and Welander, P.V. A distinct pathway for tetrahymanol synthesis in bacteria. Proc. Natl. Acad. Sci. USA, 112, 13478-13483 (2015); DOI.
- Belin, B.J., Busset, N., Giraud, E., Molinaro, A., Silipo, A. and Newman, D.K. Hopanoid lipids: from membranes to plant-bacteria interactions. Nature Rev. Microbiol., 16, 304-315 (2018); DOI.
- Damsté, J.S.S., Koenen, M., Thiel, V., Richter, N., Hopmans, E.C. and Nicole J. Bale, N.J. A comprehensive study of biohopanoid production in Alphaproteobacteria: biosynthetic, chemotaxonomical, and geobiological implications. Geobiology, 23, e70038 (2025); DOI.
- Hopmans, E.C., Smit, N.T., Schwartz-Narbonne, R., Damste, J.S.S. and Rush, D. Analysis of non-derivatized bacteriohopanepolyols using UHPLC-HRMS reveals great structural diversity in environmental lipid assemblages. Org. Geochem., 160, 104285 (2021); DOI.
- Kusch, S. and Rush, D. Revisiting the precursors of the most abundant natural products on Earth: A look back at 30+ years of bacteriohopanepolyol (BHP) research and ahead to new frontiers. Org. Geochem., 172, 104469 (2022); DOI.
- Li, X., Zhu, X., Zhong, Y., Zhang, W., Chen, F., Wang, W., Ding, W. and Zhang, Q. Characterization of HpnG as a purine nucleoside phosphorylase in bacteriohopanepolyol biosynthesis. Chin. J. Chem., 42, 2351-2356 (2024); DOI.
- Mangiarotti, A., Genovese, D.M., Naumann, C.A., Monti, M.R. and Wilke, N. Hopanoids, like sterols, modulate dynamics, compaction, phase segregation and permeability of membranes. Biochim. Biophys. Acta, Biomembranes, 1861, 183060 (2019); DOI.
- Naafs, B.D.A., Bianchini, G., Monteiro, F.M. and Sanchez-Baracaldo, P. The occurrence of 2-methylhopanoids in modern bacteria and the geological record. Geobiology, 20, 41-59 (2022); DOI.
- Pan, J.-J., Solbiati, J.O., Ramamoorthy, G., Hillerich, B.S., Seidel, R.D., Cronan, J.E., Almo, S.C. and Poulter, C.D. Biosynthesis of squalene from farnesyl diphosphate in bacteria: three steps catalyzed by three enzymes. ACS Cent. Sci. 1, 77-82 (2015); DOI.
- Santana-Molina, C., Rivas-Marin, E., Rojas, A.M. and Devos, D.P. Origin and evolution of polycyclic triterpene synthesis. Mol. Biol. Evolution, 37, 1925-1941 (2020); DOI.
- Sato, S., Kudo, F., Rohmer, M. and Eguchi, T. Characterization of radical SAM adenosylhopane synthase, HpnH, which catalyzes the 5'-deoxyadenosyl radical addition to diploptene in the biosynthesis of C35 bacteriohopanepolyols. Angew. Chem.-Int. Ed., 59, 237-241 (2020); DOI.
- Takishita, K., Chikaraishi, Y., Leger, M.M., Kim, E., Yabuki, A., Ohkouchi, N. and Roger, A.J. Lateral transfer of tetrahymanol-synthesizing genes has allowed multiple diverse eukaryote lineages to independently adapt to environments without oxygen. Biology Direct, 7, 5 (2012); DOI.
- Vitiello, G., Oliva, R., Petraccone, L., Del Vecchio, P., Heenan, R.K., Molinaro, A., Silipo, A., D'Errico, G. and Paduano, L. Covalently bonded hopanoid-Lipid A from Bradyrhizobium: The role of unusual molecular structure and calcium ions in regulating the lipid bilayers organization. J. Colloid Interface Sci., 594, 891-901 (2021); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: November 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
