Isoprenoids: 2. Retinoids (Vitamin A) and Carotenoids
That a dietary factor was involved in visual acuity was known to the ancient Egyptians and Greeks, but it was the 1930s before the importance of the carotenoids and their metabolites was recognized, and β-carotene and retinol were fully characterized. It is now recognized that vitamin A activity now resides in the metabolites retinol, retinal and retinoic acid, derived from several provitamin A carotenoids, most notably β-carotene. To honour his research contribution, the Nobel Prize for Medicine in 1967 was awarded to George Wald, who over many years showed how retinol derivatives or retinoids (named for their function in the retina) constitute the chemical basis of vision. Now, it is recognized that these retinoids and their many metabolites are required for innumerable other purposes in human metabolism from embryogenesis to adulthood, including growth and development, reproduction, cancer and resistance to infection. They are natural antioxidants with benefits to health, although some potentially harmful properties have been reported when in excess.
Carotenoids are a class of highly unsaturated terpenoids that occur in innumerable molecular forms (>1,100), and their chemistry and biochemistry are such substantial topics that I cannot at present discuss them here. They are common colourful pigments synthesised by plants, fungi and bacteria that are vital to photosynthesis in addition to innumerable other functions in the producing organisms. Only a few of these are relevant to animal metabolism, so I describe them here in brief mainly in the context of precursors for vitamin A, although there is need for carotenoids per se in animal tissues, albeit relatively minor, for vision and as antioxidants in skin. As dietary constituents, they can add ornament or warning signals to some animal species.
Other fat-soluble vitamins, tocopherols (vitamin E), vitamin K (phylloquinone) and vitamin D, are discussed in separate web pages.
1. Occurrence and Basic Metabolism of Carotenoids and Retinoids
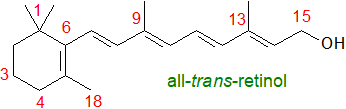 The term ‘vitamin A’ is used to denote retinol (or all-trans-retinol,
sometimes termed 'vitamin A1'), together with a family of C20 retinoids,
especially retinaldehyde and retinoic acid, derived from this ('vitamers'), which
are only found in animal tissues, where they are essential to innumerable metabolic processes.
They cannot be synthesised de novo in animals (other than some arthropods), and their biosynthetic precursors are plant carotenoids with
a β-ionone ring (provitamin A), i.e., C40 tetraterpenes of which the most efficient is β‑carotene, an orange-red pigment
that occurs in the photosynthetic tissues of plants and in seed oils.
In the human diet in the developed world, plants tend to be lesser sources than dairy products, meat, fish oils and margarines,
which provide vitamin A per se, although carrots and spinach are good sources of the provitamin.
In the U.K., all vegetable spreads must be supplemented with the same level of vitamin A (synthetic retinol or β‑carotene)
as is found in butter.
Some dietary intake of the carotenoids lutein and zeaxanthin as micronutrients is also essential.
The term ‘vitamin A’ is used to denote retinol (or all-trans-retinol,
sometimes termed 'vitamin A1'), together with a family of C20 retinoids,
especially retinaldehyde and retinoic acid, derived from this ('vitamers'), which
are only found in animal tissues, where they are essential to innumerable metabolic processes.
They cannot be synthesised de novo in animals (other than some arthropods), and their biosynthetic precursors are plant carotenoids with
a β-ionone ring (provitamin A), i.e., C40 tetraterpenes of which the most efficient is β‑carotene, an orange-red pigment
that occurs in the photosynthetic tissues of plants and in seed oils.
In the human diet in the developed world, plants tend to be lesser sources than dairy products, meat, fish oils and margarines,
which provide vitamin A per se, although carrots and spinach are good sources of the provitamin.
In the U.K., all vegetable spreads must be supplemented with the same level of vitamin A (synthetic retinol or β‑carotene)
as is found in butter.
Some dietary intake of the carotenoids lutein and zeaxanthin as micronutrients is also essential.
Following consumption in animals (including humans), carotenoids such as β-carotene in foods are solubilized with other dietary lipids in mixed micelles with the aid of bile acids, and they are absorbed in the intestines in intact form by a process facilitated by receptor proteins. Dietary retinol and retinol esters are absorbed in the same way, but the latter are first hydrolysed by pancreatic lipase; retinol-binding protein 2 (RBP2, sometimes termed 'cellular retinol-binding protein 2 (CRBP2)') is crucial to this process. Most β-carotene is metabolized in the intestines, but any that is unchanged is incorporated into chylomicrons and released into the lymphatic system and thence into the bloodstream, where some is taken up by peripheral tissues before the remainder is absorbed by the liver.
Conversion to retinoids and eventually to retinol esters occurs in the enterocytes, where dietary β-carotene is subjected to oxidative cleavage at its centre, the first step of which is catalysed by a cytosolic enzyme β‑carotene-15,15'-oxygenase‑1 (BCO1), specific for carotenes with a β‑ionone ring, to yield two molecules of all-trans-retinal, which is reversibly reduced by a retinol reductase to retinol, much of which is then esterified with palmitic acid (with some 18:0 and 18:1). Xanthophylls, which differ from carotenoids in that they contain one or more oxygen atoms, are absorbed without cleavage mainly (see below). Retinol and retinol esters in the enterocytes are exported in lymph and plasma in lipoproteins (LDL and HDL) to the liver and other tissues with any unchanged carotenoids through the agency of binding and transport proteins.
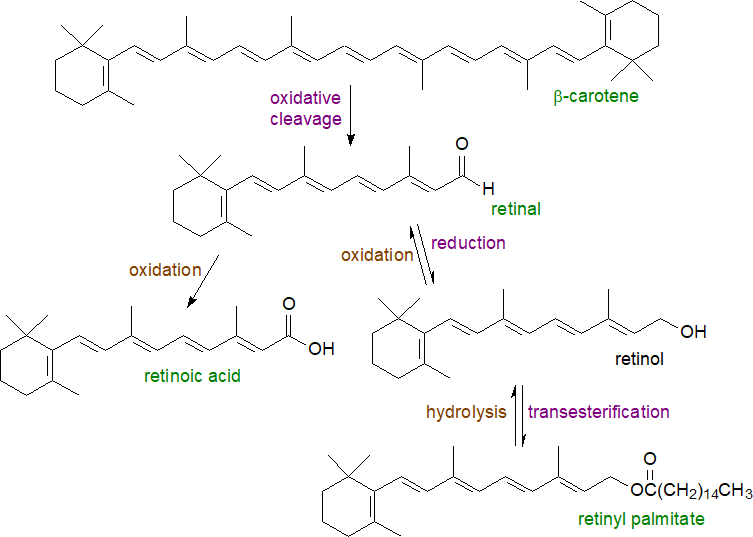 |
| Figure 1. Biosynthesis of all-trans retinoids in the intestines and liver. |
In the liver, induction of the retinol pathway starts with mobilization of the ester, followed by hydrolysis of this by retinol ester hydrolases, which include carboxylesterase ES‑10. Then, reversible oxidation of retinol to retinal is accomplished by one of several enzymes that include dehydrogenases and various cytochrome P450s, before some retinal is oxidized irreversibly to retinoic acid by further dehydrogenases (three forms). Both retinol and retinoic acid are precursors of other metabolites, which are required for particular purposes in tissues, by enzymatic modification of the functional groups and geometrical isomerization of the polyene chains. On demand, conversion of retinol to retinoic acid occurs by the same mechanisms in other tissues, although for vision, retinol esters serve directly as the substrate for the formation of the visual chromophore 11‑cis-retinal (see below). Retinyl-β-D-glucoside, retinyl-β-D-glucuronide and retinoyl-β-D-glucuronide are naturally occurring, metabolically active metabolites of vitamin A, which are found in fish and mammals. Indeed, the last acts in the same manner as all-trans-retinoic acid in some circumstances without any of the unwanted side effects.
Within the hepatocytes, retinol esters are hydrolysed in the late endosomes with release of free retinol into the cytosol, from which it can be released back into the circulation, converted to other retinoids or transferred to hepatic stellate cells for storage in lipid droplets, the main body reservoir of vitamin A. In these specialized cells, retinol is esterified to form retinyl palmitate by transfer of fatty acids from position sn-1 of phosphatidylcholine, mainly via the action of a membrane-bound lecithin:retinol acyltransferase (LRAT) in the endoplasmic reticulum. Lesser acyl-CoA dependent pathways include an acyl CoA:retinol acyltransferase and even the enzyme diacylglycerol acyltransferase 1 (DGAT1); esterification is facilitated by binding to RBP2.
 In
the aqueous environment within cells, as well as in the intestines and plasma, retinol, retinal and retinoic acid are
bound to retinoid-binding proteins (RBP), which solubilize, protect and in effect detoxify them.
These proteins have a role in facilitating retinoid transport and metabolism; some are present only in certain tissues,
and many are specific for particular retinoids and metabolic pathways.
To prevent infiltration through the kidneys, retinol and holo-RBP form an association in blood with a protein termed transthyretin (TTR),
which also serves as a thyroid hormone carrier and is essential for secretion, and normally, vitamin A circulates in plasma as a
retinol:RBP:TTR complex with a 1:1:1 molar ratio.
Unesterified retinol is the main form of the vitamin that is exported from the liver upon demand, and it is transported in blood in this
bound form in VLDL, LDL and HDL (see our web page on lipoproteins), with some directly from the
diet in the chylomicrons and their remnants.
Peripheral tissues have receptors to take up what they require, probably after hydrolysis of any esters to retinol by means
of the enzyme lipoprotein lipase.
Then, retinol dissociates from the protein as it forms a complex with a receptor (STRA6) at a target cell and diffuses through the plasma
membrane, a process driven by retinol esterification.
In
the aqueous environment within cells, as well as in the intestines and plasma, retinol, retinal and retinoic acid are
bound to retinoid-binding proteins (RBP), which solubilize, protect and in effect detoxify them.
These proteins have a role in facilitating retinoid transport and metabolism; some are present only in certain tissues,
and many are specific for particular retinoids and metabolic pathways.
To prevent infiltration through the kidneys, retinol and holo-RBP form an association in blood with a protein termed transthyretin (TTR),
which also serves as a thyroid hormone carrier and is essential for secretion, and normally, vitamin A circulates in plasma as a
retinol:RBP:TTR complex with a 1:1:1 molar ratio.
Unesterified retinol is the main form of the vitamin that is exported from the liver upon demand, and it is transported in blood in this
bound form in VLDL, LDL and HDL (see our web page on lipoproteins), with some directly from the
diet in the chylomicrons and their remnants.
Peripheral tissues have receptors to take up what they require, probably after hydrolysis of any esters to retinol by means
of the enzyme lipoprotein lipase.
Then, retinol dissociates from the protein as it forms a complex with a receptor (STRA6) at a target cell and diffuses through the plasma
membrane, a process driven by retinol esterification.
The RBP-TTR complex does not bind to retinal and retinoic acid, although these do bind to RBP on its own, and most of the low levels of retinoic acid transported in blood are bound to albumin. Local levels of retinoic acid are the result of an interplay between enzymes of synthesis, binding and catabolism, and within cells, retinoic acid binding proteins (CRABP1 and CRABP2) bind to the newly synthesised retinoic acid, increase its rate of metabolism and protect cells from an excess.
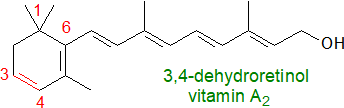 In skin, 3,4-dehydroretinoids, sometimes termed vitamin A2, are synthesized from all-trans retinoids by
the desaturase cytochrome P450 27C1 with the assistance of cellular retinol-binding proteins (retinoic acid is often added to skin care ointments).
Its derivative 3,4‑dehydroretinal is used as a visual chromophore in many cold-blooded vertebrates including lampreys, fish, amphibians
and some reptiles (see below).
In skin, 3,4-dehydroretinoids, sometimes termed vitamin A2, are synthesized from all-trans retinoids by
the desaturase cytochrome P450 27C1 with the assistance of cellular retinol-binding proteins (retinoic acid is often added to skin care ointments).
Its derivative 3,4‑dehydroretinal is used as a visual chromophore in many cold-blooded vertebrates including lampreys, fish, amphibians
and some reptiles (see below).
Cleavage of β-carotene at double bonds other than that in the centre or of a wider range of other carotenoids occurs to a limited extent by the action of a related enzyme β‑carotene-9',10'-dioxygenase (β‑carotene-oxygenase‑2 or BCO2) in mitochondria, which leads to the formation of comparable metabolites, i.e., β-apo-10′-carotenal and β-ionone, although other β-apo-carotenes of variable chain-length may be formed by enzymatic or non-enzymatic means. While these may have their own metabolic properties, there is evidence that they can be converted to acid, alcohol and thence inactive ester forms.
Although geranylgeranoic acid has structural similarities to retinoic acid and has been termed an acyclic retinoid, it has no vitamin A activity; it is synthesised in animal tissues from mevalonate and together with its 2,3‑dihydro metabolite is a potent anticancer agent in that it induces cell death in human hepatoma-derived cell lines by a mechanism of pyroptosis initiated by TLR4 signalling. It is present in some plant foods, includling turmeric, almonds, azuki beans and soybeans.
Retinol esters: A relatively small proportion of the cellular retinoids is located in membranes in tissues. Rather, retinol esters, mainly retinyl palmitate, are the main storage form of vitamin A, and they occur in many different organs, including adipose tissue and testes, but chiefly in stellate cells of the liver and pancreas. How the retinol is directed to these particular cells and enters them prior to esterification is not known. Although hepatic stellate cells are much smaller and less abundant than hepatocytes (only 5 to 8% of all liver cells), they are characterized by cytoplasmic lipid droplets that contain 90-95% of the hepatic retinoids (and up to 80% of the body pool) with other non-retinoid lipids; the lecithin:retinol acyltransferase is the only retinol ester synthase and transfers a fatty acyl moiety from membrane phosphatidylcholine to form retinol esters. When the supply of retinol in the diet is limited, hepatic stores of retinol esters are mobilized as retinol ester hydrolases maintain constant circulating retinol levels; hormone-sensitive lipase is the most efficient of these enzymes, although the adipose tissue triacylglycerol lipase and the lysosomal acid lipase can participate.
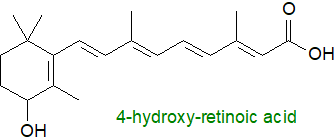 Catabolism: All-trans-retinoic acid formation is irreversible, so its synthesis and degradation
must be tightly regulated.
As a first step in catabolism, the excess is cleared by conversion to more polar metabolites through oxidation by a distinctive set of enzymes of
the cytochrome P450 family.
Secondly, the water-soluble retinoic acid metabolites, including 4-hydroxy-, 4-oxo- and 18-hydroxy-retinoic acids,
conjugate with glucuronic acid and then can be rapidly removed from circulation for elimination from the body via the kidney.
It appears to be an open question as to whether 4‑hydroxy- and 4-oxo-retinoic acids are required for any cellular processes although they may
have some biological activity in hematopoietic stem cells.
Catabolism: All-trans-retinoic acid formation is irreversible, so its synthesis and degradation
must be tightly regulated.
As a first step in catabolism, the excess is cleared by conversion to more polar metabolites through oxidation by a distinctive set of enzymes of
the cytochrome P450 family.
Secondly, the water-soluble retinoic acid metabolites, including 4-hydroxy-, 4-oxo- and 18-hydroxy-retinoic acids,
conjugate with glucuronic acid and then can be rapidly removed from circulation for elimination from the body via the kidney.
It appears to be an open question as to whether 4‑hydroxy- and 4-oxo-retinoic acids are required for any cellular processes although they may
have some biological activity in hematopoietic stem cells.
2. Retinoids and Vision
It has long been known that retinoids are essential for vision, and there is now a good appreciation of how this works at the molecular level. In the eye, uptake of retinol from the circulation is mediated by the transmembrane cell-surface STRA6 receptor of the retinal pigment epithelium (RPE), a pigmented monolayer of cells located between the photoreceptors and choroid that nourishes retinal visual cells, catalyses the release of retinol from retinol-binding proteins and transports it to the cytosol.
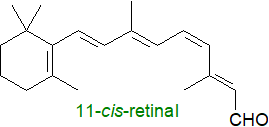 The process by which light is converted to a signal recognized by the brain, sometimes termed the
'retinoid (visual) cycle', requires a two-cell system beginning in the RPE and continuing in photoreceptor cells,
i.e., retinal rod and cone cells in the eye containing membranous vesicles that serve as light receptors.
Roughly half of the proteins in these vesicles consist of the protein conjugate, rhodopsin (a member of the superfamily of G protein–coupled
receptors (GPCRs)), which comprises a protein, opsin, covalently linked at Lys296 as a Schiff’s base to the retinoid
11‑cis-retinal with the photoreactive group.
Such is the sensitivity of this receptor that absorption of a single photon by a rhodopsin molecule is sufficient to trigger a neuronal
response.
Each step in the visual process requires specific binding or transport proteins and especially the interphotoreceptor retinoid-binding protein
(IRBP).
The process by which light is converted to a signal recognized by the brain, sometimes termed the
'retinoid (visual) cycle', requires a two-cell system beginning in the RPE and continuing in photoreceptor cells,
i.e., retinal rod and cone cells in the eye containing membranous vesicles that serve as light receptors.
Roughly half of the proteins in these vesicles consist of the protein conjugate, rhodopsin (a member of the superfamily of G protein–coupled
receptors (GPCRs)), which comprises a protein, opsin, covalently linked at Lys296 as a Schiff’s base to the retinoid
11‑cis-retinal with the photoreactive group.
Such is the sensitivity of this receptor that absorption of a single photon by a rhodopsin molecule is sufficient to trigger a neuronal
response.
Each step in the visual process requires specific binding or transport proteins and especially the interphotoreceptor retinoid-binding protein
(IRBP).
All-trans-retinol in the RPE is first converted to its ester by the enzyme lecithin:retinol acyltransferase as described above, and the products coalesce into lipid droplets, i.e., dynamic organelles termed 'retinosomes', for storage of the excess, a process driven by the protein seipin and fat storage-inducing transmembrane protein 2. At the start of the visual cycle, retinol esters and some free retinol, produced by the action of the retinol hydrolase - patatin-like phospholipase domain containing 2 (PNPLA2), are transported to the endoplasmic reticulum where a dual-purpose enzyme (RPE65 + NAD+) cleaves the O‑alkyl bond (not a conventional hydrolysis reaction) in the retinol ester and at the same time changes the geometry of the double bond in position 11 of retinol from trans to cis. The 11‑cis-retinol is then oxidized to 11-cis-retinal by 11-cis-retinal dehydrogenase (RDH5).
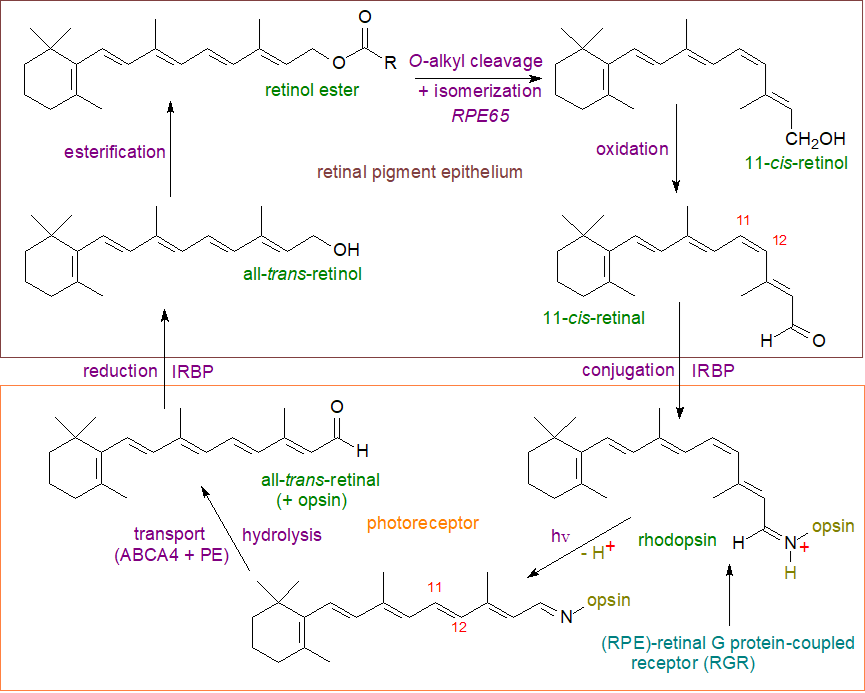 |
| Figure 2. Retinol and the visual cycle. |
The next stage of the cycle occurs in the photoreceptor, where first the 11-cis-retinal is reacted with opsin to produce the protein conjugate rhodopsin in a protonated form (Schiff base reaction). When rhodopsin is activated by light, the cis-double bond in the retinoid component is isomerized non-enzymatically by the energy of a photon to the 11‑trans form (in only 200 femtoseconds) with a change of conformation and deprotonation that in turn affect the permeability of the membrane and influence calcium transport. This results in further molecular changes that culminate in the release of opsin and all-trans-retinal, which is the trigger that sets off the nerve impulse for light to be perceived by the brain.
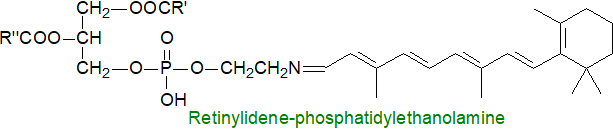
The all-trans-retinal is removed from the photoreceptor either by reduction to all-trans-retinol by the enzyme all-trans-retinol dehydrogenase 8 (RDH8) expressed in the outer segments of photoreceptors or after transport by means of the transporter ABCA4. This provides phosphatidylethanolamine (PE) for conversion to the Schiff-base adduct, i.e., N-retinylidene-phosphatidylethanolamine, which it flips from the lumen to the cytosolic leaflet of the disc membrane. Non-specific aldehyde reactions are prevented by this process because it removes potentially toxic retinoid compounds from the photoreceptors. The adduct is a transient sink that dissociates so the retinal can be reduced back to all-trans-retinol by the cytoplasmic retinol dehydrogenase (RDH8/12). All-trans-retinol exits the photoreceptor and enters the retinal pigment epithelium with the aid of retinoid-binding protein (IRBP) where it is converted back by a retinol dehydrogenase to a retinyl ester to complete the cycle and restore light sensitivity.
A second mechanism for 11-cis-retinal formation that may ensure continuous visual responsiveness in bright light utilizes the (RPE)-retinal G protein-coupled receptor (RGR), which can operate as a retinaldehyde photoisomerase in Müller glia. As the enzyme RPE65 is most efficient under low light conditions, RGR prevents the saturation of photoreceptors under high light levels and in this way facilitates vision in daylight. The isomerase, RPE65, and the photoisomerase, RGR, work together to provide a sustained supply of the visual chromophore under different levels of illumination.
As a side-reaction, some troublesome bis-retinoid adducts of PE (and further byproducts) may be produced by non-enzymatic mechanisms, and these can accumulate with age to affect vision (see our web page on phosphatidylethanolamine).
Lower organisms: Bacteriorhodopsin is the best studied of a family of opsins found in archaea, eubacteria, fungi and algae. It is a protein with seven transmembrane domains that acts as an opto-electrical transducer or light-gated ion pump to capture photon energy via its covalently bound chromophore, all-trans-retinal, converting it to 13-cis-retinal, and it moves protons against their electrochemical gradient from the cytoplasm to the extracellular space. In Archaea, it is known as the "purple membrane" and can occupy a high proportion of the surface area of the organism.
3. Retinoids in Health and Disease
As well as their role in vision, it is now realized that retinoids are required for growth and development, reproduction and resistance to infection. They are necessary for the optimum function of epithelial cells in the digestive tract, lungs, nervous system, immune system, skin and bone at all stages of life, but they are also needed for the regeneration of damaged tissues, including the heart, and they have some potential as chemo-preventive agents for cancer and for the treatment of skin diseases such as acne. Under pathological conditions, stellate cells lose their retinoid content and transform into fibroblast-like cells, contributing to the fibrogenic response. Cirrhosis of the liver is accompanied by a massive loss of retinoids, but it is uncertain whether this is a cause or a symptom, and there appears to be confusion as to when supplementation is helpful in this and other diseases of the liver. Like retinol and retinoic acid, the metabolite 9-cis-retinoic acid has value as a pharmaceutical.
With so many double bonds in conjugation, it is not surprising that carotenoids in general and retinoids in particular are efficient quenchers of singlet oxygen and scavengers of other reactive oxygen species (ROS), but any direct antioxidant properties may not be major factors in terms of general health in vivo. There is a caveat that retinoids may induce transcription of some antioxidant genes and so may act indirectly as antioxidants. In contrast, nutritional studies with dietary supplements of carotenoids have sometimes suggested that they can act as pro-oxidants, for which one explanation may be that regeneration of the parent carotenoid or retinoid from the corresponding radical cation may be limited when concentrations of reductants such as ascorbic acid are low. It is not clear how relevant the physical properties of retinoids are to biochemical processes in comparison to their effects on signalling and gene transcription.
Many of the retinol metabolites are ligands that stimulate transcription factors for receptors in the nucleus of the cell, and thus they control the expression of many genes (>500), including those necessary for the maintenance of normal cell proliferation and differentiation, embryogenesis, for a healthy immune system, and for male and female reproduction. In the innate immune system, vitamin A is required for the differentiation of cells such as macrophages, neutrophils and natural killer cells, while retinoic acid has a role in differentiating the precursors of dendritic cells. all-trans-Retinoic acid and its 9-cis-isomer are important in this context, and they are often considered the most active retinoids other than in the eye. On the other hand, there is a need for retinal other than in the eye, and in mice, oxidation of retinol to retinal is required for survival during embryogenesis, with differences in the response of males and females.
 To act upon genes, retinoic acid moves to the nucleus with the aid
of small intracellular lipid-binding proteins (CRABP2 and FABP5), which channel it to nuclear receptors, i.e.,
the retinoic acid receptors (RAR) of which there are three, RAR-α, β and γ.
These are ligand-dependent regulators of transcription, and they operate in vivo
as heterodimers with retinoid X receptors (RXR) to process the retinoic acid signal
by acting through polymorphic retinoic acid response elements (RAREs) within the promoter regions of responsive genes.
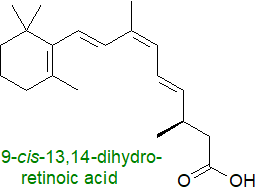
Similarly, 9-cis-retinoic acid and 9-cis-13,14-dihydroretinoic acid are high-affinity ligands for RXR in mice.
Together with retinoic acid, these are also ligands for the farnesoid X receptor (FXR), which forms a heterodimer with RXR.
The latter receptor complex is involved primarily in bile acid homeostasis, and
conversely, there are suggestions that bile acids may influence the regulation of vitamin A homeostasis.
9-cis-β,β-Carotene and 9-cis-13,14-dihydroretinol, precursors for 9-cis-13,14-dihydroretinoic acid,
have been termed 'vitamin A5'.
To act upon genes, retinoic acid moves to the nucleus with the aid
of small intracellular lipid-binding proteins (CRABP2 and FABP5), which channel it to nuclear receptors, i.e.,
the retinoic acid receptors (RAR) of which there are three, RAR-α, β and γ.
These are ligand-dependent regulators of transcription, and they operate in vivo
as heterodimers with retinoid X receptors (RXR) to process the retinoic acid signal
by acting through polymorphic retinoic acid response elements (RAREs) within the promoter regions of responsive genes.
Similarly, 9-cis-retinoic acid and 9-cis-13,14-dihydroretinoic acid are high-affinity ligands for RXR in mice.
Together with retinoic acid, these are also ligands for the farnesoid X receptor (FXR), which forms a heterodimer with RXR.
The latter receptor complex is involved primarily in bile acid homeostasis, and
conversely, there are suggestions that bile acids may influence the regulation of vitamin A homeostasis.
9-cis-β,β-Carotene and 9-cis-13,14-dihydroretinol, precursors for 9-cis-13,14-dihydroretinoic acid,
have been termed 'vitamin A5'.
In addition, the nuclear receptor peroxisome proliferator-activated receptor PPARγ forms a heterodimer with the retinoid X receptor and is stimulated by retinoic acid to recruit cofactors. This complex in turn binds to the peroxisome proliferator response element (PPRE) gene promoter, leading mainly to regulation of those genes for lipid and glucose metabolism, including some for inflammation and cancer. To add to the complexity, retinoic acid has extra-nuclear, non-transcriptional effects, such as the activation of protein kinases and other signalling pathways.
 Many of the functions
of retinoids are mediated via the action of specific binding proteins (as discussed briefly above), which control their metabolism
in vivo by reducing the available or free retinoid concentrations, by protecting them from unwanted chemical attack,
and by presenting them to enzyme systems in an appropriate conformation.
With some tissues, retinol-bound RBP in blood is recognized by the membrane protein STRA6,
which transports retinol into cells where it binds to an intracellular retinol acceptor,
cellular retinol-binding protein 1 (CRBP1) and is then able to induce a signalling cascade that targets appropriate genes.
A retinol-binding protein secreted by adipose tissue (RPB4) is involved in the development of insulin resistance and
type 2 diabetes, possibly by affecting glucose utilization by muscle tissue, with obvious application to controlling obesity.
In the eye, retinoic acid metabolism during development is controlled by binding to apolipoprotein A1.
Many of the functions
of retinoids are mediated via the action of specific binding proteins (as discussed briefly above), which control their metabolism
in vivo by reducing the available or free retinoid concentrations, by protecting them from unwanted chemical attack,
and by presenting them to enzyme systems in an appropriate conformation.
With some tissues, retinol-bound RBP in blood is recognized by the membrane protein STRA6,
which transports retinol into cells where it binds to an intracellular retinol acceptor,
cellular retinol-binding protein 1 (CRBP1) and is then able to induce a signalling cascade that targets appropriate genes.
A retinol-binding protein secreted by adipose tissue (RPB4) is involved in the development of insulin resistance and
type 2 diabetes, possibly by affecting glucose utilization by muscle tissue, with obvious application to controlling obesity.
In the eye, retinoic acid metabolism during development is controlled by binding to apolipoprotein A1.
All-trans-retinoic acid has been shown to be effective against many different types of human cancers in model systems and in some clinical trials because of how it affects cell proliferation, differentiation and apoptosis (where its relatively low toxicity at normal tissue levels is a virtue). For example, it induces complete remission in most of cases of acute promyelocytic leukaemia when administered in combination with other chemotherapy techniques. 13‑cis-Retinoic acid has been used successfully in the treatment of children with high-risk neuroblastoma to reduce the risk of recurrence and increase long-term survival rates, but the efficacy of such treatments against other types of acute myeloid leukaemia and solid tumours is poor. It is hoped that current efforts to obtain a better understanding of how it operates as an anticancer agent will lead to improved treatments, especially in relation to interactions with ceramide metabolism. Synthetic analogues of retinoic acid, termed rexinoids, which bind to retinoic X receptors, hold promise as anti-cancer drugs. By regulating lipid metabolism, inflammation and thermogenesis, retinoic acid inhibits the development and progression of non-alcoholic fatty liver disease.
Vitamin A deficiency in children and adult patients is usually accompanied by impairment of the immune system, leading to a greater susceptibility to infection and an increased mortality rate, often with growth retardation and congenital malformations. Although it is so easily prevented, vitamin A deficiency in malnourished children is the major reason for childhood mortality in the underdeveloped world, causing over 650,000 early childhood deaths annually and paediatric blindness. In adults, vitamin A deprivation affects the reproductive system, inhibiting spermatogenesis in males and ovulation in females. Unfortunately, it is not always easy to distinguish between the symptoms of vitamin A deficiency and primary defects of retinoid signalling. In contrast, an excess of vitamin A can cause liver damage and fibrosis.
4. Xanthophylls and Other Carotenoids in Human Metabolism
While most research effort has been focused on retinoids, there is an increasing interest on the actions of certain intact carotenoids in animal tissues. Xanthophylls are plant C40 tetraterpenes that differ from the carotenoids in having oxygen atoms in the ring structures (hydroxyl, oxo or epoxyl). Lutein, zeaxanthin and meso-zeaxanthin from dietary sources, such as green leafy vegetables and yellow and orange fruits and vegetables, are found specifically in membranes of the macula of the eye in humans and other primates, i.e., the functional centre of the retina in a small central pit known as the macula lutea, where they enhance visual acuity and protect the eye from high-intensity, short-wavelength (blue) light. They are powerful antioxidants in a region vulnerable to light-induced oxidative stress, and they decrease the risk of age-related macular degeneration. When exposed to oxygen, there is a change from the all-trans form to the 13-cis configuration, which changes the alignment of the molecule in the membrane and limits the penetration of molecular oxygen. Binding proteins for lutein- and zeaxanthin mediate the highly selective uptake of these carotenoids into the retina, but meso-zeaxanthin is mainly a metabolite of dietary lutein.

Many other carotenoids are absorbed from the diet and are subject to oxidative cleavage or other catabolic processes, partly in the intestines and partly in other tissues after transport in the lipoproteins. Hydroxylated xanthophylls such as lutein occur both in the free form and esterified to fatty acids (by phytyl ester synthases); the latter are hydrolysed in the intestines when consumed by animals. Carotene can be absorbed at the placental barrier and transferred to the foetus for conversion to retinoids that are needed for development purposes. In the brain, they can stimulate and maintain cognition in the elderly and assist with brain development in infants. Some carotenoids that remain intact act as antioxidants, and some may have anti-inflammatory actions by acting through established signalling pathways, including via transcription factors such as nuclear factor kappa B and nuclear factor erythroid-2-related factor 2. In relation to the immune system and cellular differentiation, they interact with nuclear receptors, such as the retinoic acid receptor/retinoid X receptor and PPARs. Carotenoids accumulate in the skin of mammals, where they have an antioxidant and photo-protective role as well as affecting the moisture content, texture and elasticity. In humans, the composition of skin carotenoids reflects that in plasma and can be measured noninvasively by optical methods.
Dietary carotenoids such as lycopene in the circulation have been associated with reduction of risk for several chronic diseases, including type 2 diabetes, certain types of cancer, atherogenesis, coronary heart disease and even lower total mortality, but their sensitivity to pH, light exposure and temperature has limited their use in therapy. In mice, zeaxanthin is an immunomodulator that has been shown to enhance the ability of CD8+ T cells to respond to tumour cells. Because of its increasing availability by synthesis or from micro-algae, astaxanthin, a red-pink pigment and member of the xanthophyll family, is attracting great interest and may have health benefits for humans.
5. Carotenoids in Plant Metabolism
Carotenoids have many vital functions in plants during photosynthesis or as precursors of plant hormones that can only be discussed briefly here; there is an online database of carotenoid structures and properties. As they have a polyene chain of 9 to 11 double bonds in conjugation, they absorb light in the gap of chlorophyll absorption and so they are additional light-harvesting pigments in plants. Their distinctive arrangement of electronic levels gives them the capacity to transfer excitation energy from the carotenoid excited state to chlorophyll in the light-harvesting complex (photosystem II), but energy can also be transferred back from chlorophyll to carotenoids as a photoprotection mechanism. The energy is transferred from chlorophyll to the polyene tail of the carotenoid where electrons are moved between the carotenoid bonds until the most balanced or lowest energy state is reached. During photosynthesis, damaging species are produced by both light and oxygen with ROS of special concern, and while there is appreciable potential for carotenoids to act as antioxidants in plants, it is uncertain how important this is in practice. The length of the polyene tail of carotenoids determines which wavelengths of light will be absorbed by the plant, and those not absorbed are reflected and so determine colouration.
Biosynthesis of carotenoids occurs via isopentenyl diphosphate and dimethylallyl diphosphate by the methylerythritol 4-phosphate (MEP) route with phytoene as a key intermediate, and the mechanism has much in common with that of the plant sterols, although this is too specialized a topic to be treated at length here. With the aim of preventing vitamin A deficiency in the populations of developing countries, conventional breeding and genetic engineering have been employed to produce some crop plants with increased carotene levels, e.g., 'golden rice', and further efforts are underway. Non-photosynthetic bacteria produce a different range of carotenoids, some with chain lengths other than C40 (C30 to C50), and at least three biosynthetic mechanisms are known depending on species that proceed via phytoene (C40), diapophytoene (C30) or squalene (C30) as intermediates.
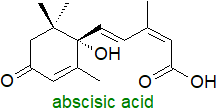 Carotenoids are precursors for two plant hormones and a diverse set of apocarotenoids including
abscisic acid, a C15 isoprenoid plant hormone synthesised in plastids from the C40 carotenoid
zeaxanthin.
A series of enzyme-catalysed epoxidations and isomerizations are followed by cleavage of the intermediate product by
a dioxygenation reaction and further oxidations to yield eventually abscisic acid.
Acting via signalling cascades, often in conjunction with oxylipins, abscisic acid
regulates innumerable metabolic processes in plants in relation to developmental processes that including growth, seed
and bud dormancy, embryo maturation and germination, cell division and elongation, floral growth and the control of stomatal closure.
It is critical for the responses to environmental stresses, that include drought, cold and heat stress, salinity and tolerance of heavy
metal ions.
Carotenoids are precursors for two plant hormones and a diverse set of apocarotenoids including
abscisic acid, a C15 isoprenoid plant hormone synthesised in plastids from the C40 carotenoid
zeaxanthin.
A series of enzyme-catalysed epoxidations and isomerizations are followed by cleavage of the intermediate product by
a dioxygenation reaction and further oxidations to yield eventually abscisic acid.
Acting via signalling cascades, often in conjunction with oxylipins, abscisic acid
regulates innumerable metabolic processes in plants in relation to developmental processes that including growth, seed
and bud dormancy, embryo maturation and germination, cell division and elongation, floral growth and the control of stomatal closure.
It is critical for the responses to environmental stresses, that include drought, cold and heat stress, salinity and tolerance of heavy
metal ions.
Strigolactones are C15 oxidation products of carotenoids that are both hormones, which regulate growth and development, and rhizosphere signalling molecules, which induce symbiosis with arbuscular mycorrhizal fungi. They adapt plant architecture to nutrient availability by controlling bud growth in stem terminals to stimulate branching, and when necessary, they terminate it.
Recommended Reading
- Blaner, W.S., Shmarakov, I.O. and Traber, M.G. Vitamin A and vitamin E: will the real antioxidant please stand up? Annu. Rev. Nutr., 41, 105-131 (2021); DOI.
- Carmona, R., Barrena, S. and Munoz-Chapuli, R. Retinoids in stellate cells: development, repair, and regeneration. J. Dev. Biol., 7, 10 (2019); DOI.
- Chen, G.X., Weiskirchen, S. and Weiskirchen, R. Vitamin A: too good to be bad? Front. Pharm., 14, 1186336 (2023); DOI.
- Choi, E.H., Daruwalla, A., Suh, S., Leinonen, H. and Palczewski, K. Retinoids in the visual cycle: role of the retinal G protein-coupled receptor. J. Lipid Res., 62, 100040 (2021); DOI - part of a Thematic Review Series: Seeing 2020: Lipids and Lipid-Soluble Molecules in the Eye.
- Demmig-Adams, B., Hodges, A.K., Polutchko, S.K. and Adams, W.W. Zeaxanthin and other carotenoids: roles in abiotic stress defense with implications for biotic defense. Plants-Basel, 14, 2703 (2025); DOI.
- di Masi, A., Leboffe, L., De Marinis, E., Pagano, F., Cicconi, L., Rochette-Egly, C., Lo-Coco, F., Ascenzi, P. and Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Aspects Med., 41, 1-115 (2015); DOI.
- Edge, R. and Truscott, T.G. Singlet oxygen and free radical reactions of retinoids and carotenoids - a review. Antioxidants, 7, 5 (2018); DOI.
- Gudas, L.J. Retinoid metabolism: new insights. J. Mol. Endocrin., 69, T37–T49 (2022) DOI - and more related articles in this special journal issue.
- Haaker, M.W., Vaandrager, A.B. and Helms, J.B. Retinoids in health and disease: A role for hepatic stellate cells in affecting retinoid levels. Biochim. Biophys. Acta, Lipids, 1865, 158674 (2020); DOI.
- Hajeer, W., Blanco, A., Miller, A.P. and Amengual, J. Recent advances in carotenoid absorption, distribution, and elimination. Biochim. Biophys. Acta, Lipids, 1870, 159619 (2025); DOI - and a series of related articles in this journal volume.
- Harrison, E.H. Carotenoids, β-apocarotenoids, and retinoids: the long and the short of it. Nutrients, 14, 1411 (2022); DOI - and more related articles in this special journal issue.
- Kono, N. and Arai, H. Intracellular transport of fat-soluble vitamins A and E. Traffic, 16, 19-34 (2015); DOI.
- Noy, N. Signaling by retinol and its serum binding protein. PLEFA, 93, 3-7 (2015); DOI.
- Reboul, R. Proteins involved in fat-soluble vitamin and carotenoid transport across the intestinal cells: New insights from the past decade. Prog. Lipid Res., 89, 101208 (2023); DOI.
- Rodriguez-Concepcion, M., Avalos, J., Bonet, M.L., Boronat, A., Gomez-Gomez, L., Hornero-Mendez, D., Limon, M.C., Meléndez-Martínez, A.J. and Olmedilla-Alonso, B. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res., 70, 62-93 (2018); DOI.
- Rühl, R., Krezel, W. and de Lera, A.R. 9-Cis-13,14-dihydroretinoic acid, a new endogenous mammalian ligand of retinoid X receptor and the active ligand of a potential new vitamin A category: vitamin A(5). Nutr. Rev., 76, 929-941 (2018); DOI.
- Saeed, A., Hoekstra, M., Hoeke, M.O., Heegsma, J. and Faber, K.N. The interrelationship between bile acid and vitamin A homeostasis. Biochim. Biophys. Acta, Lipids, 1862, 496-512 (2017); DOI.
- Sato S. and Kefalov, V.J. The retina-based visual cycle. Annu. Rev. Vision Sci., 10, 293-321 (2024); DOI.
- Uray, I.P., Dmitrovsky, E. and Brown, P.H. Retinoids and rexinoids in cancer prevention: from laboratory to clinic. Seminars Oncology, 43, 49-64 (2016); DOI.
- Yadav, A.S., Isoherranen, N. and Rubinow, K.B. Vitamin A homeostasis and cardiometabolic disease in humans: lost in translation? J. Mol. Endocrin., 69, R95-R108 (2022); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
