Eicosanoids and Other Oxylipins
An Introduction
The chemistry, biochemistry, pharmacology and molecular biology of eicosanoids and related lipids, including the octadecanoids, docosanoids and plant oxylipins, are vast, complex and occasionally contradictory subjects that continue to develop at an extraordinarily rapid rate. The only generic term that adequately covers all the relevant metabolites is oxylipin, defined by Hamberg and colleagues in 1991 (DOI) as a family of oxygenated natural products that are formed from unsaturated fatty acids by pathways with at least one step of mono- or dioxygen-dependent oxidation and so can contain hydroperoxyl, hydroxyl, epoxy, oxo and/or endoperoxide substituents. To add to this brief definition, they are usually described as being synthesised in situ when needed in response to biological stimuli while having a short half-life and acting locally via interactions with receptors or other intracellular effectors. Professors Bengt Samuelsson, Sune Bergström and John Vane were honoured by the award of the Nobel Prize for Medicine in 1982 for their discoveries in this field.
 The term
eicosanoid is used to embrace those lipid mediators
derived from C20 polyunsaturated fatty acids, and including prostaglandins, thromboxanes,
leukotrienes, hydroxyeicosatetraenoic acids
and related oxygenated derivatives.
Note that the preferred IUPAC name is ‘icosanoid’, but this is largely ignored in the scientific literature.
The main precursor is 5Z,8Z,11Z,14Z-eicosatetraenoic acid (arachidonic or 20:4(n‑6)),
although 8Z,11Z,14Z-eicosatrienoic (dihomo-γ-linolenic or 20:3(n‑6))
and 5Z,8Z,11Z,14Z,17Z-eicosapentaenoic (20:5(n‑3) or EPA) acids are also relevant
(see our web page on 'polyunsaturated fatty acids').
While these have been of special significance from a historical perspective, it is now impossible to discuss such lipids
without considering the docosanoids, i.e., protectins, resolvins and maresins
or 'specialized pro-resolving mediators', derived from 4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoic
acid (22:6(n‑3) or DHA) and other polyunsaturated fatty acids of the n‑3 family (E‑series resolvins from EPA
are eicosanoids).
Linoleate and other C18 fatty acids are precursors for octadecanoids.
Related C20 and C22 oxylipins from non-enzymic reactions (autoxidation) include the
isoprostanes.
Similarly, plant metabolites such as the jasmonates and other oxylipins derived from
9Z,12Z,15Z-octadecatrienoic (α‑linolenic or 18:3(n‑3)) acid have some comparable structural features
and functions, as do oxylipins from fungi and bacteria.
The term
eicosanoid is used to embrace those lipid mediators
derived from C20 polyunsaturated fatty acids, and including prostaglandins, thromboxanes,
leukotrienes, hydroxyeicosatetraenoic acids
and related oxygenated derivatives.
Note that the preferred IUPAC name is ‘icosanoid’, but this is largely ignored in the scientific literature.
The main precursor is 5Z,8Z,11Z,14Z-eicosatetraenoic acid (arachidonic or 20:4(n‑6)),
although 8Z,11Z,14Z-eicosatrienoic (dihomo-γ-linolenic or 20:3(n‑6))
and 5Z,8Z,11Z,14Z,17Z-eicosapentaenoic (20:5(n‑3) or EPA) acids are also relevant
(see our web page on 'polyunsaturated fatty acids').
While these have been of special significance from a historical perspective, it is now impossible to discuss such lipids
without considering the docosanoids, i.e., protectins, resolvins and maresins
or 'specialized pro-resolving mediators', derived from 4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoic
acid (22:6(n‑3) or DHA) and other polyunsaturated fatty acids of the n‑3 family (E‑series resolvins from EPA
are eicosanoids).
Linoleate and other C18 fatty acids are precursors for octadecanoids.
Related C20 and C22 oxylipins from non-enzymic reactions (autoxidation) include the
isoprostanes.
Similarly, plant metabolites such as the jasmonates and other oxylipins derived from
9Z,12Z,15Z-octadecatrienoic (α‑linolenic or 18:3(n‑3)) acid have some comparable structural features
and functions, as do oxylipins from fungi and bacteria.
It is noteworthy that the precursors for these metabolites belong to either the omega-6 (n-6) or the omega-3 (n-3) families of polyunsaturated fatty acids. As oxylipins are so numerous and operate in so many different ways, they must provide a substantial component of the reason for the essentiality of these fatty acids for the survival and well-being of animals.
Other oxygenated fatty acids occur in nature that may not be lipid mediators, and these are discussed in a further web page here..., while the Fatty Acid esters of Hydroxy Fatty Acids (FAHFA), discovered relatively recently, should perhaps be classified as oxylipins now from a functional perspective. Radical nitrogen species can react with unsaturated fatty acids to generate nitro fatty acids, which are anti-inflammatory agents, and those aldehydes arising from cleavage of fatty acid hydroperoxides must be discussed in the context of lipid mediators also. While unesterified oxylipins are of primary importance, it is becoming evident that some may operate in an esterified state, including as oxidized phospholipids, endocannabinoids and cholesterol esters (all with separate web pages on this site).
A further collective term - the epilipidome - is increasingly being applied that encompasses these and other lipid mediators, i.e., a subset of the natural lipidome formed by lipid modifications via enzymatic and non-enzymatic reactions (e.g., oxidation, nitration, sulfation and halogenation) and required to regulate complex biological actions.
These pages are intended only as a broad overview of the topic that can be understood by scientists with some knowledge of lipids in general. In this document, I introduce some basic concepts and discuss the primary rate-limiting enzyme for eicosanoid biosynthesis in animal tissues, i.e., phospholipase A2. The mechanism for catabolism of oxylipins is common to most eicosanoid classes and for convenience is discussed below. Further web pages in this series explore the chemistry, biochemistry and function of the various types of oxylipin, although I am conscious that my approach may not adequately describe the true complexity of the interactions that occur in terms of metabolism, signalling or physiology, where the balance between different oxylipins and their pro- and anti-inflammatory effects is crucial. More than 500 oxylipins have been detected in human plasma, many of unknown origin and not fully characterized, and each of these may have its own role in metabolism. Those requiring a deeper insight should consult the publications cited below and the other documents and references in this section of the website.
It should not be forgotten that many fatty acids that lack oxygenated substituents, even such simple saturated fatty acids as palmitic, can have distinctive biological functions other than their role in storage lipids and membranes.
1. The Elements
 Of those fatty acids relevant to animal metabolism, arachidonic acid has been by far the most studied, and it is special in many ways.
It is an essential fatty acid in that it cannot be synthesised
de novo in animals, and linoleic acid from the diet is required as the primary precursor.
As a major component of phospholipids such as phosphatidylinositol, it is a factor in the integrity of cellular membranes.
The four double bonds with a cis or Z-configuration mean that the molecule is highly flexible, and this helps to confer an
appropriate degree of fluidity to membranes.
Of those fatty acids relevant to animal metabolism, arachidonic acid has been by far the most studied, and it is special in many ways.
It is an essential fatty acid in that it cannot be synthesised
de novo in animals, and linoleic acid from the diet is required as the primary precursor.
As a major component of phospholipids such as phosphatidylinositol, it is a factor in the integrity of cellular membranes.
The four double bonds with a cis or Z-configuration mean that the molecule is highly flexible, and this helps to confer an
appropriate degree of fluidity to membranes.
Even when arachidonic acid is esterified, lipids can sometimes act biologically, and for example, diacylglycerols enriched in arachidonic acid and derived from phosphatidylinositol are cellular messengers. Anandamide or N‑arachidonoylethanolamine and 2‑arachidonoyl-glycerol are endogenous cannabinoids or 'endocannabinoids', which induce neurobehavioral responses like those of the phytocannabinoids from cannabis and have signalling roles in the central nervous system, especially in the perception of pain and in the control of appetite. Indeed, there are suggestions that arachidonic acid per se may have some distinctive function in animal tissues, and for example, the cellular level of unesterified arachidonic acid may be one mechanism by which apoptosis is regulated. It is reported to be a safe protective agent against blood flukes of the genus Schistosoma by inducing the tegument-associated neutral sphingomyelinase of the parasites to disrupt the membrane.
The oxygenated metabolites derived from arachidonic and related fatty acids are produced through a series of complex interrelated biosynthetic pathways that is sometimes termed the 'arachidonate or eicosanoid cascade', and the structures of some of these eicosanoids and other oxylipins are illustrated below.
 |
| Figure 1. Structures of some key oxylipins from animal tissues. |
The prostanoids (prostaglandins, thromboxanes and prostacyclins) were the first of the eicosanoids to attract major scientific interest and have ring structures in the centre of the molecule. While the hydroxyeicosatetraenes are apparently simpler in structure, they are precursors for families of more complex molecules, such as the leukotrienes and lipoxins. Most eicosanoids are synthesised enzymatically with great stereochemical precision, and this is necessary for their functions. They are highly potent in the nanomolar range in vitro in the innumerable activities that have been defined, mainly in relation to inflammatory responses, pain and fever. Most organs and cell types produce them, but with a high degree of tissue specificity, and some are even synthesised cooperatively between cells.
 While, many of the documents in this section of the website deal with these oxylipins, it should not be forgotten the primary
metabolites of both non-enzymic and enzymic oxidation are innumerable different hydroperoxides from all endogenous unsaturated fatty acids, either
in the esterified or free state, and these have their own biological properties. This has become evident in recent years especially in relation to
the phenomenon of ferroptosis, characterized by intracellular iron accumulation and an unrestricted build-up of lipid peroxides to such
an extent that cellular membranes, including the plasma membrane, are disrupted to trigger lethal ion imbalances,
membrane permeabilization and ultimately cell rupture (see our web page on
oxidized phospholipids).
While, many of the documents in this section of the website deal with these oxylipins, it should not be forgotten the primary
metabolites of both non-enzymic and enzymic oxidation are innumerable different hydroperoxides from all endogenous unsaturated fatty acids, either
in the esterified or free state, and these have their own biological properties. This has become evident in recent years especially in relation to
the phenomenon of ferroptosis, characterized by intracellular iron accumulation and an unrestricted build-up of lipid peroxides to such
an extent that cellular membranes, including the plasma membrane, are disrupted to trigger lethal ion imbalances,
membrane permeabilization and ultimately cell rupture (see our web page on
oxidized phospholipids).
There are selective mechanisms for incorporation of arachidonate into membrane phospholipids that include the formation of coA esters and remodelling by the Lands cycle before it can be released for oxylipin production. Biosynthesis of eicosanoids then involves the action of multiple enzymes, several of which can be rate limiting. In the first step, arachidonic acid is released from membrane phospholipids in tissues upon stimulation of the enzyme phospholipase A2 by various physiological and pathological factors, including hormones and cytokines. There are then three main enzymatic pathways towards eicosanoids, i.e., via cyclooxygenases (COXs), lipoxygenases (LOXs - dioxygenases) and mono-oxygenases of the cytochrome P-450 family (CYPs); the last are also used for sterol and bile acid oxidation. The COX pathway (two isoforms denoted COX-1 and COX-2) yields prostaglandins PGG2 and PGH2, which are subsequently converted into further prostaglandins, prostacyclin and thromboxanes (TXs) by characteristic synthases. The figure below summarizes in simplistic terms the various reaction pathways towards eicosanoids from phospholipid precursors (the octadecanoids result from lipoxygenases).
 |
| Figure 2. Biosynthetic pathways for eicosanoid production. |
There are several lipoxygenases that act upon different positions of arachidonic acid, mainly 5, 8, 12 and 15, to synthesise various hydroperoxyeicosatetraenoic acids (HPETEs) and thence the hydroxyeicosatetraenoic acids (HETEs) and further oxylipins. For example, leukotriene LTA4 from 5-HETE is in turn a precursor for leukotriene LTB4, cysteinyl-leukotrienes (CysLTs) and lipoxins (LXs). The cytochrome P-450 mono-oxygenase pathway yields hydroxyeicosatetraenoic acids (HETEs) and epoxides (EETs) primarily (with dihydroxy acids or DHET as secondary metabolites). While many of the requisite enzymes, precursors and products are only present in certain types of cells, the proximity of some cell types can facilitate the transfer of eicosanoids between them for further metabolism, and some of the leukotrienes are synthesised by trans-cellular mechanisms.
Most cell types can create eicosanoids from phospholipid-derived precursors in this way, although much research has been concerned with those cells that are part of the innate immune system. In addition, triacylglycerols in cytoplasmic lipid droplets of human mast cells, which are potent mediators of immune reactions and influence many inflammatory diseases, have a high content of arachidonic acid, and this can be released by adipose triacylglycerol lipase as a substrate for synthesis of eicosanoids when the cells are stimulated appropriately. Indeed, there are now suggestions that lipid droplets in all cell types (animal, plant, microorganisms) are crucial for the response mechanisms to cellular stress and act as hubs to integrate metabolic and inflammatory processes. Via their lipolytic machinery, they regulate the availability of fatty acids for production of oxylipins from polyunsaturated fatty acids and thence for the induction of signalling pathways.
Eicosanoids are generated mainly from unesterified fatty acids, not the CoA esters, and they act as such, but it is increasingly recognized that they may occur and that some may indeed be synthesised while esterified to glycerolipids. The latter may simply be inert storage molecules that are available for when a rapid response is required, but some may act as esters, especially with oxygenated forms of endocannabinoids. Ultimately, many of the eicosanoids from these reactions are directed to the cellular membranes where they can interact with receptors, which include cell-surface G-protein-linked receptors (GPCRs) and nuclear peroxisome proliferator-activated receptors (PPARs).
While the eicosanoids were the first to be identified and studied intensively and I have used them for illustrative purposes in this web page, there is now a body of evidence that docosanoids (protectins, resolvins and maresins, or collectively 'specialized pro-resolving mediators' (SPMs)) derived from the n‑3 family of fatty acids such as EPA and DHA may be equally relevant, often opposing the actions of the eicosanoids. Similarly, octadecanoids derived from linoleate have vital signalling properties. All of these are oxylipins are synthesised by related means, often using the same enzymes as for eicosanoids, and they are essential elements of this story.
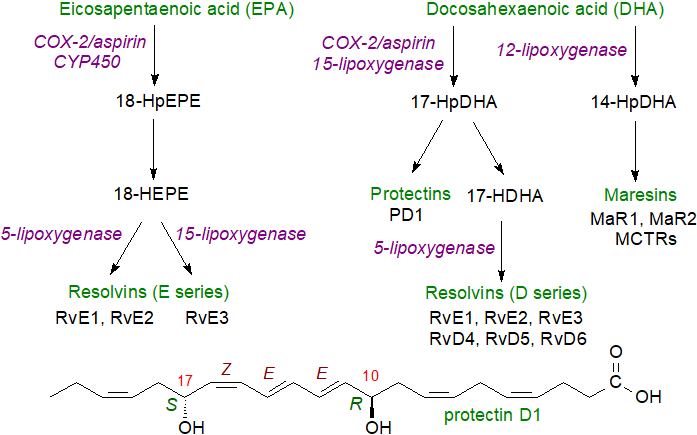 |
| Figure 3. Biosynthesis of specialized pro-resolving mediators. |
 Many parallels can be drawn between the eicosanoids in animals and the structures and functions of
plant oxylipins, which are derived primarily from C18 fatty acids like α-linolenic acid
(18:3(n-3)), the most abundant polyunsaturated fatty acid in photosynthetic tissues.
Jasmonic acid, for example, contains a cyclopentanone ring analogous to that in some prostaglandins, and its various metabolites, the jasmonates,
have signalling properties in relation to plant development and defence against pathogens and abiotic stresses of various kinds.
Plants do not have COX enzymes, but they do have lipoxygenases, which were in fact first characterized from plants,
and a variety of other oxidases and oxygenases.
Many parallels can be drawn between the eicosanoids in animals and the structures and functions of
plant oxylipins, which are derived primarily from C18 fatty acids like α-linolenic acid
(18:3(n-3)), the most abundant polyunsaturated fatty acid in photosynthetic tissues.
Jasmonic acid, for example, contains a cyclopentanone ring analogous to that in some prostaglandins, and its various metabolites, the jasmonates,
have signalling properties in relation to plant development and defence against pathogens and abiotic stresses of various kinds.
Plants do not have COX enzymes, but they do have lipoxygenases, which were in fact first characterized from plants,
and a variety of other oxidases and oxygenases.
Arachidonic acid has only rarely been encountered in higher plants, but it is a constituent of some algae, fungi and moulds. During fungal infections of plants, it is known to elicit the secretion of plant defence compounds (phytoalexins), probably after conversion to oxygenated metabolites. Fungal species such as Aspergillus, Candida and Cryptococcus spp. that are pathogenic to animals and parasitic protozoa such as Trypanosoma cruzi can synthesise the same oxylipins as are present in the host, including both eicosanoids and docosanoids, as well as some unique to the organisms by utilizing enzymes that are functionally comparable but evolutionarily distinct from those in animals.
2. Autoxidation
As well as the enzymatic reactions, lipids can be oxidized non-enzymatically by reactive oxygen and nitrogen species (ROS and RNS, respectively) in all animal and plant tissues in an uncontrolled manner by mechanisms that start with an attack by free radicals on the fatty acid components, followed by chain propagation and ultimately termination steps as discussed more fully on this website here... Such reactions occur on intact lipids in tissues, and the result is usually a complex mixture of positional and stereo isomers of hydroperoxides that can rapidly rearrange or react to form other products that may act metabolically in both the lipid-bound and free states. While these processes are often discussed in relation to their potential for harm, oxidized metabolites can have properties of value in relation to signalling and innumerable aspects of cellular homeostasis.
Those autoxidized lipids generated from ROS are discussed in a number of our web pages, and they include the oxidized phospholipids, isoprostanes and secondary oxygenated metabolites, often with reactive electrophilic carbonyl groups, such as short-chain aldehydes. Indeed any unsaturated lipid, including sterols, can be a source of oxidized metabolites. While myeloperoxidase can catalyse addition of hypochlorous acid (HOCl) to double bonds in unsaturated fatty acyl chains via radical intermediates to create chlorohydrins of significance to disease states, a more important reaction is with the vinyl ether bond in plasmalogens discussed in that web page. Analogous reactions with RNS yield nitro fatty acids.
 |
| Figure 4. Pathways leading to reactive oxygen/nitrogen species, to free radicals, and thence to lipid hydroperoxides. |
There is a delicate balance between generation of ROS and their destruction, and this determines whether ROS act as signalling molecules or cause oxidative damage to cellular components. Tissues have developed complex defensive measures involving antioxidants and antioxidant enzymes to counter the potentially harmful effects of lipid radicals and other oxidized lipids, and these are discussed in our web pages on tocopherols (vitamin E) and coenzyme Q. From a practical standpoint, ROS are a major cause of food spoilage.
Although much research has been focussed on lipid oxidation in animals because of the health implications, ROS are generated in plant cells, in mitochondria, chloroplasts, and peroxisomes, and they are important at every stage of the plant life cycle, including growth, development and senescence. In particular, they can maintain cellular metabolism during biotic and abiotic stresses of various kinds.
3. Phospholipase A2
Most of the arachidonic acid (and other polyunsaturated fatty acids) in animal tissues is esterified, mainly in membrane phospholipids, and before this can be used for eicosanoid synthesis, it must be released by the action of the enzyme phospholipase A2 (PLA2) by hydrolysis of the ester bond at the sn-2 position, which is usually enriched in this acid; the other products of the reaction are lysophospholipids. Phosphatidylinositol and the polyphosphoinositides in particular are major contributors to this process, but depending on the tissue and physiological conditions, phosphatidylcholine and phosphatidylethanolamine can be substrates for arachidonic acid release in the same way. This reaction sets in motion a cascade of cellular processes that utilize cyclooxygenases, lipoxygenases and cytochrome P450 oxidases, the key enzymes in the biosynthesis of oxylipins of all kinds.
 |
| Figure 5. Hydrolysis of phosphatidylinositol by phospholipase A2 for generation of arachidonic acid and eicosanoids. |
Many enzymes of the PLA2 type have been characterized in a superfamily with 16 sub-families (classified on the chronology of their discovery), and three main types have been identified that are relevant here: cytosolic calcium-dependent PLA2 (cPLA2), cytosolic calcium-independent PLA2 (iPLA2, Group VIA) and secreted PLA2 (sPLA2, Group V). They are all water-soluble enzymes with structures that enable association with membranes, and each has a characteristic catalytic site, a Ser-Asp dyad where the substrate binds and an interfacial surface that facilitates contact with cellular membranes (or supramolecular structures such as micelles, vesicles and liposomes in an aqueous environment).
In general, different isoforms of these and other phospholipases of this type have characteristic locations in tissues, cell types or organelles, where they each have their own substrate specificities and presumably regulatory mechanisms with diverse roles in immunity, metabolism, and other biological events. In human macrophages, which synthesise many different inflammatory metabolites from polyunsaturated fatty acids, these enzymes are highly selective for particular lipid classes and molecular species. cPLA2 has a preference for phospholipids, especially phosphoinositides containing arachidonic acid, whereas iPLA2 favours those containing EPA and sometimes linoleate, and sPLA2 prefers esterified DHA as substrate, all in position sn-2. The nature of the alkyl ester moiety in position sn-1 is sometimes relevant in that cPLA2 selects the vinyl ether phospholipid (plasmalogen) over the diester, while iPLA2 has comparable activity with both, and sPLA2 prefers diester phospholipids.
The cytosolic Ca2+-dependent phospholipase A2 (the isoform 'cPLA2α' or 'Group IVA cPLA2') has a marked preference for phospholipids containing arachidonic acid in the sn-2 position, and there is clear evidence that it is necessary for the release of this fatty acid for generation of prostanoids and related metabolites. Indeed, it is rate limiting for eicosanoid production in many tissues. It makes use of the catalytic Ser-Asp dyad to hydrolyse fatty acids, and it contains a so-called ‘C2’ domain that facilitates a calcium-dependent translocation of the enzyme from the cytosol to the membrane surface, where the phospholipid substrate and the key enzymes of eicosanoid biosynthesis are located. In fact, there are six isoenzymes with molecular masses in the range of 60 to 100kDa, which are regulated by phosphorylation, although only cPLA2α is relevant here. The specificity of cPLA2α is due to an interaction between certain of its aromatic residues with the double bonds of arachidonic acid, although also with EPA and 20:3(n-9) but perhaps surprisingly not towards DHA (which does not have a Δ5-double bond).
For this purpose, cPLA2α must translocate from the cytosol to the perinuclear and endoplasmic reticulum membranes in response to an increase in cytosolic Ca2+, where it is phosphorylated and activated by mitogen-activated protein kinases. As well as control via transcriptional regulation, the enzyme is responsive to various stimuli, such as hormones, cytokines and neurotransmitters. The lipids ceramide-1-phosphate and phosphatidylinositol 4,5‑bisphosphate bind to the enzyme, the latter in a 1:1 stoichiometry, and this is required for activation and translocation of the enzyme to the site of action. Ceramide-1-phosphate increases the inflammatory response and can inhibit wound healing by stimulating release of arachidonic acid directly via cPLA2α with translocation of this enzyme to the Golgi apparatus. Lactosylceramide stimulates the enzyme, but sphingomyelin is inhibitory.
 cPLA2α
is usually considered a pro-inflammatory enzyme in that it is the first step that leads to the prostaglandins and leukotrienes, which tend
to stimulate inflammatory processes.
In this manner, cPLA2 is responsible for the massive flux of pro-inflammatory eicosanoids that
accompanies inflammation induced by systemic flagellin or the lethal anthrax toxin, so prostaglandin synthesis via cyclooxygenase-1 can
threaten life when produced systemically rather than acutely.
On the other hand, this enzyme is responsible for the release of 15‑hydroxyeicosatetraenoic acid (15-HETE) from storage in
phospholipids of macrophages when required for the synthesis of the pro-resolving lipid mediators,
the lipoxins, i.e., it can switch the formation of eicosanoids of a pro-inflammatory type to those
that inhibit inflammation.
In healthy mitochondria, the main phospholipase responsible for Ca2+-activated release of arachidonic acid
is phospholipase A2ζ (cPLA2ζ)
cPLA2α
is usually considered a pro-inflammatory enzyme in that it is the first step that leads to the prostaglandins and leukotrienes, which tend
to stimulate inflammatory processes.
In this manner, cPLA2 is responsible for the massive flux of pro-inflammatory eicosanoids that
accompanies inflammation induced by systemic flagellin or the lethal anthrax toxin, so prostaglandin synthesis via cyclooxygenase-1 can
threaten life when produced systemically rather than acutely.
On the other hand, this enzyme is responsible for the release of 15‑hydroxyeicosatetraenoic acid (15-HETE) from storage in
phospholipids of macrophages when required for the synthesis of the pro-resolving lipid mediators,
the lipoxins, i.e., it can switch the formation of eicosanoids of a pro-inflammatory type to those
that inhibit inflammation.
In healthy mitochondria, the main phospholipase responsible for Ca2+-activated release of arachidonic acid
is phospholipase A2ζ (cPLA2ζ)
sPLA2 occurs as a diverse family of enzymes, 11 isoforms in mammals, with varying tissue and cellular distributions that hydrolyses phospholipids to release lysophospholipids and free fatty acids when there is an increase in intracellular calcium concentration. They target extracellular phospholipids such as those in extracellular vesicles, microbes, lipoproteins, surfactants, and ingested foods, as well as phospholipids in the plasma membrane of activated or damaged cells. In this context, they are inducible enzymes that enhance cPLA2 to control the magnitude and duration of elevated free fatty acid levels including that of arachidonic acid by acting primarily on the sn‑2 position of negatively charged phospholipids, although some isoforms may hydrolyse other positions. In contrast to cPLA2α, Ca2+ is required both for binding of the substrate phospholipids and for activation of most isoforms of the enzyme. A further isoform sPLA2-IID, present in lymphoid tissue and skin, has a high specificity for the mobilization of omega‑3 fatty acids and the subsequent synthesis of pro-resolving lipid mediators (SPMs) from EPA and DHA. In effect, it is an immunosuppressive sPLA2 that tips the micro-environmental lipid balance toward an anti-inflammatory state. A peroxisomal Ca2+‑independent phospholipase has only recently been identified, but it may be of special relevance to eicosanoids in that it generates arachidonoyl species, such as 2-arachidonoyl-lysophosphatidylcholine.
iPLA2 has no preference for arachidonic acid, and it may have only a minor role in eicosanoid synthesis. Rather, in tissues, it is used mainly for phospholipid re-modelling or general catabolism, where it ensures the availability of the required substrates. However, there is increasing evidence that the Ca2+‑independent iPLA2β mobilizes DHA, especially in the brain, for the biosynthesis of SPMs.
It should not be forgotten that the other metabolites from phospholipase A2 action, lysophospholipids, have their own physiological properties, while the reverse reaction in which lysophosphatidylinositol is re-acylated can occur to renew the phosphatidylinositol. As in the Lands cycle for remodelling of phospholipids, a membrane-bound O-acyltransferase (MBOAT7) with a marked preference for arachidonoyl-CoA has been characterized from neutrophils, and by its action upon lysophosphatidylinositol, this may be a means by which free arachidonic acid, eicosanoid and phosphoinositide levels are regulated. All members of the phospholipase A2 family are of course required for many other purposes as part of the normal turnover of membrane lipids, and for digestion of dietary phospholipids, host defence against bacterial infections, and the production of lysophospholipids as signalling mediators.
Enzyme coupling: As arachidonic acid is relatively mobile and can diffuse out of the cell or be re-incorporated into lipids, most eicosanoid production occurs in very close proximity to cPLA2, so enzymes that can migrate to the perinuclear and endoplasmic reticulum membranes, where this phospholipase is located, can participate preferentially in arachidonic acid metabolism. For the synthesis of prostanoids, the newly mobilized arachidonic acid must cross the membrane bilayer from the cytosolic to the lumenal side where the cyclooxygenase enzymes are located. Some passive diffusion is possible but transport by fatty acid-binding proteins (FABP) is also likely.
Other relevant enzymes: Adipose tissue lipase may hydrolyse triacylglycerols in cytoplasmic droplets of mast cells to provide unesterified arachidonic acid for eicosanoid biosynthesis, while catabolism (hydrolysis) of endocannabinoids will also release arachidonic acid. In plants and fungi, release of fatty acids by the action of various acylhydrolases on membrane complex lipids is the first step in the generation of the characteristic oxylipins required for signalling purposes in these organisms.
4. Catabolism of Eicosanoids
Efficient mechanisms for catabolism and deactivation of eicosanoids are necessary for the regulation of their metabolism. While thromboxanes and some leukotrienes have their own catabolic enzymes, there is one major type of catabolic pathway that is common to most if not all other eicosanoids and thus is a control on their signalling. The first step occurs in the cytoplasm of cells and with prostanoids consists in the oxidation of the 15S‑hydroxyl group by 15‑hydroxyprostaglandin dehydrogenase, one of a family of oxidoreductases that acts on the CH-OH group of a donor molecule with NAD+ or NADP+ as acceptor in a process mediated by solute carrier organic anion transport protein family member 2A1 (SLCO2A1). This enzyme oxidizes E-series prostaglandins, lipoxins, 15‑HETE, 5,15‑diHETE, 8,15‑diHETE and probably many others to the corresponding 15-keto compounds. A further enzyme, dehydrogenase reductase 9 (SDR9C4), recognizes a broad spectrum of oxylipins as substrates and oxidizes hydroxyl groups at carbons C9 and C13 of octadecanoids, C12 and C15 of eicosanoids (but not C5), and C14 of docosanoids. As an example, the process is illustrated for prostaglandin PGE2 with PGE-M as an end-product.
 |
| Figure 6. Catabolism of prostaglandins and many other eicosanoids. |
The second catabolic step with prostanoids consists of reduction of the Δ13 double bond by a Δ13-15-ketoprostaglandin reductase (NADPH/NADH dependent) to render it inert. This reductase was first identified against leukotriene B4 but is now known to metabolize many prostaglandins and lipoxins. Further catabolism of prostaglandins, HETEs (except for 5-HETE) and lipoxins occurs by the beta-oxidation pathway by peroxisomal enzymes, i.e., via the carboxyl end of the molecule (although some oxidation of the terminal methyl group can occur), leading to the formation of short-chain metabolites, which are excreted in the urine often following glucuronidation. 5-HETE and leukotrienes undergo beta-oxidation from the omega-terminus following an initial omega-hydroxylation, and such variations to the general mechanism are discussed in the web pages dealing with each class of oxylipin.
It is now recognized that α,β-unsaturated keto-eicosanoids generated in this way are electrophilic and may interact with nucleophilic centres in proteins and other molecules to modify their properties. In mammals, 15‑hydroxyprostaglandin dehydrogenase is a key regulatory enzyme in many physiological and pathological processes. It is considered to be a potential pharmacological target for preventing organ damage and enhancing tissue regeneration, and for resisting the complex pathology of aging-associated diseases as it accumulates in aging tissues. As a tumour suppressor, it inhibits proliferation of cancer cells, including colorectal, lung and breast cancers.
There is now evidence that some oxylipins are not processed by this route, but rather carnitine palmitoyl transferase 1 (CPT1), a mitochondrial importer of main-stream fatty acids, can remove oxylipins from cells during inflammation in vitro and in vivo for beta-oxidation. Indeed, it has been proposed that mitochondrial β‑oxidation is a regulatory metabolic checkpoint for oxylipins.
5. Analysis
As eicosanoids and other oxylipins tend to occur at low levels only in tissues, and they have such a wide range of structures of varying stereochemistry and short half-lives, analysis has become a rather specialized task at first for gas chromatography linked to mass spectrometry but increasingly for HPLC linked to tandem mass spectrometry with electrospray ionization. Many primary standards, including deuterated isomers, are now available commercially to assist in the task of comparison, calibration or validation, although gaps remain. Chiral chromatography has a role as the stereochemical (R/S) forms of oxylipins are often crucial, and this technique may be the only means of distinguishing oxylipins generated enzymatically from the racemic products of autoxidation, which can occur naturally in tissues or result adventitiously from faulty handling and storage of samples. The cis/trans geometry of double bonds is a further challenge to analysts, as this is another factor that can determine the reactivity of oxylipins.
Recommended Reading
- Alam, P., Faizan, M., Arif, Y., Azzam, M.M., Hayat, S., Afzal, S. and Albalawi, T. Reactive oxygen species: balancing agents in plants. Front. Plant Sci., 16, 1713590 (2025); DOI.
- Astudillo, A.M., Balboa, M.A. and Balsinde, J. Selectivity of phospholipid hydrolysis by phospholipase A2 enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochim. Biophys. Acta, Lipids, 1864, 772-783 (2019); DOI - and further articles in this special journal issue.
- Durand, E., Laguerre, M., Bourlieu-Lacanal, C., Lecomte, J. and Villeneuve, P. Navigating the complexity of lipid oxidation and antioxidation: A review of evaluation methods and emerging approaches. Prog. Lipid Res., 97, 101317 (2025); DOI.
- Dyall, S.C., Balas, L., Bazan, N.G., Brenna, J.T., Chiang, N., Souza, F.D., Dalli, J., Durand, T., Galano, J.M., Lein, P.J., Serhan, C.N. and Taha, A.Y. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res., 86, 101165 (2022); DOI.
- Foret, M.K., Lincoln, R., Do Carmo, S., Cuello, A.C. and Cosa, G. Connecting the 'dots': from free radical lipid autoxidation to cell pathology and disease. Chem. Rev., 120, 12757-12787 (2020); DOI.
- Hajeyah, A.A., Griffiths, W.J., Wang, Y., Finch, A.J. and O’Donnell, V.B. The biosynthesis of enzymatically oxidized lipids. Front. Endocrinol., 11, 591819 (2020); DOI.
- Jarc, E. and Petan, T. A twist of FATe: Lipid droplets and inflammatory lipid mediators. Biochimie, 169, 69-87 (2020); DOI.
- Kampschulte, N., Kirchhoff, R., Löwen, A. and Schebb, N.H. Deducing formation routes of oxylipins by quantitative multiple heart-cutting achiral-chiral 2D-LC-MS. J. Lipid Res., 65, 100694 (2024); DOI.
- Lagarde, M. and Nicolaou, A. (Editors) Oxygenated metabolism of PUFA: analysis and biological relevance. Biochim. Biophys. Acta, Lipids, (Volume 1851, Issue 4, Pages 307-518) (2015) - special issue.
- Misheva, M. and others. Oxylipin metabolism is controlled by mitochondrial β-oxidation during bacterial inflammation. Nature Commun., 13, 139 (2022); DOI.
- Murawska, G.M., Armando, A.M. and Dennis, E.A. Lipidomics of phospholipase A2 reveals exquisite specificity in macrophages. J. Lipid Res., 65, 100571 (2024); DOI.
- Murphy, R.C. and Folco, G. Lysophospholipid acyltransferases and leukotriene biosynthesis: intersection of the Lands cycle and the arachidonate PI cycle. J. Lipid Res., 60, 219-226 (2019); DOI.
- Niu, M.Y. and Keller, N.P. Co-opting oxylipin signals in microbial disease. Cell. Microbiol., 21, e13025 (2019); DOI.
- Noguchi, N., Saito, Y. and Niki, E. Lipid peroxidation, ferroptosis, and antioxidants. Free Rad. Biol. Med., 237, 228-238 (2025); DOI.
- O'Donnell, V.B. Recent updates in mammalian oxylipin biochemistry. J. Biol. Chem., 301, 110629 (2025); DOI.
- Parchem, K. and others. Oxylipin profiling for clinical research: Current status and future perspectives. Prog. Lipid Res., 95, 101276 (2024); DOI.
- Revol-Cavalier, J., Quaranta, A., Newman, J.W., Brash, A.R., Hamberg, M. and Wheelock, C.E. The octadecanoids: synthesis and bioactivity of 18-carbon oxygenated fatty acids in mammals, bacteria, and fungi. Chem. Rev., 125, 1-90 (2025); DOI.
- Schebb, N.H. and ~100 others. Technical recommendations for analyzing oxylipins by liquid chromatography-mass spectrometry. Sci. Signal., 18, 678 (2025); DOI.
- Spickett, C.M. Formation of oxidatively modified lipids as the basis for a cellular epilipidome. Front. Endocrin., 11, 602771 (2020); DOI.
- Sun, C.C., Zhou, Z.Q., Yang, D., Chen, Z.L., Zhou, Y.Y., Wen, W., Feng, C., Zheng, L., Peng, X.Y. and Tang, C.F. Recent advances in studies of 15‑PGDH as a key enzyme for the degradation of prostaglandins. Int. Immunopharm., 101, 108176 (2021); DOI.
- and in relation to the history of the topic -
- Samuelsson, B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J. Biol. Chem., 287, 10070-10080 (2012); DOI.
For tutorials on mass spectral analysis of fatty acids - see our mass spectrometry pages.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: February 2026. | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
