Ceramides
Ceramides consist of a long-chain or sphingoid base linked to a fatty acid via an amide bond. Although free ceramides are present in membranes in small amounts only, they play specialized regulatory roles in cellular metabolism that are often dependent on the chain lengths of their fatty acyl constituents. Those in skin are major components of this organ and are exceptional in having unique compositions and functions.
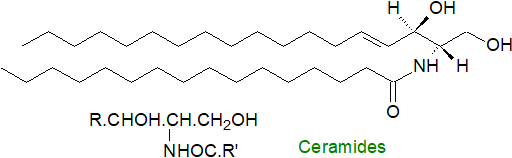
Each organism and indeed each tissue or cell type can synthesise ceramides in which there are a variety of di- and trihydroxy long-chain bases linked to fatty acids with more than 200 molecular species of ceramides characterized from mammalian cells alone. As discussed in the Introduction to the sphingolipid section, the fatty acids consist mainly of longer-chain (up to C24 or greater) saturated and monoenoic (mainly (n-9)) components, sometimes with a hydroxyl group in position 2, but other than in certain testicular cells, polyunsaturated fatty acids do not occur. In plants, 2‑hydroxy acids predominate sometimes accompanied by small amounts of 2,3‑dihydroxy acids. Although small amounts of free ceramides are produced in all tissues when required as mediators of biological reactions, most are converted rapidly to more complex sphingolipids.
A shorthand nomenclature simply combines those used conventionally for fatty acids and long-chain bases to denote molecular species of ceramides, including those as components of more complex lipids, e.g., N-palmitoyl-sphingosine is d18:1-16:0. Ceramides containing sphinganine can be termed ‘dihydroceramides’.
Ceramides have a central position as intermediates in the biosynthesis and metabolism of all sphingolipids including the complex sphingolipids in which the terminal primary hydroxyl group is linked to phosphate or carbohydrate (sphingomyelin, glycosylceramides and gangliosides - see the separate web pages), and they are also the primary source of unesterified sphingoid bases and of the vital lipid mediators sphingosine-1-phosphate and ceramide-1-phosphate.
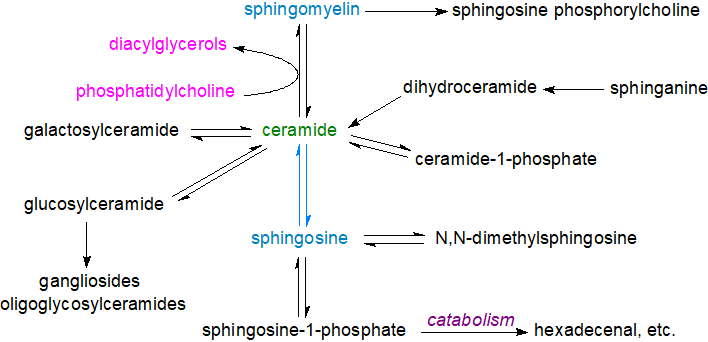 |
| Figure 1. The central role of ceramide in the biosynthesis and metabolism of sphingolipids. |
1. Ceramide Biosynthesis
Ceramide production is complex with at least three pathways. In most eukaryotic cells, biosynthesis de novo takes place in the endoplasmic reticulum with palmitoyl-CoA and serine as the precursors for an intermediate form of long-chain base component, sphinganine, which is then converted to ceramide. Alternative routes for ceramide production involve regeneration from complex sphingolipids, and in animals through sphingomyelinase action, conversion of sphingomyelin into ceramides (and vice versa) occurs in the plasma membrane, Golgi and mitochondria. Thirdly, the polar moieties of complex glycosphingolipids can be removed by various hydrolytic enzymes in the lysosomal compartment to recover the ceramides (or their component parts) in a re-cycling/catabolic process. As these biosynthetic or metabolic pathways are located in different organelles, separate pools of ceramide and sphingolipids result with differing properties.
Biosynthesis of the characteristic fatty acids of ceramides by various chain elongases (ELOVL) requires consideration, but this is discussed in our web page dealing with saturated fatty acids, although much remains to be learned of how the distinctive fatty acid compositions of ceramides and thence of complex sphingolipids are attained.
Ceramide synthesis de novo in animals: The first of these pathways is described in mechanistic terms in our web pages dealing with sphingoid bases, as some structural features of the latter (double bonds, methyl branches, etc.) are introduced only after they have been incorporated into ceramides. In brief, in animals, sphinganine is coupled to a long-chain fatty acid to form dihydroceramide by means of one of six ceramide synthases (CerS1 to 6) in the endoplasmic reticulum mainly, before the double bond is introduced into position 4 of the sphingoid base. Together with the serine palmitoyl transferase (SPT), these are the rate-limiting step in the pathway. CerS1 synthesises 18:0- and 18:1- dihydroceramide and is expressed mainly in brain with lower levels in skeletal muscle and testis. CerS2, which is ubiquitously expressed but especially in the kidney and liver, synthesises long-chain and very-long-chain ceramides and dihydroceramides (C20 to C26) and is inversely regulated by cellular cholesterol levels. CerS3 produces very-long-chain dihydroceramides mainly in testis and skin, and is responsible for the unusual ceramides in the latter. CerS4, CerS5 and CerC6 are least abundant; CerS4 is present in skin, leukocytes, and liver, where it synthesises C18- and C20-dihydroceramide, while CerS5 and CerC6 generate 14:0- and 16:0-dihydroceramides mainly in kidney and lung.
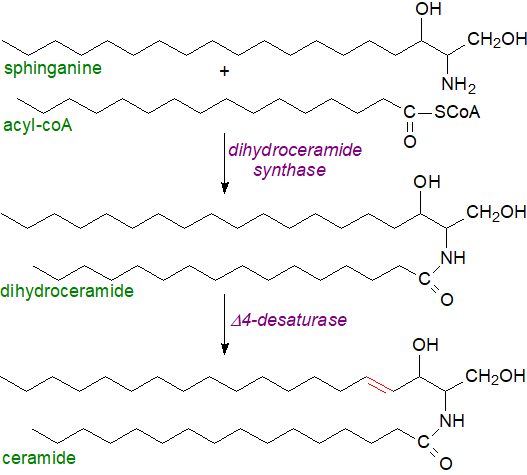 |
| Figure 2. Biosynthesis of sphingosine via ceramides |
Each ceramide synthase has six membrane-spanning domains and possesses a characteristic motif containing the structures required for catalysis and substrate binding that are essential for its activity, and they have been shown to differ primarily in an 11-residue sequence in a loop between the last two transmembrane domains. As well as separate transcriptional regulation of each of these enzymes, ceramide synthase is modulated by many different factors including reversible dimerization, while ceramide synthase 2 has a sphingosine-1-phosphate binding motif and this lipid may be inhibitory. Acyl-coenzyme A-binding protein (ACBP) facilitates the synthesis of ceramides containing very-long fatty acids and stimulates ceramide synthases 2 and 3. Three ceramide synthases, designated HYL-1, HYL-2, and LAGR-1, have been identified in the model nematode Caenorhabditis elegans.
Ceramides synthesised in the intestines are incorporated into chylomicrons for transport to other tissues; during high fat feeding C16-ceramides, which are potentially harmful, can be major products. Some ceramides are transported from the liver to other tissues in plasma lipoproteins subclasses HDL2 and HDL3, i.e., those containing apolipoprotein B. There is a suggestion that transport of ceramides via lipoproteins could be a paracrine mechanism to regulate the metabolism of other cells.
Plants, yeast and bacteria: Three ceramide synthase isoforms have been identified in Arabidopsis, designated LOH1, LOH2 and LOH3, and again these are discussed in more detail in this website in relation to the biosynthesis of long-chain bases. The yeast Saccharomyces cerevisiae has two ceramide synthases designated Lag1 and Lac1 of which Lag1 is responsible for the synthesis of ceramides containing phytosphingosine and is a factor in ageing. In contrast to animals, fungal ceramide synthases in general are capable of attaching a wide variety of fatty acids to sphingoid bases. Phytosphingolipids derived in this way are required for the establishment of a lateral diffusion barrier in the nuclear envelope. In yeasts, ceramide synthesis is regulated by the Torc2 kinase complex, which controls the steady-state levels of long-chain bases and ceramides but by mechanisms that are poorly understood. A protein Nvj2p promotes non-vesicular transport of ceramides out of the endoplasmic reticulum in yeast, but there is no CERT protein. In those bacteria that produce sphingolipids, the ceramide synthases are not related phylogenetically to those in eukaryotes and the biosynthetic steps occur in a different order from that in eukaryotes, suggesting that ceramide production in bacteria and eukaryotes evolved independently.
Ceramide transport in animals: Most of the ceramides generated in this way are rapidly utilized for synthesis of complex sphingolipids, and this ensures that ceramide concentrations in cells are regulated at appropriate levels to control their activities. These processes are discussed in detail on other web pages on this site.
In mammalian cells, most complex glycerolipids are synthesised in the endoplasmic reticulum prior to their transport to their final subcellular locations, but the process is rather different for sphingolipids. In brief, ceramide is synthesised on the cytoplasmic leaflet of the endoplasmic reticulum, but subsequent formation of complex sphingolipids occurs in the Golgi apparatus, and a key cytoplasmic protein, ceramide transporter or 'CERT' (CERamide Trafficking), mediates the transport of ceramide between these organelles in a non-vesicular manner. It has a number of functional domains, including an N-terminal phosphatidylinositol-4-monophosphate (PI(4)P)-binding or Pleckstrin homology (PH) domain, which targets the Golgi apparatus, and a C-terminal ‘START’ domain, which can recognize ceramides with the natural D-erythro stereochemistry, including dihydroceramide and phytoceramide (but not sphingosine), and holds them within a long amphiphilic cavity by hydrogen bonding with all three polar atoms of the sphingoid motif. There is a short peptide motif (FFAT) that recognizes an appropriate protein in the endoplasmic reticulum, and there is sufficient flexibility in the body of the protein to enable transfer of ceramide from the endoplasmic reticulum to the Golgi without free movement through the cytosol.
CERT-dependent ceramide transport is the rate-limiting step in the synthesis of complex sphingolipids, and it is crucial for embryogenesis and later in life to suppress cell senescence. Loss of CERT causes chronic stress in the endoplasmic reticulum and compromises the structure and operation of mitochondria.
As a neutral lipid, ceramide can flip readily across membrane leaflets, and this is necessary for the synthesis of sphingomyelin, which occurs on the lumenal side of the Golgi. The pool of ceramide utilized for synthesis of glycosylceramide is delivered to the Golgi by a separate transport mechanism that does not require ATP, while some ceramide synthesis occurs in mitochondria, although this has the potential to lead to cell death. The diet and some commensal bacteria supply further ceramides to the intestinal cells. Regulation of ceramide and subsequent sphingolipid biosynthesis is crucial as an excess of either can be toxic, while reduced synthesis can inhibit cell proliferation.
Very-long-chain ceramides containing 24:0 or 24:1 fatty acids turn over much more rapidly in animal cells than those containing 16:0 or 18:0 fatty acids because of the more rapid conversion of the former into complex sphingolipids, where they may regulate the levels and perhaps the functions of the latter. In contrast, ceramides containing d16:1 and d18:1 sphingoid bases turnover at comparable rates so do not affect the flux of ceramides through these pathways. The CERT protein is a major factor in this specificity as it extracts ceramides from membrane bilayers with a preference for those required for synthesis of complex sphingolipids. Removal of ceramide by this process provides the gradient that enables the process to continue and prevents an accumulation of ceramide in the endoplasmic reticulum that might otherwise be disruptive to the membrane and even cause cell death. While the transfer step is not dependent on ATP, the overall process requires ATP, possibly to keep PI(4)P in a phosphorylated form, and the multiple factors that control the biosynthesis of this lipid must influence sphingolipid metabolism.
Ceramide regeneration: Ceramides are produced during the catabolism of other complex sphingolipids but mainly by the action of one or other of the sphingomyelinases or of phospholipase C on sphingomyelin in animal tissues as part of the 'sphingomyelin cycle' (see the web page on this lipid for a more detailed discussion). In particular, neutral sphingomyelinase 2 is reported to be the primary driver of ceramide regulation in the plasma membrane with a critical role in maintaining ceramide homeostasis and signalling under physiological conditions. Many agonists including chemotherapeutic agents, tumour necrosis factor-alpha, 1,25-dihydroxy-vitamin D3, endotoxin, gamma-interferon, interleukins, nerve growth factor, ionizing radiation and heat stimulate hydrolysis of sphingomyelin to produce ceramide. Also, reversal of the sphingomyelin synthesis reaction may generate ceramide, and some may be produced by operation of the enzyme ceramidase in reverse (see next section). Such reactions are much more rapid than synthesis de novo, so they are of special relevance in relation to signalling by ceramides at the plasma membrane, where the steady-state level of ceramide is regulated by neutral sphingomyelinase 2.
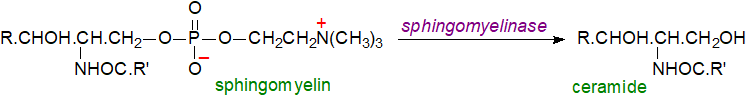 |
| Figure 3. Ceramide production from sphingomyelin. |
In this context, the acid sphingomyelinase may be necessary for the generation of the ceramides that initiate the train of events leading to apoptosis (see below) and are associated with metabolic and cardiovascular diseases. Although ceramides are mainly synthesised intracellularly, they have been identified in biological fluids such as plasma and bronchoalveolar lavage fluid in association with carrier proteins or lipid microvesicles derived from the plasma membrane. As such exogenous ceramides can induce acid sphingomyelinase activity and are able to stimulate de novo synthesis pathway, it has been suggested that a paracrine amplification loop may exist to enhance intracellular ceramide levels.
Glycosphingolipids can be hydrolysed by glycosidases to ceramides (see our web page on monoglycosylceramides) in tissues, but this process tends to be relatively minor in quantitative terms (other than in skin). The main enzymes of sphingolipid metabolism were first characterized from the yeast S. cerevisiae, and these were found to be sufficiently similar to the corresponding enzymes in mammals to facilitate their study in the latter. As discussed below, ceramides derived from synthesis de novo or by hydrolysis of complex sphingolipids can be deacylated by ceramidases to produce the sphingoid bases, which can be recycled to form ceramide.
Knowledge is slowly being acquired of how the synthesis and subsequent catabolism of ceramides are compartmentalized and regulated within cells, processes that require a complex web of at least 28 enzymes, including six ceramide synthases and five sphingomyelinases, which are all products of different genes. Each of these enzymes may produce distinctive molecular species of ceramides with their own characteristic properties.
1-O-Acylceramides, in which a fatty acid is O-esterified to position 1 of the sphingoid base component of a ceramide, are minor components of lipid droplets (LD), but they may be an inert storage form that limits ceramide-mediated apoptosis in cells. They are formed at the ER–LD interface by a multienzyme complex containing the long-chain acyl-CoA synthase ACSL5, which produces fatty acyl-CoA esters, ceramide synthases, which generate ceramides de novo, and diacylglycerol acyltransferase 2 (DGAT2), an enzyme required for triacylglycerol biosynthesis. In Vernix caseosa, the waxy substance that coats the skin of new-born babies, there is a multiplicity of such lipids: C11 to C38 ester-linked (1-O) fatty acids, saturated C12 to C39 amide-linked fatty acids and C16 to C24 sphingoid bases. Structurally related lipids are present in skin (see below).

Ceramides linked by 1-O-acyl bonds to lysine (lysylceramides) have been characterized from anaerobic marine bacteria from the Black Sea, i.e. structural analogues of lysyl-diacylglycerols of Mycobacteria.
2. Ceramide Catabolism
In animals, ceramide metabolism is controlled in part by the action of ceramidases, which catalyse hydrolysis to sphingoid bases and free fatty acids, and indeed, this is the only route to the formation of unesterified sphingosine. Five such enzymes are known in humans, classified according to their pH optima, i.e., acid (‘ASAH1’, pH ~4.5), neutral (‘ASAH2’, pH ~7), which differs between humans and other animals, and alkaline (three enzymes - ‘ACER1 to ACER3’, pH ~9), with differing cellular locations and fatty acid specificities and with the potential to affect signalling and metabolic events, including the progression of certain disease states.
The acid ceramidase is of special importance; it is located in the lysosomes and hydrolyses ceramides with small to medium chain fatty acid components (C6 to C18) most efficiently. Aberrations in its synthesis or activity is a factor in several human disease states, including the rare autosomal-recessive Farber disease, where there is a deficiency in the enzyme so ceramide accumulates; ceramide containing 26:0 in blood is considered to be a diagnostic marker. The neutral ceramidase is present in the plasma membrane and Golgi, mainly of intestinal epithelial cells and colorectal tissues, and prefers long-chain acyl components (C16 to C18). As it can catalyse the reverse reaction, this may be a means of ceramide synthesis in mitochondria. ACER1 and ACER2 are found in the endoplasmic reticulum and Golgi, respectively, and they prefer molecules with very-long-chain acyl groups. ACER3 is present in both the endoplasmic reticulum and Golgi; it has a marked preference for ceramides, dihydroceramides and phytoceramides linked to unsaturated long-chain fatty acids (18:1, 20:1 or 20:4) in vitro at least. Neutral/alkaline ceramidase has also been found in mitochondria and nuclei.
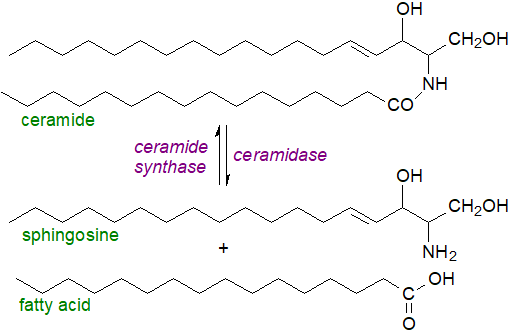 |
| Figure 4. Ceramide hydrolysis and resynthesis. |
Sphingoid bases released by the action of acid ceramidase can escape from the lysosomes and be re-utilized for ceramide biosynthesis through the action of a ceramide synthase in what has been termed the ‘salvage’ pathway; it has been estimated that it contributes from 50 to 90% of sphingolipid biosynthesis. The metabolic requirements for ceramides in tissues are discussed below, but there are reasons to believe that ceramides derived from the salvage pathway are spacially and thence functionally distinct from those synthesised de novo. Sphingoid bases released in this way can act as lipid mediators, and they can be utilized for the synthesis of the crucial metabolite sphingosine-1-phosphate. Therefore, regulation of ceramidase action is central to innumerable processes in animals.
In Arabidopsis, an alkaline ceramidase (AtACER) can hydrolyse phytosphingosine-containing ceramides, and a related enzyme from rice has a preference for d18:1Δ4-ceramide; the latter can operate in reverse to increase the content of phytoceramides with C26 and C28 fatty acid constituents. Several neutral ceramidases (AtNCERs) have been identified, but there does not appear to be an equivalent to the acid ceramidase in plants. Ceramidases are present in lower organisms such as Pseudomonas aeruginosa and slime moulds, where they are secreted proteins rather than integral membrane enzymes. A neutral ceramidase only is found in prokaryotes, including some pathogenic bacteria.
3. Physical Properties
Unsaturation in the sphingoid backbone augments intramolecular hydrogen bonding in the polar region, which permits a close packing of ceramide molecules and a tight intramolecular interaction in membranes. A further factor in this context is the length of the fatty acyl moiety, as shorter chain ceramides tend to produce a positive curvature in a lipid monolayer, while long-chain molecules oppose this and possess a marked intrinsic negative curvature that facilitates formation of inverted hexagonal phases as well as increasing the order of the acyl chains in bilayers. By their interactions with ion channels, ceramides influence the permeability of membranes and render bilayers and cell membranes permeable to solutes that vary from small- up to protein-size molecules.
 While
ceramides are minor components of membranes in general, their physical properties ensure that they are concentrated preferentially
into lateral liquid-ordered microdomains (a form of 'raft' termed
‘ceramide-rich platforms’), although again dependent upon chain-length.
These domains differ appreciably in composition from those rafts enriched in sphingomyelin and cholesterol, and ceramides containing
C12 to C18 fatty acids can in fact displace cholesterol from rafts to modify their physical properties.
Ceramides are generated within rafts by the action of acid sphingomyelinase, and one effect is to cause small rafts to merge into
larger units and modify the membrane structure in a manner that permits oligomerization of such proteins as cytokines and death receptors.
In endothelial cells of the lung, a peptide (CGSPGWVRC) interacts with C16-ceramide at the cell surface to activate acid
sphingomyelinase and ceramide production without the associated downstream apoptotic signalling (ceramide seems to act as an endothelial
cell surface receptor here).
The formation and/or secretion of exosomes require the presence of ceramides as they induce membrane curvature.
In contrast, the other signalling sphingolipids sphingosine, sphingosine-1-phosphate and ceramide-1-phosphate do not facilitate
raft formation.
While
ceramides are minor components of membranes in general, their physical properties ensure that they are concentrated preferentially
into lateral liquid-ordered microdomains (a form of 'raft' termed
‘ceramide-rich platforms’), although again dependent upon chain-length.
These domains differ appreciably in composition from those rafts enriched in sphingomyelin and cholesterol, and ceramides containing
C12 to C18 fatty acids can in fact displace cholesterol from rafts to modify their physical properties.
Ceramides are generated within rafts by the action of acid sphingomyelinase, and one effect is to cause small rafts to merge into
larger units and modify the membrane structure in a manner that permits oligomerization of such proteins as cytokines and death receptors.
In endothelial cells of the lung, a peptide (CGSPGWVRC) interacts with C16-ceramide at the cell surface to activate acid
sphingomyelinase and ceramide production without the associated downstream apoptotic signalling (ceramide seems to act as an endothelial
cell surface receptor here).
The formation and/or secretion of exosomes require the presence of ceramides as they induce membrane curvature.
In contrast, the other signalling sphingolipids sphingosine, sphingosine-1-phosphate and ceramide-1-phosphate do not facilitate
raft formation.
Through the medium of these modified rafts, ceramides can serve in signal transduction. Receptor molecules and signalling proteins are recruited and cluster within such domains, thereby excluding potential inhibitory signals, while initiating and greatly amplifying primary signals. Ceramide-rich platforms magnify both receptor- and stress-mediated signalling events and thence may influence various disease states. Such membrane domains formed by sphingomyelinases are sites for endocytic uptake of pathogens because of a concentration of pathogen receptors and signalling complexes, and in particular, these can enhance viral infections, including Norovirus, Japanese encephalitis virus, Ebola and possibly SARS-CoV-2. In contrast, elevated levels of ceramide inhibit cellular uptake of the HIV virus.
Although ceramides and diacylglycerols have structural similarities, their occurrence, location and behaviour in membranes are different. Ceramides cross synthetic lipid bilayers relatively quickly in vitro, but it is not clear whether they can flip across more complex membranes equally readily in ceramide-rich platforms in vivo. Restricted flipping could influence the signalling by ceramides in that those generated by different enzymes on each side of a membrane could have separate functions.
4. Ceramides in Health and Disease
Ceramides and the biosynthesis of complex glyco- and phospho-sphingolipids are discussed in other web pages on this site (and see the figure in the introduction to this page), so this topic need not be elaborated here. Ceramides, like other lipid second messengers in signal transduction, are produced rapidly and transiently in response to appropriate stimuli to target proteins, for example to activate certain serine/threonine protein kinases or phosphatases, or they may regulate cellular processes by influencing membrane properties. While they can be produced by synthesis de novo, activation of one of the sphingomyelinases under physiological stress or other agents is a more rapid means of generation in animal tissues at least. In fact, ceramides are formed under all conditions of cellular stress in an analogous manner in all eukaryotic organisms, and they are even produced and stored in cytoplasmic droplets. Conversely, synthesis of complex glycosphingolipids and sphingomyelin from ceramide may be a route to minimising adverse effects.
It should be noted that ceramides with different fatty acid and long-chain base compositions are formed in the various compartments or membranes of the cell by several mechanisms over differing time scales, and the metabolic properties of those ceramides containing medium-chain (up to C14), long-chain (C16 and C18) and very-long-chain (C20 and longer) fatty acids have often to be considered separately. As a generality, long-chain ceramides are considered harmful by driving metabolic dysfunction to promote various disease states, while very-long-chain ceramides are thought to be relatively benign or beneficial against disease progression. While there may be some limited scope for pharmaceutical intervention into regulating ceramide levels to ameliorate disease, there is a hope that healthier eating habits could be a better approach.
The glycoprotein CD1d presented with lipid antigens, including ceramides, deoxyceramides, and diacylglycerols, activates natural killer T (NKT) cells through an interaction with the T-cell receptor present on NKT membranes via a receptor-lipid-CD1d complex to rapidly induce cytokine production. Although these responses are relatively weak, ceramide antigens are abundant and highly regulated in cellular stress and may explain NKT cell activation in the absence of infection.
Enzyme activation/deactivation: In general, ceramides tend to modify intracellular signalling pathways to slow anabolism and promote catabolism. They trigger apoptosis as well as various protein kinase cascades, dependent on the site of generation. The mechanism of these interactions is the subject of intensive study at present, but in relation to the latter, two intracellular targets for ceramide action have been discovered, together with a family of protein kinases and at least two protein phosphatases, which may regulate glycogen synthesis, insulin resistance and the response to apoptotic stimuli. Ceramides generated by the action of sphingomyelinase and by synthesis de novo both take part in the process, while ceramidases can produce contrasting results in these and other actions of ceramides. In the plasma membrane, ceramides inhibit the enzyme phospholipase D, which catalyses the conversion of phosphatidylcholine and other phospholipids into phosphatidic acid, which is in general considered to have beneficial signalling properties in cells.
Apoptosis: Ceramides and the regulation of apoptosis and cell differentiation, transformation and proliferation have received special attention. Apoptosis is a normal process, which occurs in response to oxidative stress, in which a cell can be considered to cease its metabolism and die so its components can be recycled. It is essential for many aspects of normal development and for maintaining tissue homeostasis. There are two pathways - 'extrinsic' initiated in the plasma membrane by ligation of so-called 'death factors', such as the tumour necrosis factor-α (TNF-α), and 'intrinsic' induced by external actions in mitochondria, e.g., by DNA damage, oxidation or radiation injury. In the extrinsic pathway, acid sphingomyelinase in the plasma membrane produces clusters of ceramides with ligands and their transmembrane receptors, and these act as second messengers in the apoptotic process as they bind to proteins and regulate their phosphorylation to enable the transmission of the apoptotic signal into the intracellular space. In isolated mitochondria (intrinsic pathway), C16-ceramide causes an increase of mitochondrial outer membrane permeability followed by cytochrome c release with activation of various protein kinases, phosphatases and caspases that lead to organelle dysfunction and ultimately apoptosis. It has been proposed that the formation of distinct ceramide-rich domains within the cellular membranes may assist this process.
 As animals and plants
have multiple isoforms of ceramide synthases that are specific for the chain-length
of the base and fatty acid, it has become apparent that ceramides containing different fatty acids have distinct roles in cellular physiology.
16:0-Ceramide generated by ceramide synthase 6 is highly pro-apoptotic, while ceramides with very-long-chain fatty acids accumulate in
necroptosis, a form of apoptosis, and it takes part in apoptosis in non-neuronal tissues.
C18 ceramide has growth-arresting properties and may play a part in apoptosis in some carcinomas treated with chemotherapy agents.
On the other hand, ceramides containing 2-hydroxy acids in keratinocytes are protective against apoptosis.
A transferase has been identified that transfers the acetyl group from platelet-activating factor
to sphingosine with a high specificity, and the product, N‑acetylsphingosine - the simplest of all ceramide molecules,
signals in ways that differ from those of the parent lipids or of other ceramides.
It does not enter the salvage pathway in cancer cells in vitro and is cytotoxic.
As animals and plants
have multiple isoforms of ceramide synthases that are specific for the chain-length
of the base and fatty acid, it has become apparent that ceramides containing different fatty acids have distinct roles in cellular physiology.
16:0-Ceramide generated by ceramide synthase 6 is highly pro-apoptotic, while ceramides with very-long-chain fatty acids accumulate in
necroptosis, a form of apoptosis, and it takes part in apoptosis in non-neuronal tissues.
C18 ceramide has growth-arresting properties and may play a part in apoptosis in some carcinomas treated with chemotherapy agents.
On the other hand, ceramides containing 2-hydroxy acids in keratinocytes are protective against apoptosis.
A transferase has been identified that transfers the acetyl group from platelet-activating factor
to sphingosine with a high specificity, and the product, N‑acetylsphingosine - the simplest of all ceramide molecules,
signals in ways that differ from those of the parent lipids or of other ceramides.
It does not enter the salvage pathway in cancer cells in vitro and is cytotoxic.
Although inhibition of cell division and induction of apoptosis can be beneficial actions, failure to properly regulate these processes can have catastrophic consequences, and many disease states, including cancer, diabetes, Alzheimer's disease, Parkinson's disease and atherosclerosis, are thought to arise from deregulation of apoptosis. Ceramides have been implicated in the actions of TNF-α to trigger inflammation and the production of the proinflammatory cytokines and in the cytotoxic responses to amyloid Aβ peptide, factors in Alzheimer’s disease and neurodegeneration. Ceramides influence many aspects of the biology of aging and induce the related process of cellular senescence.
Similarly, ceramides can initiate autophagy and the related process of mitophagy, the 'maintenance' process by which cellular proteins and excess or damaged organelles are removed from cells by engulfing them in membrane-enclosed cellular compartments called autophagosomes, which are enriched in very-long-chain ceramides. While this process is beneficial in that it aids recycling of cellular nutrients, the presence of excess ceramide can lead to unnecessary apoptosis.
In contrast, the ceramide metabolite sphingosine-1-phosphate has opposing effects on cell survival and proliferation. As ceramide and sphingosine-1-phosphate are inter-convertible via sphingosine as an intermediate that is pro-apoptopic, the balance between these lipids and with ceramide-1-phosphate is obviously of great metabolic importance. It has been termed the ‘sphingolipid-rheostat’. For practical reasons, the metabolism of ceramide-1-phosphate, sphingosine-1-phosphate, ceramides and sphingoid bases is discussed separately in this website, but an integrated view is necessary for a full understanding, and this should include the conversion of ceramides to more complex sphingolipids such as sphingomyelin and glycosylceramides.
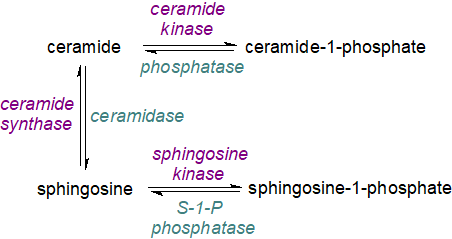 |
| Figure 5. The sphingolipid rheostat. |
Cancer: As well as Farber disease discussed above, drug therapies that influence the relative concentrations of ceramides are of interest in relation to the treatment of breast, ovarian and colorectal cancers. Thus, pathways mediated by ceramide and sphingosine-1-phosphate have been identified in both the development and progression of cancer, with the former acting to suppress tumours by inducing anti-proliferative and apoptotic responses in cancer cells and the latter serving to promote tumour growth. Thus, ceramide triggers many different tumour suppressive and anti-proliferative cellular events such as apoptosis, autophagy, senescence and necroptosis by stimulating or repressing effector molecules, while defects in ceramide generation and metabolism in cancer cells contribute to tumour survival and resistance to chemotherapy. The outcome can depend on the location and type of cancer, and ceramide synthase 1 (and the product 18:0-ceramide) is down-regulated in head and neck cancers (gliomas) and up-regulated in breast cancer. Ceramide synthase 2 is a mediator of chemoresistance and chemosensitivity. As the ceramide transfer protein (CERT) controls the ratio of ceramide to sphingomyelin in cells, it must be considered in the context of cancer.
Ceramide glycosyltransferases that catalyse ceramide glycosylation and glycosphingolipid formation regulate tumour progression, and there is a significant correlation with the poor prognosis of cancer patients. In contrast, there is a suggestion that inhibition of the acid ceramidase may be beneficial towards cancer as this enzyme is critical for the ability of polyploid giant cancer cells to generate progeny. Inhibition of the neutral ceramidase in colon cancer cells increases ceramide concentrations to reduce cancer cell survival and increase apoptosis. Lethal mitophagy is an anti-tumorigenic mechanism mediated by ceramide, where cells degrade sufficient mitochondria to kill the cancer cell in an apoptosis-independent manner.
Administration of exogenous ceramides with short-chain acyl moieties (C2, C6 or C8) encapsulated by nano-technological means is seen as a promising therapeutic approach to cancer. C6-ceramides can cause cell death in several cancers, and they are known to increase the sensitivity to other apoptosis-inducing agents, although the signalling mechanism by which this is accomplished has yet to be determined. Ceramides generated by the action of the acid sphingomyelinase may inhibit cancer development, though a virtual absence of the alkaline sphingomyelinase has been noted in colorectal cancer. A further ceramide metabolite, ceramide-1-phosphate, is anti-apoptotic and influences inflammatory responses by activating a phospholipase A2. Again, there is a need for the optimum balance between the precursor and product.
Cardiovascular disease: Although basal levels of ceramides are necessary for the production of vasoprotective metabolites such as sphingosine-1-phosphate, a chronic accumulation of ceramides can induce pro-oxidative stress pathways in endothelial cells. They can then drive cardiovascular disease and the formation of atherosclerotic plaques by promoting the oxidation of low-density lipoproteins and influencing the vascular smooth muscle cell phenotype. In mice and rats, inhibition or reduction of enzymes for ceramide synthesis by pharmacological means prevents the development of diabetes, atherosclerosis, hypertension and heart failure. In cultured cells and isolated tissues, ceramides perturb mitochondria and may contribute to heart disease by inducing cell death and inflammation; by accumulating in mitochondria of cardiomyocytes, they increase their permeability and lead to apoptosis. In humans, an elevated ratio in serum of very-long-chain to long-chain ceramides, especially of dihydroceramides and promoted by ceramide synthase 2, is considered to be a good biomarker for adverse outcomes in cardiovascular disease (and is observed in myocardial biopsies). Here once more, sphingosine-1-phosphate often has the opposite effect.
 Other
disease states: Ceramides may be less benign in many other disease states, and as discussed above
ceramide-enriched membranes can promote the entry of viruses and bacterial pathogens into cells.
Whether ceramide modulation in disease occurs as a side effect of aberrant accumulation in tissues not suited for lipid storage in response
to a pathological mechanism, such as cell stress or inflammation, or whether they participate directly in the development of disease is not
always clear.
Nonetheless, significant changes in ceramide levels and composition have been noted in many inflammatory conditions, including metabolic diseases,
irritable bowel syndrome, asthma, arthritis, multiple sclerosis, retinal degeneration and cystic fibrosis,
and the mechanistic implications are under investigation.
Those ceramides in the circulation linked to saturated fatty acids (C16 to C22) may be biomarkers of these diseases.
Other
disease states: Ceramides may be less benign in many other disease states, and as discussed above
ceramide-enriched membranes can promote the entry of viruses and bacterial pathogens into cells.
Whether ceramide modulation in disease occurs as a side effect of aberrant accumulation in tissues not suited for lipid storage in response
to a pathological mechanism, such as cell stress or inflammation, or whether they participate directly in the development of disease is not
always clear.
Nonetheless, significant changes in ceramide levels and composition have been noted in many inflammatory conditions, including metabolic diseases,
irritable bowel syndrome, asthma, arthritis, multiple sclerosis, retinal degeneration and cystic fibrosis,
and the mechanistic implications are under investigation.
Those ceramides in the circulation linked to saturated fatty acids (C16 to C22) may be biomarkers of these diseases.
In obesity and dyslipidaemia, sphingolipids such as ceramide and its metabolites induce the cellular dysfunctions that underlie diabetes in that they disrupt insulin sensitivity, and pancreatic β‑cell, vascular and mitochondrial metabolism. Thus, ceramides synthesised de novo promote apoptosis of pancreatic β-cells in both types 1 and 2 diabetes. 16:0-Ceramide has profound impacts upon adipose tissue metabolism, and it has been identified as the main mediator of obesity-derived insulin resistance, together with impaired fatty acid oxidation and hepatic steatosis. In cystic fibrosis, this ceramide accumulates at the expense of those containing long-chain and very-long-chain ceramides.
Ceramides affect neurological disorders, such as Alzheimer's disease, Parkinson's disease, drug addiction and depression, and cerebrovascular diseases such as stroke and cerebral small vessel disease. In relation to the pathology of Alzheimer's disease, high ceramide levels have been linked to its progression by contributing to amyloid beta accumulation and tau tangle formation in addition to oxidative stress and neuroinflammation, probably exacerbated by the formation of ceramide-rich domains which displace cholesterol-sphingomyelin rafts. An involvement of long- and very-long-chain ceramides, but not those with short-chains, in learning and memory has been reported, and 18:0-ceramide is a potential contributor to human aging. Excess ceramide production occurs in age-related macular degeneration and is associated with a loss of polyunsaturated fatty acids of the n-3 family; there is hope that a ceramide-targeted strategy might improve the condition. In animal models, inhibition of ceramide biosynthesis or promotion of ceramide degradation ameliorates many metabolic disorders, but in contrast, there is evidence that increasing the ceramide content of cells can inhibit inflammatory responses to bacterial lipopolysaccharides. In humans, a few rare genetic disorders of sphingoid base and ceramide synthesis or catabolism are known of which the most studied is the formation of 1-deoxy-bases and ceramides, discussed on this website here.... Those diseases related to skin ceramides are described briefly below.
In relation to bacterial infections, ceramides produced by acid sphingomyelinase promote entry of Neisseria gonorrhoeae, a pathogen primarily responsible for sexually transmitted disease, into cells. On the other hand, in a deficiency of this enzyme, there is an impaired ability to resolve infections by Salmonella typhimurium and Pseudomonas aeruginosa, but with increased resistance to lethal Mycobacterium avium in mice. Ceramides bacterial lipopolysaccharide-induced proinflammatory signalling through stabilization of TLR-4.
Dihydroceramides: The function of ceramides in animal tissues usually requires the presence of the 4,5-double bond in the long-chain base, although the trans conformation may not be essential in that synthetic ceramide containing a cis-4,5-double bond is an equally potent inducer of apoptosis at least. On the other hand, dihydroceramides in which sphinganine is the sphingoid base are now known to accumulate to a far greater extent in tissues than had previously been thought, and they can act in a different manner from the more conventional ceramides. In model membranes at least, dihydrosphingolipids have somewhat different biophysical properties that include membrane permeability and packing.
Dihydroceramides regulate autophagy by a mechanism different from that of ceramides per se, for example in autophagy-induced death of cancer cells, but they are pro-survival under conditions of physiological stress. It seems possible that the former are 'safer' when elevated concentrations of sphingosine-containing ceramides might be harmful. Further, dihydroceramides can regulate such diverse processes as the production of reactive oxygen species in mitochondria and the activity of cytochrome P450s and phosphatases. They have been implicated in the progression of such disease states as cancer, non-alcoholic fatty liver disease, diabetes and ischemia/reperfusion injury, while mutations in the human DEGS1 gene are known to cause severe neurological defects by disrupting homeostasis of the endoplasmic reticulum and lipid droplets in glial cells.
Plants: Comparatively little information is available on ceramides and cell signalling in plants, but there are suggestions that sphingolipid catabolic products may be linked to programmed cell death, signal transduction, membrane stability, host-pathogen interactions and stress responses. There is evidence that enhanced biosynthesis of ceramides with very-long-chain fatty acids and trihydroxy sphingoid bases by ceramide synthases LOH1 and LOH3 promotes cell division and growth, while in contrast, overexpression of LOH2 leads to accumulation of the ceramide 16:0 fatty acid/dihydroxy sphingoid base and results in plant dwarfing and programmed cell death. Loss of function of the ceramide kinase ACD5 in plant mitochondria and other subcellular compartments leads to ceramide accumulation and apoptosis. Ceramides aggregate in rafts in plant membranes together with other sphingolipids and sterols as in animal tissues. In the yeast S. cerevisiae, it has been reported that ceramides with different N-acyl chains and sphingoid bases and especially dihydosphingoid bases, can regulate different sets of related genes. Increased synthesis of ceramides containing saturated fatty acids and sphingoid bases is positively associated with seed germination in soybeans.
5. Skin Ceramides
The mammalian skin forms the protective barrier between the internal tissues of the host and the hostile external environment, which can include chemicals, ultraviolet light, mechanical damage and pathogenic microorganisms, while preventing the loss of water and electrolytes. It consists of stratified layers of increasingly differentiated cells or keratinocytes of which the basal layer is responsible for the renewal of the tissue but begins to migrate upwards and differentiate, while accumulating certain lipids and proteins that change the cellular architecture. Eventually, the keratinocytes lose their nucleus and become flattened structures of insoluble protein surrounded by lipids and termed ‘corneocytes’ in the outermost impermeable layer or stratum corneum. By secreting antimicrobial peptides and proteins, keratinocytes add to the defensive capability of skin against commensal microorganisms and opportunistic pathogens, and this is reinforced by lipid mediators such as free sphingoid bases and eicosanoids in the stratum corneum and free fatty acids in sebum. Our web page on waxes describes the non-polar lipids secreted onto skin by the sebaceous glands.
The stratum corneum contains high levels of ceramides (as much as 50% of the total lipids), including O-acylceramides, which exist both in the free form and linked by ester bonds to structural proteins. They are present mainly in the extracellular domains (interstices) and are accompanied by cholesterol and free fatty acids in a 1:1 ratio that may be necessary for the normal organization of the tissue into the membrane structures responsible for the efficiency of the epidermal barrier. In contrast to other tissue membranes, the lipids in the membranes of skin are arranged in two lamellar phases, which form crystalline lateral structures mainly, with repeat distances of approximately 6 and 13 nm, alongside small sub-domains of lipids in a liquid phase.
Some of these skin ceramides have distinctive structures not seen in other tissues, and many different forms are commonly recognized. Such lipids were first studied in detail in the skin of the pig as a convenient experimental model, but they now been characterized in humans and rats. They can contain the normal range of longer-chain fatty acids (a), e.g., formula 1 in the figure, and some with hydroxyl groups in position 2 (a*), e.g., formula 2, linked both to dihydroxy bases with trans-double bonds in position 4 or to trihydroxy bases. In addition, there are ω-O‑acyl ceramides in which a unique very-long-chain fatty acid component (typically C30 or C32) has a terminal hydroxyl group, and this may be in the free form or esterified to linoleate (c), e.g., formulae 3 and 4; the sphingoid base can be either di- (b) or trihydroxy (b*), e.g., formula 4; the latter is not a common feature in other sphingolipids of animal origin and can include both phytosphingosine and the unique 6‑hydroxy-4-sphingenine in human epidermis. Ceramides of type 1 in which the 1-O-hydroxyl group of the sphingoid base is acylated by a very-long-chain fatty acid (some with 2‑hydroxyl groups) are present (1‑O‑acylceramides - illustrated above), and these comprise 5% of the total ceramides in the epidermis of mice and humans and as many as 700 molecular species.
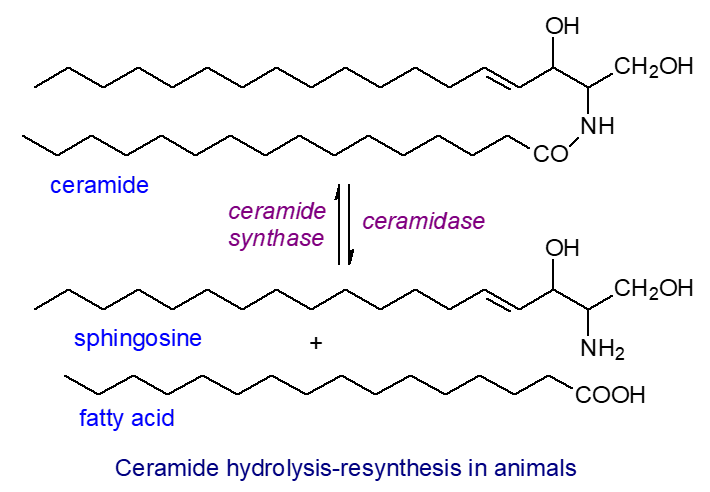 |
| Figure 6. Structural formulae of some representative skin ceramides. |
In all, 17 classes of free ceramides and 3 classes of ceramides bound covalently to protein with up to 1600 molecular species have been identified. In human skin, ceramides contain sphingoid bases that vary in chain length from C16 to C26 with C18 most abundant, followed by C20 and C22, with some differences with the layer of the skin (keratinocytes, stratum corneum, etc.). Each of the ceramide class has a characteristic distribution of these bases, but there is little difference in fatty acid composition between classes. Several molecular forms of glucosylceramide, based on the same ceramide structures, have also been identified in skin and are essential for its proper function.
The lipids have an obvious role in the barrier properties, limiting loss of water and solutes and at the same time preventing ingress of harmful substances. As the aliphatic chains in the ceramides and the fatty acids are mainly non-branched long-chain saturated compounds with a high melting point and a small polar head group, the lipid chains are mostly in a solid crystalline or gel state, which exhibits low lateral diffusional properties and low permeability at physiological temperatures. There is a report that the stratum corneum layer of the skin has a water permeability only one thousandth that of other biomembranes, for example. Natural and synthetic ceramides are now commonly added to cosmetics and other skin care preparations, and analogous emollients have been approved as repair agents.
Most steps in the biosynthesis of ceramides linked to ω-O-acylated fatty acids occur in the endoplasmic reticulum of keratinocytes. First, fatty acid synthesis of very-long-chain (and ultra-long-chain, ≥C26) acyl-CoA de novo must take place, requiring the chain-elongation enzymes ELOVL1 and ELOVL4; desaturation can occur as well as oxidation (hydroxylation) in the 2 (α) and terminal (ω) positions. The ω‑hydroxylation step requires an enzyme of the cytochrome P450 family, designated CYP4F22, of the kind used for the synthesis of hydroxy-eicosatetraenoic acids (HETE). Mutations are a cause of lamellar ichthyosis, and knockout mice deficient in the equivalent enzyme die within 8 hours of birth.
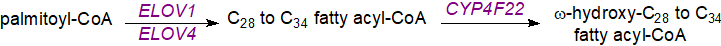 |
| Figure 7. Biosynthesis of very-long-chain ω-hydroxy fatty acids in skin. |
Ceramides are first synthesized by ceramide synthase 3 (CERS3), which has a marked preference for very-long chain fatty acids (>C26) with incorporation of the ω‑hydroxy fatty acid. This is acylated with linoleate by the action of an unusual enzyme related to the phospholipase A family, PNPLA1, which catalyses esterification by first releasing linoleate from triacylglycerols in the skin while acting as an acyltransferase to link the linoleate directly to the ω‑hydroxyl moiety of the ultra-long chain fatty acid. Linoleate for this purpose is released from triacylglycerols by PNPLA1 on the surface of lipid droplets aided by a protein ABHD5 and perilipin, which are presumably in close proximity to each other. This process is vital for proper skin barrier function and keratinocyte differentiation, as mice with defective triacylglycerol biosynthesis and metabolism, including a deficiency of the acyl-CoA synthase ACSL1, are unable to synthesis ω‑O‑acylceramides and have an impaired skin barrier. Mutations in the human PNPLA1 gene are the cause of autosomal recessive disease congenital ichthyosis.
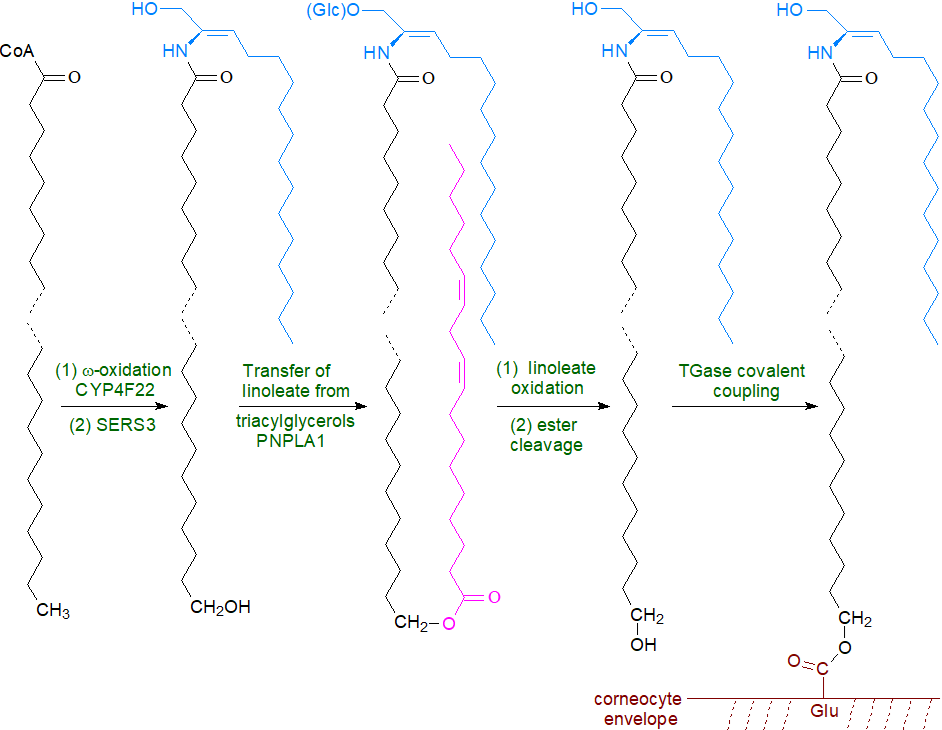 |
| Figure 8. Formation of the corneocyte lipid envelope. |
The resulting ceramides are converted to the complex sphingolipids, mainly glucosylceramide with some sphingomyelin, which are transferred with the aid of ATP-binding cassette (ABC) transporters together with degradative enzymes into the stratum corneum via organelles termed 'lamellar bodies.' These organelles must fuse with the apical plasma membrane of the outermost cell layer of the epidermis in order that their contents can be secreted. It is only then that the final step of hydrolysis of the lipid precursors occurs in the extracellular spaces of the stratum corneum, i.e., ceramides are generated from sphingomyelin by the action of acid sphingomyelinase and from glucosylceramides by β‑glucocerebrosidase. This mechanism ensures that potentially harmful ceramides never accumulate within nucleated cells. The enzyme responsible for the synthesis of 1-O-acylceramides in the stratum corneum has not yet been identified.
Eventually, ceramides with a terminal ω-hydroxyl group in the fatty acyl moiety are bound covalently to the proteins such as involucrin of the cornified envelope. Recent evidence suggests that in the first step of this process ceramides containing O-acyl linoleate in an estolide linkage are acted upon by lipoxygenases (12R‑LOX and eLOX3) to form hepoxilin-like products, which may be required in skin (see the web page on these oxylipins for a discussion of their biosynthesis). The high polarity of the main isomer, 9R,10S,13R-trihydroxy-11E-octadecenoate (a tri-HOME), facilitates its hydrolysis, initiating a procedure that leads to attachment of the free ω-hydroxyl groups of the very-long-chain fatty acid components of ceramides and glucosylceramides via ester bonds to glutamate residues of proteins exposed on the surface of corneocytes by means of transglutaminase 1 (TGase1). Ultimately, the covalently bound glucosylceramides and ceramides are further metabolized until only the long-chain ω-hydroxy acid remains attached to protein. These proteolipid structures, i.e., the corneocyte lipid envelope, form a complete lipid monolayer over the surface of each cell and are indispensable for the operation of the epidermal water barrier; they act as a template or scaffold to direct the assembly of the extruded lipids into lamellar bilayers.
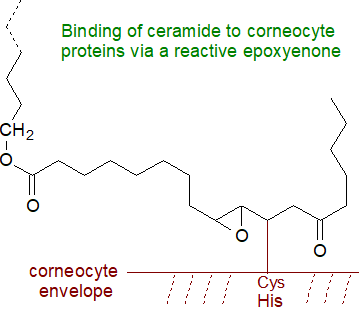 An alternative mechanism has been proposed for the final steps in which the linoleate residue attached to the
ω‑O‑acylceramide is oxidized by 12R-LOX, eLOX3 and a NAD+-dependent dehydrogenation to a highly
reactive 9,10-trans-epoxy,11E-ene,13-keto intermediate (epoxy-enone), which rather than being hydrolysed is able to link
non-enzymatically by the Michael addition reaction to the sulfur atom of cysteine or to histidine residues in proteins of the corneocyte
envelope, or by formation of a Schiff base and eventually a pyrrole derivative with a lysine residue (see our web page on
aldehydes, for discussion of such reactions).
This explanation for the role of linoleate in the process is more convincing than some alternatives.
Such epoxy-enone products can be released reversibly by sulfoxide elimination, and it is now suggested they account for approximately
60% of total protein-bound ceramides (hydroxy-cyclohexenones can be formed as artefacts by alkaline treatment).
An alternative mechanism has been proposed for the final steps in which the linoleate residue attached to the
ω‑O‑acylceramide is oxidized by 12R-LOX, eLOX3 and a NAD+-dependent dehydrogenation to a highly
reactive 9,10-trans-epoxy,11E-ene,13-keto intermediate (epoxy-enone), which rather than being hydrolysed is able to link
non-enzymatically by the Michael addition reaction to the sulfur atom of cysteine or to histidine residues in proteins of the corneocyte
envelope, or by formation of a Schiff base and eventually a pyrrole derivative with a lysine residue (see our web page on
aldehydes, for discussion of such reactions).
This explanation for the role of linoleate in the process is more convincing than some alternatives.
Such epoxy-enone products can be released reversibly by sulfoxide elimination, and it is now suggested they account for approximately
60% of total protein-bound ceramides (hydroxy-cyclohexenones can be formed as artefacts by alkaline treatment).
Among other suggestions, the epoxide intermediate can be converted by an epoxide hydrolase to a trihydroxy acid, before the ester bond is selectively hydrolysed by an esterase leaving a ceramide with the long-chain ω-hydroxy acid, which is then linked to protein by TGase1. Alternatively, a reaction with glutathione can occur to eliminate the 11,12-double bond and allow spontaneous C9-C13 cyclization to form reactive hemiketal derivatives.
In essential fatty acid deficiency, the O-acyl linoleate is replaced by oleate with concomitant acid abnormalities in the cutaneous permeability barrier. In diseased skin, there is often an altered lipid composition and organization with impaired barrier properties, and diminished levels of ceramide and changes in their compositions in the epidermis, reflecting altered sphingolipid metabolism in relation to the esterified and non-esterified omega-hydroxy-ceramides and trihydroxy bases, have been implicated in such skin disorders as psoriasis, ichthyosis and atopic dermatitis; tri-HOME and ceramides of lower molecular weight accumulate in the last of these. Much less emphasis in research has been placed upon signalling by ceramides in skin, but there is increasing evidence they may influence the regulation of the same metabolic events as in other tissues with implications for health.
6. Analysis
Ceramides can be isolated by adsorption chromatography (TLC and HPLC) and further analysed by HPLC or GC after conversion to less polar derivatives, and one widely used method for analysis of molecular species of sphingomyelin uses hydrolysis with phospholipase C to generate ceramides to simplify the technical problems. Modern mass spectrometric methods are increasingly being used for characterization and quantification, but care must be taken to distinguish dihydroceramides from the more conventional ceramides with which they can be isobaric.
Recommended Reading
- AL Mughram, M.H., Kellogg, GE. and Wattenberg, B.W. Three kingdoms and one ceramide to rule them all. A comparison of the structural basis of ceramide-dependent regulation of sphingolipid biosynthesis in animals, plants, and fungi. Adv. Biol. Reg., 91, 101010 (2024); DOI.
- Alonso, A. and Goñi, F.M. The physical properties of ceramides in membranes. Annu. Rev. Biophys., 47, 633-654 (2018); DOI.
- Bouwstra, J.A., Nădăban, A., Bras, W., McCabe, C., Bung, A. and Gooris, G.S. The skin barrier: An extraordinary interface with an exceptional lipid organization. Prog. Lipid Res., 92, 101252 (2023); DOI.
- Canals, D. and Hannun, Y.A. Biological function, topology, and quantification of plasma membrane ceramide. Adv. Biol. Reg., 91, 101009 (2024); DOI.
- Cassim, A.M., Grison, M., Ito, Y., Simon-Plas, F., Mongrand, S. and Boutte, Y. Sphingolipids in plants: a guidebook on their function in membrane architecture, cellular processes, and environmental or developmental responses. FEBS Letts, 594, 3719-3738 (2020); DOI.
- Chaurasia, B. and Summers, S.A. Ceramides in metabolism: key lipotoxic players. Annu. Rev. Physiol., 83, 303-330 (2022); DOI.
- Chitkara, S. and Atilla-Gokcumen, G.E. Decoding ceramide function: how localization shapes cellular fate and how to study it. Trends Biochem. Sci., 50, 356-367 (2025); DOI.
- Choudhary, P., Kumari, S., Bagri, K. and Deshmukh, R. Ceramide: a central regulator in Alzheimer's disease pathogenesis. Inflammopharm., 33, 1775-1783 (2025); DOI.
- Gomez-Larrauri, A., Larrea-Sebal, A., Martín, C. and Gomez-Muñoz, A. The critical roles of bioactive sphingolipids in inflammation. J. Biol. Chem., 301, 110475 (2025); DOI.
- Hammerschmidt, P. and Bruning, J.C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell. Mol. Life Sci., 79, 395 (2022); DOI.
- Jang, Y. Bioactive compounds targeting dihydroceramide and their therapeutic potential in cancer treatment. Cancers, 17, 909 (2025); DOI.
- Mu, J.M., Lam, S.M. and Shui, G.H. Emerging roles and therapeutic potentials of sphingolipids in pathophysiology: emphasis on fatty acyl heterogeneity. J. Genet. Genom., 51, 268-278 (2024); DOI.
- Parveen, F., Bender, D., Law, S.H., Mishra, V.K., Chen, C.C. and Ke, L.Y. Role of ceramidases in sphingolipid metabolism and human diseases. Cells, 8, 1573 (2019); DOI.
- Piccoli, M. and others. Sphingolipids and atherosclerosis: the dual role of ceramide and sphingosine-1-phosphate. Antioxidants, 12, 143 (2023); DOI.
- Rudan, M.V. and Watt, F.M. Mammalian epidermis: a compendium of lipid functionality. Front. Physiol., 12, 804824 (2022); DOI.
- Senkal, C.E., Salama, M.F., Snider, A.J., Allopenna, J.J., Rana, N.A., Koller, A., Hannun, Y.A. and Obeid, L.M. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab., 25, 686-697 (2017); DOI.
- Su, H., Li, Y., Li, Z.P., Li, SM., Pan, J.S., Ji, G., Lu, L. and Xu, H.C. Ceramides and associated key enzymes at the evolutionary crossroad of colorectal inflammatory immune responses and tumorigenesis. Int. J. Biol. Macromol., 322, 146916 (2025); DOI.
- Suzuki, M., Ohno, Y. and Kihara, A. Whole picture of human stratum corneum ceramides, including the chain-length diversity of long-chain bases. J. Lipid Res., 63, 100235 (2022); DOI.
- Wang, S.N., Jin, Z.H., Wu, B.Y., Morris, A.J. and Deng, P. Role of dietary and nutritional interventions in ceramide-associated diseases. J. Lipid Res., 66, 100726 (2025); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
