Sphingolipids, Membrane Rafts and Caveolae
It is not possible to fully understand the metabolic properties of sphingolipids without some knowledge of their distinctive physical location within cellular membranes. They are present only in the outer (exoplasmic) leaflet of most membrane bilayers, while anionic glycerophospholipids, including phosphatidylinositol, phosphatidylserine and phosphatidylethanolamine, occur only in the inner (cytoplasmic) leaflet under normal circumstances. The plasma membrane is a vital cellular structure that safeguards eukaryotic cells by separating the cytosolic compartments from the external environment, contributing to cell shape and protecting the cell from its surroundings. Sphingolipids are essential not only to provide a suitable membrane environment to meet these physical requirements, but also to support the protein complexes that translate extracellular signals into biochemical processes. Cholesterol is abundant in the plasma membrane, and current thinking is that it occurs predominantly in the outer leaflet of this membrane and not in roughly equal proportions in both leaflets as was once thought. Together with cholesterol and trans-membrane proteins, sphingomyelin and other sphingolipids are located in an intimate association in characteristic sub-domains or 'rafts' of most membranes in eukaryotes but of the plasma membrane in particular.
1. General Concepts
Cell membranes are a complex mosaic of domains containing many different proteins and lipids. Within these, rafts are laterally segregated regions that form transiently because of selective affinities between sphingolipids and certain membrane proteins. These act to compartmentalize and provide a platform for the latter and thereby separate different biochemical pathways, and simplistically, they are considered to be relatively rigid or solid regions embedded in a fluid region of membrane. More formal definitions are discussed below. Related invaginated structures termed 'caveolae' have many comparable properties in the plasma membrane of cells, but they are much more stable and accessible for study.
Many aspects of raft structure are uncertain and controversial, largely because of the technical difficulties for their study in membranes of living cells as opposed to model systems. Indeed, it has been argued by some that all the evidence for the existence of rafts is indirect, and that alternative explanations may be possible for the observed phenomena. On the other hand, much recent research, such as that with lipid-specific toxin fragments, supports the concept of raft domains, and any remaining controversy appears to be concerned more with the mechanism(s) behind raft formation rather than their existence with a focus on sphingolipid and protein metabolism within rafts in cells.
 While
the concept of rafts was developed for the Golgi and then the plasma membrane in animals, it has become evident that
specialized membrane domains containing signalling platforms are also present in nuclei, endoplasmic reticulum and mitochondria, while
similar nanodomains are elements of cell membranes in other eukaryotes, including higher plants, and prokaryotes.
Although raft domains are present primarily in the outer leaflets of asymmetric cell membranes,
they may be coupled to lipids in the inner leaflet by hydrogen bonding.
It has become evident that such rafts do not represent a single monolithic structure,
but a heterogeneous collection of domains differing in their protein and lipid compositions as well as in their stability with respect
to size and time, i.e., length scales of tens of nanometers and time scales of milliseconds.
Up to 50% of the plasma membrane in animal tissues may consist of rafts, and the apical membrane of epithelial cells may behave like a
large raft.
While
the concept of rafts was developed for the Golgi and then the plasma membrane in animals, it has become evident that
specialized membrane domains containing signalling platforms are also present in nuclei, endoplasmic reticulum and mitochondria, while
similar nanodomains are elements of cell membranes in other eukaryotes, including higher plants, and prokaryotes.
Although raft domains are present primarily in the outer leaflets of asymmetric cell membranes,
they may be coupled to lipids in the inner leaflet by hydrogen bonding.
It has become evident that such rafts do not represent a single monolithic structure,
but a heterogeneous collection of domains differing in their protein and lipid compositions as well as in their stability with respect
to size and time, i.e., length scales of tens of nanometers and time scales of milliseconds.
Up to 50% of the plasma membrane in animal tissues may consist of rafts, and the apical membrane of epithelial cells may behave like a
large raft.
Rafts provide a platform for most of the signalling at the plasma membrane, as well as exo- and endocytosis mechanisms, and they are in essence the main platforms for the interaction of cells with their external environment. Several types of protein are associated with rafts and caveolae, including some with essential membrane functions (caveolins, flotillins, glycosylphosphatidylinositol-linked proteins), signalling proteins (e.g., Src family kinases), G protein-coupled receptors, and others that are lipid linked (palmitoylated, myristoylated, hedgehog). Rafts regulate cellular metabolic and signalling pathways by organizing these proteins in an ordered manner on the cell surface. They are involved in such vital processes as haematopoiesis, inflammation and immunity, host interactions with infection, and the development and progression of cancer, cardiovascular disease and neurodegeneration.
The terms 'rafts', 'micro-domains', 'nano-domains' and 'transient nano-domains' in membranes are often used interchangeably, although they are not necessarily synonymous. While the term 'raft' might be considered trivial and non-scientific, I will continue to use it here for convenience and until an alternative consensus has clearly emerged.
2. Raft Structure and Organization
Definitions: A formal definition of what constitutes a membrane raft was proposed after a conference on the subject, and at the time it was accepted by the lipid community as a useful working hypothesis (Pike, L.J. J. Lipid Res., 47, 1597-1598 (2006); DOI), i.e.,
"Membrane rafts are small (10-200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes. Small rafts can sometimes be stabilized to form larger platforms through protein-protein and protein-lipid interactions."
Others (Simons, K. and Sampaio, J.L. Cold Spring Harbor Persp. Biol., 3, a004697 (2011); DOI) prefer -
"Dynamic nanoscale sterol, sphingolipid-enriched, ordered assemblies of specific proteins, in which the metastable resting state can be activated to coalesce by specific lipid–lipid, protein–lipid, and protein–protein interactions".
Simpler, and perhaps more in tune with current thinking, is that proposed by Sezgin, E. et al. (Nature Rev. Mol. Cell Biol., 18, 361-374 (2017); DOI) -
Rafts are "transient, relatively ordered membrane domains, the formation of which is driven by lipid-lipid and lipid-protein interactions".
Earlier definitions tended to depend on the methods used experimentally to produce raft preparations, i.e., their resistance to non-ionic detergents such as their insolubility in cold 1% Triton X-100. While they were once described as detergent-resistant membranes or 'DRM', this has resulted in much confusion and controversy in the literature. Such DRM certainly contain raft material, but it is now considered by many experts in the field that they should no longer be treated as rafts per se because more robust and definitive methods of studying raft formation are now available. On the other hand, it has been suggested that stable raft material can be obtained using the related polyethylene oxide detergent, Brij O20. The transient nature of the phenomena mean that technical difficulties remain in studies of raft structure and formation in living cells as opposed to model membranes, and these can confound interpretation of data and confuse the picture. Rafts are relatively small (approximately 50 nm diameter and containing roughly 3000 sphingomyelin molecules) and mobile, and they are not easy to study by microscopic methods. The rapid rate of formation-dissolution of rafts further hinders their study, although no accurate timescale has been determined.
The sphingolipid-cholesterol hypothesis: Much of the early literature on raft formation is based on the concept of specific physical interactions between sphingolipids and cholesterol in membranes, although strictly speaking this is only applicable to animal membranes (plants and bacteria are discussed below). According to this theory, the packing of the saturated acyl chains of sphingolipids with cholesterol is thermodynamically favoured over that with unsaturated acyl chains, and cholesterol is essential to the process of raft formation in the plasma membrane. If either the sphingolipid or cholesterol concentration is depleted by any means, the other follows. Indeed, there is evidence that in animal tissues sphingomyelin regulates the capacity of membranes to absorb cholesterol and thereby controls its flux between the plasma membrane and other regulatory pathways in the endoplasmic reticulum. The key to the phenomenon is that the two lipid classes form extensive intramolecular hydrogen bonds with each other, so they self-organize in membranes to form laterally segregated raft domains. Cholesterol interacts strongly with sphingomyelin and much less with glycosphingolipids, although the latter are nonetheless implicated in raft formation. It is noteworthy that epithelial cells of the kidney and stomach, which are enriched in cholesterol and sphingolipids, are highly impermeable to water and solutes.
The importance of cholesterol to the formation of rafts has led to a further definition of rafts (Kusumi, A. et al. Traffic, 21, 106-137 (2020); DOI) ‑
Raft domains in the plasma membrane are liquid-like molecular complexes/domains formed by cooperative interactions of cholesterol with saturated acyl chains as well as unsaturated acyl chains, due to saturated acyl chains' weak multiple accommodating interactions with cholesterol and cholesterol's low miscibility with unsaturated acyl chains and trans-membrane proteins.
Physical chemical studies have demonstrated that the conformation and orientation around the amide group of sphingomyelin are relatively rigid as is appropriate for an intermolecular hydrogen bond with a neighbouring sphingomyelin molecule, while cholesterol enhances the order of the central hydrocarbon chains of sphingomyelin appreciably. The mechanism of raft formation probably starts by an enhancement of the order of the central sphingomyelin alkyl chains by the rigid cholesterol molecules by restricting the chain fluctuation and thus shortening the intermolecular distances between sphingomyelin molecules to facilitate hydrogen bond formation and a hydrogen bond network in a stable ordered phase that is resistant to temperature fluctuations, even at low cholesterol concentrations. This tight packing leads to a smaller molecular surface area than would be predicted from the sum of those of the individual molecules. As a result, lateral diffusion occurs with separation of the lipids containing saturated fatty acyl chains from those that are highly unsaturated. Indeed, there is evidence that glycerolipids containing docosahexaenoic acid are incompatible with more saturated lipids, including cholesterol, and drive the segregation of the latter into raft domains, to perhaps increase raft size and stability.
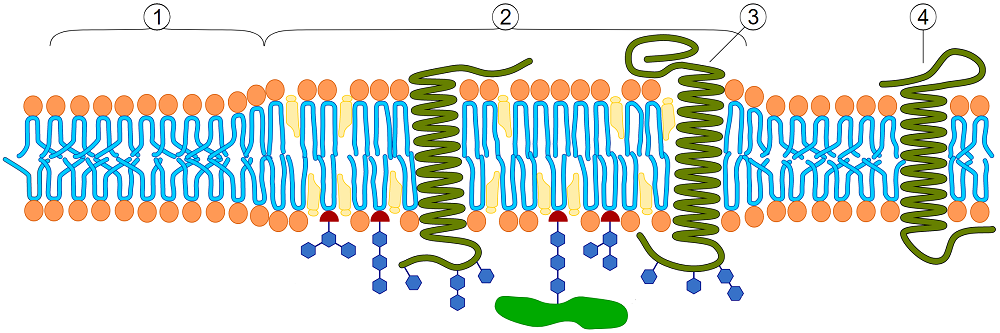
Lipid raft organization, region (1) is standard lipid bilayer, while region (2) is a lipid raft.
Illustration by Artur Jan Fijałkowskipl and reproduced from Wikipedia under a creative commons license.
As sphingolipids containing long, largely saturated acyl chains, they pack more tightly together, thus giving sphingolipids much higher melting temperatures than membrane glycerophospholipids. This tight acyl chain packing aids raft lipid organization, since the differential packing facility of sphingolipids and cholesterol in comparison with glycerophospholipids leads to a phase separation in the membrane that gives rise to sphingolipid-rich regions ('liquid-ordered' phase) surrounded by glycerophospholipid-rich domains ('liquid-disordered' phase). Such ordering is responsible for the resistance to attack by detergents. Sphingolipids tend to have more free hydroxyl groups, both in the long-chain bases and fatty acid components than glycerolipids, and these contribute to hydrogen bonding and the stability of rafts. The presence of very-long-chain fatty acid components (e.g., C26) is needed to produce thicker than normal membranes (46 versus 40 angstroms). One further relevant factor may be interleaflet coupling of liquid-ordered domains between the outer and the inner leaflets via interdigitation through such long acyl chains.
All cell organelles have characteristic membrane lipid and protein compositions, although much remains to be learned of how these are achieved. There is a gradient of lipid compositions and physical properties in cells with the endoplasmic reticulum containing low levels of raft-forming lipids, such as cholesterol and sphingolipids, while the plasma membrane is greatly enriched in these components (30-40 mol% cholesterol and 10-30% sphingolipids) and the Golgi have intermediate compositions presumably with increasing proportions from the cis to trans regions. In the secretory pathway for new lipid synthesis, it seems plausible that formation and selective transfer of laterally segregated raft domains enriched in these lipids could provide a self-organizing explanation for the observed compositions.
Lipid-protein models: There is a conflicting view of the raft hypothesis, based on studies by high-resolution secondary ion mass spectrometry, that is gaining traction. This suggests that sphingolipids are concentrated in micrometre-scale membrane domains while cholesterol is evenly distributed within the plasma membrane. In this model, sphingolipid distribution and raft formation in the plasma membrane are dependent on the organization of actin in the cytoskeleton, but not on favourable interactions with cholesterol, and the sphingolipid-cholesterol model may not hold when there are large amounts of protein in the membrane. A further confounding factor is that as many as 250 transmembrane proteins may interact directly with cholesterol at a consensus sequence termed the CRAC motif for "cholesterol recognition/interaction amino acid consensus", and this is independent of sphingolipids; many of these proteins operate in organizing signalling hubs and include the caveolins (see below). It is entirely possible that formation of lipid rafts is a multifactorial process and that no single model will provide an adequate description.
 Rather than lipid-lipid interactions,
raft formation may be driven and stabilized by lipid-protein and protein-protein interactions, and membranes
should be regarded as lipid-protein composites rather than a solution of protein in a lipid solvent.
Indeed, membrane proteins are essential for raft formation, and the reggies/flotillins, which are sometimes described as molecular scaffolding,
promote the assembly of post-translationally modified proteins such as
glycerophosphoinositol(GPI)-anchored proteins into membrane domains.
These then recruit other proteins, which can include tyrosine kinases, phosphatases and other signalling proteins,
and facilitate interactions among them.
As most proteins partition into the fluid-disordered rather than into the fluid-ordered nanodomains, raft-related proteins are in the minority
in membranes.
Rather than lipid-lipid interactions,
raft formation may be driven and stabilized by lipid-protein and protein-protein interactions, and membranes
should be regarded as lipid-protein composites rather than a solution of protein in a lipid solvent.
Indeed, membrane proteins are essential for raft formation, and the reggies/flotillins, which are sometimes described as molecular scaffolding,
promote the assembly of post-translationally modified proteins such as
glycerophosphoinositol(GPI)-anchored proteins into membrane domains.
These then recruit other proteins, which can include tyrosine kinases, phosphatases and other signalling proteins,
and facilitate interactions among them.
As most proteins partition into the fluid-disordered rather than into the fluid-ordered nanodomains, raft-related proteins are in the minority
in membranes.
Palmitoylation or myristoylation is often needed to target proteins to the inner leaflet of lipid rafts (see our web page on proteolipids), and for example, flotillin is anchored via N-terminal myristoyl and S-palmitoyl groups in the lipid bilayer where it can bind to actin and the cytoskeleton. It is now evident that these proteolipids take part in compartmentalization of the membrane and raft formation. Other proteins with trans-membrane segments are targeted to rafts by characteristic amino acid sequences that provide much of the properties of rafts and assist in maintaining their stability. By means of this interplay of lipid-based raft units with protein-mediated assembly of protein complexes, functional domains are generated with high activity in the plasma membrane. From comparisons of membrane solubility in different detergents, it appears that there may exist subsets of membrane raft domains, which differ in their molecular compositions. On the other hand, some researchers have expressed doubt that GPI-anchored proteins occur in raft domains in plasma membranes of live cells.
While lateral diffusion may explain how the lipids aggregate in rafts, the introduction of proteins might require energy so other forces must be involved unless the protein is in a lower energy state in the raft. Then the process might be driven by entropy. Another question remains as to how the raft and non-raft phases remain relatively stable, and the answer may be that there is an interaction with cortical actin cytoskeleton underneath the plasma membrane that holds the raft in place.
In an alternative model, it is suggested that that lipids cannot create stable membrane subregions independently of proteins and then recruit proteins. Instead, all membranes are organized into structural and functional zones such as receptor clusters, protein-coated pits, lamellipodia, cell junctions, and membrane fusion sites, in which a protein-lipid code directs their assembly. Rafts are only one manifestation of this concept.
Ceramides: Further stability is introduced to rafts by the action of ceramide and lysosphingolipids, which can induce coalescence into larger platforms or domains, and ceramides can be generated from sphingomyelin in the plasma membrane by the action of sphingomyelinases in a well-regulated and tuneable manner. In endothelial cells of the lung, a peptide (CGSPGWVRC) interacts with C16-ceramide at the cell surface to activate acid sphingomyelinase and ceramide production without the associated downstream apoptotic signalling; in effect, ceramide acts as an endothelial cell surface receptor. Ceramides can form gel phases in membranes and displace cholesterol from its packing with sphingomyelin with those molecular species containing fatty acids of medium chain-length (C12 to C18) as the most efficient in promoting raft formation. Such modified rafts tend to have different protein contents and thence signalling behaviour from the cholesterol-rich rafts, so some consider these ceramide-rich platforms as a third type of membrane microdomain. Another suggestion is that ceramide-rich microdomains may form within existing sphingolipid-laden lipid rafts, especially in cells exposed to excessive amounts of saturated fatty acids (e.g., in obesity) with the potential to affect cellular metabolism and contribute to cardiovascular disease.
The role of other lipids: Rafts contain some glycerophospholipids and these often consist of relatively simple molecules with one saturated and one monounsaturated acyl chain, and other lipids can apparently form microdomains in membranes that are raft-like. One such is phosphatidylglucoside, in which the diacylglycerol component contains two saturated fatty acids, so the lipid is comparable to a glycosyl-ceramide in some physical aspects. Alternatively, phosphatidylinositol 4,5-bisphosphate can interact with cationic residues of a large array of proteins together with cholesterol to form localized membrane domains. While a great deal of attention has been focused on rafts in the outer leaflet of the plasma membrane, relatively little is known of the structural organization of the corresponding inner leaflet or how the two layers interact. On the other hand, raft domains may sometimes form on the inner leaflet, and there is presumed to be interaction between the two. Both ethanolamine plasmalogens, especially those containing arachidonic acid, and phosphatidylserine are enriched in rafts as compared to the plasma membrane as a whole, and these are presumably in the inner leaflet.
There is a school of thought that gangliosides form membrane domains that differ from other raft signalling platforms (with lower cholesterol concentrations). In these, gangliosides and other oligoglycosylceramides cluster together through hydrogen donor-acceptor (cis) interactions because of the presence of hydroxyl and acetamide groups to form glycosynaptic domains, which mediate their functions.
 Plants and fungi: Lateral organization into micro-domains or rafts has been demonstrated
in the plasma membrane, Golgi apparatus and other membranes of plant cells, but it is not clear how similar these are to analogous regions
in animal tissues.
Nor is it certain that their formation is driven simply by the structures of the lipid components, or by lipid-protein interactions and the
cell wall-plasma membrane-cytoskeleton continuum.
On the other hand, while many of the lipid components in plant membranes are different from those in animal tissues in that they lack
sphingomyelin and cholesterol, they do contain structurally related molecules including plant sterols, sterol glucosides and sphingolipids, such
as glucosylceramide and the glycosylinositol phosphoceramides.
As in animal cells, such rafts may assist in positioning proteins in those regions of the cell where they influence development,
membrane trafficking and signalling.
The proteins flotillins and plant-specific remorins are seen as markers for other types of micro-domain.
Membrane micro-domains act as signalling hubs in plant pathogen-interactions and in root nodule symbiosis between plants and
arbuscular mycorrhiza (fungi) or rhizobia (bacteria).
Plants and fungi: Lateral organization into micro-domains or rafts has been demonstrated
in the plasma membrane, Golgi apparatus and other membranes of plant cells, but it is not clear how similar these are to analogous regions
in animal tissues.
Nor is it certain that their formation is driven simply by the structures of the lipid components, or by lipid-protein interactions and the
cell wall-plasma membrane-cytoskeleton continuum.
On the other hand, while many of the lipid components in plant membranes are different from those in animal tissues in that they lack
sphingomyelin and cholesterol, they do contain structurally related molecules including plant sterols, sterol glucosides and sphingolipids, such
as glucosylceramide and the glycosylinositol phosphoceramides.
As in animal cells, such rafts may assist in positioning proteins in those regions of the cell where they influence development,
membrane trafficking and signalling.
The proteins flotillins and plant-specific remorins are seen as markers for other types of micro-domain.
Membrane micro-domains act as signalling hubs in plant pathogen-interactions and in root nodule symbiosis between plants and
arbuscular mycorrhiza (fungi) or rhizobia (bacteria).
Membrane micro-domains are of special relevance in the plasmodesmata, the narrow passages through the cell walls of adjacent cells that allows communication between them, where the membranes are enriched in sterols and sphingolipids containing predominantly very-long-chain fatty acids. These micro-domains contain characteristic sets of proteins, including those anchored by glycosyl phosphatidylinositol or glycosylinositol phosphoceramides.
In the plasma membrane of the model yeast Saccharomyces cerevisiae, raft-like structures have been identified that do not contain ergosterol but are composed primarily of sphingolipids, possibly inositol phosphorylceramide, and contain glycosylphosphatidylinositol-anchored proteins. However, micro-domains enriched in both ergosterol and sphingolipids have been found in the plasma membrane of other fungi where they take part in stress responses. Yeast cells contain large (up to 5 µm) vacuoles that resemble lysosomes in other cell types, and these clearly demonstrate membrane partitioning.
Bacteria: While bacteria in general lack much of the cellular compartmentalization present in eukaryotes, as well as both the sphingolipids and sterols characteristic of rafts, they arrange various signal transduction, protein secretion and transport processes in 'functional membrane microdomains'. These are formed and operate in much the same way as rafts in eukaryotes with phase separation brought on by interactions between cardiolipin, which tends to be concentrated for interaction with particular proteins in membrane regions of high curvature where cell division is initiated, and farnesol and/or farnesol-derived polyisoprenoid lipids with flotillin-like proteins. Proteins associated with signal transduction, membrane trafficking and the regulation of metabolism are held in proximity and so increase the possibilities for interaction and the efficiency of related cell processes. Indeed, such membrane organization may be crucial in unicellular organisms, including Archaea, as this is the boundary between the organism and its environment and is vital for many cellular processes, including signal transduction and cell division.
Borrelia burgdorferi is an extracellular tick-borne bacterium (spirochete) that causes Lyme disease and is the only prokaryote known to have cholesterol-rich microdomains (as glycoside conjugates) with all the hallmarks of eukaryotic lipid rafts, although it does not have sphingolipids. The organism requires cholesterol from its host for growth and multiplication as it is unable to synthesise cholesterol itself.
Non-raft regions of membranes: A corollary of the existence of rafts, which are rich in sphingolipids and with low levels of polyunsaturated fatty acids, is that micro-domains must exist in membranes that are depleted in sphingolipids/cholesterol and enriched in polyunsaturated fatty acids (and probably any oxidized phospholipids). Indeed, the rigid structure of cholesterol and the highly flexible chains of docosahexaenoic acid are incompatible and promote the lateral segregation of membranes into rafts. Micro-domains that are enriched in polyunsaturated lipids and cholesterol/sphingolipid-poor are technically even less easy to study than rafts, but these may also contain characteristic proteins and have significant functions. It seems likely that there is no rigid defining line between raft and non-raft domains or between liquid-ordered and liquid-disordered phases, and it has been argued that the nature of the glycerophospholipid species in membranes should be considered as part of any general theory of raft formation. In addition, there are suggestions that the interface between rafts and non-raft regions may attract a specific range of proteins.
3. Rafts and Cellular Metabolism
It should be recognized that lipid rafts in general are dynamic structures, which can be formed or undergo compositional changes during signalling events and are short-lived (milliseconds or less). There may be some form of crosstalk between different raft populations, which can cause them to coalesce. Thus, in resting cells, sphingolipids may exist in small and highly dynamic domains, which on stimulation can stabilize and grow. In the process, they initiate biochemical reactions by promoting interactions between proteins as in the formation of heterodimers, which may be the activated form. Raft domains may aid membrane trafficking by facilitating the transfer of certain proteins, such as the glycosylphosphatidylinositol (GPI)-anchored proteins, from the endoplasmic reticulum or Golgi to the plasma membrane, and they take part in the formation of extracellular vesicles at the plasma membrane.
Cholesterol homeostasis: As the formation, stability and turnover of lipid rafts is critically dependent on the maintenance of a cellular gradient of cholesterol from the endoplasmic reticulum to the plasma membrane, rafts have a role in maintaining cholesterol levels in cells. They mediate the uptake of cholesterol from circulating lipoproteins and send excess back to the endosomal and other internal compartments of cells via vesicular transport in exosomes, for example. Rafts are one component of mechanism of export of excess cholesterol from cells in an ATP-dependent process carried out by ATB binding cassette transporter A1 (ABCA1), which controls cholesterol movement across the plasma membrane to an acceptor such as apo A1 in HDL for transport to the liver. Many metabolic processes in animal tissues rely on an adequate supply of cholesterol and so are influenced by raft domains in membranes, and one such is haematopoiesis, the production or renewal of blood cells and platelets, disorders of which can lead to atherosclerosis. Indeed, defective cholesterol metabolism can affect raft formation and signalling with the potential to affect many aspects of cardiovascular disease, such as the transition of macrophages into foam cells. Cholesterol-rich ion channels in caveolae (see below) are necessary for normal cardiac functions.
Signalling: Lipid rafts modulate signalling events and act as intermediaries in the flow of information from the outside to the inside of cells in many different ways according to the composition of certain subpopulations, e.g., oligoglycosphingolipids may act in the plasma membrane as glyco-signalling domains or 'glycosynapses'. The location of signalling molecules within one such micro-domain might be a control mechanism, as for example, a protein activated by phosphorylation within the raft might be prevented from interacting with an opposing phosphatase in another region of the membrane. Alternatively, interactions with particular raft lipids or with the distinctive biophysical environment could change the conformation of a resident protein and its efficiency. By concentrating all the components of particular signalling pathways within one domain, lipid rafts could promote signalling in response to stimuli, while movement of signalling molecules in and out of the raft could control whether cells are able to respond, and communication between different signalling pathways could be simplified if the relevant molecules were concentrated in the same lipid raft. In contrast, rafts might regulate signals in a negative manner by sequestering signalling molecules in a resting state.
The physical properties of rafts may be required for these interactions, and in response to receptor activation or other stimuli, sphingolipid compositions in rafts may be altered to influence membrane architecture or morphology and produce further downstream events. The epidermal growth factor must bind to its own receptor to initiate transmembrane signalling, and this can be promoted by its location in rafts. On the other hand, the ganglioside components of rafts and some other factors can be inhibitory.
 Raft formation is important in
T cells, i.e., lymphocytes derived from the thymus gland that are intimately involved in antibody production.
Lipid rafts are a vital element of membrane organization that are crucial for the initiation of signalling of these cells by
enabling efficient interaction between antigens and receptors, and they provide a platform for the interaction
of Toll-like receptors with their ligands in cells for inflammatory responses.
In the same way, immunoglobulin E and B-cell antigen receptor signalling in rafts are necessary to the immune response.
Lipid rafts enable tumour necrosis factor alpha (TNFα), one of the cytokines implicated in systemic
inflammation, to bind to its specific receptor with sphingomyelin synthase 2 as a modulating factor.
Raft formation is important in
T cells, i.e., lymphocytes derived from the thymus gland that are intimately involved in antibody production.
Lipid rafts are a vital element of membrane organization that are crucial for the initiation of signalling of these cells by
enabling efficient interaction between antigens and receptors, and they provide a platform for the interaction
of Toll-like receptors with their ligands in cells for inflammatory responses.
In the same way, immunoglobulin E and B-cell antigen receptor signalling in rafts are necessary to the immune response.
Lipid rafts enable tumour necrosis factor alpha (TNFα), one of the cytokines implicated in systemic
inflammation, to bind to its specific receptor with sphingomyelin synthase 2 as a modulating factor.
Lipid rafts have a role in the redox signalling that regulates the pathophysiology of many degenerative diseases in that large redox signalling molecules aggregate within them to produce various reactive oxygen species. NADPH oxidase is crucial in this context, and lipid rafts provide a platform to aggregate and assemble the necessary subunits of the enzyme into a single complex. In mitochondria, raft-like domains participate in the response to oxidative stress and apoptosis, while in nuclei, the signalling system that generates diacylglycerol and inositol-1,4,5-triphosphate is located in similar microdomains in the membrane.
Brain: Membranes in the brain are highly enriched in cholesterol and sphingolipids, and lipid rafts are found in most brain cells, including neurons, astrocytes and microglia, each with a characteristic lipid composition. Key neurodevelopmental processes from embryos to adulthood take place in rafts, including neural differentiation, synaptogenesis and myelination, aided by the location of signalling mechanisms. It is apparent that neurotransmitters and their receptors, in particular acetylcholine and serotonin receptors, are highly dependent upon the lipid components in raft domains. Among other events, the lipids interact with receptors to alter their conformation ("chaperone-like" effect), so regulating neurotransmitter binding and signal transduction. In glial cells, lipid rafts are a factor in neuroinflammation and the development and perpetuation of pain, including that associated with chronic debilitating conditions such as rheumatoid arthritis and diabetes. A deficiency of flotillin in cells and deficiencies in sphingomyelinase and phospholipases compromise many raft-associated cell processes and are associated with cognitive decline with age and age-related neurodegenerative conditions, such as Parkinson's and Alzheimer's diseases. It has been proposed that excessive cholesterol production and aberrant raft formation could be the primary cause of the last of these.
Cancer: Signal transduction in rafts is utilized for cell adhesion, migration, survival and proliferation, but it can support receptor-mediated apoptosis as death-promoting platforms from which apoptotic signals are launched. Higher levels of intracellular cholesterol and lipid rafts are present in cancer cells than in normal tissues. In effect, membrane rafts may act as supporting structures that enable segregation of pro- from anti-apoptotic molecules. As many oncogenic proteins are located in raftlike domains and mitogenic signalling stems from various cell surface receptors, rafts may be a factor in cancer development and progression and may be a novel target in the treatment of cancer.
Vesicle formation: Eukaryotic cells contain different types of vesicles enclosed by a membrane, including transport, endocytic, exocytic, synaptic and extracellular vesicles. Rafts promote vesicle formation by two mechanisms that induce membrane curvature; raft proteins and/or their lipids associate with coat proteins that form a budding vesicle, or vesicle budding is triggered by enzymatic generation of cone-shaped ceramides and inverted cone-shaped lysophospholipids in rafts.
Pathogen-host interactions: Rafts participate in virus attachment and recruitment to the cell surface as well as the endocytic and other mechanisms that some viruses use to enter host cells. For example, there is evidence that certain pathogens trigger the acid sphingomyelinase that releases ceramides in membrane rafts to transform them into larger units, which can mediate the internalization of bacteria, viruses and parasites into host cells to enable them to multiply to initiate programmed cell death (apoptosis) and release signalling molecules. They may assist in the budding of viruses from infected cells. Even when they are unable to enter cells, pathogens can modify rafts to disable the immune defences and cause comorbidities. They interact with rafts and caveolae enriched in cholesterol to re-organize the receptor and intracellular signalling molecules in the cell membrane to enable pathogens to mitigate immune responses and invade cells. They can then utilize cellular resources to dwell and multiply and transfer from one cell to another, again with the aid of rafts.
The lipids of viruses are derived from the host membranes, and it has been demonstrated that the lipids of the HIV virus are enriched in sphingolipids that are derived very specifically from rafts and are necessary for its infectivity, while cholesterol-rich lipid rafts act as platforms for SARS-CoV-2 (COVID-19) entry into cells and enable interaction with cellular receptors and enzymes. In contrast, rafts can assist cells to defeat infection by stimulating transcription factors and the release of cytokines. They provide a platform for many receptors and other molecules that participate in the immune response, including Toll-like receptors, T- and B-cell receptors and fragment crystallizable receptors that help to regulate viral spread and inflammation. Drugs that target lipid rafts are under consideration as a means of mitigating the symptoms of infections.
4. Caveolae
Subdomains in the plasma membrane related to rafts and termed ‘caveolae’ ("little caves") are flask-shaped invaginations 50 to 100 nm in diameter in membranes that provide a cholesterol- and sphingolipid-rich environment, which includes sphingomyelin, glycosphingolipids and gangliosides. They are found abundantly in vascular endothelial cells, adipocytes, smooth muscle cells and fibroblasts (but not in lymphocytes or mature neurons), and they can amount to 50% of the surface area of adipocytes, often in interconnected structures comprising multiple caveolar units. Not only are they present in the external cell membranes but can be found in all cellular membranes, including the endoplasmic reticulum, Golgi, mitochondria, endosomes, lipid droplets, nucleus and extracellular vesicles. Caveolae provide a reservoir of lipids to prevent membrane damage and take part in signal transduction, stress sensing, mechano-transduction and trans-endothelial transport.
They are stabilized by membrane-spanning scaffolding proteins, the caveolins, a family of hairpin-like proteins that bind strongly to cholesterol. Of these, caveolin-1 is the main structural component, i.e., a 24 kDa integral membrane protein with three palmitoylation sites at the C-terminal domain, which bind it to the inner leaflet of the plasma membrane. As well as a cholesterol binding site (CRAC), it has phosphorylation, ubiquitination and SUMOylation sites. Caveolin-2 is used in the lung, while caveolin-3 is found only in muscle, but caveolin-2 does not form caveolae independently and rather associates with caveolin-1 in hetero-oligomers. The presence of caveolins defines caveolae although there may be different classes of caveolae with different compositions, physical forms and metabolism. In contrast to rafts, caveolae are clearly visible and their limits are recognizable by electron microscopy. It has even been argued that caveolae best meet the accepted definition of a raft and should be used as a provisional standard against which other proposed raft structures should be judged. Rafts differ from caveolae in that they are self-organized.
The genesis of caveolae is a complex process of stepwise assembly. In brief, it begins in the endoplasmic reticulum with the translation of caveolins, and it proceeds via the Golgi to the plasma membrane via a secretory pathway requiring the ganglioside GM1 and glycosylphosphatidylinositol-linked proteins. The caveolin proteins are inserted into the endoplasmic reticulum co-translationally and pass to the Golgi where they self-associate to form oligomers of 12 or 14 monomers with addition of cholesterol, which binds to the CRAC site in the caveolins, and for example, human caveolin-1 complex contains 11 protomers organized into a tightly packed disc with a flat membrane-embedded surface ('8S complexes'). As this complex nears the plasma membrane, palmitoylation of the caveolins occurs by means of palmitoyl acyltransferases on three cysteines in the C-terminal domains. At the plasma membrane, they can then assemble with more caveolin oligomers to form caveolae with the aid of further structural proteins termed 'cavins' (~50 kDa), of which four occur in vertebrates. These have been shown to bind to each other in vitro and to the anionic phospholipids phosphatidylserine and phosphatidylinositol 4,5-bisphosphate, which are concentrated in the caveolae inner membranes. Within the membrane, caveolins are embedded with both amino- and carboxyl-termini facing the cytoplasm.
These phospholipids bind with the cavins and caveolins in a large caveolar coat complex in vivo, while for example, cavin-1 stabilizes caveolin-1 with the aid of adaptor proteins. The effect is to shape the caveolae, leading to invagination and stabilization of the complex, and it is assumed that the curved membrane structure is due in part to the enrichment of cone- and inverted cone–shaped lipids in the cytoplasmic leaflets of the neck and bulb subdomains, respectively. Caveolin-1 is particularly stable at the plasma membrane and only a few caveolae become internalized, leading to the suggestion that it may act to stabilize cellular membranes and reduce membrane invagination, budding and vesicle internalization.
 Other than a negligible
content of glycosyl phosphatidylinositol-linked proteins, which are not constitutively concentrated in caveolae, the lipid composition of
caveolae resembles that of rafts in general in that they contain appreciable amounts of sphingolipids and cholesterol.
However, the gangliosides GM1 and to some extent GM3 are concentrated in caveolae, while
phosphatidylserine is the main anionic lipid in the cytoplasmic leaflet and is crucial to their formation and stability.
One hypothesis that cannot yet be tested is that the neck region of caveolae with a strong positive curvature may be highly enriched in
glycosphingolipids, which may be largely absent from the lumen, as there is evidence that the caveolins interact directly with
these lipids, which may regulate signalling in caveolae.
Other than a negligible
content of glycosyl phosphatidylinositol-linked proteins, which are not constitutively concentrated in caveolae, the lipid composition of
caveolae resembles that of rafts in general in that they contain appreciable amounts of sphingolipids and cholesterol.
However, the gangliosides GM1 and to some extent GM3 are concentrated in caveolae, while
phosphatidylserine is the main anionic lipid in the cytoplasmic leaflet and is crucial to their formation and stability.
One hypothesis that cannot yet be tested is that the neck region of caveolae with a strong positive curvature may be highly enriched in
glycosphingolipids, which may be largely absent from the lumen, as there is evidence that the caveolins interact directly with
these lipids, which may regulate signalling in caveolae.
A general picture has emerged of caveolin-1 and sphingolipids forming a platform for an integrated network of interactions, which contribute to the regulation of cellular metabolism via their influence upon receptor molecules in the plasma membrane. Caveolae are known to contain particular signalling proteins, including Ras proteins, G proteins and growth factor receptors, not present in other raft microdomains. They play a part in controlling the level of free cholesterol in cells, as cholesterol distribution within the Golgi complex and plasma membrane is highly dependent on the expression of caveolin-1 and caveolae. Experimental depletion of plasmalemmal cholesterol results in the loss of caveolae.
Caveolae are central to many different cellular processes, including endocytosis, transcytosis, lipid homeostasis, cholesterol homeostasis, control of membrane composition and organization, and regulation of cellular signalling. They may act as a route by which nutrients such as folate, glucose and fatty acids are able to cross the plasma membrane, and they may have a non-signalling role in the repair of membrane damage in response to physical stress, perhaps by acting as a reservoir of structural lipids that enable membranes to flatten out under mechanical stress.
Although the mechanism is still obscure, it may be that caveolae act as transport vesicles, which detach from the plasma membrane in response to unspecified cues and move through the cytosol, a process that may assist lipid trafficking and metabolism and cellular lipid homeostasis. In this way, they can travel to intracellular organelles such as the endoplasmic reticulum or lipid droplets, where they may leave or exchange some of their cargo, and they may even return to the plasma membrane. Alternatively, they fuse with endosomes and accumulate in lysosomes for recycling of their constituents. Under conditions of oxidative stress, caveolar proteins released into the cell act to regulate the master transcriptional redox controller, nuclear factor erythroid 2-related factor 2 (NRF2) to maintain cellular susceptibility to stress-induced apoptosis. Disassociation of caveolae is a highly complex process that includes restructuring of the caveolin oligomers, ubiquitination, internalization and finally degradation.
In adipocytes, caveolae may regulate the flux of fatty acids across the plasma membrane. Insulin is the main hormone that affects metabolism in this tissue, and the receptor at the plasma membrane is located in caveolae with possible implications for diabetes, obesity and other metabolic disorders. In the skin, caveolae are required for normal development, with effects upon cell migration and proliferation as well as cellular senescence, infection and inflammation, such that disruption to caveolin metabolism can lead to the development of cutaneous pathophysiologies including cancers and psoriasis. In cancer cells, there is some evidence that changes in caveolae create a catabolic microenvironment in tumours that supports oxidative mitochondrial metabolism and so contribute to the poor survival rates for cancer patients, but they can also facilitate cancer regression by other mechanisms. Like rafts, they can facilitate the entry into cells of certain pathogens including Chlamydia, which enters host cells through membrane areas rich in cholesterol and ganglioside GM1 and co-locates with caveolins-1/2.
Membrane scaffolds: Many cell types, including hepatocytes and neurons, express caveolins without forming distinguishable caveolae, suggesting that caveolins act in cells in other ways than as structural components of caveolae. For example, caveolin 1 is present in functional non-caveolar structures or 'scaffolds' at the plasma membrane as 8S complexes. In this form, non-caveolar caveolins can determine the intracellular flow of cholesterol, sphingolipids and other lipids, and so they control many lipid-dependent processes such as the endocytosis of cargoes and sorting and transport in endocytic compartments. As a critical signalling regulator in these complexes, caveolin 1 can interact with multiple client proteins and especially the endothelial nitric oxide synthase (eNOS).
Suggested Reading
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids, 216, 114-131 (2018); DOI.
- Bukrinsky, M.I., Mukhamedova, N. and Sviridov, D. Lipid rafts and pathogens: the art of deception and exploitation. J. Lipid Res., 61, 601-610 (2020); DOI - there are several other articles in a Thematic Review Series on the biology of lipid rafts in the same journal issue.
- Cebecauer, M., Amaro, M., Jurkiewicz, P., Sarmento, MJ., Sachl, R., Cwiklik, L. and Hof, M. Membrane lipid nanodomains. Chem. Rev., 118, 11259-11297 (2018); DOI.
- Kenworthy, A.K., Han, B., Ariotti, N. and Parton, R.G. The role of membrane lipids in the formation and function of caveolae. Cold Spring Harbor Persp. Biol., 15, a041413 (2023); DOI.
- Kinnun, J.J., Bolmatov, D., Lavrentovich, M.O. and Katsaras, J. Lateral heterogeneity and domain formation in cellular membranes. Chem. Phys. Lipids, 232, 104976 (2020); DOI.
- Levental, I., Levental, K.R. and Heberle, F.A. Lipid rafts: controversies resolved, mysteries remain. Trends Cell Biol., 30, 341-353 (2020); DOI.
- Li, B.R., Qin, Y., Yu, X.J., Xu, X.W. and Yu, W.Y. Lipid raft involvement in signal transduction in cancer cell survival, cell death and metastasis. Cell Prolif., 55, e13167 (2022); DOI.
- Lim, J.E., Bernatchez, P. and Nabi, I.R. Scaffolds and the scaffolding domain: an alternative paradigm for caveolin-1 signaling. Biochem. Soc. Trans., 52, 947-959 (2024); DOI.
- Martinière, A. and Zelazny, E. Membrane nanodomains and transport functions in plant. Plant Physiol., 187, 1839-1855 (2021); DOI.
- Mitchison-Field, L.M. and Belin, B.J. Bacterial lipid biophysics and membrane organization. Curr. Opinion Microbiol., 74, 102315 (2023); DOI.
- Nieto-Garai, J.A., Lorizate, M. and Contreras, F.X. Shedding light on membrane rafts structure and dynamics in living cells. Biochim. Biophys. Acta, Biomembranes, 1864, 183813 (2022); DOI.
- Ott, T. Membrane nanodomains and microdomains in plant-microbe interactions. Curr. Opinion Plant. Biol., 40, 82-88 (2017); DOI.
- Pol, A., Morales-Paytuvi, F., Bosch, M. and Parton, R.G. Non-caveolar caveolins - duties outside the caves. J. Cell Sci., 133, jcs241562 (2020); DOI.
- Santos, F.C., Marques, J.T., Bento-Oliveira, A. and de Almeida, R.F.M. Sphingolipid-enriched domains in fungi. FEBS Letts, 594, 3698-3718 (2020); DOI.
- Stea, D.M. and D'Alessio, A. Caveolae: metabolic platforms at the crossroads of health and disease. Int. J. Mol. Sci., 26, 2918 (2025); DOI.
- Svistunov, V.O., Ehrmann, K.J., Lencer, W.I. and Schmieder, S.S. Sorting of complex sphingolipids within the cellular endomembrane systems. Front. Cell Developm. Biol., 12, 1490870 (2025); DOI.
- Viljetic, B., Blazetic, S., Labak, I., Ivic, V., Zjalic, M., Heffer, M. and Balog, M. Lipid rafts: the maestros of normal brain development. Biomolecules, 14, 362 (2024); DOI.
- Warda, M., Tekin, S., Gamal, M., Khafaga, N., Celebi, F. and Tarantino, G. Lipid rafts: novel therapeutic targets for metabolic, neurodegenerative, oncological, and cardiovascular diseases. Lipids Health Dis., 24, 147 (2025); DOI.
- Yokoyama, N., Hanafusa, K., Hotta, T., Oshima, E., Iwabuchi, K. and Nakayama, H. Multiplicity of glycosphingolipid-enriched microdomain-driven immune signaling. Int. J. Mol. Sci., 22, 9565 (2021); DOI.
- Zhukov, A. and Vereshchagin, M. Polar glycerolipids and membrane lipid rafts. Int. J. Mol. Sci., 25, 8325 (2024); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: July 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
