Lipids: Definitions, Classification and Nomenclature
A Personal View
Definitions
 General textbooks and
many web sites equate lipids with 'fats and oils' and describe them in woolly terms as a group of naturally occurring compounds, which are
soluble in such organic solvents as hydrocarbons, chloroform, benzene, ethers and alcohols.
This could describe a rather diverse range of compounds including fatty acids and their derivatives, carotenoids, terpenes, steroids,
polyketides, organo-metallics and bile acids.
It should be apparent that many of these compounds share few structural features.
While it may have some value for non-scientists, a definition of this kind is misleading, as many of the substances that are now widely
regarded as lipids, such as gangliosides, bile acid conjugates and lipopolysaccharides, may be as soluble in water as in organic solvents.
Some authors have even classified molecules such as trimethylarsine as lipids because of their solubility in organic solvents.
Unfortunately, this type of definition persists in the scientific literature, and it is the one most often encountered in a Google search.
Please do not give it further credence.
General textbooks and
many web sites equate lipids with 'fats and oils' and describe them in woolly terms as a group of naturally occurring compounds, which are
soluble in such organic solvents as hydrocarbons, chloroform, benzene, ethers and alcohols.
This could describe a rather diverse range of compounds including fatty acids and their derivatives, carotenoids, terpenes, steroids,
polyketides, organo-metallics and bile acids.
It should be apparent that many of these compounds share few structural features.
While it may have some value for non-scientists, a definition of this kind is misleading, as many of the substances that are now widely
regarded as lipids, such as gangliosides, bile acid conjugates and lipopolysaccharides, may be as soluble in water as in organic solvents.
Some authors have even classified molecules such as trimethylarsine as lipids because of their solubility in organic solvents.
Unfortunately, this type of definition persists in the scientific literature, and it is the one most often encountered in a Google search.
Please do not give it further credence.
Such an indeterminate description is perhaps understandable when it is recognized that there is no definition of a lipid that has been accepted by any international body that recommends standards or comments on nomenclature issues. IUPAC-IUB appear to have shirked the task. Many others have made suggestions over the years [1‑3], and I put forward my own preferred definition in 1987 [4]; I have recommended it from time to time for want of anything better -
Lipids are fatty acids and their derivatives, and substances related biosynthetically or functionally to these compounds.
This encompasses all the compounds we consider to be the mainstream of lipids, together with those hydrophobic compounds that function in membranes, e.g., sterols, the fat-soluble vitamins and some but not necessarily all isoprenoids and polyketides. This definition too has its limitations, although some of the more important books on lipid biochemistry that have been published in recent years have restricted their coverage to topics within this definition and not what might be considered a wider range of 'peripheral' lipid classes.
A further useful guide to lipid classification, structures and nomenclature is a paper by Fahy and colleagues [5], which is available from the Journal of Lipid Research as an open access download here... (but see update below). It is complemented by a website – Lipid Maps®, which as well as defining lipids, catalogues and illustrates a great number of them and makes available drawing and other tools as a service to lipid scientists. Of course, it also hosts the LipidWeb. I applaud their attempt to define the term 'lipid', although their definition still seems too broad for my liking and has a greater emphasis on the process than the products, i.e.,
Lipids are hydrophobic or amphipathic small molecules that may originate entirely or in part by carbanion-based condensations of thioesters (fatty acids, polyketides, etc.) and/or by carbocation-based condensations of isoprene units (prenols, sterols, etc.).
This definition may make sense to a biochemist, but what are physical chemists, food scientists, nutritionists and so forth to make of it? It can be argued that this definition appears to suggest that any organic compound not a carbohydrate, protein or nucleotide is a lipid, and while this may be a perfectly valid viewpoint, it would make my task as a chronicler of lipid science much more difficult. More seriously, there is a danger that the term 'lipid' becomes a catch-all (or even a dustbin!) for all classes of organic molecules that do not fit into other categories. Dare I say it - many polyketides come to mind as an example of the latter, as non-biochemists may find it difficult to reconcile most of these with their view of the more traditional lipids; they are not formed in higher animals so do not impact directly on human metabolism and health. I suggest that it might be better to think in terms of two main groupings under the collective lipid umbrella - 'acyl lipids' and 'isoprenoids' or 'terpenoids', with the latter including sterols, terpenes, carotenoids and many fat-soluble vitamins. I consider the first of these to be 'main-stream lipids'.
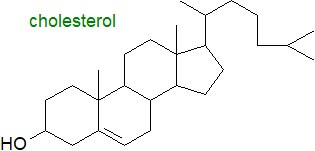 |
Cholesterol is a lipid by any definition, but especially because of its functional role in membranes. |
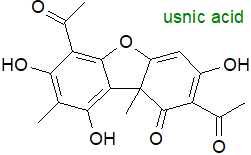 |
Usnic acid is a polyketide from lichen that is a lipid by a broad definition, which some might question. |
On reflection, I believe that both of the above definitions are too short and vague, and the second is too technical, while neither gives a significant amount of structural information. I therefore propose the following longer alternative, which is based on structural factors only and does not mention 'solubility', 'biosynthesis' or 'function'. I welcome comments for or against.
Lipids comprise a heterogeneous class of predominantly hydrophobic organic molecules of relatively low molecular weight (commonly <1000) that are defined by the presence either of linear alkyl chains, usually with even-numbers of carbon atoms and saturated or unsaturated with double bonds in characteristic positions, or of isoprene units in linear or cyclic structures. These can contain variable numbers of oxygenated substituents such as carboxylic acid, hydroxyl groups and/or other heteroatoms, such as nitrogen in amines/amides. These may be linked covalently to glycerol, carbohydrates, phosphate and other small polar entities, which can render the molecules more amphiphilic.
Classification of Lipid Structures
Fahy and colleagues [5] of the Lipid Maps® consortium have classified lipid structures according to their basic chemistry in a manner that facilitates cataloguing by computer. I expressed several reservations when this paper was first published and subsequently directly to the authors and indirectly via my blogs, mainly because the document seemed to be heavily weighted towards animal lipids, while many unique and noteworthy plant lipids were overlooked. This fault was largely remedied in a second publication [6], where the authors generously admitted their earlier bias and introduced new sub-classifications to encompass the plant glycosyldiacylglycerols including a key lipid in photosynthesis - sulfoquinovosyldiacylglycerols (although lower in their hierarchy than the glycerophospholipids). Sterols are now categorized according to their chemical structures rather than to their biological origins.
 That said,
my own preference would be to elevate more of the sub-classes to a higher level for functional reasons.
The glycosyldiacylglycerols have similar physical properties and functions in plant membranes as the glycerophospholipids,
and they should be classified in the same rank.
My preference would be for three separate classes for sphingolipids, with perhaps a fourth for gangliosides, since for consistency,
phospho-sphingolipids and glyco-sphingolipids should be treated separately by analogy with the glycerolipid classifications.
In the fatty acyl group, I would elevate eicosanoids/docosanoids (oxylipins) to a higher rank for functional reasons,
and it appears to me anomalous to leave fatty alcohols and related wax constituents in this group.
Another group in a classification limbo are the phosphoglycolipids
(as defined in this web page).
That said,
my own preference would be to elevate more of the sub-classes to a higher level for functional reasons.
The glycosyldiacylglycerols have similar physical properties and functions in plant membranes as the glycerophospholipids,
and they should be classified in the same rank.
My preference would be for three separate classes for sphingolipids, with perhaps a fourth for gangliosides, since for consistency,
phospho-sphingolipids and glyco-sphingolipids should be treated separately by analogy with the glycerolipid classifications.
In the fatty acyl group, I would elevate eicosanoids/docosanoids (oxylipins) to a higher rank for functional reasons,
and it appears to me anomalous to leave fatty alcohols and related wax constituents in this group.
Another group in a classification limbo are the phosphoglycolipids
(as defined in this web page).
The general aims of the two papers are admirable, and hopefully what I perceive as faults will be cured in time by dialogue. Lipid Maps® is of course a private consortium, not an international standards body, although I expect that their proposals will be given serious consideration by IUPAC-IUB and other international organizations in due course.
I believe that a subdivision of glycerolipids into two broad classes according to polarity or complexity is so convenient for analysts, biochemists and physical chemists that it should be given greater weight in any practical classification system, i.e., those defined in the first edition of Lipid Analysis [7].
‘Simple lipids’ are those that yield on hydrolysis at most two types of primary products per mole; ‘complex lipids’ yield three or more primary hydrolysis products per mole.
This definition has echoes of Bloor’s 'simple and compound lipoids', proposed more than 100 years ago [2]. In practice, it is often necessary to subdivide the main groups further, and the complex lipids for many purposes are best considered in terms of either the glycerophospholipids (or simply as phospholipids), which contain a polar phosphorus moiety and a glycerol backbone, or the glycolipids, which contain a polar carbohydrate moiety instead. For practical reasons, sphingolipids are often analysed separately from the glycerolipids, and they are usually treated by chemists and biochemists alike as a distinct group.
It can also be convenient to think in terms of storage lipids, e.g., triacylglycerols, versus structural lipids, i.e., those that occur and function in membranes such as most of the complex lipids, cholesterol and many of the fat-soluble vitamins.
The plasma lipoproteins are not themselves lipids but complex aggregates of lipid and proteins in which the two groups are not linked covalently. While the proteolipids, such as those with S-linked palmitic acid or N-linked myristic acid could in theory be classified as either proteins or lipids, it may be pedantic to think in such terms. In my view, it is better to consider them simply as a bridge between the two types of molecules.
Lipidomics
It was perhaps inevitable that the sciences of genomics, proteomics, metabolomics, glycomics and so forth would lead to the 'new' science of lipidomics. The first mention of this that I could find in the literature was in a paper in 2001 [8], which referred to the 'lipidome', i.e., the complete spectrum of lipids in a tissue, organelle or membrane. From 2002 onwards, publications using the term lipidomics have appeared in increasing numbers, and this is now widely regarded as an established science. The only benefit of growing older is that it gives you a longer perspective, and I regard the true beginning of the subject, if not the name, as 1969 with two papers by Wood and Harlow [9,10].
The aim of lipidomics is more than simply to analyse lipids in biological systems, and rather it is to relate lipid compositions of tissues or membranes of animals, plants or microorganisms to their physical properties, enzymes and their biology in general. A brief definition is -
The analysis of lipids on the systems-level scale together with their interacting factors.
Alternatively, the more comprehensive definition [11] may be preferred -
The full characterization of lipid molecular species and of their biological roles with respect to expression of proteins involved in lipid metabolism and function, including gene regulation.
Both definitions contain two main elements. In relation to the analysis of lipids they require a quantitative determination of all the lipids present that includes all the molecular species of each lipid class in the biological sample being studied (almost universally by the use of advanced mass spectrometry methodology). Secondly, they suggest that such analytical data must be related to the biological function of lipids through knowledge of such enzymes, genes and other factors that may relevant. It is implied that the metabolic and physical relationships of lipids to enzymes, receptors and non-lipid signalling molecules in their membrane or even whole-body environments must be considered, as in many studies of this type, the data obtained are intended to shed light on human disease states. A further aim is to eventually integrate all the various 'omics' into a single framework of cellular metabolism. The study of the epilipidome (epilipidomics) is a step towards this, as by analogy with the epigenetic modifications that can control gene expression, the relevant lipids ('epilipids') have potential signalling and regulatory effects upon innumerable metabolic processes [12,13].
Epilipidome - a subset of the natural lipidome formed by lipid modifications via enzymatic and non-enzymatic reaction (e.g., oxidation, nitration, sulfation, halogenation) required to regulate complex biological functions.
On the other hand, the topic of lipidomics has often been subdivided, and I have seen the terms 'phospholipidomics', 'sphingolipidomics', 'glycolipidomics', 'neurolipidomics', 'steroidomics', 'endocannabinoidomics' and even 'fatty acidomics' in the literature. Some of these are justified, and 'focused or targeted lipidomics' can be a sensible approach to the subject, but there is a danger that lipidomics becomes simply a 'buzz word' to describe every study that merely involves routine analyses of fatty acid compositions or of molecular species of a single lipid class in a tissue.
Some Other Useful Definitions
- Lipidon - a unique collection of co-located lipids that distinguish biological membrane nano-environments.
- Lipodystrophy - a group of genetic or acquired disorders in which the body is unable to produce and maintain healthy adipose tissue.
- Lipokine - a lipid hormone linking adipose tissue to systemic metabolism.
- Lipopathy - any disorder of lipid metabolism.
- Lipophagy - the process of selective degradation of lipid droplets by autophagy.
- Lipotoxicity - a metabolic syndrome resulting from a failure to package excess lipid into lipid droplets that leads to cell dysfunction and death.
- Lipoxidation - non-enzymatic reactions of electrophilic carbonyl species derived from oxidized lipids with proteins and other macromolecules such as DNA.
- Oxylipin - a family of oxygenated lipid mediators formed from fatty acids by pathways involving at least one step of mono- or dioxygen-dependent oxidation.
Abbreviations
I have never been a fan of abbreviations: while they may be useful for authors, they are a nuisance for readers when used in excess. If they must be used, it is better to decide on ones that are likely to become standards. Fahy et al. [5] initially suggested abbreviations that appeared to me to be needlessly complex for the main phospholipid classes, before in their 2009 paper [6] deciding to recommend the simpler two-letter forms in common use. As both formats now appear in publications, I list some of those most often encountered with the obsolete forms in brackets: phosphatidic acid - PA (GPA); phosphatidylcholine - PC (GPCho); phosphatidylethanolamine - PE (GPEtn); phosphatidylserines - PS (GPSer); phosphatidylinositol - PI (GPIns); phosphatidylinositol bis-phosphate - PIP2 (GPInsP2); phosphatidylglycerol - PG (GPGro); cardiolipin - CL; Cer - ceramides; TG - triacylglycerols; DG - diacylglycerols, MG - monoacylglycerols.
Lipid Maps later updated their lipid classifications, nomenclature and shorthand annotations as an aid to the presentation of data obtained by mass spectrometry of lipids in the hope that the scientific community will accept these as appropriate standards [14]. They have an updated version online here..
Nomenclature - Recommendations by IUPAC-IUB
The subjects of lipid nomenclature and classification are discussed at many points in this website, but especially in relation to individual lipid classes as listed here. For definitive detailed accounts, I can serve you best by pointing to the following documents, which are all available in the form of PDF files as free downloads from the journals specified below.
IUPAC-IUB Commission on Biochemical Nomenclature. The nomenclature of lipids. J. Lipid Res., 8, 523-528 (1967) or Chem. Phys. Lipids, 2, 156-167 (1968). DOI).
IUPAC-IUB Commission on Biochemical Nomenclature. The nomenclature of lipids (Recommendations 1976). J. Lipid Res., 19, 114-128 (1978) or Chem. Phys. Lipids, 21, 159-173 (1978) or Hoppe-Seyler"s Z. Physiol. Chem., 358, 617-631 (1977). DOI).
IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids. Recommendations 1997. Eur. J. Biochem., 257, 293-298 (1998) - DOI; or Carbohydr. Res., 312, 167-175 (1998) or Glycoconjugate J., 16, 1-6 (1999) or J. Mol. Biol., 286, 963-970 (1999).
References
- Rosenbloom, J. and Gies, W.L. Suggestions to the teachers of biochemistry. I. A proposed chemical classification of lipins, with a note on the intimate relation between cholesterols and bile salts. Biochem. Bull., 1, 51-56 (1911); DOI.
- Bloor, W.R. Outline of a classification of the lipids. Proc. Soc. Exp. Biol. Med., 17, 138-140 (1920); DOI.
- Gidez, L.I. The lore of lipids. J. Lipid Res., 25, 1430-1436 (1984); DOI.
- Christie, W.W. High-performance liquid chromatography and Lipids: A Practical Guide (Pergamon Press, Oxford) (1987).
- Fahy, E. and 17 others. A comprehensive classification system for lipids. J. Lipid Res., 46, 839-862 (2005); DOI - reprinted in Eur. J. Lipid Sci. Technol., 107, 337-364 (2005).
- Fahy, E. and 9 others. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res., 50, S9-S14 (2009); DOI.
- Christie, W.W. Lipid Analysis (1st edition) (Pergamon Press, Oxford) (1973).
- Kishimoto, K., Urade, R., Ogawa, T. and Moriyama, T. Nondestructive quantification of neutral lipids by thin-layer chromatography and laser-fluorescent scanning: Suitable methods for "lipidome" analysis. Biochem. Biophys. Res. Commun., 281, 657-662 (2001); DOI.
- Wood, R. and Harlow, R.D. Structural studies of neutral glycerides and phosphoglycerides of rat liver. Arch. Biochem. Biophys., 131, 495-501 (1969); DOI.
- Wood, R. and Harlow, R.D. Structural analyses of rat liver phosphoglycerides. Arch. Biochem. Biophys., 135, 272-281 (1969); DOI.
- Spener, F., Lagarde, M., Géloën, A. and Record, M. What is lipidomics? Eur. J. Lipid Sci. Technol., 105, 481-482 (2003); DOI.
- Ni, Z.X. Goracci, L., Cruciani, G. and Fedorova, M. Computational solutions in redox lipidomics - Current strategies and future perspectives. Free Rad. Biol. Med., 144, 110-123 (2019); DOI.
- Penkov, S. and Fedorova, M. Membrane epilipidome-lipid modifications, their dynamics, and functional significance. Cold Spring Harbor Persp. Biol., 16, a041417 (2024); DOI.
- Liebisch, G. and 15 others. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res., 61, 1539-1555 (2020); DOI.
Footnote: Those seeking a wider knowledge of the history of the subject may be interested in a paper from 1833 in which food substances are described as "three great divisions, viz. the saccharine, the oily and the albuminous", i.e., carbohydrates, lipids and proteins (Prout, W. On the ultimate composition of simple alimentary substances; with some preliminary remarks on the analysis of organized bodies in general. Proc. Roy. Soc., London, 2, 324-326 (1833); DOI).
A pdf ![]() file of this document is available
here..
file of this document is available
here..
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: April 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
