Gangliosides
The name ganglioside was first applied by the German scientist Ernst Klenk in 1942 to a mixture of complex glycosphingolipids newly isolated from ganglion cells of brain. Subsequently, he demonstrated that they contained as part of an oligosaccharide chain an acidic carbohydrate component, which he named "neuraminic acid" - later termed "sialic acid" from the Greek "sialon" for saliva, from which they were first isolated, but it was not until 1963 that the first ganglioside was fully characterized. Innumerable such sphingolipids are now known that differ in the nature of both the glycan (glucose, galactose, N‑acetylgalactosamine and sialic acid residues) and ceramide structures. They are present throughout the animal kingdom, from echinoderms up to higher animals, but not in plants. Such highly polar, acidic and relatively hydrophilic molecules have distinctive physical properties, and these are necessary for the many vital functions that gangliosides are now known to have in the membranes of the central nervous system and other tissues.
The biosynthetic mechanisms and metabolism of gangliosides are inextricably linked with those of non-acidic oligoglycosylceramides and sulfatides, but for reasons of practical convenience these are discussed in separate web pages.
1. Sialic acids and Gangliosides
Sialic acids: Gangliosides are oligoglycosylceramides derived biosynthetically from lactosylceramide, and they are defined by the presence of one to as many as five sialic acid residues, i.e., carbohydrate molecules with a nine-carbon backbone and a carboxylic acid group, a subclass of the superfamily of naturally occurring non‑2‑ulosonic acids. Of the many forms that have been identified, only a few are relevant to gangliosides, and the most important of these in humans is N‑acetylneuraminic acid (‘NANA’ or ‘SA’ or 'Neu5Ac' or 'NeuAc'). Less often the sialic acid component is N‑glycolylneuraminic acid (Neu5Gc), which differs by only one oxygen atom at the C-5 N-acetyl group, or it can be a Neu5Ac analogue in which the amide group is replaced by a hydroxyl group, i.e., 3-deoxy-D-glycero-D-galacto-nonulosonic acid (ketodeoxynonulosonic acid or ‘KDN’). The sialic acids are joined via α-glycosidic linkages to one or more of the monosaccharide units, e.g., via the hydroxyl group on position 2, or to another sialic acid residue. The polar head groups of the lipids carry a net-negative charge at pH 7.0 and they are acidic.
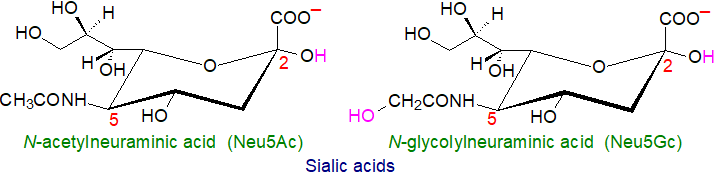
Humans lack Neu5Gc: Neu5Ac is the biosynthetic precursor of Neu5Gc, a component of gangliosides from most animals, including mice, horses, sheep and goats, via the action of the enzyme CMP-N-acetylneuraminic acid hydroxylase (CMAH). Although it is present in primates such as the great apes, NeuGc is not synthesised in humans (or birds and New World monkeys), and as it is a xeno-antigen, anti-NeuGc antibodies are produced normally in healthy humans (and after injection of NeuGc-containing glycoconjugates). The absence or irreversible inactivation of the relevant genes, but especially a critical exon in the CMAH gene, both for sialo-lipids and peptides in humans suggests that this may have been a major biochemical branch-point in human evolution that occurred ~2 to 3 million years ago after the divergence of humans and chimpanzees from a common ancestor. It may even be a factor in the superior performance of the human brain as overexpression of Neu5Gc in the brains of transgenic mice was found to result in abnormal development. It might imply the existence of a fertility barrier between us and other hominids during evolution.
While these are speculations, there is some evidence that the loss of Neu5Gc in humans had complex effects on immunity, providing greater capabilities to clear sublethal bacterial challenges. Some NeuGc can be obtained from the diet in red meat and milk, and this can be incorporated into human gangliosides to a limited extent in foetal tissues, skin and some cancers, the last under hypoxic conditions especially. In these exceptions, use of dietary Neu5Gc has been ascribed to the higher metabolic rate.
2. Structure and Occurrence of Gangliosides
Most of the common range of gangliosides are derived from the ganglio- and neolacto-series of non-acidic oligoglycosphingolipids (Table 1), and they should ideally be named systematically in the same way with the position of the sialic acid residue(s) indicated as for branched structures. A more convenient naming system uses a short-hand nomenclature proposed by Svennerholm in which M, D, T and Q refer to mono-, di-, tri- and tetrasialogangliosides, respectively, and the numbers 1, 2, 3, etc. refer to the order of migration of the gangliosides on thin-layer chromatography (TLC), e.g., the order of migration of monosialogangliosides is GM3 > GM2 > GM1 (sometimes denoted by subscripts, e.g., GM1 or GM1). To indicate variations within the basic structures, further terms are added, e.g., GM1a, GD1b, etc. This system cannot be understood intuitively by anyone unfamiliar with the analytical methodology (TLC is now used much less often), as GM3 has two carbohydrate residues, while GM2 has 3 and GM1 has 4. Although alternatives have been proposed that are more systematic in structural terms, the Svennerholm nomenclature is well established and widely used, presumably because of its relative simplicity.
Ganglio-series glycosphingolipids having 0, 1, 2 and 3 sialic acid residues linked to the inner galactose unit are termed asialo- (or 0-), a-, b- and c-series gangliosides, respectively, while gangliosides having sialic acid residues linked to the inner N‑galactosamine residue are classified as α-series gangliosides. The structures for these groups are illustrated in the section on ganglioside biosynthesis below. It is worth noting that some terminal glycan structures of gangliosides are present in glycoproteins of cells.
As of 2020, more than 200 gangliosides with variations in the carbohydrate chain had been identified in vertebrates alone. One of the most studied monosialo-gangliosides and the first to be fully characterized is ganglioside GM1a (Neu5Acα2-3(Galβ1-3GalNAcβ1-4)Galβ1-4Glcβ1Cer), a major brain ganglioside of mammals and the preferred ligand of cholera toxin, illustrated -
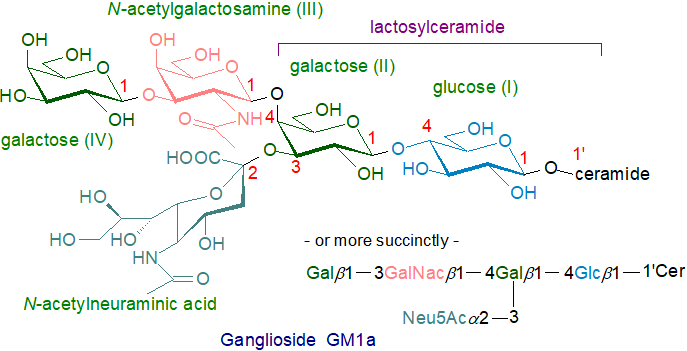
An alternative nomenclature, which is less used, is recommended by IUPAC-IUB and is based upon the ganglio (Gg) root structure (see the web page on non-acidic oligoglycosylceramides); it employs Roman numerals to designate each hexose unit and the location of the Neu5Ac along the carbohydrate chain with Arabic superscripts to designate the hydroxyl group to which this is linked. By this system, GM1a is defined as II3-α-Neu5Ac-Gg4Cer.
To add to the potential complexity, many gangliosides are known that contain O-acetylated sialic acids, such as N-acetyl-9-O-acetyl-neuraminic acid (Neu5,9Ac2), and some carry a sialic acid that forms a lactone ring. It is possible that such gangliosides are more widespread than has been reported, but they have been missed because of hydrolysis by treatment with mild alkali during the isolation procedure or the use of chromatographic conditions that include ammonia in the mobile phase, common analytical practices. Gangliosides with sulfate groups have been isolated from human, mouse and monkey kidney cells, while KDN-containing gangliosides are minor components of egg, ovarian fluid, sperm and testis of fish and of some mammalian tissues.
Brain gangliosides: Gangliosides can amount to 6% of the weight of lipids from brain, where they constitute 10 to 12% of the total lipid content (20-25% of the outer layer) of neuronal membranes. Aside from this, they are synthesised and are present at low levels (1 to 2% of the total lipids) in all animal tissues, where like the neutral oligoglycosphingolipids they are concentrated in the outer leaflet of the plasma membrane in the nanodomains known as 'rafts' or in related structures. Mammalian neurons synthesise gangliosides of the ganglio-series primarily, but oligodendrocytes in the brain produce instead myelin-forming glycosphingolipids such as galactosylceramide and sulfatide together with a minor amount of ganglioside GM4.
The brain contains as much as 20 to 500 times more gangliosides than most non-neural tissues, with three times as much in grey as in white matter. As the brain develops, there is an increase in the content of gangliosides and in their degree of sialylation with large differences between animals and tissues. During embryogenesis and the postnatal period in the human central nervous system, the total amount of gangliosides increases approximately threefold, while that of GM1 and GD1a increases 12 to 15-fold. During the same period, the hemato-series gangliosides GM3, GD3 and 9-OAc-GD3, which lack a hexosamine residue, are the predominant ganglioside species, but they are present in much lower amounts in adults and then only in some areas of the brain. GM1 concentrations decrease progressively with aging, suggesting a link with cognitive decline and neurodegenerative diseases. In mouse brain, the total amount of gangliosides is almost 8-fold greater in adults than in embryos, with a shift in composition from simple (GM3 and GD3) to more complex gangliosides. GD3 is highly expressed during development, mainly in the retina, hippocampus and cerebellum, and is necessary for cell migration and axonal extension. Gangliosides containing O‑acetylated sialic acids, such as 9-OAc-GD3, are expressed during embryonic development and in the retina and cerebellum of adult rats, but not other brain regions. It is evident that the ganglioside changes during brain maturation are correlated with many neuro-developmental milestones, and there is no doubt that gangliosides are crucial for neuronal function and brain development during infancy when there is high nutrient demand as the brain undergoes rapid restructuring.
The main gangliosides (~95%) of adult mammalian brain are ganglio series GM1, GD1a, GD1b and GQ1b, while lactosyl series gangliosides such as GM3 (sialyllactosylceramide) are found mainly in the extra-neural tissues. The remaining ~5% of minor components in brain include gangliosides GM4, GM3, GD3, GM2, GD2, Fuc-GM1, Fuc-GD1b, GT1a and GP1c, the relative proportions of which vary depending on the animal. On the other hand, modern mass spectrometric methodology (electrospray ionization ion mobility MS) has revealed a much higher degree of sialylation than was previously recognized, including a complete series of mono- to octasialylated gangliosides in the foetal frontal lobe. Subsequently, many previously unknown acetylated gangliosides were found in foetal hippocampus by this methodology. The content and composition of gangliosides in brain change with ageing, with a substantial fall in the content of lipid-bound sialic acid but an increase in the proportion of the more complex forms in terms of carbohydrate structures in the elderly.
 Gangliosides in other tissues: Among the extraneural tissues,
lactosyl series gangliosides such as GM3 (sialyllactosylceramide) and monosialogangliosides in general tend to predominate.
Relatively high concentrations of GD1a are present in erythrocytes,
bone marrow, testis, spleen and liver, while GM4 is more abundant in kidney, GM2 in bone marrow, GM1 in erythrocytes and GM3 in intestine.
In germ cells of mice, there is a switch between gangliosides of the a- and 0‑series upon differentiation when they are crossing
the blood-testis barrier.
Also in mice, primary CD4+ and CD8+ T cells express differing gangliosides, i.e., a-series and 0-series,
respectively, and dynamic changes occur during T cell development in thymus.
Skin fibroblasts and many cells of visceral organs generate gangliosides of the globo series mainly.
Similarly, globo and lacto series gangliosides are characteristic components of the stage-specific embryonic antigens (SSEA), which underlie the
development and differentiation of human embryonic stem cells.
A sialyl-lactotetraosylceramide is present in the latter and in the brains of children under the age of two, but not in tissues of adult humans.
O‑Acetylation or lactonization of the sialic acid residue adds to the number of species (acetylated gangliosides
in certain tumours may protect them from apoptosis).
Gangliosides in other tissues: Among the extraneural tissues,
lactosyl series gangliosides such as GM3 (sialyllactosylceramide) and monosialogangliosides in general tend to predominate.
Relatively high concentrations of GD1a are present in erythrocytes,
bone marrow, testis, spleen and liver, while GM4 is more abundant in kidney, GM2 in bone marrow, GM1 in erythrocytes and GM3 in intestine.
In germ cells of mice, there is a switch between gangliosides of the a- and 0‑series upon differentiation when they are crossing
the blood-testis barrier.
Also in mice, primary CD4+ and CD8+ T cells express differing gangliosides, i.e., a-series and 0-series,
respectively, and dynamic changes occur during T cell development in thymus.
Skin fibroblasts and many cells of visceral organs generate gangliosides of the globo series mainly.
Similarly, globo and lacto series gangliosides are characteristic components of the stage-specific embryonic antigens (SSEA), which underlie the
development and differentiation of human embryonic stem cells.
A sialyl-lactotetraosylceramide is present in the latter and in the brains of children under the age of two, but not in tissues of adult humans.
O‑Acetylation or lactonization of the sialic acid residue adds to the number of species (acetylated gangliosides
in certain tumours may protect them from apoptosis).
Gangliosides can cross the placental barrier into the foetus and those in milk, derived from the apical plasma membrane of secretory cells of the mammary gland, may be of nutritional value for the new-born. GD3 is the main ganglioside in human breast milk at an early stage of lactation, whereas GM3 is more abundant in the later stages (and in bovine milk); fucose-modified monosialylated and disialylated forms have been detected. Unfortunately, gangliosides are poorly characterized and quantified in foods in general.
Gangliosides from marine invertebrates (echinoderms), such as starfish and sea cucumbers, are very different in structure from those in vertebrates and do not have a shorthand nomenclature. They include forms with distinctive ceramide compositions, untypical carbohydrate residues, sialic acids within the oligosaccharide chain or with glycosyl inositol-phosphoceramide structures. The mollusc, Aplysia kurodai, lacks gangliosides but produces complex oligoglycosylceramides with 2‑aminoethylphosphonic acids and/or phosphoethanolamine groups attached that may serve as ganglioside surrogates.
Ceramide structures: In general, the ceramide structures of gangliosides tend to be relatively simple. Sphingosine is usually the main sphingoid base, accompanied by the C20 analogue in gangliosides of the central nervous system. Stearic acid (18:0) can be 80 to 90% of the fatty acid constituents in brain, accompanied by small amounts of 16:0, 20:0 and 22:0, but with little or no polyunsaturated or 2‑hydroxy acids, other than in some exceptional circumstances (e.g., some carcinomas). Palmitic acid is more abundant in gangliosides of the intestines and liver, while 2-hydroxylated fatty acids are present in higher concentrations in the last and in kidney. There are differences in the composition of the base and fatty acid components in different cells or regions of the brain. During development, the nature and concentrations of these change markedly, and for example, the ratio of C20/C18-sphingosine in GD1a of cerebellum increases 16-fold from 8-day-old to 2-year-old rats. In gangliosides outwith the nervous system, C20-sphingosine is barely detectable, and there is often a much wider range of fatty acid constituents (C14 to C24).
The nature of the ceramide component is relevant to the function of gangliosides, and changing the fatty acid component to α‑linolenic acid by synthetic means altered the properties of gangliosides dramatically in vitro, but it is the carbohydrate moiety that is of primary importance in general. In any given cell type, the number of different gangliosides may be relatively small, but their nature and compositions may be characteristic and in some way related to function.
3. Biosynthesis of Gangliosides
Biosynthesis of the ceramide and glycosylceramide precursors is discussed in separate web pages. There is evidence that the pool of glucosylceramide and thence of lactosylceramide utilized for ganglioside biosynthesis is different from that for the other neutral oligoglycosylceramides. This may explain some of the differences between the two groups in the fatty acid and sphingoid base components, which are dependent upon cell type. It is an open question how the ganglioside precursors enter the Golgi and trans-Golgi network where synthesis occurs at the luminal leaflet, but the regulation of intracellular sphingolipid traffic may be as important as the control of enzyme expression and activity in determining the final compositions of the various glycosphingolipid types.
In humans, sialic acid biosynthesis occurs by a series of reactions in the cytosol, but the Neu5Ac produced is transferred to the nucleus and activated by the cytosine 5'-monophosphate N-acetylneuraminic acid synthetase (CMAS) to form CMP-Neu5Ac, which is transported to the Golgi apparatus by a family of sialyltransferases specific for particular glycosidic linkages (α2-3, α2-6, α2-8 and α2-9).
Thereafter, in the pathways for the biosynthesis of the common series of gangliosides, membrane-spanning sialyltransferases and glycosyltransferases act sequentially as illustrated below for to produce the four main 0- (asialo), a-, b- and c‑series of gangliosides. The required enzymes are bound to the membranes of the Golgi apparatus in a sequence that corresponds to the order of addition of the various carbohydrate components. Thus, the sialyltransferase that catalyses the synthesis of the relatively simple ganglioside GM3 is located in the cis-region of the Golgi, while those that catalyse the terminal steps of ganglioside synthesis are located in the distal or trans-Golgi region. The GM3 synthase in particular, which only catalyses the transfer of Neu5Ac from cytidine monophosphate (CMP)-Neu5Ac onto the terminal galactose residue of lactosylceramide, is unique.
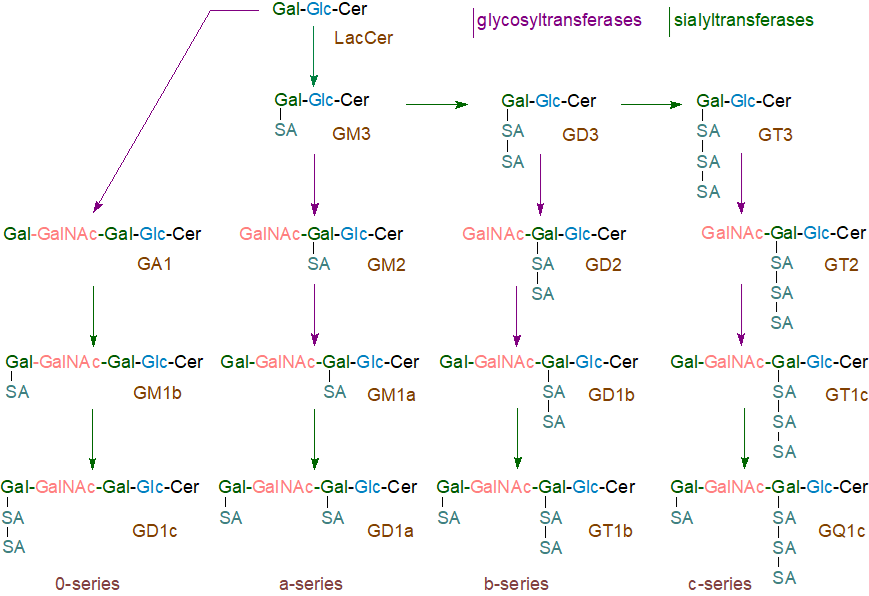 |
| Figure 1. Biosynthesis of the 0, a, b and c series of gangliosides. |
The simple ganglioside GM3 is synthesised by addition of sialic acid to lactosylceramide by CMP:LacCer α2-3 sialyltransferase (or GM3 synthase), before GD3 and GT3 are produced in turn by the action of appropriate synthases. Subsequently, GM3, GD3 and GT3 serve as precursors of more complex gangliosides by the action of further glycosyl- and sialyl-transferases. On the other hand, an alternative theory with some supporting evidence proposes that multiglycosyl-transferase complexes are responsible for the synthesis of individual gangliosides rather than a series of separate enzymes. Further sialylation of each of the a, b and c series and in different positions in the carbohydrate chain can occur to give an increasingly complex and heterogeneous range of products, such as the α‑series gangliosides with sialic acid residue(s) linked to the inner N‑acetylgalactosamine residue (not illustrated). GM4 or NeuAcα2,3Gal-ceramide, a minor component of brain and present in a few other tissues at low levels, is an exception in that galactosylceramide is its precursor. Finally, the newly synthesised gangliosides are transferred to the external leaflet of the plasma membrane via the lumenal surface of transport vesicles.
The changes that occur in ganglioside compositions of brain and other tissues in the embryonic and post-natal stages are governed mainly by changes in the expression level and activity of the glycosyl- and sialyl-transferases, although the former can be regulated by glycosylation and phosphorylation.
The presence of distinctive sialidases that differ from the catabolic lysosomal enzymes (see below) in raft-like regions of the plasma membrane bring about further changes in the composition of the cell surface gangliosides that can be characteristic for particular cell types, causing a shift from poly-sialylated species with a decrease of GM3 and formation of GM2 then GM1 by hydrolysis of terminal sialosyl residues linked either α2‑8 on another sialic acid or α2‑3 on galactose. As GM1 is resistant to most sialidases, it tends to increase in concentration relative to oligosialo gangliosides as developmental and other GM1-requiring processes come into play. This may have consequences for such cellular events as neuronal differentiation and apoptosis. Conversely, sialylation may occur in some neuronal membranes, increasing the proportions of poly-sialylated forms, as in the sialylation of GM3 by a CMP-NeuAc:GM3 sialyltransferase.
Ganglioside lactones, where the sialic acids are linked together through inner ester bonds, have been detected as minor components in brain tissues, where lactonization occurs at the plasma membrane. As this process influences the shape and properties of the original ganglioside profoundly, it is possible that lactonization-delactonization in a membrane might be a trigger for some cellular reactions. Similarly, as GD3 ganglioside can undergo O-acetylation at C9 of the outer sialic acid by the sialate O-acetyltransferase, there may be metabolic implications. Lyso-gangliosides, i.e., lacking a fatty acid moiety, are detected in some rare genetic disorders (see below).
Gangliosides added to many types of cell preparations in vitro are rapidly taken up by the cells, while gangliosides injected into animals in vivo are soon internalized by tissues. They can cross the blood-brain barrier, and via the placenta, they can enter the foetus. Although dietary gangliosides are absorbed intact by intestinal cells, they are broken down to their lipid and carbohydrate constituents for re-use, and sialic acids released by an intestinal sialidase are transported in plasma to the brain and other tissues where they influence ganglioside expression. Indeed, there is some experimental evidence that dietary gangliosides may improve cognition in animals and humans.
4. Catabolism of Gangliosides
Degradation of gangliosides takes place at the surface of intralysosomal luminal vesicles, generated by an inward budding of the endosomal membrane, and these are reached by a process of endocytosis. The principles of catabolism of glycosphingolipids in general are discussed in the web page dealing with monoglycosylceramides, and in brief, degradation occurs through the endocytosis-endosome-lysosome pathway with a requirement for an acidic pH inside the organelle. As well as the sialidases and exoglycohydrolases, the various reactions have an absolute requirement for effector molecules, termed 'sphingolipid activator proteins', including saposins (Sap) and the GM2-activator protein (GM2-AP).
In relation to gangliosides, soluble sialidases (neuraminidases) and exoglycohydrolases remove individual sialic acid and sugar residues sequentially from the non-reducing terminal unit, as illustrated for GM1, with the eventual formation of ceramide, which is then split into long-chain base and fatty acids by ceramidases. The first step is the removal of the terminal galactose residue by the GM1-β-galactosidase, assisted by GM2-AP or Sap B, before the terminal N‑acetylgalactosamine residue of GM2 is released by an N-acetyl-hexosaminidase (Hex A or Hex B) with the help of GM2AP to produce GM3, which is further degraded by an α-sialidase and Sap B to generate lactosylceramide. This is hydrolysed by β-galactosidase with assistance from Sap B or C to glucosylceramide, which is catabolized by the Sap C-assisted β-glucosidase to generate ceramide. Ultimately, this is split by acid ceramidase in the presence of Sap D to release sphingosine and free fatty acids.
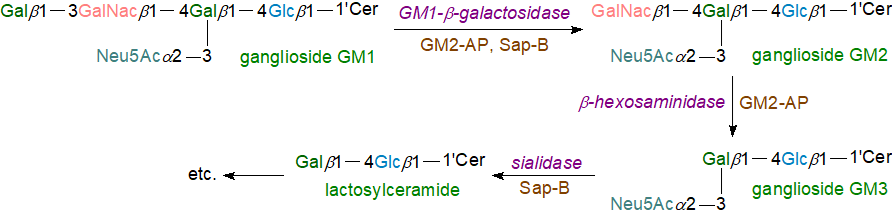 |
| Figure 2. Ganglioside degradation in the lysosomes. |
GM3 is a component of the lysosomal perimeter membrane, but in this location, it is protected from degradation by a glycocalix of the membrane facing the lysosol. Anionic lipids and especially bis(monoacylglycero)phosphate in the membranes stimulate ganglioside degradation while cholesterol is inhibitory.
This process constitutes a salvage mechanism that is necessary for the overall cellular economy, as a high proportion of the various hydrolysis products are re-cycled for glycolipid biosynthesis. By generating ceramide and sphingosine, it may be relevant to enzyme regulation and signalling by these lipids. In addition, some partial hydrolysis of gangliosides occurs in the plasma membrane as part of a biosynthetic remodelling process discussed above. Defects in catabolism can lead to the gangliosidoses discussed later.
5. Ganglioside Function
Cell surface effects: In their natural environment, gangliosides have a negative charge because of the presence of sialic acids, and this adds to the hydrophilicity of the polysaccharide constituent. This is balanced somewhat by the hydrophobic character of the ceramide moiety, so that over all the molecules are amphiphilic in nature, although they are very different from the glycerophospholipids, which are essential for the formation of membrane bilayers. Indeed, a ganglioside such as GM1 is virtually soluble in water, where it can form large aggregates through its hydrophilicity. However, the nature of the ceramide unit with its capacity to form hydrogen bonds with glycerophospholipids ensures that gangliosides are inserted in a stable manner into the outer layer of the plasma membrane.
Gangliosides are anchored in membranes by their ceramide units with the double-tailed sialoglycan components extending out from the cell surface, where they can participate in intermolecular interactions by a network of hydrogen bonds and hydrophobic interactions. For example, the glucose-ceramide bond of GM1 is oriented in the outer leaflet of the plasma membrane such that the glycan extends perpendicularly to the plane of the lipid bilayer. All gangliosides, including the simplest GM3 or Neu5Acα2-3Galβ1-4Glcβ1Cer, are structural lipids, and they have a natural propensity to segregate laterally and associate with each other and with other sphingolipids, phospholipids and cholesterol into raft nano-domains or in related structures, such as the caveolae. With the latter, the very large surface area occupied by the oligosaccharide chain imparts a strong positive curvature to the membrane. In such environments, gangliosides can interact with each other through side-by-side hydrogen bonds mediated by water molecules that act as bridges between the chains.
 Further,
molecules of GM3 and other gangliosides self-aggregate into clusters on the surface of lymphocytes of human peripheral blood,
and there is evidence that the density of these clusters in membranes governs their reactivity as antigens.
It is believed that gangliosides and other oligoglycosylceramides can cluster together through hydrogen donor-acceptor (cis) interactions
because of the presence of hydroxyl and acetamide groups to form membrane glycosynaptic microdomains, which are related to but functionally
distinct from raft signalling platforms (with lower cholesterol concentrations).
Much of the behaviour of gangliosides is determined by their location in these nanodomains, where they may operate
in cell adhesion, growth and motility through interactions with proteins and signal transduction pathways.
Further,
molecules of GM3 and other gangliosides self-aggregate into clusters on the surface of lymphocytes of human peripheral blood,
and there is evidence that the density of these clusters in membranes governs their reactivity as antigens.
It is believed that gangliosides and other oligoglycosylceramides can cluster together through hydrogen donor-acceptor (cis) interactions
because of the presence of hydroxyl and acetamide groups to form membrane glycosynaptic microdomains, which are related to but functionally
distinct from raft signalling platforms (with lower cholesterol concentrations).
Much of the behaviour of gangliosides is determined by their location in these nanodomains, where they may operate
in cell adhesion, growth and motility through interactions with proteins and signal transduction pathways.
Receptor/signalling: Gangliosides can bind to membrane proteins directly by carbohydrate-carbohydrate or carbohydrate-amino acid interactions, usually by specific ganglioside head groups, that result in changes to the location of proteins within membrane microdomains for recruitment of signalling partners or to dimerization or other effects upon receptors. In rafts and caveolae especially, gangliosides can modulate cell signalling processes by their interactions with receptors, adhesion molecules and ion channels. Cell-cell (trans) interactions occur by sialoglycans on one cell binding to complementary binding proteins (lectins) on adjacent cells, bringing about adhesion of cells and enabling regulation of intracellular signalling pathways, e.g., myelin-associated glycoprotein on myelin sheaths binds to gangliosides present on axonal membranes. Among such interactions, gangliosides in the extracellular milieu or on cell surfaces can initiate signalling by acting as ligands for the Siglec protein family, which are endogenous sialic acid-binding lectins expressed mainly on overlapping sets of immune cells or on myelinating cells in the nervous system. There are 15 members of this family in humans, each with distinct cellular locations and functions, and for example, Siglec-7 on natural killer cells binds to gangliosides GD3 and GD2 to inhibit immune signalling.
Gangliosides act as receptors for interferon, epidermal growth factor, nerve growth factor and insulin,
and they may regulate cell signalling and control growth and differentiation of cells in this way.
While intact gangliosides inhibit growth by rendering cells less sensitive to epidermal growth factor,
removal of the N‑acetyl group of sialic acid enhances this reaction and stimulates growth.
Gangliosides serve as antigens or receptors by recognizing specific molecules, including bacterial toxins, at the cell surface and
by modulating the charge density at the membrane surface (see below).
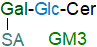 They
regulate how proteins operate within the plasma membrane such as the receptor-type tyrosine kinases and
the phosphorylation state and activity of insulin receptors in caveolae, and thence the insulin resistance of cells is controlled
by the concentration of GM3, the main ganglioside in plasma and other extraneural tissues.
This ganglioside interacts with the epidermal growth factor receptor leading to cell growth inhibition.
Of course, GM3 has a central position as the precursor of so many other gangliosides.
They
regulate how proteins operate within the plasma membrane such as the receptor-type tyrosine kinases and
the phosphorylation state and activity of insulin receptors in caveolae, and thence the insulin resistance of cells is controlled
by the concentration of GM3, the main ganglioside in plasma and other extraneural tissues.
This ganglioside interacts with the epidermal growth factor receptor leading to cell growth inhibition.
Of course, GM3 has a central position as the precursor of so many other gangliosides.
In addition, gangliosides have been shown to be cell-type specific antigens that serve in immune defence. GM3 in serum is highly enriched in membrane glycosynapses, and it forms complexes with co-localized cell signalling molecules. It serves in the innate immune system in macrophages, and it has been demonstrated that molecular species of GM3 with differing acyl-chain structures and modifications can operate as pro- and anti-inflammatory modulators of Toll-like receptor 4 (TLR4); GM3 containing very-long-chain and α-hydroxy fatty acid components increase TLR4 activation, with long-chain and mono-unsaturated (n‑9) forms of GM3 acting in the opposite way. Gangliosides and sialic acids protect cells from attack by the immune system and from autoimmunity, and they recognize and protect host organs and tissues from complement attack by binding to the complement regulatory protein factor H, which has the potential to exert strong cytotoxic and inflammation-inducing effects. In particular, sialic acids protect against complement killing of autologous cells by binding to this protein via the α2-3 linked sialic acid glycans of the GD3 ganglioside. On the other hand, breakdown of this system can lead to autoimmune diseases.
Brain: One of the first studies of a ganglioside influencing a signalling event concerned the relatively simple ganglioside GD3, which has a central role in early neurogenesis. GD3 binds to the epidermal growth factor receptor (EGFR) via a protein-carbohydrate interaction with its terminal N‑acetylneuraminic acid and a lysine residue in the transmembrane domain of the receptor and by a carbohydrate-carbohydrate interaction thereby maintaining the latter in its inert monomeric state. EGFR then binds to epidermal growth factor and stimulates the transition of the receptor from an inert monomeric to an active homodimeric form, and this in turn triggers receptor auto-phosphorylation and a signalling cascade that promotes cell proliferation. This has proven to be crucial for the regulation of the stem cell self-renewal capacity in the brain. In contrast, the neutral oligoglycosphingolipid Gb4 interacts directly with EGFR to potentiate its auto-phosphorylation with initiation of the downstream cascade. Siglec-4 (myelin-associated glycoprotein) on myelinating cells binds to gangliosides GD1a and GT1b on nerve cell axons to ensure proper operation of the axon-myelin interactions.
The occurrence of gangliosides in cell nuclei suggests a possible involvement in the expression of genes relevant
to neuronal function, and gangliosides of the gangliotetraose family are constituents of both membranes of the nuclear envelope.
Although there are appreciable differences between animal species, the techniques of molecular biology such as targeted gene deletion,
which enable target enzymes to be eliminated from experimental animals, are now leading to a better understanding of the part played
by individual gangliosides.
It is evident that they are essential to central myelination, to maintain the integrity of axons and myelin
and for the transmission of nervous impulses.
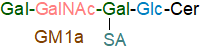 Thus, a variant of GD3, 9‑O‑acetyl GD3, acts in glial-guided neuronal migration during brain
development in the rat, while GM1 may operate in the same way in humans to determine which growth cone of unpolarized neurons becomes the axon.
By stabilizing neuronal circuits, gangliosides are required for memory, and conversely, disturbances in ganglioside synthesis can lead
to neurodegenerative disorders (see below).
GM3 in raft domains has been shown to be indispensable for the development, operation and viability of cochlear hair cells
and thence for hearing.
On the other hand, mice that express GM3 primarily and are devoid of the typical complex gangliosides of brain
suffer weight loss, progressive motor and sensory dysfunction, and deterioration in spatial learning and memory with aging.
GD3 is required for retinal structure and sight in mice.
These effects may be mediated by interactions of the negatively charged sialic acid residues of gangliosides with calcium ions,
which are critical for neuronal responses.
Thus, a variant of GD3, 9‑O‑acetyl GD3, acts in glial-guided neuronal migration during brain
development in the rat, while GM1 may operate in the same way in humans to determine which growth cone of unpolarized neurons becomes the axon.
By stabilizing neuronal circuits, gangliosides are required for memory, and conversely, disturbances in ganglioside synthesis can lead
to neurodegenerative disorders (see below).
GM3 in raft domains has been shown to be indispensable for the development, operation and viability of cochlear hair cells
and thence for hearing.
On the other hand, mice that express GM3 primarily and are devoid of the typical complex gangliosides of brain
suffer weight loss, progressive motor and sensory dysfunction, and deterioration in spatial learning and memory with aging.
GD3 is required for retinal structure and sight in mice.
These effects may be mediated by interactions of the negatively charged sialic acid residues of gangliosides with calcium ions,
which are critical for neuronal responses.
Changes in ganglioside composition can be induced by nerve stimulation, environmental factors and drug treatments. The various interconvertible ganglioside types in the plasma membrane of neurons are necessary for its development in that they regulate such processes as axonal determination and growth, signalling and repair. Gangliosides maintain myelin stability and control nerve regeneration by binding as ligands to certain myelin-associated glycoproteins. The monosialoganglioside GM1 has been shown to promote the differentiation of various neuronal cell lines in culture GM1 and takes part in the regeneration of the peripheral nervous system in adults. It strongly influences neurons by interacting with receptors such as the tropomyosin receptor kinase A (TrkA) receptor by altering its conformation to enable interaction with the nerve growth factor (NGF) ligand. It is protective towards the neural system by encouraging neural stem cell survival and proliferation, while facilitating the stability and regeneration of axons and by inhibiting neurodegeneration through autophagy, as after ischemic stroke; ultimately, it affects memory and cognition. After nerve injury, toll-like receptor 2 (TLR2) signalling is a factor in the induction of neuropathic pain; ganglioside GT1b is a TLR2 agonist that produces mechanical and thermal hypersensitivity.
Within membrane rafts, GM1 operates in several signalling systems through association with proteins that have glycolipid-binding domains, including G protein-coupled receptors, and those that modulate mechanisms such as ion transport, neuronal differentiation, immune system reactivities and neuroprotection. In general, ion homeostasis in excitable cells is required for maintenance of a steady state of cells and in the regulation of physiological functions, and in many neurological diseases, disturbances to ion concentrations and their regulation by gangliosides are a factor. They stabilize intracellular ion concentrations by regulating ion channels, and so they have a regulatory role in neuronal excitability and synaptic transmission. They also affect ion transport by binding and/or stimulating ion transporter proteins, and GM1 (with some GD1a) is tightly associated with a Na+/Ca2+ exchanger, which it potentiates. GM1 is a factor in Ca2+ and Na+ homeostasis in the nucleus and plasma membrane and in regulating platelet-derived growth factor, but unfortunately, there have been unpleasant complications when GM1 has been administered for therapeutic purposes. GD1a is sometimes considered to be a reserve pool for GM1.
Extracellular vesicles (EVs) are membrane-bound particles released by cells into the extracellular space for intercellular communication and the transfer of organelles and macromolecules between cells, including some of the latter that are potentially harmful such as misfolded proteins. Gangliosides promote or inhibit EV biogenesis depending upon the nature of their glycan headgroup with GM1 especially enhancing EV secretion, while disruption of its synthesis impairs it. By enabling EV-mediated secretion of pathogenic misfolded proteins, including mutant huntingtin, alpha-synuclein and tau, GM1 reduces the intracellular burden and is neuroprotective.
Other functions: GD3 is important for the process of apoptosis by blocking the stimulation of specific transcription factors and thence disabling the induction of antiapoptotic genes, and it is a regulator of autophagy, i.e., the degradation and/or recycling of cellular components. 9‑O‑Acetylation of the GD3 molecule prevents ganglioside oxidation and blocks its pro-apoptotic effects. Related studies with gene knockout mice have revealed that b-series gangliosides are involved in leptin secretion from adipocytes, while a-series gangliosides interact with the leptin receptor in the hypothalamus to influence the balance of energy.
Gangliosides are utilized in reproduction in both males and females, and in mice, GD1a has been shown to be necessary for oocyte maturation, monospermic fertilization and embryonic development, while GM1 takes part in sperm-oocyte interactions and sperm maturation processes. Deletion of the GM2/GD2 synthase leads to infertility in male mice with production of a novel fucosylated ganglioside containing very-long-chain polyunsaturated fatty acids. Disruptions in ganglioside metabolism have been associated with male infertility and reproductive dysfunctions, suggesting their potential as biomarkers for these conditions or as targets for therapeutic intervention.
6. Gangliosides and Disease
Bacterial toxins and viruses: In relation to adaptive immunity, a-series and 0-series gangliosides in the plasma membrane act on certain subsets of T cells as pattern-recognition receptors for invading pathogens during infections, with NeuAc as the main recognition module. Gangliosides can bind to some viruses and to various bacterial toxins, such as those from botulinum, tetanus and cholera. The best-known of these is cholera toxin, which is an enterotoxin produced by Vibrio cholerae where the specific cell surface receptor is GM1; the five B-chains of cholera toxin each bind one molecule of GM1. Interestingly, the subsequent metabolism of the ganglioside-toxin complex is dependent on the nature of the fatty acid components of the ganglioside. Toxins utilize the gangliosides to hijack an existing retrograde transport pathway from the plasma membrane to the endoplasmic reticulum, so the passage of the cholera toxin through the epithelial barrier of the intestine is mediated by GM1, possibly by endocytosis of the toxin-GM1 complex via caveolae into the apical endosome and thence into the Golgi/endoplasmic reticulum, where the complex dissociates. The consequence is persistent stimulation of adenylate cyclase by the toxin and continuous production of cAMP that leads to the severe fluid loss typical of cholera infections.
In the same way, GM2 binds to a toxin secreted by Clostridium perfringens, while GM1a is a coreceptor/attachment factor for dengue virus during infection, and the botulinum toxin binds to a complex of a polysialoganglioside with the protein synaptotagmin, which together act as a high-affinity receptor complex that is neurotoxic. Some gangliosides and GD1a especially are anti-inflammatory in that they inhibit bacterial lipopolysaccharides by preventing the activation of tumour necrosis factor (TNF) and other cytokines. In contrast, GM2 may increase cytokine production in the same circumstances, while the heat-labile toxins of Escherichia coli bind to several gangliosides in macrophages, thus inducing an inflammatory response.
Influenza viruses have two glycoproteins (hemagglutinins) in their envelope membranes that bind to cellular receptors such as gangliosides, and after entry into respiratory epithelial cells, the sialidase (neuraminidase) of the virus cleaves the sialic acid from the receptors to prevent entry of further viruses to the cell. Variations over time in the structures of these viral proteins force the development of new vaccines. The carbohydrate moiety of gangliosides binds initially to viruses, but the lipid moiety may control their intracellular transport.
Gangliosides are not found in most parasitic protozoans and worms that infect humans, but blood merozoites of the deadliest malaria parasite Plasmodium falciparum acquire GM1 and GM3 from its host during its complex binding, invasion and internalization, and it is produced in infected erythrocytes. GM3 has been detected in both the exoplasmic and cytoplasmic leaflets of the plasma membrane of this organism.
Gangliosidoses and other neurodegenerative diseases: Deficiencies in any of the ganglioside synthases can cause severe physiological problems. There may be a general rule that the mere process of lysosomal substrate accumulation in all lysosomal storage disorders impairs lysosome integrity and results in more general disruptions to lipid metabolism and to membrane structure and function, inevitably triggering pathologic mechanisms. Endogenous generation of antibodies to gangliosides is often a factor, and it has been argued that gangliosides and their sialic acids components are at the border of immune tolerance.
 As with the
non-acidic oligoglycosylceramides and ceramide monohexosides,
a number of unpleasant lipidoses have been identified in which storage of excessive amounts of gangliosides in tissues occurs
because of failures in the catabolic mechanism.
The most studied of these are the GM2 gangliosidoses, i.e., Tay-Sachs disease
(and the similar Sandhoff disease), a fatal genetic disorder found mainly in Jewish populations in which harmful quantities of
GM2 accumulate in the nerve cells in the brain and other tissues.
Lyso-GM2 (non-acylated) in plasma may serve as a marker of the disease, while a modified GM2 derivative that contains taurine in amide linkage
to the sialic acid carboxyl group has been identified in the brain of such patients.
As infants with the most common form of the disease develop, the nerve cells become distended, and a relentless deterioration
of mental and physical abilities occurs.
The condition is caused by inefficient operation of such enzymes/proteins as the GM2 activator protein or
β‑N‑acetylhexosaminidase (Hex A), which catalyses the degradation of gangliosides by removing the terminal
N‑acetylgalactosamine residue from GM2 (as illustrated above).
Biallelic mutations in GM2/GD2 synthase cause a complex form of hereditary spastic paraplegia.
As with the
non-acidic oligoglycosylceramides and ceramide monohexosides,
a number of unpleasant lipidoses have been identified in which storage of excessive amounts of gangliosides in tissues occurs
because of failures in the catabolic mechanism.
The most studied of these are the GM2 gangliosidoses, i.e., Tay-Sachs disease
(and the similar Sandhoff disease), a fatal genetic disorder found mainly in Jewish populations in which harmful quantities of
GM2 accumulate in the nerve cells in the brain and other tissues.
Lyso-GM2 (non-acylated) in plasma may serve as a marker of the disease, while a modified GM2 derivative that contains taurine in amide linkage
to the sialic acid carboxyl group has been identified in the brain of such patients.
As infants with the most common form of the disease develop, the nerve cells become distended, and a relentless deterioration
of mental and physical abilities occurs.
The condition is caused by inefficient operation of such enzymes/proteins as the GM2 activator protein or
β‑N‑acetylhexosaminidase (Hex A), which catalyses the degradation of gangliosides by removing the terminal
N‑acetylgalactosamine residue from GM2 (as illustrated above).
Biallelic mutations in GM2/GD2 synthase cause a complex form of hereditary spastic paraplegia.
A generalized GM1 gangliosidosis (an autosomal recessive and neurodegenerative disease) has been characterized in which GM1 accumulates in the nervous system leading to mental retardation and enlargement of the liver. The condition is a consequence of a deficiency of the lysosomal β‑galactosidase, which hydrolyses the terminal β-galactosyl residues from GM1 to produce GM2. Storage of substantial amounts of unwanted lipids in the lysosomal system leads to a state of cellular starvation, so that essential elements such as iron are depleted in brain tissue. Enhancement of the sialidase NEU3, which converts GM1 ganglioside to the GA1 glycolipid, confers benefits in mouse models of the disease and is a potential therapeutic target for reducing toxic GM1 ganglioside accumulation. The presence of lyso-GM1 in plasma is now seen as a useful aid to diagnosis, and it may be a causative factor by inhibiting the enzyme phosphoinositide 3-kinase (PI3K).
The Guillain-Barré syndrome is an acute inflammatory disorder, usually triggered by a severe infection, which affects the peripheral nervous system. Antibodies to gangliosides are produced by the immune system, leading to damage of the axons that can result in paralysis of the patient. In Huntington’s disease, disruption of the metabolic pathways between glycosylceramides and gangliosides occurs, especially in the composition of the ceramide component, and there is a human autosomal recessive infantile-onset epilepsy syndrome caused by a mutation to a sialyl transferase. Impaired ganglioside metabolism is also relevant to Alzheimer’s disease, because complexation with GM1 may cause aggregation of the amyloid β-protein deposits that accumulate in brain in this condition (this explanation does not appear to be universally accepted). Major disruptions to brain ganglioside content and composition are present in Amyotrophic lateral sclerosis, a devastating neurodegenerative disease marked primarily by motor neuron degeneration, while small amounts of some gangliosides accumulate as secondary storage compounds in Niemann-Pick disease.
Aberrant production of GM3 has been linked to pathophysiological changes associated with obesity, metabolic disorders and type 2 diabetes mellitus through acting upon insulin receptors, and it has a role in autoimmune disorders such as multiple sclerosis. Pathogenic variants in GM3 synthase produce an autosomal recessive disorder characterized by infantile-onset epileptic encephalopathy and profound developmental regression.
On the other hand, at normal tissue concentrations, gangliosides such as GM1 may be anti-inflammatory and neuroprotective in certain types of neuronal injury, Parkinsonism and some related diseases. In relation to Parkinson's disease, GM1 binds to α-synuclein and inhibits or eliminates fibril formation, a major cause of neurodegeneration, whereas a deficiency of GM1 in the substantia nigra may be responsible for neurodegeneration. It may be protective by preventing sphingomyelin-induced aggregation, and as the overall level of GM1 decreases during ageing, its beneficial effect decreases. In general, in ganglioside deficiencies, natural or induced, progressive inflammatory reactions take place, that lead to neurodegeneration, in part because of deterioration of the architecture of lipid rafts. For such reasons, GM1, the most accessible species, and derived molecules are under clinical investigation as potential therapeutic agents. There is no approved therapy for any gangliosidosis, although several different therapeutic strategies are being studied, including hematopoietic stem cell transplantation and gene therapy. At one time ganglioside-based drugs were widely used, but suggestions that they might be responsible for the onset of some cases of Guillain-Barré syndrome caused them to be withdrawn; these findings are now being disputed. For the moment, the blood-brain barrier remains a challenge.
Cancer: Sialylation of cell surface lipids, glycoproteins and glycoRNAs has been described as a cloak that helps tumour cells to evade immunological surveillance and retain their malignancy. Gangliosides affect cancer in many ways, and especially in the regulation of signal transduction induced by growth-factor receptors in microdomain glycosynapses in the cancer cell membranes, and in interactions with glycan recognition molecules that serve in cell adhesion and immune regulation. Depending on tissue, certain distinctive gangliosides are expressed at much higher levels in tumours than in normal healthy tissues by aberrant expression of glycosyltransferases, glycohydrolases, sialyltransferases and sialidases; GM3 is not expressed in melanocytes normally, but it is detected in 60% of primary melanomas and in 75% of metastatic melanomas. Gangliosides can be shed from the surface of tumour cells into the local environment where they can influence interactions between cancer cells, including the transition of tumours from a dormant to a malignant state (angiogenesis). When present in the circulation they can be useful diagnostic aids, and GM3 at elevated levels in the serum of patients with breast cancer may be a biomarker for the disease, while disialylated gangliosides GD2 and GD3 may indicate a neuroectoderm origin in neuroblastoma.
 Specific gangliosides can act positively or negatively towards the regulation of the malignancy of cancer
cells.
As a generality, disialyl glycosphingolipids or tandem-repeated sialic acid-structures confer malignant properties in various cancer systems and
are not merely markers.
For example, the disialo-gangliosides GD2 and GD3 are present in trace amounts only in normal tissues, but they are found at much higher
concentrations in cancer cells such as melanomas and neuroblastomas, with GD2 elevated in triple-negative breast cancer.
Perhaps surprisingly, the ceramide compositions in GD2 from patients with high-risk neuroblastoma differed appreciably from those at low risk,
suggesting a role for ceramide synthases in neuroblastoma biology.
These b‑series gangliosides play a substantial part in the malignancy of gliomas by mediating cell proliferation, migration,
invasion, adhesion and angiogenesis, and in preventing immunosuppression.
They are tumour-associated antigens, and the GD2 and GD3 synthases are seen as drug targets, although adverse effects have been noted with drugs
that reduce the expression of GD3 synthase presumably because this enzyme is required to maintain cellular homeostasis.
Metastatic melanoma cells have high levels of GD3 in comparison to poorly metastatic cells or the normal counterpart,
suggesting that GD3 may promote metastasis possibly by suppressing the anti-tumour immune response.
Specific gangliosides can act positively or negatively towards the regulation of the malignancy of cancer
cells.
As a generality, disialyl glycosphingolipids or tandem-repeated sialic acid-structures confer malignant properties in various cancer systems and
are not merely markers.
For example, the disialo-gangliosides GD2 and GD3 are present in trace amounts only in normal tissues, but they are found at much higher
concentrations in cancer cells such as melanomas and neuroblastomas, with GD2 elevated in triple-negative breast cancer.
Perhaps surprisingly, the ceramide compositions in GD2 from patients with high-risk neuroblastoma differed appreciably from those at low risk,
suggesting a role for ceramide synthases in neuroblastoma biology.
These b‑series gangliosides play a substantial part in the malignancy of gliomas by mediating cell proliferation, migration,
invasion, adhesion and angiogenesis, and in preventing immunosuppression.
They are tumour-associated antigens, and the GD2 and GD3 synthases are seen as drug targets, although adverse effects have been noted with drugs
that reduce the expression of GD3 synthase presumably because this enzyme is required to maintain cellular homeostasis.
Metastatic melanoma cells have high levels of GD3 in comparison to poorly metastatic cells or the normal counterpart,
suggesting that GD3 may promote metastasis possibly by suppressing the anti-tumour immune response.
In contrast, monosialyl gangliosides, such as GM1, GM2 and GM3, may suppress the malignancy of various cancer cells. The mechanism is thought to involve complex formation at the cell surface with membrane proteins, such as growth factor receptors and adhesion receptors like those of the integrin family, leading to the modification of cell signals mediated by these receptors.
Aberrant sialylation is found in many malignant cancers, where the levels of neuraminidases are key factors for metastasis and survival of cancer cells, and there can be a significant accumulation of unusual gangliosides containing N‑glycolyl sialic acid (Neu5Gc) in some cancers. N‑Glycolyl-GM3, normally absent from human tissues, is present in all stage II breast cancers, and it is accompanied by other less common complex gangliosides; it is sometimes considered to be a tumour-specific antigen and a target for cancer immunotherapy. The 5‑N‑deacetylated form of GM3 is expressed in metastatic melanomas but not in healthy tissue or even in primary melanomas; it is a marker for the metastatic condition and also a target for potential therapy. Increased synthesis of 9‑O‑acetyl-GD3, dependent on a sialyl-O-acetyltransferase - CAS1 Domain-Containing Protein 1, occurs in acute lymphoblastic leukaemia and in malignant melanomas, and this limits apoptosis, while O-acetylated GD2 (OAcGD2) is expressed in breast cancer and other tumours. A unique fucosyl-GM1 in which the terminal galactose is α-1,2-fucosylated at the non-reducing end is found circulating in serum of patients with several cancers and notably with small-cell lung cancer (but rarely in normal conditions), and it is considered to be a potential indicator of cancer and a further candidate for immunotherapy.
Clinical trials with an antibody to GD2 have been carried out successfully against the rare childhood cancer neuroblastoma, and the USDA has approved the use of this in combination with other drugs to treat this often-lethal cancer. However, this antibody can have painful side effects due to an interaction with GD2 on neurons, and modified antibodies, which may be safer, in combination with cytokines, chemotherapy, or immune checkpoint blocking are now being tested in clinical trials. A phase I clinical trial with an antibody to GD3 has shown promising results in patients with malignant melanoma and high-risk neuroblastomas, and antibodies to OAcGD2 and fucosyl-GM1 have been shown to be anti-tumour agents in vitro; studies with these and other antibodies are underway.
Other diseases: In epilepsy, a deficiency in the enzyme ceramide synthase 1, which produces 18:0 ceramides, leads to reduced ganglioside formation. By their presence in certain subsets of T cells, gangliosides influence allergic responses and auto-immune diseases. As gangliosides are present on the surface of vascular, vascular-associated and inflammatory cells, they may play a part in atherosclerosis and in aging.
7. Analysis
Gangliosides are not the easiest of lipids to analyse as they are most 'un-lipid-like' in many of their physical properties. They are labile to both acid and base with release of sialic acids. In the conventional Folch method for extraction of lipids from tissues, the gangliosides partition into the aqueous layer rather than with the conventional lipids in the chloroform layer. Nonetheless, methods have been devised for quantitative extraction, and gangliosides can then be sub-divided into the various molecular forms by high-performance thin-layer chromatography (TLC) or high-performance liquid chromatography. Although sometimes perceived as old technology, TLC has advantages in that immuno-staining with specific antibodies or sera from patients can be used, and efficient TLC-mass spectrometry interfaces are now available. Mass spectrometry is the main method for structural analysis, including identification and sequencing of the carbohydrate chains, with assistance from nuclear magnetic resonance spectroscopy. Ion-mobility mass spectrometry is of great value for the detection of novel structures. Conversion of individual gangliosides to ceramide derivatives for detailed analysis of molecular species is a useful ancillary technique.
Recommended Reading
- Biricioiu, M.R., Sarbu, M., Ica, R., Vukelic, Z., Clemmer, D.E. and Zamfir, A.D. Human cerebellum gangliosides: a comprehensive analysis by ion mobility tandem mass spectrometry. J. Am. Soc. Mass Spectrom., 35, 683-695 (2024); DOI - see also - DOI(1) and DOI(2).
- Breiden, B. and Sandhoff, K. Lysosomal glycosphingolipid storage diseases. Annu. Rev. Biochem., 88, 461-485 (2019); DOI.
- Fantini, J. Lipid rafts and human diseases: why we need to target gangliosides. FEBS Open Bio, 13, 1636-1650 (2023); DOI.
- Furukawa, K., Ohmi, Y., Hamamura, K., Ohkawa, Y., Hashimoto, N., Tajima, O., Kaneko, K. and Furukawa, K. GD2 is a crucial ganglioside in the signal modulation and application as a target of cancer therapeutics. Cancer Sci., 116, 862-870 (2025); DOI.
- Guo, Z.W. Ganglioside GM1 and the central nervous system. Int. J. Mol. Sci., 24, 9558 (2023); DOI.
- Herrera-Marcos, L.V., Sahali, D. and Ollero, M. 9-O Acetylated gangliosides in health and disease. Biomolecules, 13, 827 (2023); DOI.
- Hülsmeier, A.J. Glycosphingolipids in neurodegeneration - Molecular mechanisms, cellular roles, and therapeutic perspectives. Neurobiol. Disease, 207, 106851 (2025); DOI.
- Inokuchi, J., Kanoh, H., Inamori, K., Nagafuku, M., Nitta, T. and Fukase, K. Homeostatic and pathogenic roles of the GM3 ganglioside. FEBS J., 17, 5152-5165 (2022); DOI.
- IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids. Recommendations 1997. Eur. J. Biochem., 257, 293-298 (1998); DOI.
- Jin, X.F. and Yang, G.Y. Pathophysiological roles and applications of glycosphingolipids in the diagnosis and treatment of cancer diseases. Prog. Lipid Res., 91, 101241 (2023); DOI.
- Kolter, T. Ganglioside biochemistry. ISRN Biochemistry, 2012, 506160 (36 pages) (2012); DOI.
- Lunghi, G., Fazzari, M., Di Biase, E., Mauri, L., Chiricozzi, E. and Sonnino, S. The structure of gangliosides hides a code for determining neuronal functions. FEBS Open Bio, 11, 3193-3200 (2021); DOI.
- Malyarenko, T.V., Kicha, A.A., Stonik, V.A. and Ivanchina, N.V. Sphingolipids of Asteroidea and Holothuroidea: structures and biological activities. Marine Drugs, 19, 330 (2021); DOI.
- Naito-Matsui, Y. and others. Physiological exploration of the long term evolutionary selection against expression of N-glycolylneuraminic acid in the brain. J. Biol. Chem., 292, 2557-2570 (2017); DOI.
- Pedrayes, AJ., Rymen, D., Ghesquière, B. and Witters, P. Glycosphingolipids in congenital disorders of glycosylation (CDG). Mol. Gen. Metab., 142, 108434 (2024); DOI.
- Sandhoff, R. and Sandhoff, K. Emerging concepts of ganglioside metabolism. FEBS Letts, 592, 3835-3864 (2018); DOI.
- Schengrund, C.L. Sphingolipids: less enigmatic but still many questions about the role(s) of ceramide in the synthesis/function of the ganglioside class of glycosphingolipids. Int. J. Mol. Sci., 25, 6312 (2024); DOI.
- Schnaar, R.L. Gangliosides as Siglec ligands. Glycoconjugate J., 40, 159-167 (2023); DOI.
- Schnaar, R.L. and Lopez, P.H.H. (editors) Gangliosides in Health and Disease. Progress in Molecular Biology and Translational Science, Volume 156, Pages 1-462 (2018) available from Science Direct.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: October 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
