Ether Lipids
Lipids with ether bonds to long-chain alkyl or alkenyl moieties as well as ester bonds to fatty acids are common in nature as constituents of membrane lipids. Usually, the ether bond is in position sn-1 of the glycerol moiety of glycerophospholipids in animal tissues, protozoa and anaerobic bacteria, and only occasionally is it present in non-polar lipids. Ether lipids are major components in the Archaea, albeit with differing stereochemistry, but not in plants and fungi, or in facultative and aerobic bacteria (except for Myxobacteria), although they are major constituents of membranes in anaerobic bacteria. At one time, ether lipids were considered to be little more than a novelty, differing little in function from the fully acylated equivalents, although they comprise nearly 20% of the human phospholipidome. Then, findings of elevated levels of such lipids in cancer tissues, followed by the discovery of ether lipids with distinctive biological properties, such as platelet-activating factor and glycosylphosphatidylinositol anchors for proteins (with their own web pages here), stimulated research interest.
1. Basic Chemistry
 Two main types of glycerol ether bond exist in natural lipids - ether (alkyl) and vinyl (alk-1-enyl) ether as illustrated.
The double bond adjacent to the oxygen atom in the latter has the Z or cis configuration.
The terms "plasmanyl-" and "plasmenyl-" lipids for alkyl
and alk-1-enyl ethers, respectively, are recommended by IUPAC-IUB, but they have not been widely taken up in the literature.
Two main types of glycerol ether bond exist in natural lipids - ether (alkyl) and vinyl (alk-1-enyl) ether as illustrated.
The double bond adjacent to the oxygen atom in the latter has the Z or cis configuration.
The terms "plasmanyl-" and "plasmenyl-" lipids for alkyl
and alk-1-enyl ethers, respectively, are recommended by IUPAC-IUB, but they have not been widely taken up in the literature.
In animal tissues, the alkyl and alkenyl moieties in both non-polar lipids (alkyldiacylglycerols and neutral plasmalogens) and phospholipids tend to be rather simple in composition with 16:0, 18:0 and 9-18:1 predominating. Other alkyl groups may be present, but other than in fish lipids, they are found at low levels only. The trivial names - chimyl, batyl and selachyl alcohols - are sometimes used for the glycerol alkylethers (non-acylated hydrolysis products) with 16:0, 18:0 and 18:1 alkyl groups, respectively, in the sn-1 position.
Alkyl ether bonds are stable to both alkaline and acidic hydrolysis under most practical conditions, but alk-1-enyl-ether bonds open readily under acidic conditions to form aldehydes (or depending on conditions, acetal derivatives - see the Analysis section below). On hydrolysis with alkali, the ether bonds of both 1-alkyl-2/3-acyl-sn-glycerols and their 1-alk-1'-enyl equivalents are stable, and 1-alkyl- and 1-alk-1′-enylglycerol derivatives, respectively, and free (unesterified) acids are the products (the reaction with an alkyl diacylglycerol is illustrated).
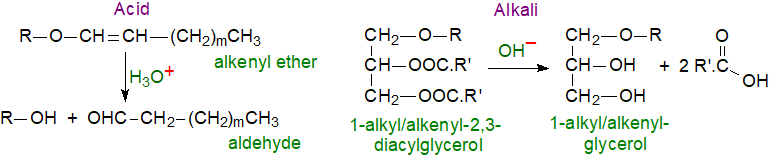 |
| Figure 1. Acidic and basic hydrolysis of ether lipids. |
2. Alkyldiacylglycerols and Neutral Plasmalogens
Ether analogues of triacylglycerols, i.e., 1-alkyldiacyl-sn-glycerols, are present at trace levels only, if at all, in most animal tissues, but they can be major components of some marine lipids. They can amount to 50% of the total lipids in dogfish (Squalus acanthias) and in ratfish (Hydrolagus colliei), and they can comprise 30% of the liver lipids of other sharks. In such species, alkyldiacylglycerols are a storage lipid, and they are located intracellularly in liver in lipid vacuoles; it has been suggested that they are part of the mechanism for density control to affect buoyancy. Similarly, 1‑alkyldiacyl-sn-glycerols can be a major component of lipids of marine invertebrates (80% of squid liver lipids), and they are present in the lipids of all corals, where it is proposed that they confer resistance to lipases. The alkyl moieties are the conventional saturated and monoenoic components, though usually with a wider range of chain lengths than in other animal tissues (ruminants may be a further exception), and in dog fish, the composition of alkyl groups is reported to be 10:0 (6%), 14:0 (2%), 16:0/16:1 (24%), 18:0 (18%), 18:1 (44%) and 22:0/22:1 (2%). In terrestrial mammals, 1-alkyldiacyl-sn-glycerols have been found in liver, adipose tissue and cancer cells, though usually in low proportions relative to triacylglycerols. Chromatographic separation of these two lipid classes is a technical challenge and is seldom reported.
Neutral plasmalogens, i.e., related compounds with vinyl ether bonds in position 1, have rarely been found at greater than trace levels in animal tissues, though again they have been detected in liver, adipose tissue and tumours. 1-Alkenyldiacylglycerols, together with the corresponding 1-alkyl lipids and ether-containing phospholipids, are reported to be major components of lipid droplets or ‘adiposomes’ in cultured CHO K2 cells. As an example, in bovine heart muscle, 1-alkyldiacyl-sn-glycerols and 1-alkenyldiacylglycerols comprised 1.6% and 0.25-0.8% of the simple lipids (mainly triacylglycerols), respectively. The compositions of the fatty acids and alkyl substituents of each of these lipids are listed in Table 1.
Table 1. Composition (wt %) of aliphatic moieties of 1-alkyldiacyl-sn-glycerols, 1-alkenyl-sn-diacylglycerols and triacylglycerols of bovine heart muscle. |
|||||
| Component | 1-Alkenyldiacylglycerols | 1-Alkyldiacylglycerols | Triacylglycerols | ||
|---|---|---|---|---|---|
| Alkenyl ethers |
Fatty acids | Alkyl ethers |
Fatty acids | Fatty acids | |
| 14:0 | 2 | 1 | 4 | 3 | 2 |
| 15br | 11 | 3 | 2 | 3 | - |
| 15:0 | 5 | - | 3 | - | 1 |
| 16:0 | 38 | 14 | 32 | 23 | 19 |
| 16:1 | 4 | 2 | - | 2 | 2 |
| 17br | 5 | 1 | 4 | 2 | - |
| 17:0 | 24 | - | 2 | 1 | 2 |
| 18:0 | 7 | 16 | 34 | 21 | 19 |
| 18:1 | - | 27 | 21 | 29 | 45 |
| 18:2 | - | 22 | - | 4 | 7 |
| 18:3(n-3) | - | 1 | - | 1 | 1 |
| 20:3(n-6) | - | 3 | - | - | - |
| 20:4(n-6) | - | 6 | - | 1 | - |
| 22:4(n-6) | - | 2 | - | 3 | - |
| 22:5(n-3) | - | 3 | - | 5 | - |
| Data from Schmid, H.H.O. and Takahashi, T. Biochim. Biophys. Acta, 164, 141-147 (1968);
DOI. A publication by Yamashita, S. and others contains a great deal of useful compositional data on plasmalogens: Molecules, 28, 6328 (2023); DOI. |
|||||
The fatty acid components of the ether lipids are more like those of the phospholipids in composition than those of the triacylglycerols (see below). In marine invertebrates, polyunsaturated fatty acids tend to be concentrated in position sn-2.
 Small amounts of methoxy-substituted glyceryl ethers have been found in alkyldiacylglycerols and alkylacyl
phospholipids in liver oils from sharks and other cartilaginous fish, as deduced by analysis of the hydrolysis products.
In addition to the methoxyl group in position 2, the main components have C16 saturated
and C16/18 monounsaturated alkyl chains with a cis double bond in position 4,
although one isomer with an alkyl group analogous to that of docosahexaenoic acid (20:6) is known.
It is claimed that they are potent antibacterial and anti-cancer agents, and that they boost the immune system.
Small amounts of methoxy-substituted glyceryl ethers have been found in alkyldiacylglycerols and alkylacyl
phospholipids in liver oils from sharks and other cartilaginous fish, as deduced by analysis of the hydrolysis products.
In addition to the methoxyl group in position 2, the main components have C16 saturated
and C16/18 monounsaturated alkyl chains with a cis double bond in position 4,
although one isomer with an alkyl group analogous to that of docosahexaenoic acid (20:6) is known.
It is claimed that they are potent antibacterial and anti-cancer agents, and that they boost the immune system.
Non-acylated 1-O-alkyl-sn-glycerols are present in trace amounts in animal tissues, including human breast milk, where it is likely that they act as an inert reservoir for biosynthesis of platelet-activating factor. Fecapentaenes are mono-alkyl-glycerols produced in animals by colonic bacteria and have five conjugated double bonds in the alkyl moiety (12 or 14 carbons in total); they have genotoxic and mutagenic potential. Although there are recent reports of the occurrence of alkyldiacylglycerols and neutral plasmalogens in higher plants, there is a suggestion that these may arise from the use of exogenous microbial lipids by plant enzymes.
3. Phospholipids with Ether-Linked Substituents
While neutral ether lipids tend to be encountered only rarely, the membrane phospholipids of many animals and bacteria usually contain appreciable proportions of molecular species with ether and vinyl ether bonds in position sn-1 as well as diacyl forms, and often the vinyl ethers or plasmalogens predominate.

In adult humans, approximately 20% of the total phospholipids have an ether linked moiety, but the proportions in different tissues vary greatly; liver phospholipids contain less than 5% of ether lipids, while spermatozoa can contain up to 40%. Other than in the heart, plasmenylethanolamine tends to be the main ether lipid, with as much as 80% of the total ethanolamine-phospholipids in some brain tissues in this form, while in kidney, skeletal muscle and retina it can amount to 20-40%. Much less of the phosphatidylcholine and commonly little or none of the other phospholipids, such as phosphatidylinositol (but see below) or phosphatidylserine, contain ether moieties. In phosphatidylcholine of most tissues, a higher proportion is often of the O-alkyl rather than the O‑alkenyl form (neutrophils contain over 40% as the O‑alkyls), but the reverse tends to be true in heart lipids.
In bovine heart muscle once more, alkylacyl-, alkenylacyl and diacyl phosphatidylcholines comprised 1%, 16% and 24% of the phospholipids, respectively, and the corresponding proportions in phosphatidylethanolamine were 0.5%, 11% and 16%, respectively. The compositions of the fatty acids and alkyl substituents of phosphatidylcholines are listed in Table 2.
Table 2. Composition (wt %) of aliphatic moieties of alkylacyl-, alkenylacyl- and diacyl-forms of phosphatidylcholine of bovine heart muscle. |
||||||
| Component | Alkenylacyl- | Alkylacyl- | Diacyl- | |||
|---|---|---|---|---|---|---|
| 1-Alkenyl ethers |
Fatty acids position 2 |
1-Alkyl ethers |
Fatty acids position 2 |
Fatty acids position 1 |
Fatty acids position 2 |
|
| C14-15 | 6 | - | 10 | - | 1 | - |
| 16:0 | 62 | 2 | 5 | 11 | 62 | 5 |
| 16:1 | 2 | 1 | - | 1 | 3 | 3 |
| 17br | 8 | - | 9 | - | 7 | - |
| 17:0 | 3 | - | 1 | - | 2 | - |
| 18:0 | 11 | 1 | 10 | 2 | 14 | - |
| 18:1 | 7 | 12 | 18 | 13 | 8 | 29 |
| 18:2 | - | 53 | - | 51 | 3 | 46 |
| 18:3(n-3) | - | 1 | - | 1 | - | 1 |
| 20:3(n-6) | - | 6 | - | 5 | - | 5 |
| 20:4(n-6) | - | 21 | - | 14 | - | 10 |
| 20:5(n-3) | - | 1 | - | - | - | - |
| 22:4(n-6) | - | 2 | - | 2 | - | 1 |
| 22:5(n-3) | - | 1 | - | 1 | - | - |
| Data again from Schmid, H.H.O. and Takahashi, T. Biochim. Biophys. Acta, 164, 141-147 (1968); DOI, and this paper has data on the comparable phosphatidylethanolamines. | ||||||
The corresponding phosphatidylethanolamines differ in having higher proportions of 18:0 components in position sn-1 and much more arachidonate (20:4(n‑6)) in position sn-2, and in brain and retina, 20:4, 22:4 and 22:6 are the main fatty acid constituents in the latter position. In that they contain high proportions of longer-chain alkyl constituents (C20 to C24) in plasmanylethanolamine, neutrophils differ from other cell types. Appreciable amounts of diplasmalogens (1,2-di-(O-1'-alkenyl)) have been reported in the phosphatidylethanolamine in epididymal spermatozoa from rabbits. The nematode Caenorhabditis elegans, now considered a useful model for studies of ether lipid function, contains significant amounts of alkylacyl and alkenylacyl phosphatidylethanolamines but not of other phospholipids; it has mainly C18 constituents in both positions of the glycerol moiety. In addition to phospholipids, small amounts of alkylacyl- and alkenylacyl-monoglycosyldiacylglycerols are present in the central nervous system of animals.
In anaerobic bacteria, the most common plasmalogens are constituents of phosphatidylethanolamine, phosphatidylglycerol, phosphatidylserine and cardiolipin, but many other phospholipids and even glycosyldiacylglycerols have been found with vinyl ether bonds. Plasmalogens do not occur in aerobic or facultative anaerobic bacteria (except Myxobacteria), fungi or plants, and it has been suggested that during evolution there has been an appearance, disappearance and then reappearance of plasmalogens, a theory supported by differences in the biosynthetic mechanisms between bacteria and animals. In addition to ether-containing phosphatidylethanolamine and phosphatidylglycerol, a sulfate-reducing bacterium, Desulfatibacillum alkenivorans synthesises cardiolipins containing one (monoether/triester) to four (tetraether) ether-linked saturated straight-chain or methyl-branched alkyl chains. The annamox bacteria, which contain ladderane fatty acids, are unusual in that the alkyl moieties are in position sn‑2 of their phospholipids.
4. Biosynthesis of Ether Lipids
Animals: The biosynthesis of glycerol ethers including plasmalogens has been studied in animal tissues mainly, but the very different biosynthetic mechanism in anaerobic bacteria is now known to be equally interesting. In animals, there are important differences in how ether lipids are synthesised in comparison to the corresponding diacyl-phospholipids (see the appropriate web pages). Some of the first steps are carried out by enzymes associated with the membranes of peroxisomes, an organelle in constant interaction with various other organelles via contact sites and normally associated with the catabolism of lipids, with later steps being completed in the endoplasmic reticulum.
A pool of fatty acids imported from the cytosol by the peroxisomal ABCD proteins, especially ABCD3, or produced intra-peroxisomally by β-oxidation of CoA esters of very long-chain fatty acids is used by the enzyme fatty acyl-CoA reductase 1 (FAR1) anchored to the external surface of the peroxisome to generate most of the fatty alcohols that are the precursors for the alkyl moieties of ether lipids. FAR1 is reported to be the rate-limiting enzyme in plasmalogen biosynthesis, with a marked substrate specificity for CoA esters of 16:0, 14:0 and 9-16:1, and it is regulated by a feedback mechanism that senses the amount of plasmalogens in the inner leaflet of the plasma membrane and controls the stability of FAR1. A second such enzyme, FAR2, is known but serves only in C20 alcohol synthesis in the meibomian glands. The long-chain fatty alcohols from FAR1 are transported across the peroxisomal membrane in a protein-independent manner via a flip-flop mechanism.
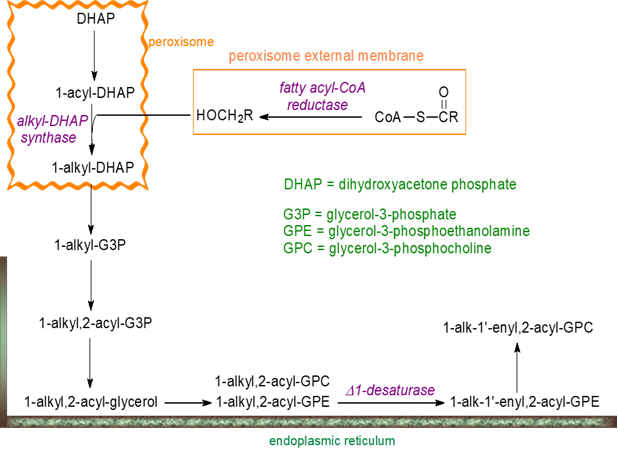 |
| Figure 2. Biosynthesis of ether lipids and plasmalogens in animal tissues. |
Glycerol 3-phosphate is imported into the lumen of the peroxisome and converted into dihydroxyacetone phosphate (DHAP), which is first esterified with a long-chain acyl-CoA ester by means of a glyceronephosphate O-acyltransferase (GNPAT), before the ether bond is introduced by exchanging the acyl group for a long-chain alcohol, a reaction catalysed by an alkyl-DHAP synthase (alkylglyceronephosphate synthase, AGPS). The mechanism comprises a sequence of reactions with first 1‑acyl-DHAP binding to AGPS, then reaction of acyl-DHAP with the cofactor flavin adenine dinucleotide (FAD) for release of a fatty acid containing both oxygen atoms from the ester linkage in acyl-DHAP, binding of the fatty alcohol to AGPS, and finally formation of 1‑alkyldihydroxyacetone phosphate (1‑alkyl-DHAP). A notable feature of this reaction is that the oxygen atom comes from the alcohol moiety not glycerol.
At this point, the intermediate is transferred from the peroxisome via a protein ACBD5 with an acyl-CoA binding domain to a protein VAP‑B on the cytosolic face of the endoplasmic reticulum, with the VAP-ACBD5 complex acting as a tether to link the two organelles. All subsequent reactions occur at the endoplasmic reticulum and following reduction of the ketone group at the sn-2 position to make 1-alkyl-sn-glycero-3-phosphate by an acyl/alkyl-DHAP reductase requiring NADPH as a cofactor, a fatty acyl moiety is introduced by a distinctive alkyl/acyl-glycero-3-phosphate acyltransferase to yield 1-alkyl-2-acyl-sn-glycero-3-phosphate. A phosphohydrolase removes the phosphate group, and the resulting 1-alkyl-2-acyl-sn-glycerol is converted to the ethanolamine/choline phospholipid by the enzyme systems used to generate the diacyl species (see the appropriate web pages).
1-Alkyl-2-acyl-sn-glycero-3-phosphoethanolamine is the main substrate for an oxygen-dependent Δ1′-desaturase to yield the plasmalogen 1‑alk-1′-enyl-2-acyl-GPE. The first enzyme of this kind to be characterized was a protein designated CarF in the aerobic bacterial species Myxococcus xanthus, and this led to the discovery of the human orthologue TMEM189 as the long-sought plasmanylethanolamine desaturase (PEDS1). Incidentally, this aerobic pathway is present in the closest unicellular relatives of animals.
1-Alk-1′-enyl-2-acyl-GPE is the precursor for the corresponding choline lipid, first by the removal of phosphoethanolamine by an ethanolamine plasmalogen-specific phospholipase C to produce 1-alkenyl-2-acyl-sn-glycerol, which is then converted to 1-alk-1′-enyl-2-acyl-sn-glycero-3-phosphocholine by a choline-phosphotransferase. In the liver, a phosphatidylethanolamine N-methyltransferase can catalyse the same conversion. It should be noted that this pathway is very different and is separated spatially from that producing diacyl-phosphatidylethanolamines (or phosphatidylcholines) via the CDP-ethanolamine pathway. A further route to glycerol ethers and plasmalogens involves phosphorylation of alkylglycerols with an alkylglycerol kinase. The mechanism for biosynthesis of the 2‑methoxy ethers in sharks has yet to be established.
As with other phospholipids, the final fatty acid compositions of ether lipids are attained by remodelling processes as in the Lands cycle. This can occur by re-acylation after removal of the fatty acids of position sn-2 by the action of a phospholipase A2 with formation of lysophospholipids (which may act as signalling molecules), and in macrophages, for example, cytosolic-group IVC phospholipase A2γ (cPLA2γ) is the key enzyme. Much of the arachidonate and other polyunsaturated fatty acids are obtained from diacyl phospholipids by exchange reactions catalysed by CoA-independent transacylases. Many of the enzymes for the last stages of the synthesis of ether lipids are shared with those for the diacyl forms, so some crosstalk between the two pathways can occur to control the quantitative balance between them in each tissue. Eventually, plasmalogens are transported to the post-Golgi compartment in a manner that is dependent on ATP (but not vesicular transport) and requires P4-type ATPase ATP8B2 for preferentially location in the inner leaflet of the plasma membrane. Alkyl-linked ether lipids are transported up to twofold faster than vinyl-linked species.
Presumably, 1-alkyl-2-acyl-glycerols derived from phospholipids are the main source of the 1-alkyl-2,3-diacyl-sn-glycerols in animal cells, although 1‑alkylglycerols can be acylated in position sn-2 by a variety of acyltransferases, while acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) is reported to be the enzyme responsible for introducing the fatty acid into position sn-3 of alkylacylglycerols.
Diet: Although it seems possible that plasmalogens might be hydrolysed by acid lipase in the intestines, this does not seem to happen to a significant extent, possibly because of buffering by other food constituents although the ester bonds to fatty acids are hydrolysed. Plasmenyl lipids are hydrolysed to the lyso form in the intestines and are absorbed as such before some emerge unchanged in the lymph, while some are re-esterified with arachidonic acid preferentially with some base-exchange from ethanolamine to choline in enterocytes. 1‑O‑Alkyl- and 1-O-alkenyl-sn-glycerols in the diet are rapidly absorbed from the gastrointestinal tract from which they are transported to other tissues and utilized for synthesis of a full range of ether-containing lipids (3-O-alkyl-sn-glycerols are absorbed equally rapidly but are then oxidized to fatty acids). Dietary 1‑O‑alkyl-sn-glycerols can be converted to the alkenyl lipids, as this step in biosynthesis occurs after those in peroxisomes.
Biosynthesis in bacteria: A quite different pathway for the biosynthesis of ether lipids is known to exist in anaerobic bacteria, and details have now emerged. It is known that sn‑glycerol-3-phosphate, rather than dihydroxyacetone phosphate, is the initial precursor for biosynthesis of the common range of diacylphospholipids before the ether lipids are produced from these. In Clostridium perfringens, a two-gene operon encoding a multi-domain complex containing reductase and dehydratase enzymes has been identified that is responsible for plasmalogen biosynthesis by reduction of the ester bond in position sn-1 of the diacylphospholipid intermediate to a vinyl ether. The relevant genes have been detected in many different obligate and facultative anaerobic bacteria, including a number from the human gut.
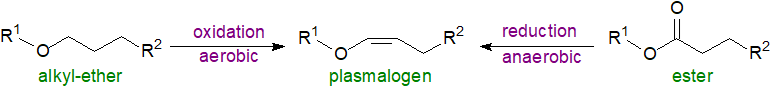 |
| Figure 3. Plasmalogen biosynthesis - aerobic versus anaerobic. |
Aerobic bacteria such as the gram-negative myxobacterium Myxococcus xanthus synthesise appreciably amounts of ether lipids with C14 iso-methyl-branched acyl and alkyl/alkenyl chains, both as phospholipids and alkyldiacylglycerols, in fruiting bodies where they assist in spore formation. In general, the biosynthetic mechanism is similar to that for animals, and although many of the relevant enzymes have yet to be characterized, the oxygen-dependent Δ1′-desaturase responsible for plasmalogen synthesis was described from this species first.
5. Catabolism of Ether Lipids in Animals
The fatty acid in position sn-2 of an alkylacyl phospholipid, including platelet-activating factor, is first released by the action of a phospholipase A2 before the O-alkyl linkage is cleaved oxidatively by a microsomal alkylglycerol monooxygenase (AGMO) present in liver and intestinal tissue that uses molecular oxygen and tetrahydrobiopterin (H4biopterin) as a co-factor. The aliphatic product is a fatty aldehyde, which can be further oxidized to the corresponding acid by a fatty aldehyde dehydrogenase. Release of the polyunsaturated fatty acids during the process has implications for oxylipin production, and in female mice, a cell type-dependent role of alkylglycerol monooxygenase in limiting prostanoid biosynthesis has been observed.
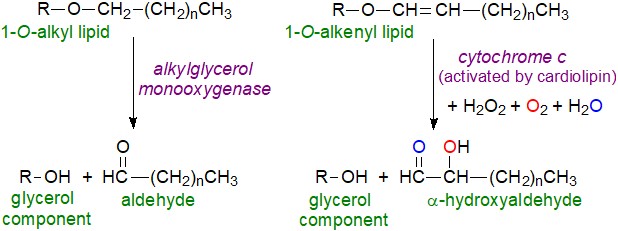 |
| Figure 4. Catabolism of ether lipids. |
After removal of the fatty acid moieties, two lysoplasmalogenases, TMEM86A and TMEM86B (especially the latter), have been characterized that can hydrolytically cleave a plasmalogen ether bond to form a long-chain fatty aldehyde. Alternatively, the vinyl ether bond can be cleaved by a very different mechanism in which the key enzyme is cytochrome c, better known for its role in the respiratory chain of mitochondria. This must first be activated to become a peroxidase by an interaction with cardiolipin, then, after a complex series of reactions, the products are a lysophospholipid and an α‑hydroxyaldehyde. Elegant mass spectrometric studies with stable isotopes demonstrated that the carbonyl oxygen is derived from water while that of the α‑hydroxyl group comes from molecular oxygen (or possibly from oxidized cardiolipin). As the resulting lysophospholipid is likely enriched in arachidonic or docosahexaenoic acids, this process may also influence oxylipin production. The findings are relevant to Alzheimer's disease (see below), as it has long been known that α-hydroxyaldehydes accumulate in the brains of affected patients.
Plasmalogens and heme peroxidases: Myeloperoxidase, an abundant protein in leukocytes such as neutrophils, monocytes and macrophages, has unwanted catabolic effects upon plasmalogens. On activation, the enzyme converts hydrogen peroxide and chloride ions to hypochlorous acid (HOCl), the primary purpose of which is to aid the innate immune response to kill invading pathogens (e.g., bacteria, yeasts, fungi and parasites). If this is poorly controlled, there is an adventitious reaction with other cellular constituents as hypochlorous acid reacts with the vinyl ether bond of choline and ethanolamine plasmalogens to generate lysophospholipids with the fatty acid component in position sn-2 and 2-chloro-fatty aldehydes. The latter can be reduced in tissues to 2‑chloro-fatty alcohols or oxidized to 2-chloro-fatty acids, which may react further to give many other metabolites. In addition, HOCl can react with the polar head group of ethanolamine-containing lipids to generate chloramines, while 2‑bromo-aldehydes result in the same way from an eosinophil peroxidase in eosinophils.
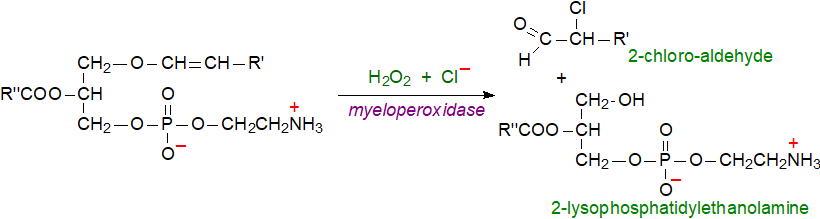 |
| Figure 5. Reaction of plasmalogens with reactive oxygen species from myeloperoxidase. |
Activated neutrophils and monocytes together with infarcted myocardium and human atherosclerotic lesions can then produce significant amounts of 2‑chlorohexadecanal and related lipids for which many deleterious pro-inflammatory effects have been demonstrated. In particular, they react rapidly with thiol groups including those of glutathione and proteins to form conjugates in the same manner as with other aldehydes. 2-Chloro-fatty acids induce a process known as NETosis in which neutrophils produce extracellular traps (NETs) as a defence against bacterial pathogens, but these can be harmful in relation to many different human diseases with increased levels in tissues being correlated with mortality in animal models of sepsis and in humans, possibly because of disruption to the epithelial barrier. The other products of the reaction, lysophospholipids, are cytotoxic and pro-atherogenic. It seems likely that the proposed protective action of plasmalogens as antioxidants is in competition with these damaging reactions. As HOCl is widely used as a disinfecting and antibacterial agent in commercial cleaning products, these may represent a source for concern if mishandled.
6. Functions of Ether Lipids
Membranes: Within a membrane, the acyl chain in plasmalogens is oriented perpendicularly to the membrane surface in marked contrast to the sn‑2 acyl chain of diacyl glycerophospholipids where there is a bent conformation, while the head-group lacks a carbonyl oxygen in the sn-1 position and is much more lipophilic. Plasmalogen thus takes up less area per lipid molecule in a membrane plane than a diacyl counterpart. As a result, there is stronger intermolecular hydrogen bonding between head groups, leading to changes to the arrangement of lipids within membranes with a high propensity to form an inverse hexagonal-II phase (non-bilayer), which is a requirement for membrane fusion. While this is true for the predominant phosphatidylethanolamines, the phosphatidylcholines tend to occur as lamellar phases. Hexagonal-II phases occur at lower temperatures than for the diacyl analogues and as they have a larger dipole moment, plasmalogen-containing cell membranes are less fluid than those deficient in plasmalogens, i.e., they form more compressed, thicker and rigid lipid bilayers in comparison with the diacyl equivalents, a property that is relevant for the compact membrane structures present in myelin. In spite of the relatively high concentrations of polyunsaturated fatty acids, they have a tendency to accumulate in membrane raft domains, i.e., regions of membranes enriched in cholesterol and sphingolipids where many signalling proteins are concentrated, to influence the compartmentation and activities of membrane enzymes.
Apart from being structural components of cell membranes, plasmalogens are required for many other purposes. The available information is based partly on their distribution in various types of cell and partly on their physical properties, but also on the effects of changes that occur in plasmalogen metabolism in certain mutant cells. Their distribution in ion channels embedded in membranes may be a relevant factor, as they can regulate these either directly via their physical location in membranes or indirectly as second messengers, which control innumerable aspects of cell physiology. A study with genetic mutants of the nematode C. elegans demonstrated that ether lipids were required for optimal fertility, lifespan, survival at cold temperatures and resistance to oxidative stress.
 Signalling: Plasmalogens serve as a store of polyunsaturated fatty acids that can be liberated
from membranes that are stimulated physiologically.
Thus, at least two plasmalogen-selective enzymes of the phospholipase A2 type degrade plasmalogens to release arachidonic and
docosahexaenoic acids from position sn-2 for eicosanoid or docosanoid production, respectively, as part of signalling mechanisms.
Arachidonic acid from this source is necessary for synthesis of the endocannabinoid anandamide
in brain, but in general as a precursor for other lipid mediators, this process is regarded as a pro-inflammatory response of plasmalogens.
In contrast, intact plasmalogens are considered to be anti-inflammatory, when present in membranes at optimum levels.
They may have a more direct role in signalling by interaction with Toll-like receptor 4, certain G-protein-coupled receptors (GPCRs),
and peroxisome proliferator-activated receptors (PPAR).
It is not clear whether alkyl-ether lipids act in the same way in any metabolic aspect, as the two types are rarely differentiated in
studies.
Signalling: Plasmalogens serve as a store of polyunsaturated fatty acids that can be liberated
from membranes that are stimulated physiologically.
Thus, at least two plasmalogen-selective enzymes of the phospholipase A2 type degrade plasmalogens to release arachidonic and
docosahexaenoic acids from position sn-2 for eicosanoid or docosanoid production, respectively, as part of signalling mechanisms.
Arachidonic acid from this source is necessary for synthesis of the endocannabinoid anandamide
in brain, but in general as a precursor for other lipid mediators, this process is regarded as a pro-inflammatory response of plasmalogens.
In contrast, intact plasmalogens are considered to be anti-inflammatory, when present in membranes at optimum levels.
They may have a more direct role in signalling by interaction with Toll-like receptor 4, certain G-protein-coupled receptors (GPCRs),
and peroxisome proliferator-activated receptors (PPAR).
It is not clear whether alkyl-ether lipids act in the same way in any metabolic aspect, as the two types are rarely differentiated in
studies.
The other product of phospholipase A2 hydrolysis of plasmalogens is a lysoplasmalogen (i.e., lacking a fatty acyl group), which can be re-acylated or further degraded to aldehyde and phosphoglycerol moieties. As they are known to activate cAMP-dependent protein kinase, lysoplasmalogens may have a signalling function, and they are reported to assist the maturation and stimulation of semi-invariant natural killer T (iNKT) cells in the thymus. An alkenyl phosphatidylethanolamine is an endogenous agonist for the nuclear hormone receptor RORγt (retinoic-related orphan receptor gamma), which is involved in the inflammatory response.
It has been established that plasmenylcholine, which is abundant in linoleate in heart mitochondria, is a substrate for the transacylase tafazzin and may be required for the remodelling of cardiolipin. Plasmalogens are involved in lipid droplet formation and maintenance, and in brown adipose tissue, they regulate thermogenesis by mediating mitochondrial fission.
Oxidation: Claims that plasmalogens protect membranes against oxidative stress by acting as sacrificial antioxidants in vivo have been difficult to substantiate. Singlet oxygen interacts more rapidly with plasmalogens than with other lipids in vitro to yield hydroperoxyacetals and dioxetanes, which break down spontaneously under normal conditions to aldehyde and lysophospholipid products, which are capable of further generation of reactive species. Indeed, there are counter-suggestions that polyunsaturated fatty acids protect plasmalogens against oxidative damage, although there is evidence from studies in rat brain and retina that plasmalogens act as endogenous antioxidants in these tissues at least. The oxidation by-products of plasmalogens other than by singlet oxygen are less toxic than the free aldehydes and hydroperoxides produced by oxidation at other unsaturated centres, and they may not propagate oxidation. When polyunsaturated fatty acids in plasmalogens are oxidized, hydroperoxide groups generated in position 7 of the fatty acid in position sn-2 can react intramolecularly with the vinyl ether bond to convert it to an epoxide.
It has become evident that the biosynthesis of ether-linked phospholipids influences ferroptosis, an iron-dependent type of non-apoptotic cell death induced by excess accumulation of peroxidized phospholipids. This seems to explain why saturated fatty acids have been implicated in promoting ferroptosis, as they are reduced by FAR1 to the alcohol precursor of ether lipids, i.e., the plasmanyl forms which exacerbate ferroptosis, with subsequent addition of polyunsaturated fatty acids into position sn-2 of phospholipids, although the latter are not the important factor in this instance. Another factor is the action of the desaturase TMEM189 to generate the vinyl ether bond in plasmenyl phospholipids, which affect ferroptosis differently in various cell types and may be dependent upon the concentrations of plasmalogens, mainly plasmenylethanolamine, in the inner leaflet of the plasma membrane. One suggestion is that at high levels of plasmenylethanolamine, TMEM189 is down-regulated to protect against ferroptosis, while at lower levels, down-regulation of TMEM189 may not change the sensitivity to ferroptosis significantly.
Ether lipids and disease: In animal studies, dietary supplementation with plasmalogens is reported to be beneficial towards health in general with anti-inflammatory effects in response to ageing including the amelioration of cognitive decline and other pathological conditions, some of which have no other treatment. Changes in the ether content of lipids has been noted in many disease states, although it is not always clear whether these are causal, and plasmalogen replacement therapy with dietary supplements of ether lipids can restore plasmalogen levels. As ethanolamine plasmalogens enriched in arachidonic acid in position sn-2 make up a high proportion of the lipids in cardiomyocytes, they may act as a reservoir of the eicosanoid precursor to influence heart pathologies. Plasmalogens affect aspects of cholesterol metabolism, and it has been established that dysregulation of plasmalogen homeostasis inhibits cholesterol biosynthesis by reducing the stability of squalene monooxygenase, a key enzyme in cholesterol biosynthesis. High concentrations in male reproductive tissues suggest that plasmalogens have a role in spermatogenesis and fertilization, while in the eye, deficiencies in plasmalogens can lead to cataract formation, 'glaucoma-like' optic nerve abnormalities and developmental defects.
It has long been known that greatly elevated levels of ether lipids are found in cancers, and there is a strong correlation with the promotion of aggressive disease. The Δ1′-desaturase TMEM189 is upregulated and promotes breast and other cancers by inhibiting autophagy-regulated ferroptosis, and its inhibition is a potential therapeutic treatment. The peroxisomal alkylglyceronephosphate synthase is up-regulated appreciably in cancer cells, leading to substantial changes in the content and composition of many lipids including signalling lipids such as lysophosphatidic acid and eicosanoids, which favour the development of cancers. Synthetic ether analogues of lysophospholipids are being tested as anticancer agents.
 In
Alzheimer’s disease, there is a significant loss of ethanolamine plasmalogens in the cerebral and cerebellar white matter and cerebral
grey matter of brain tissue that can be correlated with the progression of the illness, although it is not yet certain whether this is a cause
or consequence.
One hypothesis is that the oxidative stress conditions in this disease induce oxidative damage to ether lipids with a
loss of polyunsaturated fatty acids and of antioxidant and neuroprotective capacity.
The resulting changes in composition of the neural cell membranes alter the processing of amyloid-beta and cause other metabolic defects that
favour the progression of the disease.
Similarly, a loss of plasmalogens has been reported in patients with Parkinson’s disease and multiple sclerosis and in some psychiatric disorders,
which may be accompanied by cognitive disturbances and aberrant behaviour.
In
Alzheimer’s disease, there is a significant loss of ethanolamine plasmalogens in the cerebral and cerebellar white matter and cerebral
grey matter of brain tissue that can be correlated with the progression of the illness, although it is not yet certain whether this is a cause
or consequence.
One hypothesis is that the oxidative stress conditions in this disease induce oxidative damage to ether lipids with a
loss of polyunsaturated fatty acids and of antioxidant and neuroprotective capacity.
The resulting changes in composition of the neural cell membranes alter the processing of amyloid-beta and cause other metabolic defects that
favour the progression of the disease.
Similarly, a loss of plasmalogens has been reported in patients with Parkinson’s disease and multiple sclerosis and in some psychiatric disorders,
which may be accompanied by cognitive disturbances and aberrant behaviour.
Plasmalogens are reported to promote the neurogenesis associated with improvement of learning and memory in mice, and there is an intriguing report that human centenarians have elevated concentrations of ether and vinyl ether phospholipids in their plasma.
In the human peroxisomal disorder Rhizomelic Chondrodysplasia Punctata, which is characterized clinically by defects in eye, bone and nervous tissue, there are defects in the biosynthesis of plasmalogens. There are comparable reports of plasmalogen deficiency in Zellweger’s syndrome and Niemann–Pick type C disease, where there are known to be peroxisomal dysfunctions. Neutral ether lipids but not the analogous phospholipids accumulate in the tissues of those who suffer from Wolman’s disease, another rare genetic disorder caused by a deficiency in lysosomal acid lipase, while elevated plasmalogen levels have been detected in visceral fat of obese patients. Impaired ether metabolism has been implicated in Sjögren-Larsson syndrome, a rare inherited metabolic disease characterized by ichthyosis and intellectual impairment, where a very substantial increase in alkylglycerols and the alcohol precursors has been observed in the lipids of the stratum corneum of the skin relative to normal controls. These were mainly non-polar alkyl-diacylglycerols and free non-esterified alkylglycerols, but with very little of the plasmalogens.
Studies of these phenomena are now being aided by using genetically modified mice lacking certain enzymes for the biosynthesis of ether lipids, and the recent identification of the 1-O-alkyl desaturase responsible for the last step in plasmalogen biosynthesis makes possible the engineering of host cells such as yeasts for studies of how and why ether lipids operate in cells. Production of larger quantities of plasmalogens for oral consumption by this means may have pharmacological potential.
There is a tradition in Scandinavian folk medicine for the use of shark liver oils, which are rich in ether lipids, for the treatment of cancers and other ailments, including wound healing, gastric ulcers and arthritis, and there may be some substance to the claims that are under investigation. The alkylglycerol constituents, and the 2-methoxy constituents especially, are considered to be the important ingredients. The mechanism for such effects is uncertain, but they may bypass the peroxisome step, which is rate-limiting in plasmalogen biosynthesis. In addition, they increase the permeability of membranes and there is evidence they may interact directly with the enzyme protein kinase C, which is vital for signal transduction.
7. Other Ether Lipids
Many other types of natural lipids with ether bonds have been reported, some very different in structure from those discussed above. These include cholesterol ethers and vinyl ethers, glycerol thio-ethers and dialkylglycerophosphocholines, which have been found in bovine heart. In mammalian tissues, platelet-activating factor is an ether lipid related to phosphatidylcholine and has its own web page here (as do many other ether lipids). Seminolipid or 1‑O‑hexadecyl-2-O-hexadecanoyl-3-O-β-D-(3'-sulfo)-galactopyranosyl-sn-glycerol, is a vital constituent of the lipids of testis and spermatozoa, and small amounts of ether analogues of galactosyldiacylglycerols, including seminolipid, have been found in brain and nervous tissue. The glycosylphosphatidylinositol (GPI) component of GPI-anchored proteins generally contain a 1-O-alkyl-2-O-acyl-sn-glycerol residue. Highly unusual glycosyldiacylglycerols with ether bonds are occasionally reported from sponges, although these probably originate in symbiotic bacteria. Noladin ether, an analogue of 2‑arachidonylglycerol, has been detected in porcine brain and is an endogenous agonist of a cannabinoid receptor.
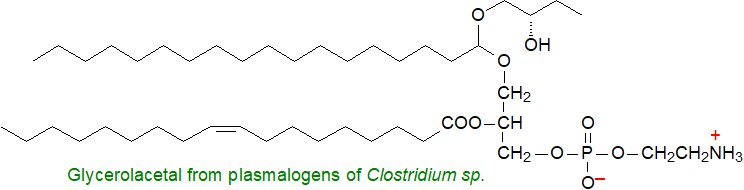
Di- and tetra-alkyl ether lipids of the Archaea are unique lipids based on 2,3-dialkyl-sn-glycerol backbones and also have their own web page. To add to the list, an unusual galactoglycerolipid with phytol ether-linked to position 1 of glycerol has been partially characterized from algae and cyanobacteria, and it may occur at trace levels in some higher plants. Several members of the bacterial genus Clostridium contain glycerol-acetals that are derived biosynthetically from plasmenylethanolamine, while bacterial proteolipids contain a diacylglycerol unit linked from position sn-3 via a thio ether bond to a protein. Cyclic ether lipids, e.g., epoxy and furanoid fatty acids and betaine lipids in which the polar moiety is linked to glycerol via an ether bond are discussed in separate web pages.
In recent years, an unusual group of branched dialkyl glycerol tetraether lipids has been discovered in peat bogs and soils. In general, they consist of octacosane (C28) alkyl units with either 13,16-dimethyl- or 5,13,16-trimethyl substituents, and others with 4 to 6 methyl groups attached to the n-alkyl chains and/or with 0 to 2 cyclopentyl moieties in the alkyl chain. They are non-isoprenoid in nature, and they differ from those of the Archaea in that they have a 1,2‑di‑O‑alkyl-sn-glycerol rather than the 2,3‑di‑O-alkyl-sn-glycerol configuration typical of the latter. In the most abundant form illustrated, the glycerol moieties are in an ante-parallel arrangement and the methyl groups in each alkyl chain have a syn conformation with respect to each other.

These branched membrane lipids were first characterized in anaerobic soil bacteria of the genus Acidobacteria, but many bacterial genera are now known to have the required genes. The nature of the intact lipids from which they are derived has yet to be fully elucidated, although some components have been identified with glucuronosyl or glucosyl units attached to the glycerol ether backbone. Related membrane-spanning diabolic acids are present, and these are now known to be the precursors for the ether bonds by the action of glycerol ester reductases.
8. Analysis of Ether Lipids
Pure ether lipids are rarely easy to separate physically from the fully acylated forms, and their presence in tissues is usually inferred from isolation of their hydrolysis products or from spectroscopic methods, although 1‑alkyldiacylglycerols and neutral plasmalogens can be separated from triacylglycerols by exacting thin-layer chromatography techniques. It is usually necessary to convert phospholipids to non-polar derivatives, either by modifying or removing the polar head group, before alkylacyl-, alkenylacyl- and diacyl-glycerides can be isolated by TLC or HPLC. In the native state, ether-containing phospholipids of all types can be quantified readily by 31P NMR spectroscopy, although mass spectrometry is often the preferred methodology nowadays for more comprehensive analyses.
 Another common approach to analysis consists in isolation and derivatization of
the alkyl or alkenyl moieties for analysis by gas chromatography-mass spectrometry.
When acidic transesterification methods are used to prepare fatty acid methyl esters from lipid extracts that contain a proportion
of vinyl ether bonds, free aldehydes are generated (see Figure 2 above) that are rapidly converted to dimethyl acetals and can be analysed
as such along with the methyl ester derivatives of the fatty acid components; they elute just before the comparable methyl esters on GC.
Alkylglycerols can be analysed as the trimethylsilyl ethers or isopropylidene derivatives by GC-MS.
Another common approach to analysis consists in isolation and derivatization of
the alkyl or alkenyl moieties for analysis by gas chromatography-mass spectrometry.
When acidic transesterification methods are used to prepare fatty acid methyl esters from lipid extracts that contain a proportion
of vinyl ether bonds, free aldehydes are generated (see Figure 2 above) that are rapidly converted to dimethyl acetals and can be analysed
as such along with the methyl ester derivatives of the fatty acid components; they elute just before the comparable methyl esters on GC.
Alkylglycerols can be analysed as the trimethylsilyl ethers or isopropylidene derivatives by GC-MS.
Suggested Reading
- Almsherqi, Z.A. Potential role of plasmalogens in the modulation of biomembrane morphology. Front. Cell Developm. Biol., 9, 673917 (2021); DOI.
- Balsinde, J. and Balboa, M.A. Plasmalogens in innate immune cells: from arachidonate signaling to ferroptosis. Biomolecules, 14, 1461 (2024); DOI.
- Curran, C.S., Remaley, A.T. and Torabi-Parizi, P. Plasmalogens as biomarkers and therapeutic targets. J. Lipid Res., 66, 100925 (2025); DOI.
- Dean, J.M. and Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell, 9, 196-206 (2018); DOI.
- Dorninger, F., Werner, E.R., Berger, J. and Watschinger, K. Regulation of plasmalogen metabolism and traffic in mammals: The fog begins to lift. Front. Cell Developm. Biol., 10, 946393 (2022); DOI.
- Faria, R.L., Prado, F.M., Junqueira, H.C., Fabiano, K.C., Diniz, L.R., Baptista, M.S., Di Mascio, P. and Miyamoto, S. Plasmalogen oxidation induces the generation of excited molecules and electrophilic lipid species. PNAS Nexus, 3, pgae216 (2024); DOI.
- Fontaine, D., Figiel, S., Félix, R., Kouba, S., Fromont, G., Mahéo, K., Potier-Cartereau, M., Chantôme, A. and Vandier, C. Roles of endogenous ether lipids and associated PUFAs in the regulation of ion channels and their relevance for disease. J. Lipid Res., 61, 840-858 (2020); DOI.
- Gallego-García, A., Monera-Girona, A.J., Pajares-Martínez, E., Bastida-Martínez, E., Pérez-Castano, R., Iniesta, A.A., Fontes, M., Padmanabhan, S. and Elías-Arnanz, M. A bacterial light response reveals an orphan desaturase for human plasmalogen synthesis. Science, 366, 128-132 (2019); DOI.
- Jackson, D.R., Cassilly, C.D., Plichta, D.R., Vlamakis, H., Liu, H., Melville, S.B., Xavier, R.J., and Clardy, J. Plasmalogen biosynthesis by anaerobic bacteria: identification of a two-gene operon responsible for plasmalogen production in Clostridium perfringens. ACS Chem. Biol., 16, 6-13 (2021); DOI.
- Jenkins, C.M., Yang, K., Liu, G., Moon, S.H., Dilthey, B.G. and Gross, R.W. Cytochrome c is an oxidative stress–activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage J. Biol. Chem., 293, 8693-8709 (2018); DOI.
- Kimura, T., Kimura, A.K. and Epand, R.M. Systematic crosstalk in plasmalogen and diacyl lipid biosynthesis for their differential yet concerted molecular functions in the cell. Prog. Lipid Res., 91, 101234 (2023); DOI.
- Papin, M., Bouchet, A.M., Chantôme, A. and Vandier, C. Ether-lipids and cellular signaling: A differential role of alkyl- and alkenyl-ether-lipids? Biochimie, 215, 50-59 (2023); DOI.
- Paul, S. and others. Modulation of endogenous plasmalogens by genetic ablation of lysoplasmalogenase (Tmem86b) in mice. J. Lipid Res., 66, 100808 (2025); DOI.
- Rangholia, N., Leisner, T.M. and Holly, S.P. Bioactive ether lipids: primordial modulators of cellular signaling. Metabolites, 11, 41 (2021); DOI.
- Sailer, S., Keller, M.A., Werner, E.R. and Watschinger, K. The emerging physiological role of AGMO 10 years after its gene identification. Life-Basel, 11, 88 (2021); DOI.
- Schröter, J. and Schiller, J. Chlorinated phospholipids and fatty acids: (patho) physiological relevance, potential toxicity, and analysis of lipid chlorohydrins. Oxidative Med. Cell. Longevity, 8386362 (2016); DOI.
- Vítová, M., Palyzová, A. and Rezanka, T. Plasmalogens - Ubiquitous molecules occurring widely, from anaerobic bacteria to humans. Prog. Lipid Res., 83, 101111 (2021); DOI.
- Wang, H.L., Tan, C.P., Liu, Y.F. and Xu, Y.J. Alkylglycerol: Not abundant but promising functional lipid. Trends Food Sci. Technol., 153, 104701 (2024); DOI.
- Wu, Y., Deng, Y.R., Angelov, B. and Angelova, A. Plasmalogen as a bioactive lipid drug: from preclinical research challenges to opportunities in nanomedicine. FASEB Bioadv., 7, e70028 (2025); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: January 2026 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
