Fatty Acids: Natural Cyclic
Carbocyclic (alicyclic and aromatic) fatty acids occur naturally in plants, especially certain seed oils, and microorganisms, but only rarely in animal tissues, other than the prostaglandins, which are described elsewhere in these web pages as are the plant jasmonates. Cyclopropane fatty acids are occasionally reported from marine animals such as sponges, and some at least may be produced by the animals themselves, although others are probably synthesised by symbiotic bacteria. While cyclic fatty acids can be formed as artefacts from conventional unsaturated fatty acids during food processing, the structures, compositions and biochemistry of natural cyclic fatty acids only are considered here.
1. Cyclopropane Fatty Acids in Microorganisms
Lactobacillic acid or cis-11R,12S-methylene-octadecanoic acid was first isolated from the phospholipids of the bacterium Lactobacillus arabinosus and is a cyclopropane analogue of cis-vaccenic acid, the main unsaturated fatty acid in this organism and its biosynthetic precursor.
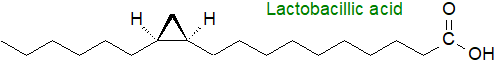
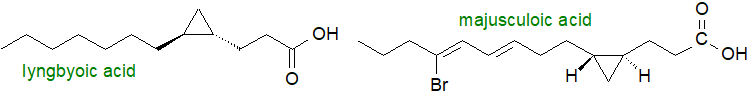
It has since been found in a wide range of bacterial species, both gram-negative and gram-positive, from strict anaerobes to obligate aerobes, often accompanied by cis-9,10-methylene-hexadecanoic acid and other homologues (C14 to C20 in chain-length). Cyclopropane fatty acids are present in at least one extremophile, Aquifex aeolicus, and some of the branched-chain mycolic acids contain cyclopropane rings of either the cis or trans configuration. While 9,10‑methylene-5-hexadecenoic acid and 11,12-methylene-5-octadecenoic acid have been isolated from the cellular slime mould Polysphondylium pallidum, they are known to be metabolites of ingested microbial fatty acids. Unusual cyclopropyl fatty acids with the ring of the trans configuration in the 4,5‑position, not to mention conjugated double bonds, bromine rings and methyl branches, have been found in various cyanobacteria of marine origin, and majusculoic and lyngbyoic acids from marine cyanobacteria have the structures illustrated.
3-Hydroxy-lactobacillic acid is the major amide-linked component of an ornithine-containing lipid of Mesorhizobium ciceri, and a unique fatty acid with multiple cyclopropane rings (attached to a nucleoside) and termed jawsomycin is synthesised by the bacterium Streptoverticillium fervens by a polyketide pathway. Some organisms, including the protozoal parasites Leishmania sp., contain cis-9,10-methylene-octadecanoic acid (dihydrosterculic acid), the cyclopropane analogue of oleic acid, often together with homologous fatty acids that are related biosynthetically, while 17-methyl-cis-9,10-methylene-octadecanoic acid occurs in another protozoan, Herpetomonas megaseliae.
Although cyclopropane rings are fully saturated, they are chemically reactive because of their highly strained nature. On catalytic hydrogenation, ring opening occurs with the formation of two methyl-branched fatty acids, a reaction that has proved useful for characterization purposes in conjunction with mass spectrometry, although 3-pyridylcarbinol esters are now recognized as the most useful derivatives for mass spectrometric characterization. Cis/trans configurations of cyclopropane rings can be distinguished by 1H‑NMR spectroscopy.
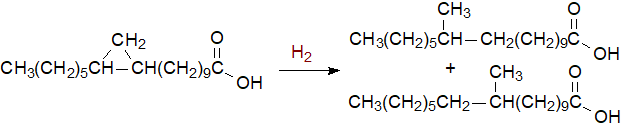 |
| Figure 1. Hydrogenation of a cyclopropane ring. |
The cyclopropane ring is also opened under relatively mild conditions by reaction with bromine, mercuric acetate or acidic reagents such as boron trifluoride-methanol. In contrast, cyclopropyl fatty acids are stable on silver nitrate chromatography, when they elute together with saturated fatty acids, and they are unaffected by bases and by the oxidizing agents commonly used to locate double bonds.
The first step in the biosynthesis of the cyclopropane ring in a fatty acid in bacteria is similar to that for certain methyl-branched fatty acids with addition of a methyl group to the double bond from S-adenosylmethionine, which is converted to S‑adenosylhomocysteine in the process. Experiments with double bonds labelled with deuterium confirm that the hydrogen atoms in the fatty acid are retained, so the subsequent cyclization step involves a loss of hydrogen from the methyl group.
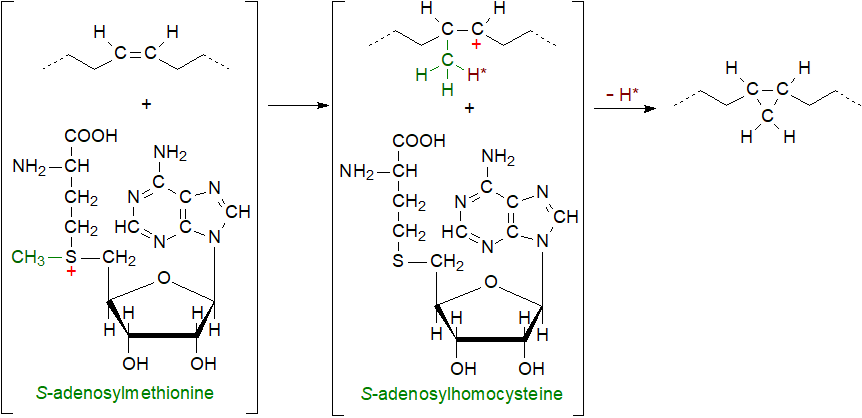 |
| Figure 2. Biosynthesis of cyclopropyl fatty acids. |
Most phospholipid synthesis occurs during the exponential growth period, but cyclopropane ring formation reaches its maximum at the end of exponential growth and in the early part of stable growth and is energetically expensive as synthesis of each S‑adenosylmethionine molecule requires three ATP equivalents. It is noteworthy that the substrate for the reaction is a monoenoic fatty acid in position sn-2 of phosphatidylethanolamine usually (or rarely position sn-1 depending on species), not the free acid or coenzyme A ester. In Pseudomonas aeruginosa, the cyclopropane fatty acid synthase is a stable homodimer that preferentially modifies phosphatidylglycerol lipid substrates, and gene expression is upregulated during the transition to stationary phase and in response to environmental stress. Escherichia coli is a further exception in that phosphatidylglycerol is the preferred substrate, and cyclopropanation occurs in many different lipids and in both positions of the glycerol moiety independently of the polar head group in the symbiotic soil bacterium Sinorhizobium meliloti.
In E. coli and the gastric pathogen Helicobacter pylori, one function of cyclopropane fatty acids is to reduce the permeability of the cell membranes, and so they protect from acid shock as can occur during passage of ingested organisms through the stomach. More generally, cyclopropanation regulates cell membrane fluidity, stability and permeability to protons, with effects upon bacterial growth, stress and antibiotic resistance; it is necessary for the virulence of Salmonella enterica and of Mycobacterium tuberculosis.
2. Fatty Acids with Terminal Ring Structures in Microorganisms
11-Cyclohexylundecanoic acid was first isolated as a minor component of butter fat, but it is almost certainly produced by bacteria in the rumen, which are later digested, and the fatty acids are released for uptake into the tissues of the host animals.
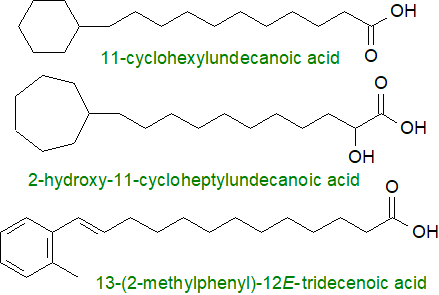 |
| Figure 3. Some fatty acids with terminal ring structures from bacteria. |
It has now been found together with homologous fatty acids in several bacterial species, including some that are tolerant of extreme environmental conditions. Certain Bacillus species produce 11-cyclohexylundecanoic and 13-cyclohexyltridecanoic acids normally, but they can synthesise fatty acids with terminal C4 to C7 rings, when provided with appropriate exogenous precursors. The biosynthetic mechanism for synthesis of the terminal cyclohexane ring involves conversion of sugars to shikimic acid and thence to cyclohexanecarboxylic acid, which serves as the primer for the fatty acid synthase.
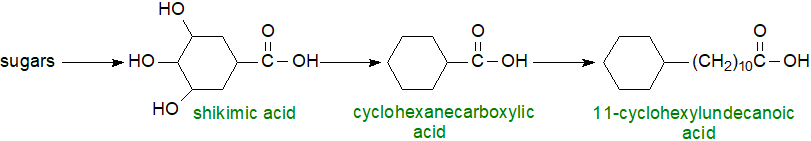 |
| Figure 4. Biosynthesis of 11-cyclohexylundecanoic acid |
Perhaps more surprising is the finding of a variety of ω-cycloheptyl fatty acids in bacteria of the genus Alicyclobacillus; 11‑cycloheptylundecanoic (and the 2‑hydroxy analogue) and 13‑cycloheptyltridecanoic acids, together with three minor homologues, comprise nearly 80% of the fatty acids of A. cycloheptanicus. Many aspects of the biosynthesis of such fatty acids are still unclear, but the expected precursor, cycloheptanecarboxylic acid, is produced in the organisms from phenyl acetic acid by a complex sequence of reactions. Four ω-phenyl fatty acids (with C10 to C14 saturated chains) are minor components of the lipids of a halophilic Bacillus species from Bulgaria, while 13-(2-methylphenyl)-12E-tridecenoic acid has been isolated from two Streptomyces sp.
In contrast, three highly unsaturated fatty acids with benzene rings located centrally in the chain, i.e., cinnamic acid analogues, have been identified in the pathogenic Gram-negative Chromobacterium violaceum. These act as signalling molecules to influence the differentiation and secondary metabolism of Gram-positive Streptomyces via a receptor that normally responds to γ-butenolide quorum signalling molecules.
Ladderane fatty acids: These are arguably the most unusual fatty acids in nature (first described by J.S.S. Damsté and colleagues, DOI), and they are present in 'anammox' bacteria of the phylum Planctomycetes, which have an important role in the nitrogen cycle of the ocean and derive their energy by oxidizing ammonia via the anaerobic combination of the substrates ammonia and nitrite into nitrogen gas in a specific organelle termed an anammoxosome. The molecular structures are composed of three to five linearly concatenated cyclobutanes attached to the terminal carbon of a short- to medium-chain fatty acid, or via a cyclohexane ring when there are three cyclobutane rings. They occur in the membranes of the organisms as components of glycerophospholipids, although related structures are found as alcohols or as alkylglycerols of various kinds, or with 5, 6 or 7‑membered rings attached to the fused cyclobutane rings. Studies of the NMR spectra showed that all the rings are fused by cis-ring junctions, resulting in a staircase-like arrangement of the fused cyclobutane rings, defined in the first example illustrated as a [5]‑ladderane or 20‑[5]‑ladderanoic acid; chemical syntheses have been achieved to facilitate study.

The biosynthetic mechanism for such highly strained and energy-rich structures is slowly being unravelled, and there is evidence that it may differ from any known pathway. So far, a distinctive acyl carrier protein (denoted as ‘amxACP’) and a variant of FabZ (‘amxFabZ’), the (3R)-3‑hydroxyacyl-acyl carrier protein dehydratase known from Type II fatty acid synthesis in bacteria, have been identified. amxACP is produced in a distinctive gene cluster (cluster I) that synthesises ladderane fatty acids, while a second gene cluster (cluster III) produces another acyl carrier protein (KsACPII) and is responsible for synthesis of canonical fatty acids such as palmitic. The high concentration of ladderane lipids in the anammoxosome (50% of the membrane lipids) produces a dense membrane with reduced permeability that may inhibit passive diffusion of protons across the membrane, a process that might otherwise be detrimental to the organisms, because of their relatively slow metabolism.
Identification of most terminally cyclic fatty acids is straightforward by gas chromatography-mass spectrometry as the 3‑pyridylcarbinol ester and pyrrolidide derivatives, although 4,4-dimethyloxazolines (DMOX) can been used when authentic spectra are available for comparison. The ladderane lipids present special problems, as they tend to isomerize with formation of 6‑membered rings when heated, as during analysis by gas chromatography-mass spectrometry. LC-MS with APCI or electrospray detection presents fewer problems and has enabled the identification of phospholipids containing both ladderane fatty acids and their ether analogues.
3. Cyclopropane and Cyclopropene Fatty Acids from Plants
A fatty acid containing a cyclopropene ring was first isolated from the seed oil of Sterculia foetida and was characterized as 8-(2-octyl-cyclopropen-1-yl)-octanoic acid (9,10-methylene-octadec-9-enoate or 'sterculic acid'). Shortly afterwards, a related fatty acid with one fewer carbon atom was characterized from a related seed oil, i.e., 7-(2-octyl-cyclopropen-1-yl)-heptanoic acid (9,10-methylene-heptadec-9-enoate or 'malvalic acid').

These two fatty acids have since been found in many seed oils from plant families of the order Malvales (Sterculiaceae, Malvaceae, Bombaceae and Tiliaceae), and they are they are now known to be present in leaves, roots and shoots. Generally, both fatty acids are present together in concentrations that vary up to 60% in seed oils, depending on species, and they are usually accompanied by small amounts of the cyclopropanoid analogues, i.e., dihydrosterculic and dihydromalvalic acids. Two further cyclopropenoid fatty acids have been characterized from seed oils, i.e., 9,10-methylene-octadec-9-en-17-ynoate or 'sterculynic acid' and 2‑hydroxysterculic acid, with the latter a possible intermediate in the bioconversion of sterculic to malvalic acid by an alpha-oxidation mechanism. Cyclopropene fatty acids are troublesome impurities in commercial cottonseed oil, Gossypium hirsutum, which contains 1.1% malvalic and 0.4% sterculic acids, and they must be removed by refining processes before the oil can be used in animal feed.
Cyclopropene fatty acids produce unwanted biological effects when ingested by animals by inhibiting desaturases, leading to an accumulation of saturated fatty acids, and it is possible that there is an irreversible reaction between the ring structures and thiol moieties on the enzymes. This has been noted in many animal models, but it is most troublesome in animal husbandry, and when poorly refined cottonseed oil is fed to laying hens, the resultant eggs have a rubbery texture and the white of the yolk can have a pink coloration. On the other hand, their ability to inhibit stearoyl-CoA desaturase may be of clinical value as an adjuvant in diseases such as cancer, non-alcoholic steatohepatitis and skin disorders, and they may have a protective role in retinal diseases such as age-related macular degeneration. As cyclopropene fatty acids inhibit desaturation during the synthesis of fatty acids and pheromones in insects and so may protect plants against insect attack, it is possible that they have evolved in plants for this purpose.
Much remains to be learned of the biosynthesis of cyclopropenoid fatty acids, but the mechanism begins with conversion of oleate to dihydrosterculate and thence by desaturation to sterculate. The cyclopropane synthase in S. foetida has much in common with the bacterial enzyme described above and catalyses the addition of a methylene group from S‑adenosylmethionine across the double bond. In this instance, the primary substrate for the enzyme is oleic acid in position sn-1 of phosphatidylethanolamine (rather than position sn-2 as in bacteria).
The cyclopropene ring is highly strained and is therefore very reactive; in particular, it reacts readily with thiol groups and other sulfur compounds, and one of the first tests for cyclopropene-containing oils was a pink coloration formed on solution in carbon disulfide (the Halphen test). The facile addition reaction with methanethiol illustrated has been used as an aid to mass spectrometric characterization.
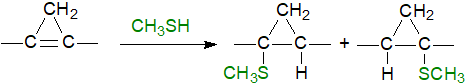 |
| Figure 5. Reaction of methanethiol with a cyclopropene ring. |
Acid-catalysed transesterification to prepare methyl ester derivatives brings about rapid destruction of the cyclopropene ring, but base-catalysed transesterification can be used safely. Silver nitrate reacts rapidly with cyclopropene fatty acids, so silver ion chromatography cannot be employed for isolation purposes, although the reaction has been used as an aid to structural analysis by mass spectrometry.
The cyclopropanoid dihydrosterculic acid is the major carbocyclic fatty acid in the seed oils of Litchi chinensis and Euphorbia longana, while lactobacillic acid is a substantial component of the seed oil of Byrsocarpus coccineus (accompanied by small amounts of two branched-monoenes that may be intermediates in its biosynthesis). (1S,2S)-trans- and (1S,2R)-cis-2-octylcyclopropyl-1-carboxylic acids are the main odorants in frankincense, a resin from the bark of Boswellia species.
In the early days of gas chromatography when packed columns were standard, cyclopropenoid fatty acids decomposed on the columns and could not be analysed directly. With modern wall-coated open-tubular columns, this is not a problem and quantitative analysis as the methyl esters can be accomplished by GC, and reversed-phase HPLC is a useful alternative. Identification of cyclopropenoid fatty acids is nowadays straightforward by gas chromatography-mass spectrometry in the form of the 3‑pyridylcarbinol ester, and DMOX or pyrrolidide derivatives.
4. Fatty Acids with Other Ring Structures from Plants
ω-Cyclopentenyl fatty acids: Fatty acids with a terminal cyclopent-2-enyl ring are found in seed oils from many species of the plant family Flacourtiaceae. 'Chaulmoogra' oil is extracted from seeds of Hydnocarpus (Taraktogenus) kurzii and is used in folk medicine as a treatment for leprosy. The three most common fatty acids of this type are 11-cyclopent-2-enyl-undecanoic (hydnocarpic), 13-cyclopent-2-enyl-tridecanoic (chaulmoogric) and 13‑cyclopent-2-enyltridec-6-enoic (gorlic) acids. All have the (R)-(+) stereochemistry at carbon 1 of the cyclopent-2-enyl ring.
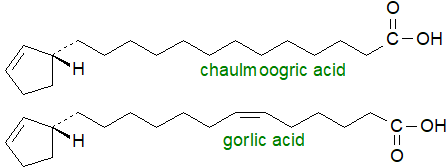
15-Cyclopent-2-enylpentadec-9-enoic (hormelic) acid is a minor component of most oils of this type, while 13-cyclopent-2-enyltridec-4-enoic acid is the main component of the seed oil of H. anthelmintica, which also contains trace levels of 11‑cyclopentylundecanoic acid. These fatty acids are found in all lipid classes in the seeds, but mainly in the triacylglycerols. Although little is known of the biosynthetic pathway for the biosynthesis of these fatty acids, the evidence points to cyclopentenylglycine as the precursor of cyclopent-2-enylcarboxylic (aleprolic) acid, which is then subjected to chain-elongation and desaturation by conventional enzyme systems.
ω-Phenyl fatty acids: Seed oils of the Araceae (Aroid) family, such as Arum maculatum, contain fatty acids with terminal phenyl moieties. 13‑Phenyltridecanoic acid is the major component, accompanied by C17 and C21 homologues and analogous fatty acids with double bonds in position 9 of the alkyl chain (similar fatty acids have been found in a few bacterial species (see above)). They are readily characterized by mass spectrometry and nuclear magnetic resonance spectroscopy.

Phenylpropanoids, such as coumaric, ferulic acids and caffeic illustrated, are part of a diverse family of organic acids that are synthesised by plants from the amino acids phenylalanine and tyrosine via cinnamic acid as an intermediate. They are common constituents of plant surface waxes, and they are one of the building blocks of lignin, often forming a covalent bridge to cellulose, and of sporopollenin. Chlorogenic acid, an ester of caffeic acid with quinic acid, is a constituent of fruits and vegetables, including coffee, with antioxidant and potential pharmaceutical properties. A different type of cyclic fatty acid, the resorcinolic acids, are formed in plants by polyketide synthases. The simplest and best known of these is salicylic acid or aspirin, a key plant hormone (as well as a pharmaceutical), but the anacardic acids or 2-alkyl-6-hydroxybenzoic acids from cashew nuts have long-chain 'tails' derived biosynthetically by condensation of the CoA esters of normal fatty acids (16:0, 18:0 and 16:1(n-5) to 24:1(n-5)) with polyketide intermediates. Related fatty acids from the leaves of Ginkgo biloba are known as ginkgolic acids and are highly toxic.

There are many more related acidic and non-acidic metabolites, including alkylphenols, alkylcatechols, alkylresorcinols, cardol, cardanol, olivetolic acid and urushiol. Many of these phenolic compounds are plant defence agents by participating in plant signalling to drive immune responses and acting as a physical or chemical barrier to prevent invasion. The unsaturated anacardic acids confer resistance against insect pests, bacteria and fungi, while an unusual phenylpropanoid diacylglycerol, 1‑O‑p‑coumaroyl-3-O-feruloylglycerol, is associated with rust resistance in wheat. In insects, anacardic acids cause infertility by inhibiting the biosynthesis of prostaglandins and other eicosanoids.
5. Cyclic Fatty Acids in Animals
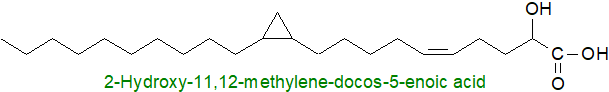
Cyclic fatty acids have been reported from several marine invertebrates, and for example, 19,20-methylene-hexacosanoic acid is a minor component of the sponge Calix niceaensis. The marine sponge Plakortis simplex contains unusual prenylated galactosylceramides (plakosides) in which both the fatty acid and long-chain base components contain a cis-cyclopropane ring in position 11 of the chain, i.e., 2‑hydroxy-11,12-methylene-docos-5-enoate. Although the biosynthetic origin of many sponge fatty acids is obscure, it is known that some can arise from further metabolism of ingested microbial lipids, and many of the lipid components in this species are derived from symbiotic bacteria. For example, a double bond in position 5 is typical of sponges (demospongic acids), and it would be surprising if this feature was not introduced within the tissues of the animal. The same may be true of comparable fatty acids from the fresh-water invertebrate, Acathogammarus grewingkii, i.e., cis-11,12-methylene-5-eicosenoate, cis,cis-11,12-14,15-bis-methylene-5-eicosenoate and their homologues.
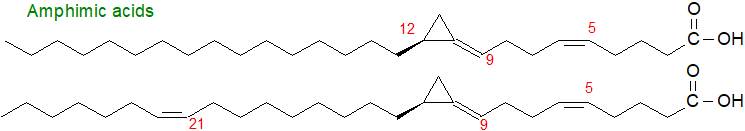
Unusual C27 to C30 unsaturated fatty acids have been isolated from an Australian sponge, Amphimedon sp., which are distinctive in possessing a cyclopropylidene group and have been termed 'amphimic acids', two of which are illustrated below. Again, apart from the ring structure they are obviously closely related to the demospongic acids.
Several eicosanoids (lactones) with cyclopropane rings adjacent to an oxygenated substituent have been isolated from
corals, other marine invertebrates and red algae.
In these organisms, the cyclopropane ring is of the trans configuration, and the biosynthetic mechanism differs from that for
more conventional cyclopropyl fatty acids.
Unusual cyclopropyl fatty acids with trans ring structures in the 4,5‑position (and with iso- and anteiso-methyl
branches) have been isolated from the sponge Pseudospongosorites suberitoides, and once more, it is possible that they originate in
cyanobacteria that are symbiotic with the sponge.
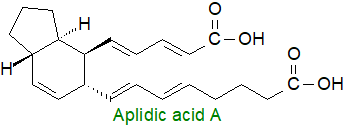 Marine colonial tunicates such as Didemnum sp. contain α,ω‑dicarboxylic acids
with a centrally located bicyclic structure (hexahydro-indene) that have been termed 'aplidic acids'.
Marine colonial tunicates such as Didemnum sp. contain α,ω‑dicarboxylic acids
with a centrally located bicyclic structure (hexahydro-indene) that have been termed 'aplidic acids'.
While 9,10-methylene-hexadecanoic acid has been found at low levels in some bovine tissues, e.g., the heart, and traces of this this and other isomers/homologues, including odd-chain components are present in human plasma and adipose tissue, it seems likely that they have their origin in bacteria at some point in the food chain, e.g., during silage production for ruminants, or in the intestinal microbiome (see the cyclohexyl fatty acids above). Cyclopropyl fatty acids can bind to peroxisome proliferator activated receptors (PPAR) in a similar manner to monoenoic analogues and so influence lipid metabolism in host animals, although it has been suggested that further work is necessary to validate these findings in human systems and in animal models of disease. cis-3,4-Methylene-heptanoylcarnitine is a relatively abundant medium-chain acylcarnitine in human blood and may be to be a product of incomplete beta-oxidation of long-chain cyclopropyl fatty acids. Cyclopropanoid fatty acids found in nematodes probably originate in bacteria in their diet.
Recommended Reading
- Almahli, H. Cyclopentenyl fatty acids: history, biological activity and synthesis. Curr. Topics Med. Chem., 17, 2903-2912 (2017); DOI.
- Badami, R.C. and Patil, K.B. Structure and occurrence of unusual fatty acids in minor seed oils. Prog. Lipid Res., 19, 119-153 (1981); DOI.
- Bao, X., Thelen, J.J., Bonaventure, G. and Ohlrogge, J.B. Characterization of cyclopropane fatty-acid synthase from Sterculia foetida. J. Biol. Chem., 278, 12846-12853 (2003); DOI.
- Biala, W. and Jasinski, M. The phenylpropanoid case - it is transport that matters. Front. Plant Sci., 9, 1610 (2018); DOI.
 Buist, P.H.
Exotic biomodification of fatty acids.
Nat. Prod. Rep., 24, 1110-1127 (2007); DOI.
Buist, P.H.
Exotic biomodification of fatty acids.
Nat. Prod. Rep., 24, 1110-1127 (2007); DOI.- Christie, W.W. 13-Phenyltridec-9-enoic and 15-phenylpentadec-9-enoic acids in Arum maculatum seed oil. Eur. J. Lipid Sci. Technol., 105, 779-780 (2001); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Debédat, J., Pastor, L., Knotts, T.A., Pitman, J.G., Griffett, K. and Adams, S.H. Cyclopropane xenolipids resemble monounsaturated fatty acids and modulate peroxisome proliferator-activated receptors. J. Lipid Res., 66 100896 (2025); DOI.
- Destaillats, F. and Angers, P. On the mechanisms of cyclic and bicyclic fatty acid monomer formation in heated edible oils. Eur. J. Lipid Sci. Technol., 107, 767-772 (2005); DOI.
- Geng, J. and others. Current status of cyclopropane fatty acids on bacterial cell membranes characteristics and physiological functions. Microbial Path., 200, 107295 (2025); DOI.
- Laroche, M., Imperatore, C., Grozdanov, L., Costantino, V., Mangoni, A., Hentschel, U. and Fattorusso, E. Cellular localisation of secondary metabolites isolated from the Caribbean sponge Plakortis simplex. Marine Biol., 151, 1365-1373 (2007); DOI.
- Moss, F.R., Shuken, S.R., Mercer, J.A.M., Cohen, C.M., Weiss, T.M., Boxer, S.G. and Burns, N.Z. Ladderane phospholipids form a densely packed membrane with normal hydrazine and anomalously low proton/hydroxide permeability. Proc. Natl. Acad. Sci. USA, 115, 9098-9103 (2018); DOI.
- Pelaez, R., Pariente, A., Perez-Sala, A. and Larrayoz, I.M. Sterculic acid: the mechanisms of action beyond stearoyl-CoA desaturase inhibition and therapeutic opportunities in human diseases. Cells, 9, 140 (2020); DOI.
- Sébédio, J.L. and Grandgirard, A. Cyclic fatty acids: natural sources, formation during heat treatment, synthesis and biological properties. Prog. Lipid Res., 28, 303-336 (1989); DOI.
For tutorials on mass spectral analysis of fatty acids - see our mass spectrometry pages.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
