Glycosyldiacylglycerols
The glycosyldiacylglycerols of higher plants, yeasts and many lower organisms are membrane constituents with innumerable vital functions. In many ways, they are equivalent to and may substitute for phosphoglycerolipids, especially when the supply of phosphorus is restricted, as they have a common 1,2‑diacyl-sn-glycerol backbone but with polar carbohydrate rather than phosphate moieties in position sn-3. As mono- and digalactosyldiacylglycerols together with sulfoquinovosyldiacylglycerols are key components of chloroplasts, they are essential for life on Earth with an intimately part in the process of photosynthesis, while maintaining chloroplast morphology and responding to abiotic stresses. Related lipids are cell wall constituents of bacteria, and they are the anchor element of the lipoteichoic acids. Together, they are probably the most abundant lipid classes in the biosphere. Other than the sulfolipid seminolipid, glycosyldiacylglycerols are minor components of animal tissues, where they have been somewhat neglected by scientists.
1. Mono- and Digalactosyldiacylglycerols from Plants
Monogalactosyldiacylglycerols and digalactosyldiacylglycerols (together with the plant sulfolipid and phosphatidylglycerol) are the main lipid components of the various membranes of chloroplasts and related organelles. The predominant structures are 1,2-di-O-acyl-3-O-β-D-galactopyranosyl-sn-glycerol and 1,2-di-O-acyl-3-O-(α-D-galactopyranosyl-(1→6)-O-β-D-galactopyranosyl)-sn-glycerol, i.e., the galactose of monogalactosyldiacylglycerols is in the β-anomeric configuration to diacylglycerol, whereas the second galactose in the digalactosyldiacylglycerols is in the α-anomeric configuration. These structures are conserved from cyanobacteria to green algae to vascular plants.
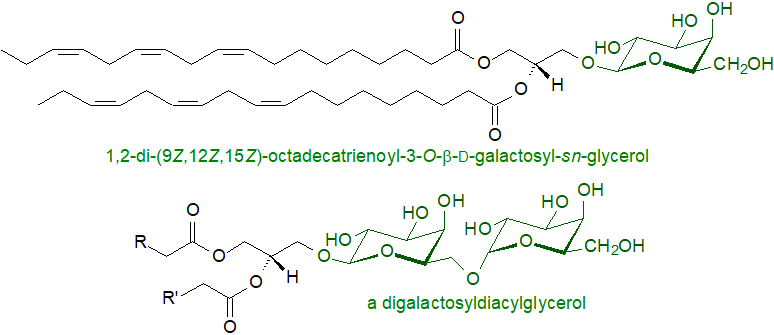 |
| Figure 1. Representative structures of mono- and digalactosyldiacylglycerols. |
Galactosyldiacylglycerols ('galactolipids') are the most abundant lipids in all photosynthetic tissues, including those of higher plants, algae and certain bacteria, and mono- and digalactosyldiacylglycerols amount to 27% and 31%, respectively, of spinach chloroplast glycerolipids (or 50 and 30%, respectively, of the lipids in thylakoids), where they are accompanied by 6% sulfoquinovosyldiacylglycerol and 9% phosphatidylglycerol. In photosynthetic tissues, monogalactosyldiacylglycerols are located exclusively in plastid membranes, but digalactosyldiacylglycerols can be present in extra-plastid membranes under some conditions, including the plasma membrane where they are located on the inner leaflet. Although they do not occur in plant mitochondrial membranes under normal growth conditions, digalactosyldiacylglycerols can accumulate until they amount to 18% of the total during phosphate deprivation. In non-photosynthetic tissues of plants, the proportion of these glycosyldiacylglycerols is usually much lower, although flowers contain appreciable amounts, and in pollen, galactolipids are concentrated in the plasma membrane. The relative proportions of the two galactolipids and the ratio of galactolipids to phospholipids are stable when plants are grown under favourable conditions, but they can change markedly when these are subjected to stress.
In higher plants, the galactolipids of photosynthetic tissues contain a high proportion of polyunsaturated fatty acids, up to 95% of which in some genera can be α‑linolenic acid (18:3(n-3)), and the most abundant molecular species of mono- and digalactosyldiacylglycerols have 18:3 at both sn-1 and sn-2 positions of the glycerol backbone. In microalgae of marine origin, these lipids can contain more highly unsaturated fatty acids, including eicosapentaenoic (20:5(n-3)) and docosahexaenoic (22:6(n-3)) acids.
Plants such as the pea, which have 18:3 as almost the only fatty acid in the monogalactosyldiacylglycerols, have been termed "18:3 plants". Other species, with the 'model' plant Arabidopsis thaliana as an example, contain appreciable amounts of 7,10,13-hexadecatrienoic acid (16:3(n‑3)) in the monogalactosyldiacylglycerols, and they are termed "16:3 plants". A further distinctive feature is that 16:3 is located entirely at the sn-2 position of the glycerol backbone (see Table 1). Palmitic acid tends to be found mainly in digalactosyldiacylglycerols, usually in small amounts and then in position sn-1, although the positional distribution varies somewhat. In non-photosynthetic tissues, such as tubers, roots or seeds, the Cl8 fatty acids are usually more saturated in that they tend to contain more linoleate (18:2(n-6)) (cf., the data for wheat flour lipids).
Table 1. Composition (mol %) of fatty acids in positions sn-1 and sn-2 of mono- and digalactosyldiacylglycerols and of sulfoquinovosyl-diacylglycerols from leaves of A. thaliana and from wheat flour. |
||||||
| Position | Fatty acids | |||||
|---|---|---|---|---|---|---|
| 16:0 | 16:3(n‑3) | 18:0 | 18:1 | 18:2 | 18:3(n‑3) | |
| Arabidopsis thaliana [1] | ||||||
| MGDG | ||||||
| sn-1 | 2 | 1 | trace | trace | 4 | 93 |
| sn-2 | trace | 70 | trace | trace | 1 | 28 |
| DGDG | ||||||
| sn-1 | 15 | 2 | trace | 2 | 3 | 76 |
| sn-2 | 9 | 3 | trace | trace | 4 | 83 |
| SQDG | ||||||
| sn-1 | 23 | - | 2 | 2 | 6 | 68 |
| sn-2 | 63 | - | trace | 2 | 3 | 32 |
| Wheat flour [2] | ||||||
| MGDG | ||||||
| sn-1 | 11 | - | 1 | 5 | 81 | 1 |
| sn-2 | trace | - | trace | 9 | 83 | 7 |
| DGDG | ||||||
| sn-1 | 26 | - | 2 | 4 | 63 | 4 |
| sn-2 | 2 | - | trace | 7 | 83 | 7 |
| [1] Browse, J. et al. Biochem. J., 235, 25-31
(1986); DOI. [2] Arunga, R.O. and Morrison, W.R. Lipids, 6, 768-776 (1971); DOI. |
||||||
"Eukaryotic versus prokaryotic": Galactolipids have been classified into two groups based on their structures. One has mainly C18 fatty acids at the sn-1 position of the glycerol backbone, and only C16 fatty acids, such as 16:3(n-3), at the sn-2 position, and it has been termed a "prokaryotic" structure (as it is characteristic of cyanobacteria (Table 3) and an erroneous assumption that the prokaryotic pathway in plastids has originated from an endosymbiotic event). The other class has C16 or C18 fatty acids at the sn-1 position but only C18 fatty acids, especially 18:3(n‑3), in the sn-2 position, and this has been termed a "eukaryotic" structure as it is present in most glycerolipids, including the phospholipids, of all eukaryotic cells. The exception is phosphatidylglycerol, which is synthesised in chloroplasts via the "prokaryotic" pathway only. Some plants contain both "eukaryotic" and "prokaryotic" structures in the monogalactosyldiacylglycerols, and in fact, Arabidopsis has roughly equal amounts synthesised by each pathway. However, it is now known that the terms "eukaryotic/prokaryotic" are misnomers, as genomic studies have shown that the two steps of acylation in cyanobacteria and chloroplasts utilize enzymes that have no phylogenetic relationship. The structural differences in the diacylglycerol moieties of galactolipids from various algae and higher plants originate in compartmentalization of the biosynthetic pathways or precursors within cells between the chloroplasts and endoplasmic reticulum, each compartment having its own distinctive enzymes (as discussed next).
2. Biosynthesis of Glycosyldiacylglycerols in Plants
Chloroplasts or plastids are a major site for lipid biosynthesis in plants. These are organelles enclosed by an envelope consisting of a double membrane with outer and inner layers surrounding an intermembrane space. A further internal membrane, the thylakoid membrane, is arranged in tight stacks or grana, connected by stromal lamellae, and surrounded by an aqueous region filled with stroma, a matrix containing chlorophyll, soluble enzymes and starch granules. Like mitochondria, chloroplasts contain their own DNA separate from the cell nucleus, an argument in favour of their evolution from an ancient cyanobacterium engulfed by an early eukaryotic cell. The major function of chloroplasts and the thylakoid membrane in photosynthesis is discussed below.
| Figure 2. Representative structure of a chloroplast and thylakoid membrane. Reproduced under a Creative Commons License from Kelvinsong and Wikipedia. |
Fatty acid biosynthesis and metabolism and glycerolipid biosynthesis are intimately connected. In A. thaliana, production of chloroplast lipids begins with fatty acid synthesis in the chloroplast stroma by a multi-subunit fatty acid synthase. Ultimately, some acyl-carrier protein-bound 16:0 is released as such, but most is extended to form 18:0, which is desaturated to 18:1 by a stearoyl-ACP desaturase. In the chloroplast envelope, 16:0-ACP and 18:1-ACP newly synthesised in the plastid are utilized for production of phosphatidic acid and subsequently via phosphatidic acid phosphatase of 1,2‑diacyl-sn-glycerols, which contain only oleic acid in position sn-1 and palmitic acid in position sn-2. This is achieved by the stepwise action of two acyltransferases, ATS1 (GPAT or glycerol-phosphate acyltransferase) in the stroma and ATS2 (LPAAT or lyso-phosphatidic acid acyltransferase) in the inner envelope membrane of the chloroplast. The palmitic acid in position sn-2 then serves as a substrate for desaturases to produce 16:3.
Fatty acids are released from the acyl-carrier protein as CoA esters by acyl-ACP thioesterases and these are in turn hydrolysed to free acids for transport across the chloroplast membranes with the aid of the FAX1 fatty acid export protein (and probably other transporters) to the cytosol for re-conversion to CoA esters for phosphatidylcholine synthesis in the endoplasmic reticulum. In the endoplasmic reticulum pathway for galactosyldiacylglycerol synthesis, this phosphatidylcholine is utilized as the precursor to yield 1,2-diacylglycerols with C18 fatty acids in position sn-2 and a C18 or C16 fatty acid in position sn-1. There is extensive trafficking of phosphatidylcholine, diacylglycerols, and fatty acids (and possibly of phosphatidic acid and lysophospholipids) between the various cellular compartments, requiring active transport mechanisms across the cytoplasm or via contact sites between the chloroplast envelope and the endoplasmic reticulum, and there may also be transport of lipids via contact sites between mitochondria and chloroplasts and between the inner and outer chloroplast membranes. Phosphatidylcholine is a minor component of the chloroplast membranes, but it must originate in the ER as there is no synthesis in the chloroplast.
The acyl moieties of the precursors and products are desaturated in situ by fatty acid desaturases (FAD5, 6, 7 and 8 in the chloroplast and FAD2 and 3 in the ER) to produce the eventual fatty acid and molecular compositions (see our web page on biosynthesis of plant polyunsaturated fatty acids). Thus, the final galactolipid compositions are governed by the relative activities of the various enzyme systems in different cellular organelles and the rates of exchange between each.
The basic biochemical mechanisms of galactolipid synthesis require the synthesis of 1,2-diacyl-sn-glycerols either by dephosphorylation of phosphatidic acid in the chloroplasts (so-called "prokaryotic" diacylglycerols) or of phosphatidylcholine, produced by the complex traffic of fatty acids from the chloroplast to the endoplasmic reticulum and then back to the chloroplast (so-called "eukaryotic" diacylglycerols), the latter from phosphatidic acid generated via the action of a phospholipase D or of phosphatidic acid phosphatase. In A. thaliana, the chloroplast phosphatases LPPγ and LPPε1 hydrolyse extraplastidic phospholipids to phosphatidic acid. There are indeed two biosynthetic pathways, but the synthesis of specific molecular species of lipids in each can be explained by the specificities of the two acyltransferases producing the phosphatidic acid precursor in the chloroplasts and the endoplasmic reticulum (ER). The "eukaryotic/prokaryotic" terminology should now be discarded, and the pathways named from the organelle in which the lipids are synthesised (the old terms are so entrenched that this may take some time). The biosynthetic route to galactolipids in eukaryotes has strongly diverged from that in extant cyanobacteria, which have a very different biosynthetic mechanism (see below).
 |
| Figure 3. Biosynthesis of mono- and digalactosyldiacylglycerols in plants. |
A monogalactosyldiacylglycerol synthase, located in the inner envelope membrane of the chloroplast, must first be activated by phosphatidic acid, a key signalling molecule in plants, although phosphatidylglycerol may have a role. This enzyme catalyses the reaction between the diacylglycerols and uridine 5‑diphosphate(UDP)-galactose (produced in the cytoplasm) to form monogalactosyldiacylglycerols. In the outer envelope membrane, a further enzyme system catalyses the addition of another galactose unit from UDP-galactose to produce digalactosyldiacylglycerols. It is noteworthy that the MGDG synthase changes the configuration of the α‑galactose in UDP-galactose to the β‑form, but the DGDG synthesis preserves the α‑configuration.
There are now known to be three different sets of lipid galactosyltransferases or monogalactosyldiacylglycerol synthases that catalyse the first galactosylation step in the plastid envelope of A. thaliana. The first, designated MGD1, is an inner envelope membrane-associated protein of chloroplasts responsible for most galactolipid biosynthesis in green tissues, and it is indispensable for the biogenesis of thylakoid membranes and for embryogenesis. Under conditions of phosphate limitation and in non-photosynthetic tissues such as roots and pollen, two further isoforms (MGD2 and MGD3) located in the outer envelope of plastids are more active, but these are not needed for chloroplast biogenesis or plant development when there is sufficient nutrient.
There are two digalactosyldiacylglycerol synthases, DGD1 and DGD2; the first is responsible for most digalactosyldiacylglycerol synthesis under normal conditions, while DGD2 is more important and is upregulated during phosphate deficiency and supplies this lipid as a substitute for phospholipids in extraplastidial membranes. As DGD1 is located on the chloroplast outer membrane, the precursor monogalactosyldiacylglycerols must either be transported across the membrane or be synthesised by MGD2, and it has been established that the N‑terminal sequence of DGD1 is essential to this process and indeed for the integration of the chloroplast galactolipid synthesis machinery within the plant cell. Transfer of digalactosyldiacylglycerols into mitochondria during phosphate deprivation probably occurs at a contact site in the endoplasmic reticulum, possibly after remodelling of glycerolipids in the tonoplast membranes, which contain a phospholipase D.
As discussed briefly above, many plants, including Arabidopsis, tomato, tobacco and spinach, which have been the subject of most experimental study, may contain either 16:3 or 18:3 acyl moieties in position sn-2 of MGDG, with the implication that phosphatidic acid synthesised in both plastids and the endoplasmic reticulum is directed towards MGDG biosynthesis. The '16:3 plants' in contrast to '18:3 plants', such as legumes and monocots (e.g., grasses), which have 18:3 only at position sn-2 of MGDG, are presumably derived from phosphatidic acid synthesised only in the endoplasmic reticulum. As the digalactosyldiacylglycerols contain very little 16:3, the DGDG synthase must utilize specific molecular species of monogalactosyldiacylglycerols as substrates.
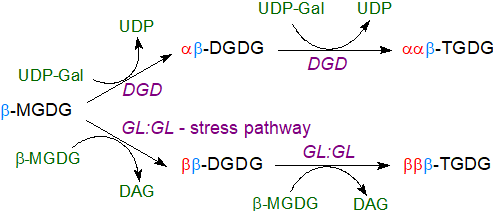
Trigalactosyldiacylglycerols have been found in pumpkins and potatoes, and tri- and tetragalactosyldiacylglycerols occur in oats and rice bran. There are two biosynthetic mechanisms that can be distinguished by the anomeric configuration of the additional galactose unit (and have differing evolutionary origins). Successive galactosylations by the DGDG synthase (DGD) generate oligogalactolipids exclusively of the α‑configuration, and these are constitutive lipids found mainly in non-vegetative tissues.
An alternative pathway for the biosynthesis of di- and oligogalactosyldiacylglycerols in the outer chloroplast membrane does not use UDP-galactose as the donor, but rather a transferase that transfers galactose from one galactolipid to another with concomitant formation of diacylglycerols, i.e., it is a galactolipid:galactolipid galactosyltransferase (GL:GL) with the capacity to produce tri- and tetragalactosyldiacylglycerols. This process is a factor in freezing tolerance as it commences only after the onset of freezing stress. In this mechanism, the digalactosyldiacylglycerol produced is different in respect of the glycosidic linkage, i.e., ββ‑DGDG rather than αβ-DGDG, while trigalactosyldiacylglycerols have the βββ-TGDG structure, and βαβ‑oligoglycolipids can be generated in some circumstances.
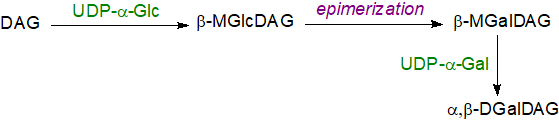 |
| Figure 4. Biosynthesis of oligoglycosyldiacylglycerols in plants. |
Mono- and digalactosylmonoacylglycerols (lyso derivatives) are found from time to time in small amounts in plant tissues. Usually, the sn‑1 isomer is identified, but acyl migration could occur quickly to give this, the more thermodynamically stable isomer. It is not clear whether these lyso-compounds play a part in galactolipid turnover and fatty acid re-modelling.
Catabolism: Acylhydrolases are present in plants that rapidly remove fatty acids from both positions of galactolipids, and α- and β‑galactosidases complete the breakdown. Most of the acylhydrolases ('patatin'-like) can hydrolyse phospholipids also, although at least one is selective for galactolipids. Two isoforms of a non-specific phospholipase C from plants are known that can hydrolyse mono- and digalactosyldiacylglycerols, as well as phospholipids, with generation of 1,2‑diacyl-sn-glycerols, i.e. AtNPC6 (A. thaliana) and OsNPC1 (Oryza sativa).
3. Other Non-acidic Glycosyldiacylglycerols from Plants
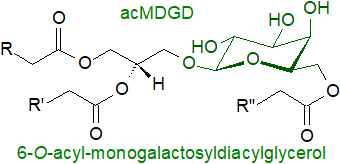 Head-group and oxylipin acylation:
Monogalactosyldiacylglycerols in which the galactose unit is acylated,
usually in position 6, and sometimes accompanied by acylated digalactosyl analogues have been detected in green tissues of all the main groups
of land plants, but they are most abundant under abiotic stress conditions, such as mechanical wounding,
bacterial infection and freezing/thawing.
The new fatty acid can be one normally present in these lipids or it can be a plant oxylipin,
such as 12‑oxo-10,15c-phytodienoic acid (12-oxo-PDA or 'OPDA') or dinor-OPDA.
Although the purpose of these acylated forms has still to be determined, it is now apparent that head-group acylation of mono- and
digalactosyldiacylglycerols is a common stress response in plants, but the nature of the additional fatty acid is
determined by the stress condition with conventional fatty acids more common during freezing and oxylipins on wounding or bacterial infection.
Under such conditions, acylphosphatidylglycerol can be formed but to a lesser extent.
Head-group and oxylipin acylation:
Monogalactosyldiacylglycerols in which the galactose unit is acylated,
usually in position 6, and sometimes accompanied by acylated digalactosyl analogues have been detected in green tissues of all the main groups
of land plants, but they are most abundant under abiotic stress conditions, such as mechanical wounding,
bacterial infection and freezing/thawing.
The new fatty acid can be one normally present in these lipids or it can be a plant oxylipin,
such as 12‑oxo-10,15c-phytodienoic acid (12-oxo-PDA or 'OPDA') or dinor-OPDA.
Although the purpose of these acylated forms has still to be determined, it is now apparent that head-group acylation of mono- and
digalactosyldiacylglycerols is a common stress response in plants, but the nature of the additional fatty acid is
determined by the stress condition with conventional fatty acids more common during freezing and oxylipins on wounding or bacterial infection.
Under such conditions, acylphosphatidylglycerol can be formed but to a lesser extent.
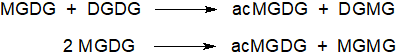
A phylogenetically conserved enzyme has been identified as responsible for the accumulation of acyl-monogalactosyldiacylglycerols in A. thaliana, i.e., a cytosolic protein closely associated with the chloroplast outer membrane and termed 'acylated galactolipid associated phospholipase 1' (AGAP1). Acylated monogalactosylmonoacylglycerol (acMGDG) is formed mainly by transfer of a glycerol-linked fatty acid of DGDG to the galactose of MGDG, producing digalactosylmonoacylglycerol (DGMG) as a by-product, although some acMGDG can be produced by transfer of an acyl group from one MGDG to the galactose of another with formation of monogalactosylmonoacylglycerol (MGMG) as the by-product. The fatty acid group can be transferred from either position sn‑1 or sn‑2 of the glycerol moiety with a slight preference for position sn‑1.
Oxylipins such as OPDA are found esterified to positions sn-1 and 2 of the glycerol moiety of mono- and digalactosyldiacylglycerols and sometimes to galactose in Arabidopsis and termed 'arabidopsides', while similar lipids containing divinyl ether fatty acids and termed 'linolipins' occur in other plants, but they are discussed further in our web page on plant oxylipins.
Other less-common forms: A digalactosyldiacylglycerol from plants but with an alpha rather than a beta linkage between the two galactopyranosyl units has been found in Launaea capitata (Asteraceae) and was reported to be anti-inflammatory and neuroprotective in animal cells in vitro, while in other species, environmental stresses can induce the accumulation of digalactosyldiacylglycerols in which both the galactose units have the β‑configuration. 1,2-Di-O-acyl-3-O-β-D-glucopyranosyl-sn-glycerol has been found in rice bran, but not in the chloroplasts, where it occurs with the corresponding galactolipids in an approximate ratio of 1:2. Interestingly, the two forms differ appreciably in their fatty acid compositions.
Triglycosyldiacylglycerols containing a high proportion of glucose have been reported from rice, but the structures do not appear to have been confirmed definitively. Although glucosyldiacylglycerols have been found in some other plants, they are always rather minor components. An interesting oligoglycosyl glycerolipid has been found in mung beans with a terminal rhamnose unit and unusually an alkyl group in position sn-2 (in animal glycerolipids, the ether moiety is invariably in position sn-1 - see our web pages on Ether lipids). An unusual galactoglycerolipid with phytol ether-linked to position 1 of glycerol has been partially characterized from algae and cyanobacteria, and it may occur at trace levels in some higher plants.
 Oat
seeds contain a novel form of digalactosyldiacylglycerol with an estolide linkage, i.e., in which 15-hydroxylinoleic acid is esterified to
position sn-2 of the glycerol moiety, and the hydroxyl group of the fatty acid is esterified with linoleic acid.
Further tri- and tetragalactosyldiacylglycerols with up to three estolide-linked fatty acids have now been identified.
Oat
seeds contain a novel form of digalactosyldiacylglycerol with an estolide linkage, i.e., in which 15-hydroxylinoleic acid is esterified to
position sn-2 of the glycerol moiety, and the hydroxyl group of the fatty acid is esterified with linoleic acid.
Further tri- and tetragalactosyldiacylglycerols with up to three estolide-linked fatty acids have now been identified.
Seaweeds (multicellular brown/red algae) contain the conventional range of galactolipids, including sulfoquinovosyldiacylglycerol discussed below, though often with distinctive fatty acid compositions as might be expected of marine organisms, and some red algae, such as Gracilaria sp., contain glycosyldiacylglycerols that are highly enriched in arachidonic acid, i.e., 57% of the 20:4/20:4 molecular species and 18% of the 16:0/20:4 combination in the monogalactosyldiacylglycerols. In addition, some algae contain monoglucopyranosyldiacylglycerols. The phytoplankton Chrysochromulina polylepis contains monogalactosyldiacylglycerol linked via the sugar moiety and an ester bond to a chlorophyll pigment, while one marine alga contains an aminoglycoglycerolipid, avrainvilloside or sn‑1,2‑dipalmitoyl-3-(N-palmitoyl-6'-desoxy-6'-amino-α-D-glucosyl)-glycerol, and the non‑N‑acylated form.
4. Sulfoquinovosyldiacylglycerol and Other Acidic Glycosyldiacylglycerols in Plants
Sulfoquinovosyldiacylglycerol (SQDG) or 1,2-di-O-acyl-3-O-(6'-deoxy-6'-sulfo-α-D-glucopyranosyl)-sn-glycerol (quinovose = 6‑deoxyglucose), the plant sulfolipid, is the single glycolipid most characteristic of all photosynthetic organisms, including higher plants, algae, chloromonads and cyanobacteria. It is a sulfonolipid (carbon-sulfur bond) as opposed to a lipid sulfate such as seminolipid discussed below. In contrast to the neutral galactosyldiacylglycerols, it is an anionic lipid with a negative charge on the head group like phosphatidylglycerol for which it can partly compensate when synthesis of the latter is inhibited (and vice versa). It is most abundant in the photosynthetic tissues where it is part of the photosystem II (PSII), four molecules per monomer, and cytochrome b6f complexes, and in the PSII from the cyanobacterium Thermosynechococcus elongatus, two molecules of SQDG are located at the monomer-monomer interface where they stabilize the dimeric structure. Together with phosphatidylglycerol, it is required to maintain the anionic charge in the thylakoid membrane during photosynthesis.

In higher plants, the concentration of SQDG is very variable (2% or so of the total glycerolipids), but the proportion in the thylakoid membranes is higher at ~7%. In many, including A. thaliana (Table 1), the sn-1 position is enriched in 18:3 and the sn-2 position in 16:0, a very different pattern from the mono- and digalactosyldiacylglycerols (or from the phospholipids), but it is noteworthy that there is no 16:3. Trace levels of monoacyl (lyso or SQMG) analogues have been detected in parsley and spinach leaves. In cyanobacteria, the comparable compositions differ appreciably (see Table 3 below).
Biosynthesis of the sulfoquinovose head-group utilizes a unique set of enzymes that have no other purpose in plants. While much remains to be learned regarding the details of the biosynthetic pathway, it involves synthesis of UDP-sulfoquinovose from UDP-glucose and sulfite by UDP-SQ synthase (SQD1), a soluble enzyme in the chloroplast stroma, followed by the transfer of sulfoquinovose to position sn-3 of 1,2-diacyl-sn-glycerols by SQDG synthase (SQD2) in the chloroplast inner envelope membrane (where the anionic phospholipid phosphatidylglycerol is synthesised). The process occurs entirely in the plastids, although diacylglycerols transferred from the endoplasmic reticulum can be used as substrates.
Other than in photosynthetic organisms (cyanobacteria - see below), sulfoquinovosyldiacylglycerol has only been found in a few bacteria, mainly of the genus Rhizobium, which have a symbiotic relationship with plants in root nodules and may have obtained the required genes by horizontal gene transfer, although it was found to comprise half the lipids of the halophilic eubacteria Planococcus sp. and Haloferax volcanii. Surprisingly, it has been detected in a sea urchin (Scaphechinus mirabilis), where the fatty acid components are mainly saturated and monoenoic (C14 to C24).
An acylated derivative of this sulfolipid, 2'-O-acyl-sulfoquinovosyldiacylglycerol has been found in the unicellular alga Chlamydomonas reinhardtii, i.e., with an additional acyl group attached to the 2'-hydroxyl of the sulfoquinovosyl head group. While the fatty acids of sulfoquinovosyldiacylglycerol were mostly saturated in this organism, the 2’-acylated analogue contained mainly unsaturated fatty acids with an 18-carbon fatty acid with four double bonds linked to the head group. In this lipid in the diatom P. tricornutum, the 2' fatty acid is 20:5. The lake ball-forming green alga Aegagropilopsis moravica (family Pithophoraceae) contains an ether analogue of lysosulfoquinovosyldiacylglycerol, i.e., sulfoquinovosylchimyl alcohol.
Catabolism: SQDG is an important reservoir of organic sulfur in the biosphere that must be conserved. The first step in catabolism is de-esterification by lipases, followed by one of two sulfoglycolytic pathways to produce C3-sulfonates. Some bacteria possess a sulfoglycolytic sulfoquinovose monooxygenase pathway that cleaves the sulfur-carbon bond with excretion of inorganic sulfur (mainly as sulfite) to complete the sulfur cycle.
 Glucuronosyldiacylglycerol:
When A. thaliana and other plants are stressed by being deprived of phosphate, a second anionic glycosyldiacylglycerol is produced,
i.e., 1,2-diacyl-3-O-α-glucuronosyl-sn-glycerol, which may have the same function as sulfoquinovosyldiacylglycerol
in protecting plants from the effects of this stress, presumably by maintaining the negative charge on the membranes (see next section).
Biosynthesis requires the sulfoquinovosyldiacylglycerol synthase SQD2 located in the chloroplast envelope and transfers glucuronic acid from
its UDP conjugate to diacylglycerols, so it is not surprising that the molecular species compositions of the two lipids are almost identical.
This lipid is present at low levels only in plants grown under normal conditions, but its concentration is greatly elevated when phosphorus
is limiting at the expense of phosphatidylinositol and phosphatidylethanolamine, now known to be a wide-spread phenomenon in higher plants
and algae, often together with production of betaine lipids in the latter.
In the tomato (Solanum lycopersicum), glucuronosyl-diacyl/monoacylglycerols and their acylated derivatives have been characterized.
Although this lipid had been reported earlier from bacteria, fungi and algae, little is known of its metabolism or why it is necessary
in these organisms.
Glucuronosyldiacylglycerol:
When A. thaliana and other plants are stressed by being deprived of phosphate, a second anionic glycosyldiacylglycerol is produced,
i.e., 1,2-diacyl-3-O-α-glucuronosyl-sn-glycerol, which may have the same function as sulfoquinovosyldiacylglycerol
in protecting plants from the effects of this stress, presumably by maintaining the negative charge on the membranes (see next section).
Biosynthesis requires the sulfoquinovosyldiacylglycerol synthase SQD2 located in the chloroplast envelope and transfers glucuronic acid from
its UDP conjugate to diacylglycerols, so it is not surprising that the molecular species compositions of the two lipids are almost identical.
This lipid is present at low levels only in plants grown under normal conditions, but its concentration is greatly elevated when phosphorus
is limiting at the expense of phosphatidylinositol and phosphatidylethanolamine, now known to be a wide-spread phenomenon in higher plants
and algae, often together with production of betaine lipids in the latter.
In the tomato (Solanum lycopersicum), glucuronosyl-diacyl/monoacylglycerols and their acylated derivatives have been characterized.
Although this lipid had been reported earlier from bacteria, fungi and algae, little is known of its metabolism or why it is necessary
in these organisms.
5. Photosynthesis and Other Functions of Glycosyldiacylglycerols in Plants
Photosynthesis: Chloroplasts are double-membrane organelles that are present only in plants and algae and perform oxygenic photosynthesis, a process by which sunlight is absorbed and its excitation energy transferred efficiently to reaction centres surrounded by light-harvesting complexes containing many different proteins that enhance the absorption of light. These are located in the extensive internal membrane system of chloroplasts, the thylakoid membrane. The galactosyldiacylglycerols and sulfoquinovosyldiacylglycerols are major lipid components of the chloroplast membranes in plants (Table 2), and there is evidence that their biosynthesis is coordinated with the synthesis of chlorophyll and the proteins utilized in photosynthesis. Further factors that regulate galactolipid synthesis include light, plant hormones, redox state, phosphatidic acid levels and many stress conditions, including drought.
Table 2. Lipid class composition (mol %) of membranes in plants and cyanobacteria. |
|||||||
| MGDG | DGDG | SQDG | PG | PI | PC | Others | |
|---|---|---|---|---|---|---|---|
| Chloroplast thylakoid membrane (a) | 53 | 27 | 7 | 7 | 2 | - | 4 |
| Chloroplast inner envelope (a) | 49 | 30 | 5 | 8 | 1 | 6 | 1 |
| Chloroplast outer envelope (a) | 17 | 29 | 6 | 10 | 5 | 32 | 1 |
| Non‑green plastid (b) | 32 | 27 | 6 | 9 | 4 | 20 | 2 |
| Synechocystis sp. | 54 | 18 | 15 | 13 | - | - | - |
| (a) Spinach (b) cauliflower buds Table adapted from Kobayashi, K. J. Plant Res., 129, 565-580 (2016); DOI. |
|||||||
Because of its small head group, monogalactosyldiacylglycerol has a cone-like geometry with galactose at the point and the two fatty acyl chains oriented towards the base. Therefore, in aqueous systems, it tends to form a hexagonal-II phase, with the polar head group facing towards the centre of micellar structures rather than forming a bilayer, while in contrast, digalactosyldiacylglycerols with two galactose moieties in the head group have a more cylindrical shape so form lamellar phases and thence bilayers in a similar manner to phosphatidylcholine. The ratio of these two lipids must be under tight control for proper membrane function. As with other biomembranes, the thylakoid membrane has an asymmetric distribution of glycolipids between the two leaflets, often with much of the digalactosyldiacylglycerols on the luminal leaflet, where hydrogen bonding effects of its polar head group balance the repulsive electrostatic contributions of the charged lipids phosphatidylglycerol and sulfoquinovosyldiacylglycerol. There is a suggestion that the polar head groups of the latter could assist the movement of protons along the luminal membrane surface to the ATPase.
Phosphatidylcholine is only found on the outer leaflet of the outer envelope of chloroplasts and resembles that in the endoplasmic reticulum in composition, in line with the suggestion above that these two membrane systems operate together and may be connected at contact sites.
 There are two
families of reaction centres that use light to reduce molecules by providing electrons - photosystem I
in chloroplasts and in green-sulfur bacteria and photosystem II in chloroplasts and in non-sulfur purple bacteria - and
these are located in the thylakoid membranes of plants, algae and cyanobacteria, or in the cytoplasmic membrane of photosynthetic bacteria.
In photosystem I, ferredoxin-like iron-sulfur cluster proteins are used as terminal electron acceptors,
while photosystem II transfers electrons to a quinone terminal electron acceptor.
Both types of reaction centre are present in chloroplasts and cyanobacteria, and they work together to form a metabolic system
that extracts electrons from water while creating oxygen as a by-product.
In brief, when exposed to light, one molecule of the pigment chlorophyll, with phytol
as a covalently bound lipid component, absorbs one photon and loses one electron, which is passed to a quinone molecule.
This starts a flow of electrons down an electron transport chain that leads to the reduction of NADP to NADPH and generates a proton gradient
across the chloroplast membrane for ATP synthesis.
The chlorophyll molecule then regains the electron it lost when a water molecule is split by photolysis catalysed by photosystem II
with release of oxygen.
There are two
families of reaction centres that use light to reduce molecules by providing electrons - photosystem I
in chloroplasts and in green-sulfur bacteria and photosystem II in chloroplasts and in non-sulfur purple bacteria - and
these are located in the thylakoid membranes of plants, algae and cyanobacteria, or in the cytoplasmic membrane of photosynthetic bacteria.
In photosystem I, ferredoxin-like iron-sulfur cluster proteins are used as terminal electron acceptors,
while photosystem II transfers electrons to a quinone terminal electron acceptor.
Both types of reaction centre are present in chloroplasts and cyanobacteria, and they work together to form a metabolic system
that extracts electrons from water while creating oxygen as a by-product.
In brief, when exposed to light, one molecule of the pigment chlorophyll, with phytol
as a covalently bound lipid component, absorbs one photon and loses one electron, which is passed to a quinone molecule.
This starts a flow of electrons down an electron transport chain that leads to the reduction of NADP to NADPH and generates a proton gradient
across the chloroplast membrane for ATP synthesis.
The chlorophyll molecule then regains the electron it lost when a water molecule is split by photolysis catalysed by photosystem II
with release of oxygen.
The galactosyldiacylglycerols are essential for photosynthesis. After crystallization, the photosystem I complex of cyanobacteria was found to contain one molecule of monogalactosyldiacylglycerol and three of phosphatidylglycerol, while the photosystem II complex contains up to 25 lipid molecules, including eleven moles of monogalactosyldiacylglycerols, four of digalactosyldiacylglycerols, three or four of sulfolipid and five of phosphatidylglycerol. These glycolipids are required for crystallization of the light-harvesting complex II in pea chloroplasts (again together with phosphatidylglycerol). When exposed to light, photosystem II monomers are assembled into dimeric complexes in the thylakoid membrane, in which the sulfolipid confers stability by forming hydrogen-bonds between its head group and amino acid residues. Dimer formation protects them from proteolysis under high light conditions and increase their thermal stability. Photosystem I monomers aggregate to form a trimer, and there is evidence that the non-bilayer-forming properties of monogalactosyldiacylglycerols enable this lipid to stabilize the trimeric complex and bind together the various extrinsic proteins in the complex to modulate their folding and conformation for maximum efficiency. In contrast, in purple phototrophic bacteria, there are no glycosyldiacylglycerols, and cardiolipin and phosphatidylglycerol are major components of the light-harvesting 1-reaction centre complexes.
In addition, galactolipids are necessary to induce the transcription of photosynthesis-associated nuclear-encoded genes and of photosynthesis-associated plastid-encoded genes and for the accumulation of the resulting proteins in developing chloroplasts.
Other functions: Individual glycolipids are associated in a highly characteristic way with various membrane proteins, where the ability of monogalactosyldiacylglycerols to form inverted micelles may again be relevant, for example, to assist the transport of proteins and other nutrients across membranes. As they are concentrated in the peribacteroid membrane surrounding nitrogen-fixing rhizobia in the nodules of legumes, they may be needed for the exchange of ammonium and nutrients, in this instance between the symbiotic bacteria and the host cell. The glycosyl moiety of digalactosyldiacylglycerols plays a special role in the plant immune response towards bacterial infection (systemic acquired resistance), as it is required to produce salicylic acid and nitric oxide while monogalactosyldiacylglycerols regulate downstream signals. The axial hydroxyl group at C4 of galactose is necessary for certain of these interactions and may explain why galactolipids are favoured over those containing glucose. As with the neutral galactosyldiacylglycerols, the negatively charged sulfoquinovosyldiacylglycerol is essential for the operation of the thylakoid membrane in plants, where it is located mainly on the inner leaflet and may assist the process of protein insertion and passage through the membranes.
Under phosphate-limiting conditions, as discussed briefly above, galactosyldiacylglycerols assist in conserving this vital nutrient in plants and photosynthetic microorganisms and maintain membrane homeostasis by acting as a replacement for phospholipids, which undergo a remodelling process involving hydrolysis to diacylglycerols with release of phosphate for other purposes immediately prior to glycolipid formation. Sulfoquinovosyldiacylglycerols then provide the required negative charge to membranes with a minimum demand for phosphate, although glucuronosyldiacylglycerols can then assume greater importance, leaving a small amount of phosphatidylglycerol, which has a comparable function, as the only phospholipid present. It is evident that there is a reciprocal relationship between the concentrations of the anionic lipids and of phosphatidylglycerol in all photosynthetic organisms. In mitochondrial membranes during phosphate deprivation, a transmembrane lipoprotein (MTL) complex, normally used for the export of phosphatidylethanolamine, exchanges glycerolipids between mitochondria and plastids with the result that digalactosyldiacylglycerols replace cardiolipin in part at least.
Under other abiotic stresses, the degree of unsaturation of the fatty acid constituents of the galactosyldiacylglycerols in photosynthetic membranes can vary to maintain the correct degree of fluidity, possibly through changes in the ratio of the ER to chloroplast pathways. Lower temperatures tend to lead to a higher degree of unsaturation, while higher temperatures lead a higher proportion of saturated fatty acids. There can also be changes in the ratio of mono- to digalactosyldiacylglycerols under conditions of temperature stress, drought or elevated salt levels.
6. Glycosyldiacylglycerols of Bacteria
Photosynthetic bacteria: Cyanobacteria are oxygenic photosynthetic bacteria (Gram negative) that are distinct from most other bacteria in innumerable ways, including their lipid compositions, as they contain appreciable amounts of mono- and digalactosyldiacylglycerols together with sulfoquinovosyldiacylglycerol in which the configuration of the anomeric head groups is identical to that of the corresponding plant lipids (see Table 2). Indeed, the membrane architectures of cyanobacteria and chloroplasts in higher plants are rather similar, and cyanobacteria possess thylakoid membranes with comparable lipid compositions and other properties. As discussed briefly above, there is a theory that an ancestral cyanobacterial cell, which was photosynthetically active, was engulfed by a eukaryotic organism to become the precursor of the first plant cell, the composition of which has been largely conserved throughout evolution. As in higher plants and algae, galactolipids are produced in greater relative amounts during phosphate deprivation with phosphatidylglycerol decreasing in concentration and sulfoquinovosyldiacylglycerol increasing.
As can be seen from the data in Table 3, the overall fatty acid compositions of the lipids of the cyanobacterium Synechocystis PCC6803 resemble those of photosynthetic tissues in higher plants, although the polyunsaturated fatty acids (C18) are concentrated in position sn-1 in this instance with saturated fatty acids (C16) in position sn-2. The only phospholipid present in appreciable amounts is phosphatidylglycerol, and compositional data are listed on the relevant web page (see Table 2 above also).
Table 3. Composition (mol %) of fatty acids in positions sn-1 and sn-2 of mono- and digalactosyl- and sulfoquinovosyldiacylglycerols from Synechocystis PCC6803*. |
|||||||
| Position | Fatty acids | ||||||
|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3** | 18:4 | |
| MGDG | |||||||
| sn-1 | 14 | 4 | tr | - | 8 | 54 | 20 |
| sn-2 | 94 | 2 | tr | 2 | tr | tr | tr |
| DGDG | |||||||
| sn-1 | 16 | 4 | 2 | 2 | 8 | 50 | 18 |
| sn-2 | 94 | 2 | 2 | tr | - | - | - |
| SQDG | |||||||
| sn-1 | 34 | 8 | 2 | 10 | 16 | 28 | tr |
| sn-2 | 92 | tr | 4 | tr | tr | tr | - |
| Grown at 22°C; ** mainly 18:3(n-3); tr = trace. Data from Wada, H. and Murata, N. Plant Physiology, 92, 1062-1069 (1990); DOI. On average, cyanobacteria contain ~52% MGDG, ~15% DGDG and ~9% SQDG, together with ~22% phosphatidylglycerol and ~1% minor components (Petroutsos, D. et al. (2014); DOI). |
|||||||
Although the nature of the lipids is highly conserved in plants and photosynthetic bacteria, the biosynthetic mechanisms are somewhat different. Cyanobacteria contain trace amounts of a monoglucosyldiacylglycerol in which the glucosyl group is in the β-conformation, i.e., 1,2‑diacyl-3-O-(β‑D-glucopyranosyl)-sn-glycerol, while in Bacillus subtilis it amounts to 10% of the total lipids. It is now known that the production of monoglucosyldiacylglycerol in most cyanobacteria is the first step in biosynthesis of galactosyldiacylglycerols before an epimerization reaction occurs to the carbon-4 hydroxyl group to convert it to the galactosyl form (although a few species contain a bacterial monogalactosyldiacylglycerol synthase obtained by horizontal transfer that enables direct transgalactosylation). The second galactose unit is added as such to the monogalactosyl product by a digalactosyldiacylglycerol synthase with UDP-galactose as the carbohydrate donor.
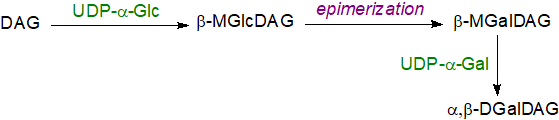 |
| Figure 5. Biosynthesis of galactolipids in cyanobacteria. |
Monoglucosyldiacylglycerols have been detected in Synechococcus sp. PCC 7002 (stereochemistry not defined), and mutants in which the epimerase has been 'knocked out' accumulate this lipid in the thylakoid membranes only, although the organisms continue to photosynthesise, if less efficiently. While the role of digalactosyldiacylglycerols (and of sulfoquinovosyl-diacylglycerol) in the photosynthetic apparatus in these organisms is discussed briefly above, it should be noted that there is an absolute requirement for digalactosyldiacylglycerol in Synechococcus elongatus PCC 7942. Its loss cannot be compensated by other lipids, including glucosylgalactosyldiacylglycerol, so the second galactose molecule must be crucial.
Many anoxic photosynthetic bacteria contain monogalactosyldiacylglycerols, but digalactosyldiacylglycerols are found only rarely. One exception is the occurrence of digalactosyldiacylglycerols as major membrane components of free-living and bacteroid forms of Bradyrhizobium japonicum, which normally live symbiotically with plants in root nodules. In addition to monogalactosyldiacylglycerols, the green photosynthetic bacterium Chlorobium tepidum contains rhamnosylgalactosyldiacylglycerols, the biosynthesis of which is by a different mechanism with a unique UDP-galactose diacylglycerol transferase.
Sulfoquinovosyldiacylglycerol assists anoxygenic photosynthesis in the purple bacterium Rhodobacter sphaeroides, where it is required to bind monomers of the reaction centre-light harvesting 1 (RC-LH1) core complex to form a dimer via arginine residues on an additional polypeptide known as PufX, although monomers lacking the lipid are still able to photosynthesise.
Other bacteria: A wide variety of glycosyldiacylglycerols are found in non-photosynthetic bacteria; those with one to three glycosyl units linked to sn‑1,2‑diacylglycerol are most common, although others with up to five glycosyl units are found. For example, αGal1→2αGlc- and αGal1→6αGal1→2αGlc-diacylglycerols are often detected in Lactobacilli, while mono- and diglucosyldiacylglycerols are present in the opportunistic pathogen Enterococcus faecalis. These lipids can differ from the plant glycosyl diacylglycerols in that glucose is much more common than galactose, and a UDP-glucose:1,2-diacylglycerol-3-β-D-glucosyl transferase that can transfer one or more sugars to create mono-, di- or polyglycosylated diacylglycerols has been characterized from Bacillus subtilis. There is a related enzyme in Mesorhizobium loti that can utilize both UDP-galactose and UDP-glucose. In this instance, processive glycosyltransferases are responsible for the transfer of each sugar moiety, and they are able to catalyse three transferase steps, each using the glycosyldiacylglycerol produced by the earlier step as a substrate. The digalactosyldiacylglycerols in this organism differ from the normal plant lipid in that both galactose units are of the β‑conformation. As noted earlier, Rhizobium and its relatives produce sulfoquinovosyldiacylglycerols. The fatty acid components of these lipids are mainly saturated, monoenoic and branched-chain or cyclopropanoid, but many anaerobic bacteria contain alk‑1'‑enyl moieties (plasmalogens) in position sn-1 of the glycosyldiradylglycerols.
The human pathogen Clostridium (Clostridioides) difficile contains such diradylglycerols in mono-, di- and tri-hexosyl forms together with a unique aminohexosyl-hexosyl species, which are important for critical cell functions, including sporulation, cell size and morphology, membrane fluidity, and resistance to some antimicrobial agents. In C. difficile, these can amount to 50% of the total membrane lipids, and glycosyltransferases designated UgtA and UgtB responsible for their synthesis have been characterized.
 The nature of the glucose linkages is variable, and some Streptococci contain mono- and
diglucosyldiacylglycerols, with the diglucoside unit having an α‑(1→2) linkage as in kojibiose, and so can be termed
‘kojibiosyldiacylglycerols’.
Related lipids together with diglucosyl-1-monoacyl-sn-glycerol and the phosphoglycolipid
glycerophosphoryldiglucosyldiacylglycerol are present in S. mutans.
S. pneumoniae contains glucopyranosyl- and galactoglucopyranosyl-diacylglycerols,
while this and other bacteria contain comparable lipids with a fatty acyl group attached to a carbohydrate moiety (usually in position 3 or 6).
Rhodobacter sphaeroides contains
1,2-di-O-acyl-3-O-[α‑D-glucopyranosyl-(1→4)-O-β‑D-galactopyranosyl]glycerol
and three other glycosyldiacylglycerols with 11‑18:1 as more than 80% of the fatty acid constituents.
α-Glucosyl-(1→3)-α-mannosyl-diacylglycerol produced in sub-nanomolar concentrations
by Rhizobium leguminosarum may be required for the induction of symbiosis-related processes.
The nature of the glucose linkages is variable, and some Streptococci contain mono- and
diglucosyldiacylglycerols, with the diglucoside unit having an α‑(1→2) linkage as in kojibiose, and so can be termed
‘kojibiosyldiacylglycerols’.
Related lipids together with diglucosyl-1-monoacyl-sn-glycerol and the phosphoglycolipid
glycerophosphoryldiglucosyldiacylglycerol are present in S. mutans.
S. pneumoniae contains glucopyranosyl- and galactoglucopyranosyl-diacylglycerols,
while this and other bacteria contain comparable lipids with a fatty acyl group attached to a carbohydrate moiety (usually in position 3 or 6).
Rhodobacter sphaeroides contains
1,2-di-O-acyl-3-O-[α‑D-glucopyranosyl-(1→4)-O-β‑D-galactopyranosyl]glycerol
and three other glycosyldiacylglycerols with 11‑18:1 as more than 80% of the fatty acid constituents.
α-Glucosyl-(1→3)-α-mannosyl-diacylglycerol produced in sub-nanomolar concentrations
by Rhizobium leguminosarum may be required for the induction of symbiosis-related processes.
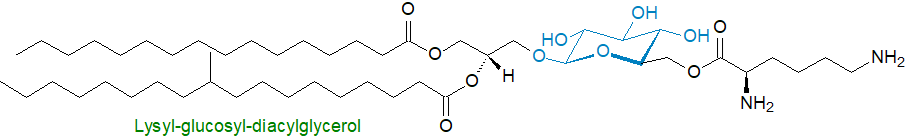
The bacterium Streptococcus agalactiae synthesises mono-and diglucosyldiacylglycerols, together with appreciable amounts of lysyl-glucosyl-diacylglycerol (1‑palmitoyl, 2-10-methylstearoyl) and a diglucosyl analogue, cationic lipids, which are factors in the pathogenesis of neonatal meningitis and contribute to infection by enabling traversal of the endothelial blood-brain barrier. Together, these glycolipids promote virulence by inhibiting immune cell clearance in the host.
Some microorganisms accumulate galactofuranosyl-diacylglycerols rather than the galactopyranosyl form, and a variety of unusual glycosyldiacylglycerols with differing carbohydrate moieties, or with differences in the glycosidic bonds from those in higher plants, have been found, including Bifidobacterium longum subs. infantis from the intestinal tract of infants, which contains a galactofuranosyl-diacylglycerol with a novel acetal linkage to glycerol; this was found to suppress the innate immune response in the host. Eggerthella lenta from the human gut microbiome produces a similar lipid, with a 1-alkenyl,2-acyl-sn-glycerol backbone, together with a derived lysoglycoglycerolipid containing a 16:0 acetal linked to the β-D-galactofuranose as a signalling lipid that upregulates inflammatory cytokines in the host.
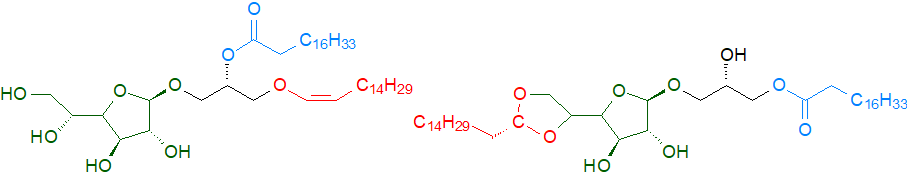 |
| Figure 6. Galactofuronosyl 1-alkenyl,2-acyl-sn-glycero-lipid and derived lyso form from E. lenta (DOI). |
Amongst others, Micrococcus luteus synthesises mono- and dimannosyldiacylglycerols, while β-gentiobiosyl-diacylglycerols with long-chain iso- and anteiso-fatty acid constituents have been isolated from Mycobacteria. Other bacteria contain N‑acetylgalactosamine linked to a diacylglycerol (Bacillus megaterium) or a long-chain fatty acid linked similarly via glucosamine (Thermus thermophilus). As might be expected, even greater complexity exists in the triglycosyldiacylglycerols from bacteria. In mechanistic terms, the biosynthesis of these lipids is analogous to that in higher plants described above.
Glycosyldiacylglycerols with a glycerophosphate group linked to a carbohydrate moiety (phosphoglycolipids and glycophospholipids) are known from other bacteria, and for example, the Gram-negative aerobe Thermus thermophilus from a high temperature environment contains several complex glycosyldiacylglycerols and glycophospholipids with an alkylamine unit attached to the carbohydrate moiety.
In some Gram-positive bacteria such as Staphylococcus aureus, lipoteichoic acid is anchored in the membranes by a diglucosyldiacylglycerol moiety (gentiobiosyl) and 8 mol% of the free glycolipid are present. The ratio of mono- to diglucosyldiacylglycerol may be a significant factor in determining bilayer stability as only the latter will form a bilayer. The human pathogen Enterococcus faecalis produces diglucosyldiacylglycerol as a membrane component and as a lipoteichoic acid precursor in a secreted biofilm, which is involved in adherence to host cells and virulence in vivo. There is increasing interest in such lipids as it has been demonstrated that galactosyldiacylglycerols from Borrelia burgdorferi, the causative agent of Lyme disease, take part in the antigen response via interactions with receptors.
Certain bacteria, fungi and algae, including Mycobacteria, contain the ionic 1,2-diacyl-3-O-α-glucuronosyl-sn-glycerol (glucuronosyldiacylglycerol) among their membrane lipids, and this may have a functional relationship to sulfoquinovosyldiacylglycerol as discussed above. Anaerobic sulfate-reducing Desulfatibacillum alkenivorans produces glucuronosyl-acylalkylglycerols when grown under conditions of phosphorus limitation. A conjugate of glucuronosyldiacylglycerol with taurine is known (see our web page on other sulfolipids). Of course, the bacterial lipids have very different fatty acid compositions from those in algae or in higher plants. In addition, glucosylglucuronyl- and galacturonyldiacylglycerols have been detected in bacteria.
The complex diether isoprenoid glycerolipids (discussed elsewhere) from the extreme halophilic bacteria of the Archaea family exist in the form of glycosyldiacylglycerols, both as neutral lipids and in sulfated form, with two to four glycosyl units attached to the glycerols.
7. Mono- and Diglycosyldiacylglycerols from Animal Tissues
Mono- and digalactosyldiacylglycerols are now known to be ubiquitous if minor components of brain and other nervous tissues, usually amounting to only 0.1 to 0.6% of the total lipids, and they can occur in trace amounts in other tissues such as kidney. As they are minor components relative to the glycosphingolipids and can be inadvertently destroyed during some of the isolation procedures for the analysis of the latter, they are often overlooked in studies of animal glycolipids. Some might categorize them as forgotten lipids.
They exist in diacyl, alkylacyl and alkenylacyl forms and contain mainly saturated and monoenoic fatty acid components, with 16:0, 18:0 and 18:1 comprising 90% or more of the total; the alkyl moieties consist of 70% or more of 16:0. The molecular compositions of monohexosyl lipids present in sciatic nerve, spinal cord, brain stem and cerebrum in rats and mice are very similar, although the absolute amounts differ between tissues and species. The basic structure of the monogalactosyldiacylglycerol of mammalian brain is the same as that of plants, i.e., it is 1,2‑di‑O-acyl-3-O-β-D-galactopyranosyl-sn-glycerol (and the 1-alk(en)yl-2-acyl form), although the alkenylacyl form was not detectable in one recent study of rat spinal cord lipids. In fish brain, only the diacyl form is found, and it can be accompanied by related lipids in which the position 6 of the galactose unit is acylated, or in which an aldehyde is linked to the carbohydrate moiety via an acetal linkage. In contrast, relatively little is known of the digalactosyl equivalent, although it has been fully characterized (from a human carcinoma) and is distinctive in having a Galα1‑4Gal linkage rather than Galα1‑6Gal as in plants, i.e., it is 1-O-alkyl-2-O-acyl-3-O-(β‑galactosyl(1-4)α-galactosyl)-sn-glycerol.
The biosynthesis of the galactosyldiacylglycerols has been studied in vitro with HeLa cells, and it has been demonstrated that UGT8, a ceramide galactosyltransferase in the endoplasmic reticulum, is able to catalyse MGDG biosynthesis from diacylglycerol and alkylacylglycerol precursors. Why these galactolipids are required is still a matter for conjecture, but they are probably used for myelination, cell differentiation and intracellular signalling in brain.
Related lipids but with glucose rather than galactose have been characterized from saliva, bronchial fluid and gastric secretions. The lipid portion is 1‑O‑alkyl-2-O-acyl-sn-glycerol, with the fatty acid and alkyl constituents again being predominantly saturated. The carbohydrate moiety can consist of up to 8 glucose units, with six being the most abundant. Although present at low levels only in absolute terms, they can comprise as much as 20% of the total lipids in saliva, where they may be part of a defence mechanism against microbial attack.
Unusual glycolipids containing sugar moieties linked to both positions sn-2 and 3 of glycerol together with an O-alkyl ether chain at position sn-1 were isolated from the sponge Myrmekioderma sp. and named 'myrmekiosides'. A lipid with two xylose units linked to glycerol and a vinyl ether linked alkyl group was found in the sponge Trikentrion loeve, and comparable lipids have been isolated from soft corals. Like the crasserides, it is possible that these are produced by symbiotic bacteria rather than being of animal origin, but this has yet to be investigated.
There are reports that plant galactolipids have antitumour, antimicrobial, antiviral, immunosuppressive and anti-inflammatory activities in animals in addition to their nutritional value, but their potential has yet to be tapped because of a limited availability in quantity with high purity.
8. Seminolipid
Seminolipid or sulfogalactosylglycerolipid or 1-O-hexadecyl-2-O-hexadecanoyl-3-O-β-D-(3'-sulfo)-galactopyranosyl-sn-glycerol is a lipid sulfate (and so differs from the plant sulfolipid) and as the trivial name suggests was first found in mammalian spermatozoa and testes, where it can amount to 3% of the total lipids (10% of the lipids of testicular germ cells) and 90% of the glycolipids; it is located primarily in the outer leaflet of the plasma membrane. It is now known to be present at low levels in many other animal tissues, especially those rich in glycolipids such as myelin and other nervous tissues.
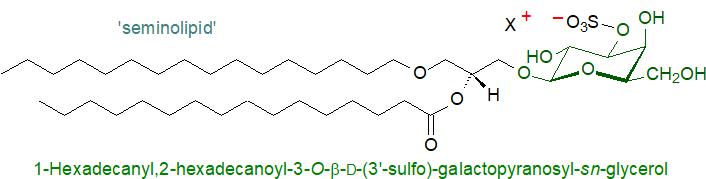
This lipid in male reproductive tissues is unusual in several ways, not least in that it exists largely as a single molecular species, i.e., with an ether-linked C16 alkyl group in position sn-1 and palmitic acid in position sn-2. Thus, it is fully saturated and co-exists with other phospholipids that are highly unsaturated. Depending on the tissue and animal, there is some limited variation in the lipid moiety, which can contain alkylacyl-, diacyl- and dialkylglycerol moieties, and the relative proportions and compositions can change somewhat with aging. In the mouse at least, different molecular species are located in different regions of the spermatogenesis apparatus, i.e., the major one (16:0‑alkyl-16:0-acyl) is in tubules, while 16:0‑alkyl-14:0-acyl and 14:0‑alkyl-16:0-acyl species are in spermatocytes mainly with the 17:0-alkyl-16:0-acyl combination in spermatids and spermatozoa. While there is some limited variation in the chain-lengths of the aliphatic components, they are usually saturated. Fish brain is an exception, where the diacyl form predominates with 16:0 and 18:1 fatty acids.
The alkyl moiety is essential for spermatogenesis and is synthesised within testes, requiring the fatty acyl-CoA reductase FAR1 (see our web page on ether lipids). The polar head group is identical to that of the cerebroside sulfate in myelin while the lipid component has physical properties akin to those of ceramides, and indeed many other parallels can be drawn between the biosynthesis, metabolism and function of seminolipid and sphingolipid sulfates. Seminolipid is synthesised by sulfation of its precursor, galactosylalkylacylglycerol, by the action of 3‑phosphoadenosine-5'-phosphosulfate:cerebroside 3-sulfotransferase, i.e., the same enzyme and sulfate donor as for the synthesis of the analogous sphingolipid (3'‑sulfo-galactosylceramide), and the process is reversed by the corresponding sphingolipid enzyme, i.e., arylsulfatase A, the enzyme missing in patients suffering from metachromatic leukodystrophy. Indeed, the glycolipid precursor is synthesised by a sphingolipid enzyme, i.e., ceramide galactosyltransferase. In live germ cells, there appears to be no turnover of seminolipid, and degradation only occurs during apoptosis, when the reaction must go back at least to the glycerolipid backbone. In consequence of this biosynthetic/degradative relationship with the brain lipid, it has been suggested that seminolipid levels in sperm might be used as a predictor of neurological status.
There is abundant evidence from experiments with genetically modified animals that seminolipid is essential for germ cells, and for spermatogenesis in testes and thence male fertility. In particular, it is responsible for the formation of a lactate transporter assembly to take up the critical energy source for spermatocytes. It participates in the formation of lipid rafts in the sperm head and contributes to the shape and stability of sperm cell membranes. In inactivated or immature spermatozoa, membrane cholesterol induces a tilt in the glycosphingolipid receptor, rendering it unavailable, but cholesterol efflux during maturation of spermatozoa causes a change in seminolipid conformation to expose sugar residues for recognition by lectins in the zona pellucida of the egg. In the mammalian ovum, it is involved the binding of sperm and zona pellucida. While it is evident that cell surface seminolipid molecules are needed for germ cell differentiation and in interactions with other cell types, little mechanistic information seems to be available.
9. Analysis
The main neutral galactolipids in plants present no noteworthy difficulties for analysis. They are easily separated from phospholipids by adsorption chromatography, usually by making use of the fact that they are soluble in acetone in contrast to phospholipids. Because of its highly polar acidic nature, sulfoquinovosyldiacylglycerol presents more analytical problems, but methods have been devised for its analysis that make use of adsorption or ion-exchange chromatography. Glycosyldiacylglycerols tend to be present in animal tissues at such low levels that isolation and analysis presents real difficulties. Indeed, they are usually ignored by scientists with an interest in glycosphingolipids because the methodology used to concentrate the latter can be destructive to O-acyl lipids. Azure A, a cationic dye that reacts with anionic lipids, is often employed to quantify seminolipid in reproductive tissues, although it lacks sensitivity and specificity. Electrospray-ionization tandem mass spectrometry is now widely used for structural analyses and in lipidomic studies.
Recommended Reading
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Cook, R., Lupette, J. and Benning, C. The role of chloroplast membrane lipid metabolism in plant environmental responses. Cells, 10, 706 (2021); DOI.
- Gasulla, F., García-Plazaol, J.I., López-Pozo, M. and Fernández-Marín, B. Evolution, biosynthesis and protective roles of oligogalactolipids: Key molecules for terrestrial photosynthesis? Environm. Exp. Bot., 164, 135-148 (2019); DOI.
- Goddard-Borger, E.D. and Williams, S.J. Sulfoquinovose in the biosphere: occurrence, metabolism and functions. Biochem. J., 474, 827-849 (2017); DOI.
- Hölzl, G. and Dörmann, P. Chloroplast lipids and their biosynthesis. Annu. Rev. Plant Biol., 70, 51-81 (2019); DOI.
- Hori, K., Nobusawa, T., Watanabe, T., Madoka, Y., Suzuki, H., Shibata, D., Shimojima, M. and Ohta, H. Tangled evolutionary processes with commonality and diversity in plastidial glycolipid synthesis in photosynthetic organisms. Biochim. Biophys. Acta, Lipids, 1861, 1294-1308 (2016); DOI.
- LaBrant, E., Barnes, A.C. and Roston, R.L. Lipid transport required to make lipids of photosynthetic membranes. Photosynth. Res., 138, 345-360 (2018); DOI.
- Lavell, A.A. and Benning, C. Cellular organization and regulation of plant glycerolipid metabolism. Plant Cell Physiol., 60, 1176-1183 (2019); DOI.
- Liu, X., Duan, L.Q. and Song, J.M. Phosphorus limitation induces membrane lipid remodeling in aquatic phytoplankton. Marine Environ. Res., 212, 107526 (2025); DOI.
- Ohba, Y., Motohashi, M. and Arita, M. Characterization of UGT8 as a monogalactosyl diacylglycerol synthase in mammals. J. Biochem., 177, 141-152 (2025); DOI.
- Okazaki, Y., Nishizawa, T., Takano, K., Ohnishi, M., Mimura, T. and Saito, K. Induced accumulation of glucuronosyldiacylglycerol in tomato and soybean under phosphorus deprivation. Physiologia Plantarum, 155, 33-42 (2015); DOI.
- Sato, N. and Awai, K. "Prokaryotic Pathway" is not prokaryotic: noncyanobacterial origin of the chloroplast lipid biosynthetic pathway revealed by comprehensive phylogenomic analysis. Genome Biol. Evolution, 9, 3162-3178 (2017); DOI.
- Schmid, K.M. Lipid metabolism in plants. In: Biochemistry of Lipids, Lipoproteins and Membranes, 6th Edition, pp. 113-147 (ed. N.D. Ridgway and R.S. McLeod, Elsevier, Amsterdam) (2016) - see Science Direct.
- Slomiany, B.L., Murty, V.L.N., Liau, Y.H. and Slomiany, A. Animal glycoglycerolipids. Prog. Lipid Res., 26, 29-51 (1987); DOI.
- Song, Y., Lwe, Z.S.Z., Wickramasinghe, P.A.D.B.V. and Welti, R. Head-group acylation of chloroplast membrane lipids. Molecules, 26, 1273 (2021); DOI.
- Tanphaichitr, N. and others. Properties, metabolism and roles of sulfogalactosylglycerolipid in male reproduction. Prog. Lipid Res., 72, 18-41 (2018); DOI.
- Yoshihara, A. and Kobayashi, K. Lipids in photosynthetic protein complexes in the thylakoid membrane of plants, algae, and cyanobacteria. J. Exp. Botany., 73, 2735-2750 (2022); DOI.
- Yu, L.H., Zhou, C., Fan, J.L., Shanklin, J. and Xu, C.C. Mechanisms and functions of membrane lipid remodeling in plants. Plant J., 107, 37-53 (2021); DOI.
- Yuan, Y. and Zeng, W. An overview of multifaceted applications and the future prospects of glyceroglycolipids in plants. J. Agric. Food Chem., 72, 22420-22432 (2024); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: October 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
