Lipids in Action - Their Biological Functions
Energy - Membranes - Signalling
For many years, lipids were considered to be intractable and uninteresting oily materials required only to serve as sources of energy or as the building blocks of cell membranes. These properties are indeed vital and continue to be studied. On the other hand, for much of the last century, lipids were certainly not considered to be appropriate candidates for such crucial molecular tasks as intracellular signalling or local hormonal regulation. In 1929, George and Mildred Burr demonstrated that linoleic acid was an essential dietary constituent for animals, but it was many years before the importance of this finding was recognized by biochemists in general. In the 1960s, it was established that certain glycosphingolipids were blood group determinants, and with the discovery in 1964 that the essential fatty acid arachidonate was the biosynthetic precursor of the prostaglandins with their effects on inflammation and other disease states, the scientific world in general began to realize that lipids were much more interesting than they had previously thought. I find it astonishing that there is no consensus on the definition of a lipid, but you can find my opinion here...
 From the mid-1950s to the 1970s,
there arose an awareness of the distinctive biochemistry of phosphatidylinositol and its metabolites and eventually their
involvement in signalling processes, and another milestone was achieved in 1979 with
the discovery of a further intact phospholipid with a dynamic function, i.e., platelet-activating factor.
Since then, virtually every individual lipid class has been found to have some unique role in tissues other than as a source of energy
or as a simple construction unit of a cellular membrane.
Indeed, it is now recognized that lipids in membranes operate in innumerable ways in the trafficking of cellular constituents, in the
regulation of membrane protein activity and in signalling, in addition to being structural components at the interface between
all living cells and their external environment.
From the mid-1950s to the 1970s,
there arose an awareness of the distinctive biochemistry of phosphatidylinositol and its metabolites and eventually their
involvement in signalling processes, and another milestone was achieved in 1979 with
the discovery of a further intact phospholipid with a dynamic function, i.e., platelet-activating factor.
Since then, virtually every individual lipid class has been found to have some unique role in tissues other than as a source of energy
or as a simple construction unit of a cellular membrane.
Indeed, it is now recognized that lipids in membranes operate in innumerable ways in the trafficking of cellular constituents, in the
regulation of membrane protein activity and in signalling, in addition to being structural components at the interface between
all living cells and their external environment.
All multi-cellular organisms use chemical messengers to send information between organelles and to other cells, and as relatively small hydrophobic molecules, lipids are excellent candidates for this purpose. Unesterified fatty acids and their oxygenated metabolites (oxylipins) have well-defined structural features, such as cis-double bonds or oxygen atoms in particular positions of an alkyl chain, so can carry information by binding selectively to specific receptors. They can infiltrate membranes or be translocated across them to carry signals to other cells. During transport in the circulation, they are usually bound to proteins and their effective solution concentrations are very low, so they are inert until they reach their site of action and encounter the appropriate receptor.
Storage lipids, such as triacylglycerols, in their cellular context are chemically inert in that they only rarely have biological effects per se, and indeed, esterification with fatty acids may be a method of de-activating other lipids such as steroidal hormones until they are required for use in tissues. In contrast, polar phospholipids have both hydrophobic and hydrophilic sites that can bind via various mechanisms to membrane proteins and influence their efficiency, while glycosphingolipids carry complex carbohydrate moieties that amongst many functions have a part to play in the immune system. Lipids have been implicated in many human disease states, including cancer and cardiovascular disease, sometimes in a beneficial and at others in a detrimental manner. In short, every scientist should now be aware that lipids are just as fascinating as all the other groups of organic molecules that make up living systems.
In this web document, the biology and metabolism of some key lipids in animals, plants and microorganisms are summarized to give a brief overview, but much more information is available in this website on those pages dealing with each lipid class.
Fatty Acids and Oxylipins
Fatty acids are one of the defining constituents of all the main lipid classes, and they are in large part responsible for the physical state and metabolism of the latter, as discussed in the next sections below. They are vital for life in that they constitute integral components of all tissue membranes, and because they have the highest energy density of all energy substrates. As precursors for many lipid mediators, they modulate innumerable metabolic reactions, while through post-translational acylations they control the membrane location and efficient operation of many proteins (proteolipids), but they also have relevance in non-esterified form, i.e., as free (unesterified) fatty acids. They are released from triacylglycerols in fat depots during fasting to provide a source of energy and of structural components for cells.
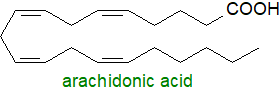 Fatty acids interact with living systems in innumerable ways, and it has long been known that linoleic and linolenic acids
are essential fatty acids
in that they cannot be synthesised by animals and must come from plants via the diet.
They are precursors of arachidonic, eicosapentaenoic and docosahexaenoic acids, which are components of all membrane lipids and precursors of
oxylipins, a family of oxygenated fatty acids formed by mono- or dioxygen-dependent oxidation.
Linoleic acid is a necessary component of skin ceramides and is essential in its own right,
not simply as a precursor of higher polyunsaturated fatty acids.
Fatty acids interact with living systems in innumerable ways, and it has long been known that linoleic and linolenic acids
are essential fatty acids
in that they cannot be synthesised by animals and must come from plants via the diet.
They are precursors of arachidonic, eicosapentaenoic and docosahexaenoic acids, which are components of all membrane lipids and precursors of
oxylipins, a family of oxygenated fatty acids formed by mono- or dioxygen-dependent oxidation.
Linoleic acid is a necessary component of skin ceramides and is essential in its own right,
not simply as a precursor of higher polyunsaturated fatty acids.
Dietary fatty acids of short and medium chain-length (< C14) are not usually esterified in tissues but are oxidized rapidly as a source of 'fuel' to support all the events necessary to keep organisms operative, while longer-chain fatty acids are present largely in esterified form in triacylglycerols or structural lipids in tissues. Although all lipids are in a state of dynamic flux, membrane lipids are largely conserved in content and composition except under conditions of extreme stress. Triacylglycerols are the primary storage form of long-chain fatty acids for energy and structural purposes, and hydrolysis with release of the free acid components can occur quickly when these are required for metabolic reasons; they are then transported in an appropriate form to the heart, liver and other tissues. Polyunsaturated fatty acids are major constituents of the phospholipids, where they affect the physics of membranes by decreasing their rigidity. On the other hand, the presence of saturated and monoenoic acids ensures that there is a correct balance between rigidity and flexibility. Indeed, saturated and 2‑hydroxy fatty acids in sphingolipids give additional rigidity and hydrogen-bonding stability to the sub-domains of membranes termed 'rafts'.
Unesterified fatty acids can act as second messengers required for the translation of external cellular signals, as they are produced rapidly after binding of relevant agonists to plasma membrane receptors. Within cells, fatty acids can act to amplify or otherwise modify signals to influence such enzymes as protein kinases, phospholipases and many more. They can regulate gene expression, mainly targeting genes that encode proteins with roles in fatty acid transport or metabolism via their influence on transcription factors, e.g., peroxisome proliferator-activated receptors (PPARs) in the nuclei of cells. Such effects can be unique to particular fatty acids, and for example, unesterified arachidonic acid per se is part of a mechanism by which apoptosis (programmed cell death) is regulated.
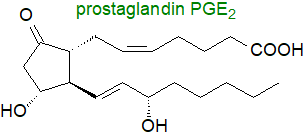 The essential fatty acids, linoleic and linolenic acids and their longer-chain polyunsaturated metabolites such as
arachidonic acid, can be found in most lipid classes in animals, and they are the precursors of many different types of
eicosanoids (C20) and other oxylipins, including the
hydroxyeicosatetraenes ('HETE'),
prostanoids (prostaglandins, thromboxanes and prostacyclins)
and leukotrienes, while docosahexaenoic acid is the precursor of
docosanoids (C22 - specialized pro-resolving mediators - resolvins, protectins
and maresins).
These are produced enzymatically with great stereochemical precision by cyclooxygenases (COXs), lipoxygenases (LOXs) and epoxygenases of the
cytochrome P-450 family (CYPs) mainly from the free fatty acids.
Related isoprostanes are formed by non-enzymic means (autoxidation) from the same precursors
in esterified form in situ in lipids within membranes.
While these oxylipins are usually treated separately in biochemical textbooks, it should not be forgotten that they are in fact fatty acids.
The essential fatty acids, linoleic and linolenic acids and their longer-chain polyunsaturated metabolites such as
arachidonic acid, can be found in most lipid classes in animals, and they are the precursors of many different types of
eicosanoids (C20) and other oxylipins, including the
hydroxyeicosatetraenes ('HETE'),
prostanoids (prostaglandins, thromboxanes and prostacyclins)
and leukotrienes, while docosahexaenoic acid is the precursor of
docosanoids (C22 - specialized pro-resolving mediators - resolvins, protectins
and maresins).
These are produced enzymatically with great stereochemical precision by cyclooxygenases (COXs), lipoxygenases (LOXs) and epoxygenases of the
cytochrome P-450 family (CYPs) mainly from the free fatty acids.
Related isoprostanes are formed by non-enzymic means (autoxidation) from the same precursors
in esterified form in situ in lipids within membranes.
While these oxylipins are usually treated separately in biochemical textbooks, it should not be forgotten that they are in fact fatty acids.
Some oxylipins are occasionally present in esterified form in phospholipids (and in glycosyldiacylglycerols in plants), and they may even operate as lipid mediators in this form (see our web page on oxidized phospholipids). The eicosanoids and docosanoids are highly potent at nanomolar concentrations in the regulation of innumerable processes in cells, often in relation to inflammatory responses, pain and fever. Some of these are pro-inflammatory and others are anti-inflammatory, so the balance between the two groups must be optimized for the maintenance of health. Fatty acids are the biosynthetic precursors of many insect pheromones and of secondary metabolites in plants, and it is surely of evolutionary significance that plant hormones, such as the jasmonates, are derived from the essential fatty acids and have structural similarities to prostaglandins.
Many other fatty acids are not essential in animal tissues in the above sense but nonetheless are vital to tissues even when they are saturated or monoenoic in nature. For example, palmitic acid is a required biosynthetic precursor of the sphingoid bases and thence of all sphingolipids, while palmitic and myristic acids are covalently linked constituents of proteolipids and are required to direct them to appropriate membrane locations. On the other hand, excessive amounts of these in the human diet are often regarded as harmful (I will not enter into this debate).
Tri-, Di- and Monoacylglycerols
Virtually all the natural fats and oils of commerce from olive oil to lard consist of triacylglycerols, but here we are concerned with their interactions and metabolism in tissues. As discussed briefly above, triacylglycerols are the main storage lipid in animal and plant cells where they occur as discrete droplets surrounded by a protective monolayer of phospholipids and hydrophobic enzymes. Such ‘lipid droplets’ are now considered to be functional organelles. When required, fatty acids are released by hydrolysis reactions catalysed by lipases under the influence of hormones. One specialized form of adipose tissue, brown fat, is highly vascularized and rich in mitochondria, which oxidize fat so rapidly that heat is generated. This is required in young animals especially and in those recovering from hibernation. Triacylglycerols are the main lipid component in the only material designed in nature entirely as a food, i.e., milk, although triacylglycerols in seeds could perhaps be considered as 'food' for the developing plant embryo until it is capable of photosynthesis.
Triacylglycerols are not merely deposits of lipids. Subcutaneous depots serve as insulation against cold in many terrestrial animals, as is obvious in the pig, which is surrounded by a layer of fat, and for marine mammals. In the latter and in fish, the lipid depots are less dense than water and so aid buoyancy with the result that less energy is expended in swimming. More surprisingly perhaps, triacylglycerols together with the structurally related glyceryl ether diesters and wax esters are the main components of the sonar lens used in echolocation by dolphins and some whales. Despite their hydrophobic nature, triacylglycerols are the major transport lipid in plasma in association with lipoproteins where their chemical inertness is a virtue.
1,2-Diacyl-sn-glycerols are formed as intermediates in the biosynthesis of triacyl-sn-glycerols and of complex glycerolipids, and they operate as second messengers in many cellular processes, modulating biochemical mechanisms by activating members of the protein kinase C family of enzymes. They are formed together with the inositol phosphates by the action of the enzyme phospholipase C on phosphatidylinositol and polyphosphoinositides mainly. Their influence extends to the pathophysiology of cancer and other disease states.
2-Monoacylglycerols are produced when triacylglycerols are digested in the intestines of animals, but they are re-esterified before they are transported elsewhere in the body. In general, monoacylglycerols are minor components of tissues, which are never permitted to accumulate because their strong detergent properties would have a disruptive effect on membranes.
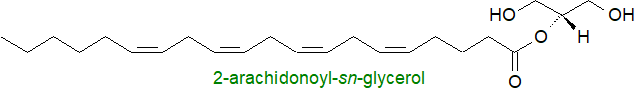
On the other hand, 2-arachidonoylglycerol, another product of phosphatidylinositol metabolism in animal tissues is an endogenous ligand for cannabinoid receptors, i.e., it is an endogenous cannabinoid or endocannabinoid and mediator of inflammatory responses. In the intestines, 2‑oleoylglycerol signals as a 'fat sensor'.
Waxes
Waxes form a thin hydrophobic layer over all the green tissues of plants that is both a chemical and a physical barrier, with wax esters usually as major components accompanied by many different aliphatic compounds. This layer serves many purposes; it limits the diffusion of water and solutes, while permitting a controlled release of volatiles that may deter pests or attract pollinating insects, it provides protection from disease and predators and helps the plants resist drought. Waxes provide waterproofing and protection on the external surface of insects in the same way.

Waxes are storage lipids in marine organisms and in the seeds of the jojoba plant. Bees use wax to produce the rigid structures of honeycombs, while the uropygial (preen) glands of birds secrete waxes, which they use to provide waterproofing for feathers.
Some Other Simple Lipids
Before a fatty acid can be metabolized in tissues, it must usually be activated by conversion to a coenzyme A ester or acyl-CoA, with the fatty acid group linked to the terminal thiol moiety. The thiol ester is a highly energetic bond that permits a facile transfer of the acyl group to receptor molecules such as free hydroxyl or amine groups during the biosynthesis of virtually all lipid classes. Acyl-carnitines assist the transport and metabolism of fatty acids in and out of mitochondria, where they are oxidized for energy production. In so doing, carnitine maintains a balance between free and esterified coenzyme A in cells.
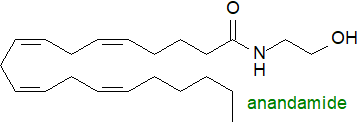 Long-chain N-acylethanolamines are ubiquitous trace constituents of animal and human
cells with important pharmacological properties controlled by the nature of the fatty acid constituent.
Anandamide or N‑arachidonoylethanolamine has attracted interest
because of it exerts marked effects through binding to and stimulating cannabinoid receptors.
Like 2‑arachidonoylglycerol, discussed above, it is an ‘endocannabinoid’.
In contrast, oleoylethanolamine is a regulator of food intake with potential as an anti-obesity drug,
while palmitoylethanolamine is an anti-inflammatory agent, and stearoylethanolamine is a pro-apoptotic agent.
Changing the nature of the amide moiety changes how it acts in cells, and the simple oleamide
molecule or cis-9,10-octadecenamide, isolated from the cerebrospinal fluid of sleep-deprived cats, has been identified as the signalling
molecule responsible for causing sleep.
Many more simple fatty acid derivatives of amines and amino acids are now known, and their biological activities are slowly being revealed.
Long-chain N-acylethanolamines are ubiquitous trace constituents of animal and human
cells with important pharmacological properties controlled by the nature of the fatty acid constituent.
Anandamide or N‑arachidonoylethanolamine has attracted interest
because of it exerts marked effects through binding to and stimulating cannabinoid receptors.
Like 2‑arachidonoylglycerol, discussed above, it is an ‘endocannabinoid’.
In contrast, oleoylethanolamine is a regulator of food intake with potential as an anti-obesity drug,
while palmitoylethanolamine is an anti-inflammatory agent, and stearoylethanolamine is a pro-apoptotic agent.
Changing the nature of the amide moiety changes how it acts in cells, and the simple oleamide
molecule or cis-9,10-octadecenamide, isolated from the cerebrospinal fluid of sleep-deprived cats, has been identified as the signalling
molecule responsible for causing sleep.
Many more simple fatty acid derivatives of amines and amino acids are now known, and their biological activities are slowly being revealed.
Sterols
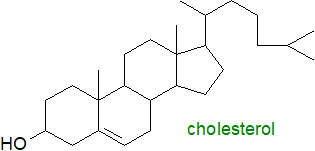 Cholesterol is a ubiquitous component of all animal tissues,
and a biosynthetic precursor of bile acids, vitamin D and steroidal hormones, but much of it is located in membranes.
It occurs in the free form and esterified to long-chain fatty acids (cholesterol esters) in animal tissues, including the plasma lipoproteins.
The main physical task of cholesterol is to modulate the fluidity of membranes by interacting with their phospholipid components, such as
phosphatidylcholine and sphingomyelin, so increasing the degree of order by promoting a 'liquid-ordered phase'.
More intimate protein-cholesterol interactions may regulate certain membrane proteins such as ion channels.
While it may have negative connotations in the popular press, cholesterol is crucial for life.
Cholesterol is a ubiquitous component of all animal tissues,
and a biosynthetic precursor of bile acids, vitamin D and steroidal hormones, but much of it is located in membranes.
It occurs in the free form and esterified to long-chain fatty acids (cholesterol esters) in animal tissues, including the plasma lipoproteins.
The main physical task of cholesterol is to modulate the fluidity of membranes by interacting with their phospholipid components, such as
phosphatidylcholine and sphingomyelin, so increasing the degree of order by promoting a 'liquid-ordered phase'.
More intimate protein-cholesterol interactions may regulate certain membrane proteins such as ion channels.
While it may have negative connotations in the popular press, cholesterol is crucial for life.
In plants, cholesterol tends to be a minor component only of a complex phytosterol fraction that includes campesterol, β-sitosterol, stigmasterol, Δ5‑avenasterol and brassicasterols, while yeasts and fungi have ergosterol as their main sterol component. Plant sterols can regulate membrane fluidity and permeability, and they modulate membrane-bound enzymes in a similar manner to cholesterol in animal membranes. Some bacteria produce structurally related lipids, the hopanoids, which have been termed 'sterol surrogates'.
Cholesterol is a polyisoprenoid molecule or triterpene, and many more related terpenoids, including tocopherols, retinoids and dolichols, are important to life as vitamins, antioxidants, cofactors, and so forth; many of these are discussed in separate web pages on this site.
Complex Lipids in Membranes
Cellular membranes are semi-permeable barriers that enclose and define cells and their sub-cellular compartments by separating and protecting the interiors from their external environment, although they can deform to enable budding, fission and fusion. They control the transport of materials, including signalling molecules, between cells and organelles, and indeed, many biochemical reactions occur within membranes, including energy production and biosynthesis of cellular components. It is evident that the lipid compositions of membranes have evolved to provide a barrier to control the diffusion of ionic solutes and other molecules into cellular compartments where they may not be required. At the same time, the membrane environment for each organelle is distinct and provides a stable molecular platform for metabolic events and for signalling purposes. Cellular membranes are the first site for receipt of extracellular signals, they recruit and stimulate effector molecules, and they are then the launch pad for these molecules throughout the cell.
A characteristic feature of membrane lipids is that they contain both hydrophobic and hydrophilic constituents, i.e., they are amphiphilic. As such, they are weak surfactants, and they tend to form aggregates in bilayer or hexagonal-II arrangements in aqueous media in the normal temperature ranges that prevail in living cells. In natural membranes, there is a mixture of lipid types, which determine that bilayer structures predominate with the same principles governing the distributions of lipids in membranes of animals, plants and microorganisms, although their compositions are very different.
Glycerophospholipids, such as phosphatidylcholine, phosphatidylethanolamine and so forth, together with the sphingolipids, such as sphingomyelin and the glycosphingolipids, and cholesterol (sterols) are structural elements of all cellular membranes in Eukaryotes. In the conventional model of the plasma membrane, polar lipids form a bilayer with the polar head groups oriented towards the aqueous phase while the hydrophobic fatty acyl moieties are arranged internally.
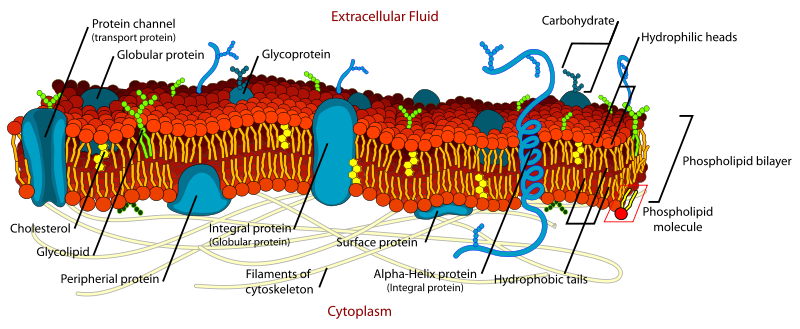
Figure 1. Schematic representation of a cellular membrane. Released into the public domain by its author, LadyofHats,
and Wikipedia Creative Commons and gratefully acknowledged.
It should be noted that lipids are only part of the bilayer, as proteins, such as enzymes, transport systems or signalling receptors, span or intercalate into the bilayer and take up much of the membrane surface. Proteins interact via their basic amino acid residues with the ionic groups of polar lipids via electrostatic interactions, generating a net charge that is mainly negative or zwitterionic, and through interactions by Van der Waals forces between their hydrophobic amino acids and the fatty acid components of lipids. The lipids surrounding a membrane protein are often crucial for its tertiary structure and how it operates, either because of direct interaction between a lipid and a protein or because of the physical properties of the membrane matrix surrounding the protein, e.g., its fluidity, affect how it acts indirectly. Defined lipid species are required to stabilize protein structures, to control the insertion and folding of proteins in membranes, and even for the assembly or polymerization of enzyme complexes with direct effects on their functioning. Lipids determine the plasticity of membranes and regulate the composition and efficient operation of their protein constituents with each organelle within a cell having a distinctive composition that is appropriate for its particular role.
These membrane structures are not static, and free movement is possible within each leaflet (lateral diffusion) and between leaflets (vertical or flip-flop diffusion), while lipid molecules can rotate around their principal axis (rotational diffusion). The lateral and rotational diffusions are responsible for the liquid characteristics of membranes, with the constraint that the hydrophobic chains remain parallel to each other and perpendicular to the surface of the bilayer. In addition, elements of the inner membrane layer are linked to the underlying cytoskeleton.
The distribution of lipids in each of the membrane leaflets is asymmetric with phosphatidylcholine and sphingolipids located in the outer leaflet of the plasma membrane in animals, while phosphatidylethanolamine and anionic phospholipids such as phosphatidylinositol (and polyphosphoinositides) and phosphatidylserine occur primarily in the inner leaflet. Cholesterol is believed to occur in roughly equal proportions in both faces, where it modulates the fluidity of membranes by its interaction with phospholipids. A membrane translocation machinery, which consumes large amounts of energy, is required to maintain this asymmetry. Each glycerophospholipid with its own polar head group and characteristic fatty acid composition modifies the fine structure of a membrane in a unique manner and contributes to its overall properties. Phosphatidylcholine is often the most abundant lipid in membranes, and it has a cylindrical shape, which does not induce curvature. On the other hand, an increased concentration of cone-shaped lipids on one side and inverted cones on the other side of a membrane will bring about curvature, which may be required for membrane transport and fusion processes. Each membrane and membrane leaflet requires a certain lipid composition, including characteristic molecular species of every lipid class, to maintain structural integrity and thence function.
 In Eukaryotes, a balance
between saturated, monoenoic and polyunsaturated fatty acid constituents is required to maintain the optimum degree of fluidity of a given membrane.
Docosahexaenoic acid adopts a more flexible and compact conformation than fatty acids with few double bonds with an average length only
60% of that for oleic acyl chains, and this in turn increases the conformational disorder of saturated chains in mixed-chain phospholipids.
To add to the complexity, the position of double bonds in the acyl chains can affect the motion of transmembrane proteins, the adsorption
of peripheral proteins, and some mechanical aspects of membrane structure.
When ether and plasmalogen forms of lipids are considered, membranes can contain a thousand molecular species of
phospholipids, and it is obviously impossible to quantify the relative contributions of each of these to the physical and biological
properties of membranes, so some general assessments only are possible.
In bacterial membranes, branched chain and cyclopropane fatty acids modify the fluidity in an analogous manner.
In Eukaryotes, a balance
between saturated, monoenoic and polyunsaturated fatty acid constituents is required to maintain the optimum degree of fluidity of a given membrane.
Docosahexaenoic acid adopts a more flexible and compact conformation than fatty acids with few double bonds with an average length only
60% of that for oleic acyl chains, and this in turn increases the conformational disorder of saturated chains in mixed-chain phospholipids.
To add to the complexity, the position of double bonds in the acyl chains can affect the motion of transmembrane proteins, the adsorption
of peripheral proteins, and some mechanical aspects of membrane structure.
When ether and plasmalogen forms of lipids are considered, membranes can contain a thousand molecular species of
phospholipids, and it is obviously impossible to quantify the relative contributions of each of these to the physical and biological
properties of membranes, so some general assessments only are possible.
In bacterial membranes, branched chain and cyclopropane fatty acids modify the fluidity in an analogous manner.
While the need to form stable bilayers is a primary prerequisite for all membranes, there is also a requirement for a potential ability of the lipids to form non-bilayer structures for some membrane-associated cell processes, and short-lived non-bilayer structures with some lipid components are probably formed in the processes of fusion and fission of lipid bilayers and for cell division. The activities of certain membrane-associated proteins can be modulated by lipids that do not form lamellar layers.
Many proteins are directed to membranes by covalent linkages to lipids, such as the glycosyl phosphatidylinositol anchors, or by modification with myristoyl, palmitoyl, prenyl or sterol moieties (proteolipids - see below). The sphingolipids together with cholesterol arrange themselves into sub-domains or 'rafts' (see below) with certain membrane enzymes, and they act to compartmentalize these for optimal efficiency. In an alternative model, it is suggested that that lipids cannot create stable membrane subregions independently of proteins and then recruit proteins but are assembled together into structural and functional zones through a protein-lipid code.
Glycerophospholipids
Phospholipids have multiple roles in cells apart from establishing structural barriers as membrane components. They provide a matrix for the assembly and subsequent efficiency of a wide variety of enzymes, they participate in the synthesis of macromolecules, and they act as molecular signals to influence metabolic events. Anionic lipids like phosphatidylinositol and its phosphorylated derivatives, which are concentrated on the cytoplasmic leaflet of membranes, exert a control on the electronic charge at the membrane-cytosol interface and consequently on many aspects of membrane trafficking, including vacuole formation, transport and fusion. Lipids of this kind are associated with particular organelles in cells, where in combination with other signalling molecules they can recruit effector proteins that are required for each cellular compartment.
Phosphatidylcholine is a zwitterionic lipid and is usually the most abundant phospholipid in membranes of animals and plants, constituting a high proportion of the outer leaflet of the plasma membrane, and it is an integral component of the lipoproteins in plasma. It can serve as a source of diacylglycerols as signalling molecules, while the plasmalogen form may provide much of the arachidonate for eicosanoid production (though secondary to phosphatidylinositol). As phosphatidylcholine is the biosynthetic precursor of sphingomyelin and thence of many signalling molecules, it has an influence on innumerable metabolic pathways.
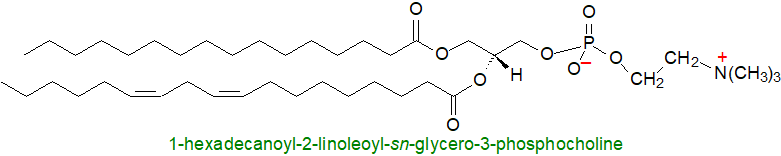
Platelet-activating factor or 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, is a closely related lipid, and among the innumerable functions that have been documented, it effects the aggregation of platelets at concentrations as low as 10-11M, it is a mediator of inflammation, and it is part of the mechanism of the immune response.
Phosphatidylethanolamine is another major component of membranes, especially in bacteria, with distinctive physical properties because of its small head group and hydrogen bonding capacity. In the model bacterium Escherichia coli, it supports transport by the lactose permease, and other transport systems may require or be stimulated by it. In animal and plants, it acts as a 'chaperone' during the assembly of membrane proteins to guide the folding path for the proteins and to aid in the transition from the cytoplasmic to the membrane environment.
 Phosphatidylinositol is an acidic or anionic phospholipid,
a high proportion of which in animal membranes consists of the 1‑stearoyl,2-arachidonoyl species,
which is of considerable importance in cells.
Phosphatidylinositol mono-, di- and triphosphates, reversibly phosphorylated on the 3, 4, or 5 positions of inositol, influence innumerable
cellular processes such as signalling and trafficking, and specific isomers are often identified with particular cellular compartments.
Thus, phosphatidylinositol 5-phosphate can be phosphorylated to generate phosphatidylinositol 4,5-bisphosphate and thence to
phosphatidylinositol 3,4,5-trisphosphate, which activates pathways required for cell growth and survival.
They are the primary precursors of 1,2-diacyl-sn-glycerols as signalling molecules (see above), together with
water-soluble inositol phosphates, which are required for many other purposes in cells.
The production of these various metabolites are elements in phosphoinositide and phosphatidylinositol cycles.
In addition, phosphatidylinositol and its metabolites are the main source of arachidonic acid for eicosanoid
and endocannabinoid biosynthesis.
In all eukaryotes, phosphatidylinositol can serve as an anchor to link proteins covalently to the external leaflet of the plasma membrane via
complex glycosyl bridges, i.e., glycosyl-phosphatidylinositol(GPI)-anchored proteins,
to enable their efficient operation in the extracellular environment.
Phosphatidylinositol is an acidic or anionic phospholipid,
a high proportion of which in animal membranes consists of the 1‑stearoyl,2-arachidonoyl species,
which is of considerable importance in cells.
Phosphatidylinositol mono-, di- and triphosphates, reversibly phosphorylated on the 3, 4, or 5 positions of inositol, influence innumerable
cellular processes such as signalling and trafficking, and specific isomers are often identified with particular cellular compartments.
Thus, phosphatidylinositol 5-phosphate can be phosphorylated to generate phosphatidylinositol 4,5-bisphosphate and thence to
phosphatidylinositol 3,4,5-trisphosphate, which activates pathways required for cell growth and survival.
They are the primary precursors of 1,2-diacyl-sn-glycerols as signalling molecules (see above), together with
water-soluble inositol phosphates, which are required for many other purposes in cells.
The production of these various metabolites are elements in phosphoinositide and phosphatidylinositol cycles.
In addition, phosphatidylinositol and its metabolites are the main source of arachidonic acid for eicosanoid
and endocannabinoid biosynthesis.
In all eukaryotes, phosphatidylinositol can serve as an anchor to link proteins covalently to the external leaflet of the plasma membrane via
complex glycosyl bridges, i.e., glycosyl-phosphatidylinositol(GPI)-anchored proteins,
to enable their efficient operation in the extracellular environment.
A further acidic lipid, phosphatidylserine, contributes substantially to non-specific electrostatic interactions in the inner leaflet of membranes. This normal distribution is disturbed during platelet activation and in the process of cellular apoptosis when the lipid is transferred from the inner to the outer leaflet of the plasma membrane and acts as an "eat me" signal to scavenger cells. Phosphatidylserine chelates with calcium to act as the foundation for bone growth, and it is a cofactor for many enzymes, including protein kinase C, which is involved in signal transduction.
Cardiolipin or diphosphatidylglycerol is a unique acidic phospholipid with four acyl groups, and in the mitochondria of cells, its primary location, it has an intimate association with those enzymes concerned with oxidative phosphorylation. Indeed, it is integrated into their quaternary structure, where it is an essential component of the interface between the enzymes and their environment and may stabilize the catalytic sites. In higher plants, cardiolipin is an integral constituent of the photosystem II complexes, which provide energy to cells, and where it may be required for the maintenance of their structures and thence efficient operation. Phosphatidylglycerol is the precursor for cardiolipin, and is a minor component of animal membranes but is much more abundant in plants and bacteria.
Phosphatidic acid is generally a minor component of cells, but it is an intermediate in the biosynthesis of all other phospholipids. It is known to be a signalling molecule in animal cells by binding to certain proteins, and it is utilized for this purpose in higher plants where it is formed rapidly in response to stresses of all kinds.
Bis(monoacylglycero)phosphate has a unique stereochemistry and occurrence in the endosomal membranes of cells, where it is relatively resistant to enzymatic hydrolysis.
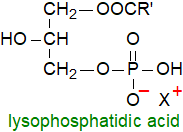 Lysophospholipids, i.e., with only one mole of fatty acid per mole of lipid, were long
thought to be merely intermediates in the biosynthesis of phospholipids that if allowed to accumulate were potentially disruptive to cells
as powerful surfactants.
This view has changed, and lysophosphatidic acid is of great interest as it has been shown
to be a signalling molecule that is dependent on receptor mechanisms.
It is produced by a wide variety of cell types with most mammalian cells expressing receptors for it, and it activates of protein kinases,
adenyl cyclase and phospholipase C and much more.
Interest was stimulated by a finding that lysophosphatidic acid is elevated significantly in the plasma of ovarian cancer patients
compared to healthy controls, so that it may represent a useful marker for the early detection of the disease.
Other lysophospholipids, including the sphingolipid analogue, sphingosine-1-phosphate (below), are also signalling molecules.
Lysophospholipids, i.e., with only one mole of fatty acid per mole of lipid, were long
thought to be merely intermediates in the biosynthesis of phospholipids that if allowed to accumulate were potentially disruptive to cells
as powerful surfactants.
This view has changed, and lysophosphatidic acid is of great interest as it has been shown
to be a signalling molecule that is dependent on receptor mechanisms.
It is produced by a wide variety of cell types with most mammalian cells expressing receptors for it, and it activates of protein kinases,
adenyl cyclase and phospholipase C and much more.
Interest was stimulated by a finding that lysophosphatidic acid is elevated significantly in the plasma of ovarian cancer patients
compared to healthy controls, so that it may represent a useful marker for the early detection of the disease.
Other lysophospholipids, including the sphingolipid analogue, sphingosine-1-phosphate (below), are also signalling molecules.
Glycosyldiacylglycerols
Mono- and digalactosyldiacylglycerols and sulfoquinovosyldiacylglycerol (a sulfonolipid) are major components of membranes of chloroplasts and related organelles, and indeed these are the most abundant lipids in all photosynthetic tissues, including those of higher plants, algae and cyanobacteria. They may substitute in part for phospholipids, especially when phosphorus is limiting, although the ability of monogalactosyldiacylglycerols to form inverted micelles may be a factor in determining membrane structure and for interactions with proteins. The thylakoid membrane where photosynthesis occurs in plants has an asymmetric distribution of glycolipids, with much of the digalactosyldiacylglycerol on the luminal leaflet, where it may assist the movement of protons along the membrane surface to the ATPase. While many different functions have been ascribed to these lipids, they are certainly essential for photosynthesis.
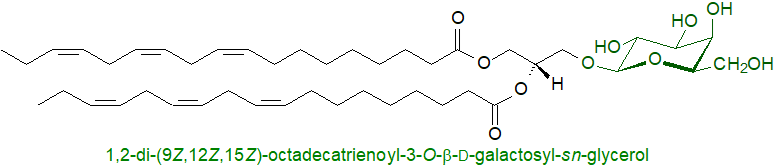
Although glycosyldiacylglycerols have been found in animal tissues, they are usually present in rather small amounts, and their role in mammalian membranes is poorly understood. On the other hand, the lipid sulfate seminolipid or 1-O-hexadecyl-2-O-hexadecanoyl-3-O-β-D-(3'-sulfo)-galactopyranosyl-sn-glycerol, which was first found in mammalian spermatozoa and testes, is known to be necessary for spermatogenesis and is a component of myelin in the central nervous system.
Sphingolipids
Sphingolipids are characterized by the presence of a long-chain or sphingoid base, such as sphingosine, to which a fatty acid is linked by an amide bond and usually with the primary hydroxyl group attached to complex phosphoryl or carbohydrate moieties. They operate in tissues in innumerable ways that are quite distinct from those of the complex glycerolipids. While sphingomyelin, a sphingophospholipid, has structural similarities to phosphatidylcholine, it has very different physical and biological properties, and the complex oligoglycosylceramides and gangliosides have no true parallels among the glycerolipids. The complex glycophosphosphingolipids (phytoglycosphingolipids) in plants do not resemble the complex sphingolipids in animals.
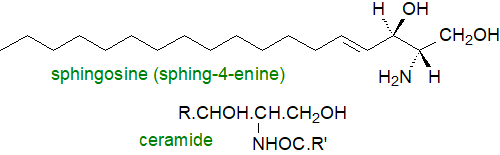
Free sphingoid bases are found at trace levels only in tissues, but they are mediators of many cellular events; they inhibit the enzyme protein kinase C, and they are inhibitors of cell growth, although they stimulate cell proliferation and DNA synthesis. Some of the structural features of the long-chain bases are only introduced after they are esterified with long-chain fatty acids to form ceramides, which are the primary precursors of the complex sphingolipids, but also have a role in cellular signalling and in the regulation of apoptosis and of cell differentiation, transformation and proliferation. In contrast, sphingosine-1-phosphate is a vital sphingosine metabolite that promotes cellular division (mitosis) as opposed to apoptosis, so that the balance among the former and ceramide, ceramide-1-phosphate and sphingosine levels in cells is critical. In fact, the biosynthesis and catabolism of sphingolipids involves numerous metabolites, many of which have unique and characteristic activities in cells. In animals the relationships between these metabolites have been rationalized in terms of a 'sphingomyelin cycle', in which each of the various compounds has a characteristic metabolism. Some comparable sphingolipid pathways occur in plants (although not via sphingomyelin).
Sphingomyelin is by far the most abundant sphingolipid in animal tissues. As well as serving as a source of crucial cellular metabolites, it is a building block of membranes and like its glycerolipid analogue and biosynthetic precursor phosphatidylcholine, it tends to be most abundant in the plasma membrane of cells where it occurs in the outer leaflet. The sphingolipids in general contain high proportions of longer-chain saturated and monoenoic fatty acids, often accompanied by high proportions of 2-hydroxy but not polyunsaturated fatty acids.
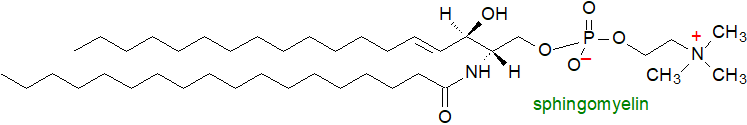
Sphingomyelin and other sphingolipids together with cholesterol are often located in an intimate association in sub-domains or 'rafts' (or related structures termed 'caveolae') of membranes, but notably in the plasma membrane. These are laterally segregated regions that form because of selective affinities between sphingolipids and membrane proteins. As sphingolipids containing long saturated acyl chains, they pack more tightly together, thus giving sphingolipids much higher melting temperatures than glycerophospholipids. This tight acyl chain packing is essential for raft lipid organization, since the differential packing facility of sphingolipids and cholesterol in comparison with glycerophospholipids leads to spontaneous phase separation in the membrane, thus giving rise to the sphingolipid-rich regions ('liquid-ordered' phase) surrounded by glycerophospholipid-rich domains ('liquid-disordered' phase). The ordered phases are relatively resistant to attack by detergents, a property that was once used to define them. One result of this process is that rafts contain a variety of different proteins, including glycosyl-phosphatidylinositol (GPI)-anchored proteins and tyrosine receptor kinases. These enable rafts to operate in a stable and optimal manner. Comparable micro-domains or rafts that are enriched in sphingolipids (other than sphingomyelin), sterols and proteins have been detected in the plasma membrane of plant cells.
Monoglycosylceramides or cerebrosides are common constituents of membranes of animals and plants. Galactosylceramide is the principal glycosphingolipid in brain tissue and myelin, while glucosylceramide is a major constituent of skin lipids and is the source of the unusual complex ceramides that are found in the stratum corneum. In plants, glucosylceramides can elicit defence responses against fungal attack, and they may assist plants to withstand stresses brought about by cold and drought.
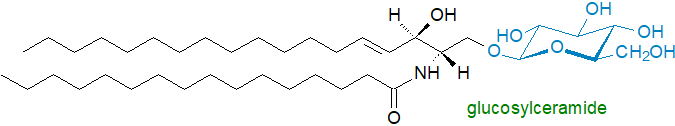
The membranes of animals contain a wide range of complex oligoglycosylceramides for which glucosylceramide and thence lactosylceramide are the biosynthetic precursors. Forms with several hundred different head groups that differ in the numbers, types and arrangements of the carbohydrate moieties have been characterized, and most of these occur on the external leaflet of the plasma membrane in rafts, where they are components of the body's immune defence system, both as cellular immunogens and as antigens. Certain glycosphingolipids determine the antigenicity of blood group determinants, while others bind to toxins or bacteria. Some operate as receptors for cellular recognition, and they can be specific for particular tissues or tumours.
Glycosphingolipid sulfates are highly polar acidic molecules that are involved in the transport of sodium and potassium ions and osmoregulation in animal tissues, and they may protect the intestinal mucosa against digestive enzymes. Gangliosides are complex oligoglycosylceramides containing sialic acid residues and are highly polar and acidic. As cell-type antigens that control the growth and differentiation of cells, they play a significant part in the interactions between cells in the immune defence system. They are necessary for myelination in brain and other nervous tissues, where they are most abundant. As gangliosides act as receptors for interferon, epidermal growth factor, nerve growth factor, insulin and many other metabolites, they can regulate cell signalling. Certain gangliosides bind to some bacterial toxins, and they mediate interactions between microbes and host cells during infections.
Proteolipids and Lipoproteins
Proteins that contain covalently bound fatty acids or other lipid moieties, such as isoprenoids, cholesterol and glycosylphosphatidylinositol, are widespread in nature. The term proteolipid can be used to define such complexes and to differentiate them from the plasma lipoproteins, which have very different structures, occurrence and functions. Two main types of protein with a fatty acid modification have been described, i.e., those with only myristoyl and those with predominantly palmitoyl moieties with differing types of covalent linkage, amide or thiol ester, respectively. The prenylated lipids contain an isoprenoid group, farnesyl or geranylgeranyl, linked via a sulfur atom (thiol ether bond) to the protein, while the so-called "hedgehog” proteins, which are required for cellular development, are modified covalently by both cholesterol and N-palmitoyl moieties. The hormone ghrelin and the Wnt proteins, participating also in the development of animal tissues, are O-acylated.
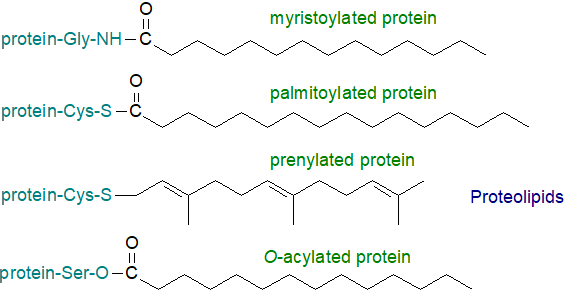
It is now clear that such modifications can determine the activities of proteins and target them to particular subcellular membrane domains, including the rafts in plasma membranes. Thus, both myristoylated and palmitoylated proteins are targeted to rafts (as are the GPI-anchored proteins), but prenylated lipids are not unless they are also S/N-acylated. It is noteworthy that many signalling proteins are modified by lipids in this way with implications for events at cell surfaces.
Lipoproteins are complex aggregates of lipids and proteins (not bound covalently) that render the lipids compatible with the aqueous environment of body fluids and enable their transport throughout the body of all vertebrates, beginning with uptake of dietary lipids in the intestines. Within the circulation, these aggregates are in a state of constant flux, changing in composition and physical structure as the peripheral tissues take up the various components before the remnants return to the liver. The most abundant lipid constituents are triacylglycerols, free cholesterol, cholesterol esters and phospholipids (phosphatidylcholine and sphingomyelin mainly), although fat-soluble vitamins and some antioxidant molecules are transported in the same way. Free (unesterified) fatty acids and lysophosphatidylcholine are bound to the protein albumin in plasma by hydrophobic forces.
Ideally, the lipoprotein aggregates should be described in terms of the different protein components or apoproteins (or ‘apolipoproteins’), as these determine the overall structures and metabolism, and the interactions with receptor molecules in liver and peripheral tissues. However, the practical methods that have been used to separate different lipoprotein classes for study have determined the nomenclature. Thus, the main groups are classified as chylomicrons (CM), very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL) and high-density lipoproteins (HDL), based on the relative densities of the aggregates on ultracentrifugation. Lipoproteins deliver nutrients to the peripheral tissues and are the key to maintaining a healthy balance of cholesterol, triacylglycerols (or their fatty acid constituents) and other lipids within the body.
Other Lipids
Many more lipids that are essential for life occur in nature than can be described in this brief account, and while lipopeptides, lipopolysaccharides, betaine lipids, fat-soluble vitamins (A, D, E and K), rhamnolipids, arsenolipids and so forth are not discussed in this document, you will encounter them elsewhere on this website. There are a host of lipids that are unique to certain organisms from bacteria to marine invertebrates and beyond that are not described above, and readers will find information on the chemistry and biochemistry of many of these in other web pages on this site.
Suggested Reading
I trust that this brief commentary and the various pages in this website will provide an insight into the importance of lipids to the health and well-being of all living creatures and will stimulate further reading. All the various documents dealing with individual lipid classes here have reading lists attached that should help readers obtain much more information, but the following books and review articles would form a good basis for any lipid library -
- Leonard, T.A., Loose, M. and Martens, S. The membrane surface as a platform that organizes cellular and biochemical processes. Developmental Cell, 58, 1315-1332 (2023); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Ding, W.L., Gu, J.Y., Xu, W.Y., Wu, J., Huang, Y.W., Zhang, S. and Lin, S.X. The biosynthesis and applications of protein lipidation. Chem. Rev., 124, 12176-12212 (2024); DOI.
- Dyall, S.C. and others. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res., 86, 101165 (2022); DOI.
- Farese, R.V. and Walther, T.C. Essential biology of lipid droplets. Annu. Rev. Biochem., 94, 447-477 (2025); DOI.
- Gunstone, F.D., Harwood, J.L. and Dijkstra, A.J. (Editors), The Lipid Handbook (3rd Edition). (CRC Press, Boca Raton) (2007) - see CRC Press.
- Gurr, M.I., Harwood, J.L., Frayn, K.N., Murphy, D.J. and Michell, R.H. Lipids: Biochemistry, Biotechnology and Health (6th Edition). (Wiley-Blackwell) (2016).
- Hannun, Y.A., Merrill, A.H. and Luberto, C. The bioactive sphingolipid playbook. A primer for the uninitiated as well as sphingolipidologists. J. Lipid Res., 66, 100813 (2025); DOI.
- Nicolson, G.L. The Fluid-Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta, Biomembranes, 1838, 1451-1466 (2014); DOI.
- Penkov, S. and Fedorova, M. Membrane epilipidome-lipid modifications, their dynamics, and functional significance. Cold Spring Harbor Persp. Biol., 16, a041417 (2024); DOI.
- Reinisch, K.M., De Camilli, P. and Melia, T.J. Lipid dynamics at membrane contact sites. Annu. Rev. Biochem., 94, 479-502 (2025); DOI.
- Ridgway, N.D. and McLeod, R.S. (Editors) Biochemistry of Lipids, Lipoproteins and Membranes (6th Edition). (Elsevier, Amsterdam) (2016) - see Science Direct - now in a 7th edition (2021).
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: July 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
