Carnitine, Acylcarnitines and β-Oxidation of Fatty Acids
The quaternary ammonium compound and amino acid carnitine (L-3-hydroxy-4-aminobutyrobetaine or L-3-hydroxy-4-N-trimethylaminobutanoic acid) and its acyl esters (acylcarnitines) have many important functions in animals, plants and some microorganisms. In particular, they are essential for the oxidative catabolism of long-chain fatty acids by shuttling them across the mitochondrial membrane from the cytosol into the mitochondrial matrix for β‑oxidation and cellular energy production and thence for maintaining energy homeostasis in the human body. Further, carnitine binds acyl residues resulting from the intermediary metabolism of amino acids (branched-chain especially) to aid their elimination, and it regulates the acyl-CoA/CoA ratio, thereby controlling the activity of several mitochondrial enzymes.
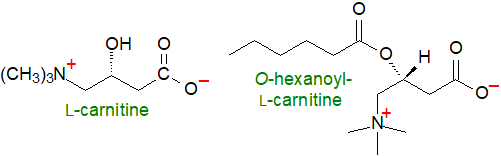
Carnitine is involved in innumerable aspects of metabolism in animals and plants that include mitochondrial homeostasis, neuroprotection, epigenetic regulation, regulation of inflammation and the immune system, and signal transduction, but here the focus is on lipids and their oxidation.
1. Carnitine Synthesis and Dietary Uptake
In animal tissues, L-carnitine concentrations are relatively high, typically between 0.2 and 6 mmol/kg with almost all in the heart and skeletal muscle, and it is synthesised de novo in animal cells by a multi-step process with N-trimethyl-lysine derived from protein degradation as the primary precursor and butyrobetaine as an intermediate. As it is likely that insufficient is produced by this means, most must come from the diet other than in strict vegetarians, and plasma carnitine levels are positively correlated with the dietary intake. The major sources of carnitine for humans are meat, fish and dairy products, which can supply 2 to 12 μmol per day per kg of body weight, as opposed to 1.2 μmol per day per kg of body weight of carnitine synthesised endogenously. For some individuals with genetic or medical disorders and for pre-term infants, who cannot make enough, carnitine is a conditionally essential nutrient.
Dietary carnitine is taken up from the intestinal lumen into the enterocyte by an active transport mechanism, but it passes across the serosal membrane into the circulation by simple diffusion. In some laboratory animals, such as the rat and guinea pig, much of it is acetylated prior to export from the intestines. Synthesis of carnitine occurs in the kidney, liver and brain, and it is transported to other tissues in the circulation before it is taken up by tissues by carnitine transporters at the plasma membrane that are tissue-specific. In the kidney, carnitine and butyrobetaine are reabsorbed efficiently by transport by a high affinity carnitine transporter termed the 'organic cation transporter novel 2 (OCTN2)', located in the renal brush border membrane and necessary for regulation of carnitine homeostasis. Urinary loss is thereby minimized, although excess amounts can be eliminated when necessary.
D-Carnitine does not occur naturally but may be found in some synthetic preparations; it does not participate in the key biological processes but can sometimes interfere with them.
2. Carnitine, Acylcarnitines and Fatty Acid Transport into Mitochondria
The substrates for oxidation and most of the energy production in cells are unesterified fatty acids, which originate from three main sources: exogenous fatty acids that enter cells from the circulation, either from the diet or by mobilization from other tissues, those that arise via synthesis de novo from acetyl-CoA, and those released within the cell by the hydrolysis of phospholipids and triacylglycerols. First, they must be transported to the mitochondria where the enzymes of β-oxidation are located and fatty acids are oxidized, as there is only a minimal contribution from the peroxisomal system for which the preferred substrates are very long-chain fatty acids.
Intracellular unesterified fatty acids must first undergo thio-esterification to coenzyme A, a process catalysed by acyl-CoA synthases and consuming the equivalent of two ATP, with formation of acyl-CoAs, i.e., the activated form of fatty acids. These are usually bound to proteins with characteristic acyl-CoA binding domains and depending upon on the energy status of the cell, they are directed to particular metabolic pathways such as storage or energy production by many different proteins including fatty acid binding proteins and sterol carrier protein 2. Long-chain fatty acyl-CoA thioesters cannot enter the mitochondrial matrix in animals because they are not able to pass through the inner mitochondrial membrane. Instead, carnitine assists the transport and metabolism of fatty acids into mitochondria, and in so doing, carnitine maintains a balance between free and esterified CoA, as an excess of acyl-CoA intermediates is potentially toxic to cells.
In mammals, carnitine acts through the reversible esterification of its 3-hydroxyl group by fatty acyl-CoA esters with subsequent translocation of the acylcarnitines produced from one cellular compartment to another. Carnitine acyltransferases are a family of enzymes responsible for production of acylcarnitines, and among them, they have specificities that cover fatty acids differing in chain-length, branching and other structural features, over the entire range of acyl chain lengths, depending on the cellular location and metabolic purpose, and they enable reversible shuttling of acyl groups between free CoA and carnitine. Thus, carnitine is required to transport fatty acyl groups into mitochondria and to remove any surplus of acyl groups, as well as to export acetyl- and other short-chain acyl groups from peroxisomes via the actions of a short-chain acyl-CoA-specific carnitine acetyltransferase and a medium chain-specific carnitine octanoyltransferase. In consequence, acylcarnitines constitute an appreciable component of the tissue carnitine pool, and tissue and plasma concentrations of carnitine and acylcarnitines are together maintained within relatively narrow limits by a variety of mechanisms, which influence in turn innumerable aspects of carbohydrate and lipid metabolism, including the regulation of insulin secretion by pancreatic β-cells and the determination of tissue insulin sensitivity.
Mammalian carnitine acetyltransferase is found in the mitochondrial matrix, peroxisomal matrix, endoplasmic reticulum and nucleus. In mitochondria, this enzyme regulates the ratio of acetyl-CoA to free CoA to prevent depletion of the latter. When overproduction of acetyl-CoA occurs in muscle during intensive exercise, this process ensures the replenishment of free CoA to facilitate pyruvate metabolism, but when exercise ends, transacetylation of acetylcarnitine provides acetyl-CoA for the Krebs cycle. In peroxisomes, carnitine acetyltransferase aids the export of products of peroxisomal β‑oxidation.
Several enzymes are involved in the various processes that are required to transport long-chain fatty acids (>C8) into mitochondria. Activation of fatty acids to form highly polar thiol esters, i.e., acyl-CoA, occurs on the outer mitochondrial membrane, but to enable transport across the inner mitochondrial membrane, the acyl group is transferred to carnitine with formation of acylcarnitines, which can enter the mitochondria with the assistance of translocases. The transport system consists of three main proteins, the first of which is the enzyme carnitine palmitoyltransferase I (CPTI) present in the mitochondrial outer membrane that forms a complex with a long-chain acyl-CoA synthetase and the voltage-dependent anion channel, while the second transport component is carnitine:acylcarnitine translocase (CACT), an integral inner membrane protein, which forms a complex with carnitine palmitoyltransferase II (CPTII) located on the matrix side of the inner membrane. CPTI differs from CPTII in that it contains an additional domain of about 160 amino acids at its N-terminal that interacts with malonyl-CoA.
Via a porin channel, acylcarnitine crosses the inner mitochondrial membrane in exchange for a carnitine molecule in the opposite direction, thus ensuring that the mitochondrial carnitine concentration remains constant. This reaction is a rate-controlling step for the β-oxidation of fatty acids. Inside the mitochondria, carnitine and acyl-CoA are then regenerated from the internalized acylcarnitines by CPTII before the acyl-CoA is catabolized in two-carbons units by β‑oxidation by the mechanism described below with generation of acetyl-CoA as the main product in normal circumstances.
 |
| Figure 1. The function of carnitine in mitochondrial long-chain fatty acid oxidation. |
This is a greatly simplified account of the process, and at least 25 proteins take part both in the transport and β-oxidation aspects, some of which are organized into at least three functional subdomains, one associated with the outer mitochondrial membrane, one with the inner mitochondrial membrane and the other in the matrix. There are three isoforms of CPTI, each present in characteristic tissues, CPTIA in liver and kidney, CPTIB in heart and skeletal muscle and CPTIC in the brain, and while CPTIA and CPTIB are the main enzymes for the transfer of long-chain fatty acids into mitochondria for oxidation, the lesser-known CPTIC may be a sensor of lipid metabolism in neurons. Malonyl-CoA generated by acetyl-CoA carboxylase - isoform 2 (ACC2) binds with high affinity to each of the CPTI isoforms and acts like a switch to control the sensitivity to this key inhibitory molecule and regulate the transfer of fatty acids into the mitochondrial matrix and thence their oxidation. When the energy demand is high, inhibition of the isoform ACC2 of acetyl-CoA carboxylase increases fatty acid oxidation, while inhibition of the isoform ACC1 decreases fatty acid synthesis. Carnitine palmitoyltransferases are also present in peroxisomes where some similar reactions may occur.
In contrast, short-chain fatty acids at least up to octanoate can permeate the inner mitochondrial membrane in non-esterified form, and they are activated to their CoA-derivatives in the mitochondrial matrix for use in energy-dependent mitochondrial processes.
3. β-Oxidation of Fatty Acids in Mitochondria
The main pathway for the degradation of fatty acids is mitochondrial β-oxidation with acetyl-CoA as the end-product, a key metabolic means for energy homoeostasis in issues such as the liver, heart and skeletal muscle. During fasting, this is of special importance as most tissues other than the brain can thereby utilize fatty acids directly to generate energy. The liver can use this mechanism to convert fatty acids into ketone bodies via acetyl-CoA and acetoacetyl-CoA as an additional source of energy for all tissues, but especially for the brain. Related enzymes and mechanisms are present in all eukaryotes and most prokaryotes. Because of the structural diversity of natural fatty acids, many seemingly homologous enzymes are known for each step of the β-oxidation cycle, including auxiliary enzymes for isomerase and reductase reactions together with acyl-CoA thioesterases, which remove any dead-end products.
Considered simplistically, four enzymes are involved in the cycle of β-oxidation of a long-chain saturated fatty acid as its CoA ester in mitochondria. The first step consists in the formation of a double bond between the C2 and C3 by the enzyme acyl-CoA-dehydrogenase to produce a trans-Δ2-enoyl-CoA while flavin adenine dinucleotide (FAD) is reduced to FADH2. Next, the trans-2 double bond is hydrated stereospecifically by an enoyl-CoA hydratase to form an L‑3‑hydroxyacyl-CoA, which is in turn oxidized by a hydroxyacyl-CoA dehydrogenase to produce a 3-ketoacyl-CoA with nicotinamide adenine dinucleotide (NAD) reduced to NADH. The cycle is completed when the keto intermediate is cleaved between C2 and C3 by thiolysis by a 3-ketoacyl-CoA thiolase to produce acetyl-CoA and the CoA ester of a fatty acid two carbons shorter than the original. A new cycle then commences, and the reaction continues until all the fatty acid is converted to acetyl-CoA units.
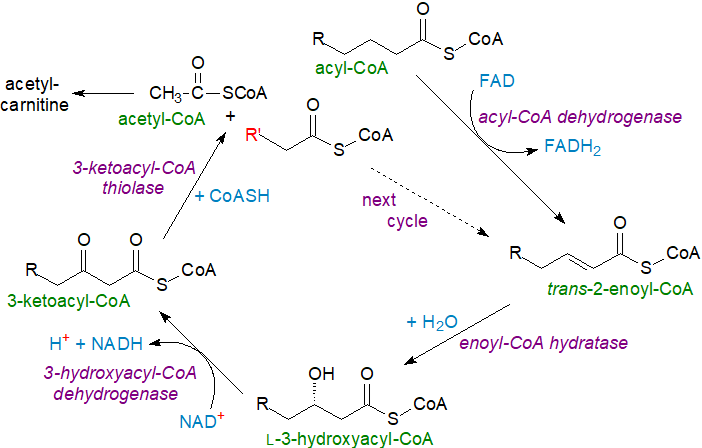 |
| Figure 2. Beta-oxidation of long-chain saturated fatty acids. |
Different isoforms of the enzymes of β-oxidation exist with affinities for fatty acids of different chain lengths, including four acyl-CoA dehydrogenases, and for efficient oxidation to occur, these isoforms must work cooperatively. A further feature of interest is that the last three enzymes in the cycle with a specificity for long-chain fatty acids form a trifunctional enzyme complex on the inner mitochondrial membrane. With odd-chain fatty acids, additional enzymes are required for complete oxidation, and propionyl-CoA produced in the last cycle can be metabolized to succinyl-CoA, which can then enter the citric acid cycle.
With unsaturated fatty acids, the β-oxidation cycle is interrupted when a cis-double bond in position 3 is reached, and three further enzymes are required before the process can be completed. For example, with linoleoyl-CoA (illustrated), three cycles of β‑oxidation yield a 3Z,6Z‑12:2 intermediate, which must be isomerized by an enoyl-CoA isomerase to form 2E,6Z-12:2-CoA. This can now undergo one cycle of β‑oxidation to yield 4Z-10:1-CoA, which is acted upon by an acyl-CoA dehydrogenase to produce 2E,4Z-10:2-CoA before this in turn is the substrate for an NADPH-dependent 2,4‑dienoyl-CoA reductase to form 3E-10:1-CoA. After further reaction with an enoyl-CoA isomerase to yield 2E-10:1-CoA, four cycles of β-oxidation occur to yield acetyl-CoA as the final product.
 |
| Figure 3. Beta oxidation of linoleoyl-CoA. |
Then, acetyl-CoA is used directly for the generation of energy by the tricarboxylic acid cycle, or it can be converted to acetylcarnitine via the action of carnitine acetyltransferase for transport out of the mitochondria to be utilized elsewhere.
Feed-back regulation occurs as each of the enzymes of β-oxidation is inhibited by its own fatty acyl-CoA product, while the reaction is regulated allosterically by the ratios of NADH/NAD+ and acetyl-CoA/CoA; a rise in either ratio is inhibitory. Transcriptional regulators of the enzymes include the peroxisome proliferator-activated receptors (PPARs) and a transcription factor coactivator PGC-1a (and involve the retinoid X receptor), but the details of the process are tissue dependent.
During cold exposure in mammals, thermogenesis is a protective measure against a reduction in ambient temperature, and recent research suggests that cold stimulates adipocytes in white adipose tissue to release unesterified fatty acids that activate the nuclear receptor HNF4α, which is required for acylcarnitine production in the liver. This organ then undergoes a metabolic switch to produce acylcarnitines, which are transported in plasma to brown adipose tissue to serve as a fuel for thermogenesis. At the same time, uptake of acylcarnitines into white adipose tissue and liver is blocked. However, the quantitative contribution of acylcarnitines from the liver to thermogenesis in brown adipose tissue has still to be determined.
Energy produced: The importance of β-oxidation of fatty acids is seen from the fact that the process generates twice as much energy (39 KJ g‑1) as can be obtained from glucose (15 KJ g-1). Energy is produced at each stage of the process, and stepwise shortening of acyl-CoA generates one molecule of FADH2 and NADH for every two-carbon unit released, while each acetyl-CoA molecule yields 3 molecules of NADH, 1 molecule of FADH2 and 1 of GTP via the tricarboxylic acid cycle. In total, the degradation of one molecule of palmitic acid produces approximately 130 molecules of ATP.
4. Ketone Bodies
During periods of fasting and some other physiological conditions, water-soluble molecules that may contain a ketone group, such as acetoacetate, β‑hydroxybutyrate, and the spontaneous breakdown product of acetoacetate, acetone, are produced from acetyl-coA by the liver after oxidation of fatty acids. These are known as "ketone bodies" and the process as "ketogenesis", and they are readily transported to extrahepatic tissues and converted back to acetyl-CoA, which then enters the citric acid cycle and is oxidized in mitochondria for energy (ATP production). In the brain, they are a major energy source and they provide acetyl-CoA for fatty acid synthesis, but they also support energy demand in oxidative organs, such as the heart and skeletal muscle, especially during exercise. In the liver, ketone bodies are produced from fatty acids and other precursors under conditions of intense gluconeogenesis, as when the liver glycogen stores have been depleted, and ketosis is defined as the metabolic state in which circulating ketone bodies are elevated above normal levels. They are produced in excess during untreated (or inadequately treated) type 1 diabetes mellitus.

A key enzyme in the anabolic utilization of ketone bodies is cytosolic acetoacetyl-CoA synthetase, which catalyses the coupling of acetoacetate to CoA in a reaction dependent on ATP to produce acetoacetyl-CoA. This can be utilized to produce acetyl-CoA for fatty acid biosynthesis, or it can be used directly to produce HMG-CoA for sterol synthesis, processes that bypass citrate and ATP citrate lyase. When present, ketone bodies are better substrates for lipid synthesis than glucose and indeed can suppress the use of this for lipogenesis. While there is interest in ketogenic diets as a potential means of weight loss, any effects are likely to be small. In addition to energy production, ketones support whole-body physiology and are signalling molecules that serve as ligands for G protein coupled receptors and modifiers of gene expression by regulating DNA post-translational modifications. Dysregulation of ketone metabolism contributes to the pathologies of some cardiometabolic diseases and can lead to insulin resistance.
5. Peroxisomal β-Oxidation in Animals
To complete the picture, some β-oxidation of fatty acids occurs in peroxisomes in animals, which interact continuously with other subcellular organelles including lipid droplets, lysosomes, the endoplasmic reticulum, and mitochondria, by means of tethering proteins that enable efficient transfer of metabolites. In relation to lipid metabolism, peroxisomes are most active for catabolism of very-long-chain (>C24), methyl-branched and dicarboxylic fatty acids, some of which cannot be degraded in mitochondria, as well as being responsible for an essential step in the biosynthesis of ether lipids.
Despite similarities in the reactions, mitochondria and peroxisomes have different catalytic proteins and electron transport chains, and the orientations of metabolites relative to the enzymes differ; the reaction is not coupled to ATP synthesis. For example, acyl-CoA oxidase (three isoforms - ACOX1, 2 and 3), the first enzyme in peroxisome β‑oxidation transfers hydrogen to oxygen to produce H2O2 while carrying out a dehydrogenation reaction to produce the first α,β-unsaturated intermediate, trans-2-enoyl-CoA. i.e., molecular oxygen rather than the electron transfer flavoprotein is the electron donor. Two bi-functional proteins (DBP and LBP (dicarboxylic acids only)), which may contain cis-3,trans-2-enoyl-CoA epimerases, carry out the next two steps of hydration (enoyl-CoA hydratase) then dehydrogenation (β‑hydroxyacyl-CoA dehydrogenase) to produce 3-keto intermediates. Finally, two β‑ketothiolase (with a CoA-acyltransferase) generate acetyl CoA and a new acyl-CoA two atoms shorter, which continues through the cycle as far as octanoate, which can then be subjected to mitochondrial β-oxidation.
 |
| Figure 4. Peroxisomal beta-oxidation of fatty acids. |
As peroxisomes lack the enzymes of the citric acid cycle, any acetyl-CoA produced cannot be degraded to CO2 and H2O but rather must be transferred to mitochondria either as acetylcarnitine or acetate for this to occur.
Monomethyl branched-chain fatty acids are rapidly synthesised and then catabolized in brown adipose tissue by ACOX2 in peroxisomes to support energy dissipation. Some fatty acids with multiple methyl branches, e.g., those derived from phytol such as phytanic acid, are not amenable to β‑oxidation, but they can be degraded by α‑oxidation in peroxisomes; in the genetic disorder Refsum disease, this system is impaired and phytanic acid accumulates in the plasma lipids. Peroxisomal oxidation can prevent the accumulation of VLCFAs in tissues in neurological diseases. Plasma 24:0- and 26:0-lysophosphatidylcholines are biomarkers for the diagnosis of aberrant peroxisomal β-oxidation and can include the Zellweger spectrum disorders and X-linked adrenoleukodystrophy.
In a further minor reaction, fatty acids can undergo ω-oxidation with the eventual production of dicarboxylic acids, and carnitine esters of these can be present in the plasma of patients with peroxisome biogenesis disorders.
6. Carnitine, β-Oxidation and Health
Deficiencies in any of the enzymes associated with the metabolism of carnitine and acylcarnitines that inhibit the formation of the latter can cause an accumulation of acyl-CoA of certain chain-lengths, and these be toxic if they are not removed by some means; acylcarnitines with highly polyunsaturated components are less cardiotoxic than are saturated and monounsaturated analogues. As the acylation state of carnitine in the plasma reflects the composition of the cytosolic acylcarnitine pool, this serves as a diagnostic marker for the equilibrium between acyl-CoA and acylcarnitine species. When unusual acylcarnitines are identified in biological fluids in patients at much higher concentrations than in healthy individuals, the chain lengths can be indicative of certain enzymic disorders in the β‑oxidation pathway and enable recognition of the disease involved. There can be consequent effects upon the compositions of the simple and complex lipids (lipidome) within tissues.
Inherited defects of fatty acids oxidation are transmitted as autosomal recessive traits in humans, and more than thirty inherited metabolic diseases can be identified by screening for the presence of acylcarnitines in the blood and urine of new-born infants, although thankfully none of these is common (1 in ~10,000 live births), and that found most often is medium-chain acyl-CoA dehydrogenase deficiency. The clinical manifestations can vary from multi-organ failure in the neonate with a fatal outcome to late-onset symptoms associated with significant disabilities. Dysfunction of the carnitine transporter OCTN2 is the cause of Primary Carnitine Deficiency (PCD), which is characterized by systemic loss of carnitine with severe clinical manifestations, including fertility issues in both males and females. L-Carnitine deficiency is often seen in chronic haemodialysis patients, and in consequence, it has been termed a conditionally essential metabolite ('metabimin').
 In adults,
these defects are usually characterized by the accumulation of medium-chain and long-chain (3-hydroxy) carnitine derivatives,
and they are associated with lipotoxicity in several organs that can lead to life-threatening complications and comorbidities.
Acylcarnitines produced as products of incomplete mitochondrial fatty acid oxidation have been detected in obesity,
type 2 diabetes, cardiovascular disease, encephalopathy, and diseases of the eye, while patients with peroxisomal biogenesis disorders,
such as Zellweger syndrome or X-linked adrenoleukodystrophy, or with acidemias have abnormal profiles of circulating acylcarnitines.
Often, the disorders result in underproduction of acetyl-CoA and dysfunction of the Krebs cycle.
In addition, the gut microbiota can convert carnitine into trimethylamine and trimethylamine N-oxide with the latter associated with
cardiovascular risks, including atherosclerosis, heart attacks and stroke.
In adults,
these defects are usually characterized by the accumulation of medium-chain and long-chain (3-hydroxy) carnitine derivatives,
and they are associated with lipotoxicity in several organs that can lead to life-threatening complications and comorbidities.
Acylcarnitines produced as products of incomplete mitochondrial fatty acid oxidation have been detected in obesity,
type 2 diabetes, cardiovascular disease, encephalopathy, and diseases of the eye, while patients with peroxisomal biogenesis disorders,
such as Zellweger syndrome or X-linked adrenoleukodystrophy, or with acidemias have abnormal profiles of circulating acylcarnitines.
Often, the disorders result in underproduction of acetyl-CoA and dysfunction of the Krebs cycle.
In addition, the gut microbiota can convert carnitine into trimethylamine and trimethylamine N-oxide with the latter associated with
cardiovascular risks, including atherosclerosis, heart attacks and stroke.
As β-oxidation is utilized for energy production in breast cancer, targeting this pathway is considered to be a potential strategy for treatment, and as carnitine palmitoyltransferase isoforms, especially CPT1A, are over-expressed in certain cancers, they are likewise seen as potential drug targets. In particular, the carnitine shuttle system is a key regulator of ferroptosis in cancer cells. Dysfunction of the carnitine cycle in general can influence tumorigenesis and progression of the disease by altering intracellular oxidative and inflammatory status or regulating tumour metabolism.
In addition to its role in energy metabolism with some antioxidant properties, L-acetyl-carnitine can cross the blood-brain barrier, where it modulates brain neurotransmitters, such as acetylcholine, serotonin and dopamine, and acts on neurotrophic factors by modifying the activity of the relevant genes. It is marketed as a drug to alleviate neuropathic pain, and there are suggestions that it may help with cognitive and mood disorders. Carnitine is important to lipid metabolism in brain in general, where fatty acid oxidation is less significant though still relevant, and there is interest in its use for neuroprotection in disorders that include traumatic and other brain injuries and Alzheimer's disease. Although there appear to be few controlled clinical studies in humans, supplements of acetyl-carnitine are reported to improve the cognitive abilities of patients in the milder, early stages of Alzheimer's Disease. On the other hand, elevated levels of palmitoyl-L-carnitine are produced in aged mice, and they may cause mitochondrial disruption and contribute to the pathology of neurological diseases. Acylcarnitines are utilized in the synthesis of lipids in the brain and thence regulate membrane compositions.
The use of L-carnitine ('levocarnitine') and short-chain acylcarnitines (acetylcarnitine and propionylcarnitine) as proprietary dietary supplements is widespread because of reports that acetylcarnitine deficiency might lead to neurologic and psychiatric diseases. Carnitine supplementation has helped some patients who have angina secondary to coronary artery disease or who have severe heart arrhythmias, which quickly deplete their stores of carnitine, and they may be useful in many forms of metabolic liver diseases and heart muscle disease. Acetyl-carnitine is more easily absorbed from the gut, crosses the blood-brain barrier more readily, and as a bonus supplies acetate, which may boost mitochondrial metabolism both for energy and acetylcholine production. While the potential of carnitine, acetyl-carnitine and other esters as therapeutic agents is perhaps controversial, there is no doubt that they are lifesaving in patients with rare genetic disorders of carnitine metabolism such as PCD.
7. Carnitine and β-Oxidation in Plants, Yeasts and Bacteria
Peroxisomal β-oxidation is the main mechanism for the degradation of fatty acids in plants and yeasts such as Saccharomyces cerevisiae, and a process that is mechanistically similar occurs in bacteria but by an enzyme cycle in the cytosol.
Although it has long been known that carnitine per se is present at very low levels in the tissues of many plants, including seeds and leaves, it was only recently that the presence of acylcarnitines was demonstrated definitively. Free L-carnitine is present in plants in µg g-1 amounts, while the acylcarnitine content is 17 to 38 ng g-1. Why acylcarnitines are required is still relatively obscure, but they may take part in a carnitine shuttle system in mitochondria analogous to that in animal cells, and they have been associated with anabolic pathways of lipid metabolism during development, including the biosynthesis of membrane and storage lipids. There is some evidence that acylcarnitines participate in plastidial export of fatty acids synthesised de novo, or in the import of fatty acids into the endoplasmic reticulum for synthesis of certain glycerolipids. With Arabidopsis thaliana seedlings subjected to salt stress, they are reported to enhance recovery. The yeast Candida albicans can synthesise carnitine, and while it is not clear whether this is possible in bacteria, they can acquire it from the environment or from metabolic precursors, as it is used in protection against environmental stresses in some species.
In many bacteria, the reactions of the β-oxidation cycle can be carried out by multiple proteins (FadA to FadM), with FadE as the acyl-CoA dehydrogenase, FadB as the dual enoyl-CoA hydratase and 3-hydroxylacyl-CoA dehydrogenase, and FadA as the 3-ketoacyl-CoA thiolase (see Fig 1. above), but in Escherichia coli, a trifunctional enzyme complex, a heterotetramer of FadA-like (β-subunit) and FadB-like (α-subunit) catalyses three steps; FadR is the key regulator. As in eukaryotes, further enzymes are necessary for oxidation of unsaturated and methyl-branched fatty acids. The various products of such enzymes in the microbiome of the human gut are currently of great interest, as they have the potential to impact host immunity and metabolic responses. Cyanobacteria seem to lack a β‑oxidation pathway, and they may remove excess by producing a range of unusual secondary metabolites into which fatty acids are incorporated.
8. Analysis
Acylcarnitines are polar hydrophilic molecules that require special precautions for extraction and analysis, and butanol saturated with water is usually recommended for extracting them from tissues. They are zwitterionic, so tend to elute with phospholipids such as phosphatidylcholine in many chromatographic systems. Many of the technical problems have been solved (see the reviews cited below), and mass spectrometric methods are suited to routine screening of large numbers of samples of biological fluids from neonates, as they permit a considerable degree of automation, both of the analytical steps and of the gathering and interpretation of data, although there appears to be a need for a standardized methodology. The Human Metabolome Database has information on 215 molecular species of acylcarnitines detected in human tissues.
Recommended Reading
- Bene, J., Szabo, A., Komlosi, K. and Melegh, B. Mass spectrometric analysis of L-carnitine and its esters: potential biomarkers of disturbances in carnitine homeostasis. Curr. Mol. Med., 20, 336-354 (2020); DOI.
- Bergstrom, J.D. The lipogenic enzyme acetoacetyl-CoA synthetase and ketone body utilization for denovo lipid synthesis, a review. J. Lipid Res., 64, 100407 (2023); DOI.
- Brejchova, J., Brejchova, K. and Kuda, O. Metabolic pathways of acylcarnitine synthesis. Physiol. Res., 73, S153-S163, (2024); DOI.
- Dakhili, S.A.T., Yang, K.Y., Stenlund, M.J. and Ussher, J.R. The multifaceted roles of ketones in physiology. Exp. Physiol., in press (2025); DOI.
- Dambrova, M. and others. Acylcarnitines: nomenclature, biomarkers, therapeutic potential, drug targets, and clinical trials. Pharm. Rev., 74, 506-551 (2022); DOI.
- Jacques, F., Rippa, S. and Perrin, Y. Physiology of L-carnitine in plants in light of the knowledge in animals and microorganisms. Plant Sci., 274, 432-440 (2018); DOI.
- Mazza, T., Scalise, M., Console, L., Galluccio, M., Giangregorio, N., Tonazzi, A., Pochini, L. and Indiveri, C. Carnitine traffic and human fertility. Biochem. Pharm., 230, 116565 (2024); DOI.
- Panov, A.V. The structure of the cardiac mitochondria respirasome is adapted for the β-oxidation of fatty acids. Int. J. Mol. Sci., 25, 2410 (2024); DOI.
- Ranea-Robles, P. and Houten, S.M. The biochemistry and physiology of long-chain dicarboxylic acid metabolism. Biochem. J., 480, 607-627 (2023); DOI.
- Simcox, J. and others. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab., 26, 509-522.e6 (2017); DOI.
- Szrok-Jurga, S., Turyn, J., Hebanowska, A., Swierczynski, J., Czumaj, A., Sledzinski, T. and Stelmanska, E. The role of acyl-coA β-oxidation in brain metabolism and neurodegenerative diseases. Int. J. Mol. Sci., 54, 13977 (2023); DOI.
- Vianey-Saban, C., Guffon, N., Fouilhoux, A. and Acquaviva, C. Fifty years of research on mitochondrial fatty acid oxidation disorders: The remaining challenges. J. Inher. Metab. Dis., 46, 848-873 (2023); DOI.
- Wanders, R.J.A., Baes, M., Ribeiro, D., Ferdinandusse, S. and Waterham, H.R. The physiological functions of human peroxisomes. Physiol. Rev., 103, 957-1024 (2023); DOI.
- Wang, X.J., Yang, C.X., Huang, C. and Wang, W. Dysfunction of the carnitine cycle in tumor progression. Heliyon, 10, e35961 (2024); DOI.
- Wang, Z.Y., Hou, X., Shang, .GH., Deng, G.A., Luo, K. and Peng, M. Exploring fatty acid β-oxidation pathways in bacteria: from general mechanisms to DSF signaling and pathogenicity in Xanthomonas. Curr. Microbiol., 81, 336 (2024); DOI.
- Wang, Z.Y., Su, C.L., Zhang, Y.S., Shangguan, S.F., Wang, R.M. and Su, J. Key enzymes involved in the utilization of fatty acids by Saccharomyces cerevisiae: a review. Front. Microbiol., 14, 1294182 (2024); DOI.
- Xiang, F., Zhang, Z.M., Xie, J.C., Xiong, S.H., Yang, C., Liao, D.F., Xia, B.H., Lin, L.M. Comprehensive review of the expanding roles of the carnitine pool in metabolic physiology: beyond fatty acid oxidation. J. Transl. Med., 23, 324 (2025); DOI.
- The website L-carnitine at Oregon State University is also recommended.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: November 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
