Ceramide-1-Phosphate
Ceramide-1-phosphate, a sphingoid structural analogue of phosphatidic acid, is one of the metabolites in the 'sphingomyelin cycle' or 'sphingolipid rheostat' in animals (see below) and also functions in plants. As a bioactive phosphosphingolipid involved in regulating cell growth and survival, and inflammation, it often acts in opposition to the effects of another sphingolipid mediator, i.e., ceramide.
1. Structure and Biosynthesis in Animals
Ceramide-1-phosphate (or C1P or Cer1P) is present in animal tissues at a level comparable to that of sphingosine-1-phosphate (0.5-1μM in peripheral blood), and it is presumed to be located at the cytosolic leaflet of cellular membranes. Relatively high concentration of palmitoylated (C16) ceramide-1-phosphate have been observed in macrophages, mast cells and neutrophils. It is formed from ceramide by the action of a ceramide kinase (CERK), which is related to but distinct from the sphingosine kinases that synthesise sphingosine-1-phosphate, and there is evidence that the ceramide precursor is derived primarily from sphingomyelin by the action of sphingomyelinases and only at trace levels from other sphingolipids. For this purpose, a specific pool of ceramide containing 16:0 and 18:0 fatty acid components is transported to the site of synthesis by the ceramide transport protein (CERT) for conversion to ceramide-1-phosphate by CERK, the first defined biosynthetic route to this lipid in mammalian cells.
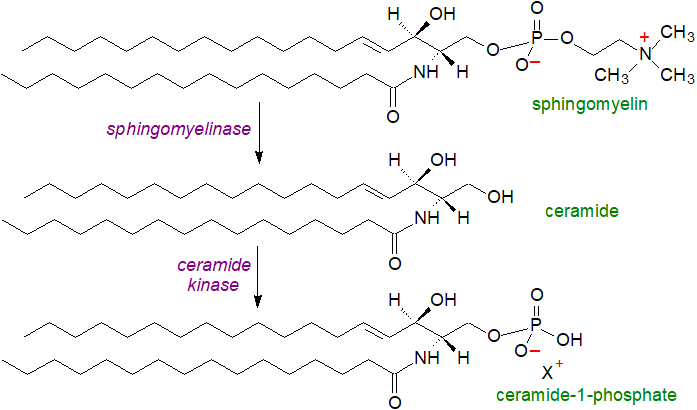 |
| Figure 1. Biosynthesis of ceramide-1-phosphate. |
CERK is associated mainly with membranes, especially the trans-Golgi network at the cytosolic face, but it has been detected in the cytosol, nucleus, perinuclear membranes and plasma membrane. It utilizes ATP as the phosphate donor, it has an absolute requirement for calcium ions, and it can be stimulated by interleukin 1‑beta (IL-1β); the enzyme is optimally active at neutral pH, and regulation is by phosphorylation/dephosphorylation processes. While it was first detected in brain synaptic vesicles and in human leukaemia (HL 60) cells, it has since been found in many other tissues such as brain, heart, skeletal muscle, kidney and liver. CERK is specific for natural ceramides with the erythro configuration and a 4,5-trans double bond in the base component, which is esterified to long-chain fatty acids (C16 mainly). It has a calmodulin-binding motif, but an interesting relationship to glycerophospholipid metabolism is evident in that a molecule of phosphatidylinositol 4,5-bisphosphate binds selectively to CERK via a Pleckstrin homology domain and may then direct the enzyme to particular membranes within the cell.
A membrane-bound transport protein, i.e., ceramide-1-phosphate transfer protein or CPTP (once thought to be a glycolipid transfer protein, GLTPD1 or CPTP), is essential for the biosynthesis of ceramide-1-phosphate and for most of its functions. It maintains a constant level of the lipid in the Golgi membrane and transfers it by a non-vesicular mechanism to the plasma membrane and other cellular compartments as required. Although this protein is mainly present in the cytosol, it is found in association with nuclear membranes, while at the plasma membrane, it may be recognized by an interaction with phosphatidylserine.
Other biosynthetic routes to ceramide-1-phosphate exist, as mice in which the CERK enzyme has been deleted have normal levels of the metabolite, and it has recently been demonstrated that diacylglycerol kinase ζ has a limited ability to phosphorylate ceramides, but a further mechanism is assumed to exist
 A component of the venom from the spider Loxosceles reclusus
and related species is an enzyme of the sphingomyelinase D type, also present in some bacteria but not mammalian cells, which converts
sphingomyelin to ceramide 1,3-cyclic phosphate (and not ceramide-1-phosphate as first reported) and causes a severe inflammatory response,
which can even result in death.
The reaction proceeds by an intramolecular transphosphatidylation and not by hydrolysis.
As there is no effective treatment for anyone bitten by these spiders, sphingomyelinase/phospholipase Ds are seen as attractive targets
for therapeutic intervention.
A component of the venom from the spider Loxosceles reclusus
and related species is an enzyme of the sphingomyelinase D type, also present in some bacteria but not mammalian cells, which converts
sphingomyelin to ceramide 1,3-cyclic phosphate (and not ceramide-1-phosphate as first reported) and causes a severe inflammatory response,
which can even result in death.
The reaction proceeds by an intramolecular transphosphatidylation and not by hydrolysis.
As there is no effective treatment for anyone bitten by these spiders, sphingomyelinase/phospholipase Ds are seen as attractive targets
for therapeutic intervention.
Catabolism: The reverse reaction to produce ceramide is accomplished by phosphatases, suggesting that ceramide and ceramide-1-phosphate are readily interconvertible in cells. Those enzymes to have been implicated include a ceramide-1-phosphate phosphatase, phosphatidate phosphohydrolase, and the lysosomal acid sphingomyelinase.
2. Function in Animals
It is now known that ceramide-1-phosphate serves many different purposes, some of which are confined to certain cell types and are very different from those of other sphingolipid metabolites. In contrast to sphingosine-1-phosphate it is not secreted as such by transporters in intact cells, although it is released by leaky or damaged cells and it is present in exosomes exported into plasma, so it is able to interact with sites at the plasma membrane of other cells. Its physical properties determine that it is fusogenic and increases the fusibility of vesicle membranes,and while it does not participate in raft formation in membranes, it may cluster in segregated domains, which faciltate interations with proteins.
Since ceramide-1-phosphate and ceramide are antagonistic, as discussed below, a correct balance between the concentrations of the two metabolites is crucial for cell and tissue homeostasis as are the relative concentrations of sphingosine-1-phosphate and long-chain bases, since all are mutually convertible as part of the 'sphingolipid rheostat' (discussed further in relation to ceramides and sphingosine-1-phosphate). A consequence of distortion to this balance in any direction may be metabolic dysfunction or disease, as the enzymes for synthesis and catabolism must be coordinated efficiently to ensure that cells operate normally.
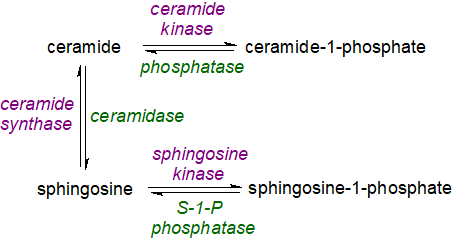 |
| Figure 2. The sphingolipid rheostat. |
Ceramide-1-phosphate is a regulator of cell growth and survival, and it is a mitogenic agent that stimulates DNA synthesis and cell division in rat fibroblasts by phosphorylation (activation) of various cell signalling kinases,. Like sphingosine-1-phosphate, it is a potent inhibitor of apoptosis and a promoter of cell survival, and in macrophages, ceramide‑1-phosphate blocks apoptosis through inhibition of the enzyme acid sphingomyelinase, which generates the pro-apoptotic molecule ceramide. It also inhibits serine palmitoyltransferase, the main regulatory enzyme in the biosynthesis of long-chain bases and thence of ceramides. By inhibiting caspases and preventing DNA fragmentation in macrophages, it prevents apoptosis. Ceramide-1-phosphate in plasma has a role in the recruitment of stem/progenitor cells to damaged organs and may promote their vascularization with the potential to be used in regenerative medicine. Ceramides inhibit cell proliferation stimulated by this means by up-regulation of the lipid phosphate phosphatase that leads to dephosphorylation of ceramide-1-phosphate.
Ceramide-1-phosphate is a mediator of inflammation by stimulating the release of arachidonic acid through activation of the cytosolic phospholipase A2α (cPLA2α), which is the initial rate-limiting enzyme in the production of the inflammatory prostaglandins and leukotrienes via the release of arachidonic acid. For this purpose, it binds to a Ca2+-dependent phospholipid binding domain, as opposed to indirectly via a receptor mechanism; the effect is to translocate the enzyme from the cytosolic compartment to the intracellular membranes where phospholipid substrates for eicosanoid production such as phosphatidylcholine are located. There is evidence that ceramide kinase and phospholipase A2 are closely linked within the same membranes, mainly the trans-Golgi network, following recruitment of the latter enzyme from the cytosol. As an example, by blocking the receptor for 5‑oxo-eicosatetraenoic acid (5-oxo-ETE), ceramide-1-phosphate promotes the inflammatory phase of wound repair and inhibits the proliferation and remodelling steps. As the transport protein CPTP transfers ceramide-1-phosphate between membranes, it makes a contribution to the regulation of eicosanoid production. There may be synergy with sphingosine-1-phosphate, which induces up-regulation of the enzyme cyclooxygenase-2 (COX‑2).
 Conversely,
ceramide-1-phosphate has anti-inflammatory properties when produced in some cell types or tissues in that it inhibits the release of
pro-inflammatory cytokines and blocks activation of the pro-inflammatory transcription factor NF-κB and the formation of tumour necrosis
factor α (TNFα), which can be contributors to the deleterious effects of septic shock if produced to excess.
It thus again has the opposite effect to ceramides.
It increases their release of anti-inflammatory interleukin-10 and induces cell migration in human and mouse macrophages; macrophage migration
across the peritoneum into the lymphatic vessels is important for the resolution of inflammation.
Phosphatidic acid, which is structurally similar to ceramide-1-phosphate, is able to displace the
latter from its membrane-binding site (possibly a receptor) to block macrophage migration, suggesting a role for the former in the regulation
of macrophage-mediated inflammatory responses.
Conversely,
ceramide-1-phosphate has anti-inflammatory properties when produced in some cell types or tissues in that it inhibits the release of
pro-inflammatory cytokines and blocks activation of the pro-inflammatory transcription factor NF-κB and the formation of tumour necrosis
factor α (TNFα), which can be contributors to the deleterious effects of septic shock if produced to excess.
It thus again has the opposite effect to ceramides.
It increases their release of anti-inflammatory interleukin-10 and induces cell migration in human and mouse macrophages; macrophage migration
across the peritoneum into the lymphatic vessels is important for the resolution of inflammation.
Phosphatidic acid, which is structurally similar to ceramide-1-phosphate, is able to displace the
latter from its membrane-binding site (possibly a receptor) to block macrophage migration, suggesting a role for the former in the regulation
of macrophage-mediated inflammatory responses.
Inhibition of acid sphingomyelinase and the subsequent reduction of ceramide levels by ceramide-1-phosphate production may be required for the resolution of inflammation and infection in the lung. The tick-borne bacterium Anaplasma phagocytophilum was found to elevate cellular levels of ceramide-1-phosphate to promote fragmentation of the Golgi to enable bacterial proliferation, conversion to the infectious form and thence severe infection.
There is some evidence that ceramide-1-phosphate binds to and activates a plasma membrane receptor that is different from the receptors for sphingosine-1-phosphate, but this has not been identified. As sphingosine-1-phosphate functions mainly via G-protein-coupled receptors, this is now thought to be true also for ceramide-1-phosphate, e.g., in macrophage migration, although the latter can act by binding directly to its target molecules such as phospholipase A2 (see above). A direct interaction with a receptor at the cell surface may not always occur, and although ceramide-1-phosphate added exogenously induces cellular responses in vitro, some of these effects may be a result of ceramide generated on the plasma membrane via hydrolysis. There are apparently contradictory effects during adipogenesis in that although ceramide kinase is upregulated during differentiation of pre-adipocytes into mature adipocytes, exogenous ceramide-1-phosphate reduces adipogenesis by acting through a putative Gi protein-coupled receptor. CERK may regulate the biogenesis of lipid droplets, and in relation to obesity, experiments with animal models have shown that deletion of this enzyme suppresses the inflammatory cytokines associated with high-fat diets and returns insulin signalling to normal.
Ceramide-1-phosphate has a negative role in cancer development and metastasis. Both sphingosine-1-phosphate and ceramide-1-phosphate are potent chemo-attractants for a variety of cell types with effects upon the trafficking of normal and malignant cells, but especially of normal hematopoietic stem/progenitor cells. In particular, ceramide-1-phosphate has been shown to promote cancer cell growth, migration and survival, although it is not yet known whether it participates in inflammation-associated cancer. It is possible that ceramide-1-phosphate secreted into plasma may trigger signalling cascades at the plasma membrane of cells and modulates the expression of genes that favour tumour promotion and dissemination. CERK is overexpressed in breast cancer and is associated with poor prognosis, and it is involved in the migration and invasion of cancer cells in the pancreas and lung. It is hoped that pharmacological control of this enzyme may inhibit or prevent cancer metastasis.
3. Ceramide-1-Phosphate in Plants and Bacteria
Phytoceramide-1-phosphate with 2-hydroxy fatty acids as the N-acyl constituents and phytosphingosine as the sphingoid base is formed in plant tissues, such as Arabidopsis thaliana, where it is generated from ceramide by a ceramide kinase (CERK/ACD5) and from the membrane constituents glycosylinositol phosphoceramides (mainly the species with two sugar residues) by a specific phospholipase D. In undamaged tissues, it is produced at such low levels that it is not easily measured in vivo. ACD11 is a CPTP homologue in A. thaliana that is a transporter for phytoceramide-1-phosphate and ceramide-1-phosphate but not for other plant sphingolipids. Ceramide kinase has not been detected in yeast.
Phytoceramide-1-phosphate may be important for growth and for removal of excess ceramide to make plants more resistant to environmental stresses such as the response to cold; phosphorylated ceramides accumulate rapidly if transiently upon cold shock treatment, and CERK/ACD5 promotes seed germination at low temperatures. Similarly, the balance between phytoceramide and phytoceramide-1-phosphate may be crucial in modulating the process of apoptosis in plants as the latter may be pro-survival, but a ceramide-1-phosphate phosphatase that might convert ceramide-1-phosphate to ceramide has not yet been identified, and the mechanisms for signalling effects in plants have still to be identified. Arabidopsis mutants that lack ceramide kinase have impaired defence mechanisms and display spontaneous apoptosis. Stem rot caused by the fungus Sclerotinia sclerotiorum is one of the most severe diseases affecting the growth and production of Brassica napus, and during infection, ceramide kinase is induced to produce higher levels of ceramide-1-phosphate and enhanced resistance to the pathogen.
The oral pathogen Porphyromonas gingivalis and many species of the genus Bacteroides contain ceramide-1-phosphate or dihydroceramide analogues in which both the long-chain base and the fatty acid component have iso-methyl branches while the fatty acid can have a 3-hydroxyl group. The ceramide kinase from Caulobacter crescentus is evolutionarily distinct from comparable eukaryotic enzymes, and in this and other bacterial species, the product ceramide-1-phosphate is a precursor for more complex sphingophospholipids such as ceramide phosphoglycerate.

The pathogenic bacterium Anaplasma phagocytophilum elevates host levels of ceramide-1-phosphate to promote Golgi fragmentation and thence bacterial proliferation and increased infection.
4. Analysis
Analysis of the various components of the sphingomyelin cycle, including ceramide-1-phosphate, can now be carried out in a comprehensive manner by high-performance liquid chromatography in conjunction with tandem mass spectrometry and electrospray ionization. A popular method in which a strong alkaline treatment is used to cleave interfering glycerolipids must be followed by a neutralization step, otherwise there can be a gross overestimation of ceramide-1-phosphate levels.
Suggested Reading
- Bornancin, F. Ceramide kinase: The first decade. Cellular Signalling, 23, 999-1008 (2011); DOI.
- Camacho, L., Ouro, A., Gomez-Larrauri, A., Carracedo, A. and Gomez-Muñoz, A. Implication of ceramide kinase/C1P in cancer development and progression. Cancers, 14, 227 (2022); DOI.
- Dutilleul, C., Chavarria, H., Rézé, N., Sotta, B., Baudouin, E. and Guillas, I. Evidence for ACD5 ceramide kinase activity involvement in Arabidopsis response to cold stress. Plant Cell Environ., 38, 2688-2697 (2015); DOI.
- Gomez-Larrauri, A., Larrea-Sebal, A., Martín, C. and Gomez-Muñoz, A. The critical roles of bioactive sphingolipids in inflammation. J. Biol. Chem., 301, 110475 (2025); DOI.
- Hait, N.C. and Maiti, A. The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Med. Inflamm., 4806541 (2017); DOI.
- Kida, T., Itoh, A., Kimura, A., Matsuoka, H., Imai, H., Kogure, K., Tokumura, A. and Tanaka, T. Distribution of glycosylinositol phosphoceramide-specific phospholipase D activity in plants. J. Biochem., 161, 187-195 (2017); DOI.
- Lachmayr, H. and Merrill, A.H. A brief overview of the toxic sphingomyelinase Ds of brown recluse spider venom and other organisms and simple methods to detect production of its signature cyclic ceramide phosphate. Mol. Pharmacol., 105, 144-154 (2024); DOI.
- Maceyka, M. and Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature, 510, 58-67 (2014); DOI.
- Presa, N., Gomez-Larrauri, A., Dominguez-Herrera, A., Trueba, M. and Gomez-Muñoz, A. Novel signaling aspects of ceramide 1-phosphate. Biochim. Biophys. Acta, Lipids, 1865, 158630 (2020); DOI.
- Yamaji, T. and Hanada, K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic, 16, 101-122 (2015); DOI.
- Yamazaki, A., Kawashima, A., Honda, T., Kohama, T., Murakami, C., Sakane, F., Murayama, T. and Nakamura, H. Identification and characterization of diacylglycerol kinase ζ as a novel enzyme producing ceramide-1-phosphate. Biochim. Biophys. Acta, Lipids, 1868, 159307 (2023); DOI.
- Zhang, Y., Zhang, X., Lu, M. and Zou, X. Ceramide-1-phosphate and its transfer proteins in eukaryotes. Chem. Phys. Lipids, 240, 105135 (2021); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: August 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
