Coenzyme A, Acyl Carrier Protein and
Functionally Related Molecules
 Before a
fatty acid can be metabolized in tissues by being esterified, oxidized or subjected to synthetic modification,
it must usually be activated by conversion to a coenzyme A (CoA) ester or acyl-CoA in which the carboxyl
group is linked to the terminal thiol moiety of CoA to form a thiol ester.
This can be the simplest fatty acid of all, i.e., acetyl-CoA, or one of the long-chain fatty acids.
Thiol esters have large negative, standard free energies of hydrolysis and are subject to much less resonance stabilization than oxygen
esters, so transfer of the acyl group to receptor molecules with formation of O‑esters is highly favoured energetically.
This is true for the most primitive organisms, such as Archaea, through to humans, and it has been estimated that CoA and its thioester
derivatives take part in about 4% of all cellular reactions in bacteria and eukaryotes.
It must be discussed together with the acyl carrier protein, which has the same functional moiety and is
also utilized in fatty acid biosynthesis and metabolism.
In 1953, F.A. Lipmann was a recipient of the Nobel Prize in Physiology and Medicine "for his discovery of co-enzyme A and its
importance for intermediary metabolism."
Before a
fatty acid can be metabolized in tissues by being esterified, oxidized or subjected to synthetic modification,
it must usually be activated by conversion to a coenzyme A (CoA) ester or acyl-CoA in which the carboxyl
group is linked to the terminal thiol moiety of CoA to form a thiol ester.
This can be the simplest fatty acid of all, i.e., acetyl-CoA, or one of the long-chain fatty acids.
Thiol esters have large negative, standard free energies of hydrolysis and are subject to much less resonance stabilization than oxygen
esters, so transfer of the acyl group to receptor molecules with formation of O‑esters is highly favoured energetically.
This is true for the most primitive organisms, such as Archaea, through to humans, and it has been estimated that CoA and its thioester
derivatives take part in about 4% of all cellular reactions in bacteria and eukaryotes.
It must be discussed together with the acyl carrier protein, which has the same functional moiety and is
also utilized in fatty acid biosynthesis and metabolism.
In 1953, F.A. Lipmann was a recipient of the Nobel Prize in Physiology and Medicine "for his discovery of co-enzyme A and its
importance for intermediary metabolism."
CoA participates in innumerable catabolic and anabolic reactions, including those for the metabolism of carbohydrates, proteins, ethanol and xenobiotics as well as lipids with acetyl-CoA and succinyl-CoA as the two most abundant acyl-CoAs in mammalian cells and tissues. Of these, acetyl-CoA is of special importance for the delivery the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. As a cellular antioxidant, CoA protects protein thiols from over-oxidation when cells are exposed to oxidative or metabolic stress. However, only the role of these metabolic agents in the first steps in lipid biosynthesis can be described in this web page. Alternative acyl activating agents are known in some bacteria.
1. Coenzyme A - Structure and Occurrence
Coenzyme A biosynthesis: CoA (or CoASH) itself is a complex and highly polar molecule, consisting of adenosine 3',5'‑diphosphate linked to 4‑phosphopantothenic acid and thence to β‑mercaptoethylamine, which takes a direct part in acyl transfer reactions. The adenosine 3’,5’‑diphosphate moiety serves as a recognition site as many proteins have nucleotide-binding folds, and it increases the affinity for CoA binding to enzymes.
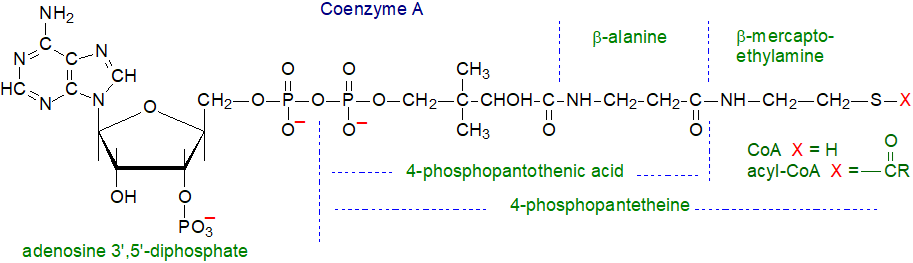
The genes encoding the enzymes for CoA biosynthesis have been identified, and the structures of many proteins in the pathway have been determined. Although there are sequence differences in these enzymes between prokaryotes and eukaryotes, they assemble coenzyme A in five steps from pantothenic acid, cysteine and ATP in much the same way. Pantothenic acid (vitamin B5) per se can only be synthesised by microorganisms (including gut microflora) and a few plants, and animals must acquire it largely from the diet. In animals, CoA biosynthesis is initiated in the cytosol of cells, and the enzyme pantothenate kinase, several isoforms of which are known, catalyses the first and rate-limiting step. Although the reactive sulfhydryl group is not part of the pantothenate moiety, the steric configuration of pantothenic acid is necessary for recognition by enzymes.
In mitochondria and peroxisomes, the concentrations of CoA are reported to lie in the range 2-5 mM and 0.7 mM, respectively, while that in the cytosol is much lower (0.05 to 0.14 mM), and these are strictly controlled by nutrients, hormones, metabolites and cellular stresses. A deficiency in pantothenic acid is sometimes observed during severe malnutrition, and it may be a factor in the brain in Huntington’s disease.
Although acyl-dephospho-CoA esters lacking the 3’‑phosphate group on the ribose moiety have been detected in tissues, there is at present no explanation for their presence. The anaerobic sulfate-reducing bacterium Desulfobacula toluolica produces CoA analogues with inosine instead of adenosine as the nucleoside.
2. Formation of Coenzyme A Esters in Animals and Plants
Animals: Intracellular free fatty acids arising from synthesis de novo or from the diet must be activated by thioesterification by a fatty acyl-CoA synthetase (fatty acid:CoA ligase) before they can be utilized for the synthesis of triacylglycerols, wax esters, long-chain aldehydes and alcohols, and complex lipids, or for covalent modification of proteins by myristoylation or palmitoylation in reactions catalysed by many different N‑acyltransferases. Indeed, CoA is intimately associated with most reactions of fatty acids, i.e., those with elongases and desaturases, dehydrogenases, acyl-CoA thioesterases, carnitine-palmitoyltransferases and lipid and protein acyltransferases. These enzymes are discussed in greater detail in most of the web pages in the Lipid Essentials section of this website. As all of these pathways are often present within a single subcellular compartment, there must be a high level of organization of the various enzymes.
Acyl CoA synthetases (ACLSs) produce CoA esters of fatty acids through a process that is energy-dependent and requires ATP and CoA. It is a two-stage reaction, requiring magnesium ions in the first step, with formation of an acyl-AMP intermediate (acyl-adenylate - structure illustrated in Section 6 below), which reacts with CoA in the second step to produce acyl-CoA. In the process, ATP is consumed, and AMP and pyrophosphate are produced.
 |
| Figure 1. Biosynthesis of coenzyme A esters. |
In humans, five acyl-CoA synthetases have been identified with specificities for fatty acids in different chain-length groups, i.e., short-chain (2 to 3 carbons), medium-chain (4 to 12 carbons), long-chain (12 to 22 carbons), so-called ‘bubble-gum’ (14 to 24 carbons) and very-long-chain (18 to 26 or more carbons) fatty acid substrates. They are membrane-bound enzymes and are distinguished by two highly conserved sequence elements, an ATP/AMP binding motif, which is common to enzymes that form an adenylated intermediate, and a fatty acid binding motif. In all forms of life, multiple isoforms of these enzymes are known to be present, and six have been identified in the yeast genome while there are at least 26 in the human genome in the five families.
The human long-chain acyl-CoA synthetases (ACSL1, 3, 4, 5 and 6) (EC 6.2.1.3) convert fatty acids of 12 to 20 carbons to their corresponding CoA esters, with each isoform differentiated by its substrate preference, regulatory mechanism, binding partner, expression pattern across cell/tissue types, and subcellular location. These factors control the ability of an individual ACSL to channel fatty acids towards different metabolic fates.
ACSL1 is expressed ubiquitously, and its subcellular location varies appreciably among cells, i.e., in mitochondria and endoplasmic reticulum (ER) of hepatocytes, in mitochondria of adipocytes and cardiomyocytes, and in association with lipid droplets in adipocytes and hepatocytes. It utilizes long-chain fatty acids (C14 to C18) only and is anchored to membranes by a single N‑terminal transmembrane domain. In highly oxidative tissues, ACSL1 is located on the outer mitochondrial membrane and directs fatty acids into mitochondria for β-oxidation. In the liver, the same isoform in the ER interacts with many cellular proteins, of which some are concerned with esterification, including acyl-CoA binding proteins and ceramide synthase isoforms, and some are in peroxisomes for oxidation purposes. It is required to provide linoleate in skin for the triacylglycerol precursors that are utilized for ω‑O‑esterification to the ceramides, which are vital components of its barrier to the environment. It is induced by interferon type I (IFN-I) to generate a reservoir of phosphatidic acid containing saturated fatty acids as a means of reducing their potential lipotoxicity.
ACSL3 is most active in brain, skeletal muscle and reproductive tissues and favours palmitic acid, but it does utilize unsaturated fatty acids to a limited extent. ACSL4 predominates in brain, adrenal gland, testis, ovary, with reproductive tissues using arachidonic and adrenic acids preferentially as its substrates. It is used for the remodelling of those phospholipids that incorporate arachidonate, for example in macrophages, and it may be connected to inflammatory responses. By mopping up any excess of this acid produced by the action of phospholipase A2, ACSL4 may reduce or regulate eicosanoid biosynthesis. In contrast, it may be a factor in ferroptosis by triggering phospholipid peroxidation with the expression level or enzymatic activity of ACSL4 a potential indicator of cellular susceptibility. ACSL5 is widely expressed in mammalian liver, small intestine, adipose tissue, spleen, uterus, lung and skeletal muscle and utilizes C16 to C20 fatty acids, mainly saturated and monoenes, as substrates in the cytoplasm to form acyl-CoA for synthesis of complex lipids and other products in the ER or for β‑oxidation in mitochondria with effects upon circulating lipids and insulin resistance. As the major intestinal ACSL isoform, it is used during the absorption of fatty acids from dietary triacylglycerols and their subsequent metabolism.
There are two variants of ACSC6, i.e., ACSL6V1 and V2, which differ appreciably in their chain-length preferences, with ACSL6V1 selecting octadecapolyenoic acids (C18) such as linoleate, whereas ACSL6V2 strongly prefers docosapolyenoic acids (C22). ACSL6V2 is utilized to enrich docosahexaenoic acid (DHA) in membrane lipids in the brain and retina and is vital for neurological health; reduction in the activity of this enzyme in the aging brain may be a cause of cognitive decline in the elderly. In spermatids, ACSL6 supplies this fatty acid together with docosapentaenoic acid (22:5(n-3)) for lipid synthesis and is required for normal spermatogenesis and fertility in the mouse.
There is appreciable sequence homology between the very-long-chain acyl-CoA synthetases and certain fatty acid transport proteins in animals, so it is possible that they participate in the transport of fatty acyl moieties across membranes. Inevitably, the influence of these enzymes on CoA levels and compositions has a bearing on many disease states, including metabolic disease and cancer. Long-chain acyl-CoA esters bind to certain hormone receptors and have a signalling function, while many of observations regarding free fatty acids in nuclear signalling may be attributable to acyl-CoA esters.
 CoA and CoA esters are required
for many processes in lipid metabolism in addition to esterification, including chain elongation and desaturation (some reactions in plants are
exceptions), and they promote cellular growth and proliferation as discussed in other web pages here.
They have antioxidant properties that enhance cell survival.
During fasting or starvation, intracellular long-chain fatty acids mobilized from adipose tissue reserves are catabolized as fuel by the
mitochondrial β‑oxidation pathway, and they must be first be converted to the CoA esters prior
to synthesis of carnitine derivatives for translocation into mitochondria.
Medium-chain fatty acids can enter mitochondria without carnitine transport, but they must be still activated before β‑oxidation
can occur.
Similarly, peroxisomes in animal cells have a distinct fatty acid β-oxidation system
with a separate set of enzymes, including as many as three acyl-CoA oxidases.
The acyl-CoA oxidase 1 catalyses the β‑oxidation of straight-chain acyl-CoAs, while acyl-CoA oxidase 2 is involved in the oxidation
of the sidechain of bile-acid precursors, and acyl-CoA oxidase 3 catalyses the oxidation of methyl branched-chain CoA esters.
CoA and CoA esters are required
for many processes in lipid metabolism in addition to esterification, including chain elongation and desaturation (some reactions in plants are
exceptions), and they promote cellular growth and proliferation as discussed in other web pages here.
They have antioxidant properties that enhance cell survival.
During fasting or starvation, intracellular long-chain fatty acids mobilized from adipose tissue reserves are catabolized as fuel by the
mitochondrial β‑oxidation pathway, and they must be first be converted to the CoA esters prior
to synthesis of carnitine derivatives for translocation into mitochondria.
Medium-chain fatty acids can enter mitochondria without carnitine transport, but they must be still activated before β‑oxidation
can occur.
Similarly, peroxisomes in animal cells have a distinct fatty acid β-oxidation system
with a separate set of enzymes, including as many as three acyl-CoA oxidases.
The acyl-CoA oxidase 1 catalyses the β‑oxidation of straight-chain acyl-CoAs, while acyl-CoA oxidase 2 is involved in the oxidation
of the sidechain of bile-acid precursors, and acyl-CoA oxidase 3 catalyses the oxidation of methyl branched-chain CoA esters.
Acyl-CoA binding proteins: As they have both polar and hydrophobic molecular components, CoA esters of long-chain fatty acids have strong detergent-like physical properties with critical micellar concentrations of 5 to 42 µM, depending on their chain length and degree of unsaturation, and they have the potential to be disruptive towards cells. The intracellular concentrations of free CoA and of acyl-CoA esters are tightly controlled by feedback inhibition of the acyl-CoA synthetase and by various extracellular stimuli, including nutrients, hormones and cellular metabolites. They are buffered by acyl-CoA binding proteins in the cytoplasm, such as the protein family designated ACBP or DB1 of which there are seven members in humans. These are approximately 10 kDa proteins that bind long-chain CoA esters with high specificity and affinity via a highly conserved domain and reduce their effective concentration by up to 104-fold. ACBP is an intracellular acyl-CoA transporter, and it is required for lipid biosynthesis and remodelling, β‑oxidation and protein acylation in eukaryotes. It is necessary for the synthesis of ceramide and thence of other sphingolipids in mammals by activating ceramide synthase 3. In mice, ACBP modifies dietary behaviour by its effects upon food intake and obesity.
Plants: In the genome of the model plant Arabidopsis thaliana, a superfamily of 63 genes encoding acyl-activating enzymes has been identified that can be divided into seven subclades of which one has been characterized biochemically, i.e., long-chain acyl-CoA synthetase enzymes (LACS1-9 or ACOS1-9). These can all catalyse the formation of acyl-CoA thioesters from the common range of fatty acids found in plants, though with some selectivity for particular substrates and with differing subcellular locations. LACS1-4 and LACS8, which are located in the ER, have a stronger preference for 16- over 18-carbon fatty acids, but LACS5 in this organelle differs in that it has a specificity for 16:1 and 18:2 unsaturated over saturated fatty acids. LACS6 and LACS7, believed to occur in peroxisomes, have a preference for eicosenoic acid (20:1), while LACS9 in the chloroplasts favours oleic acid and 16-carbon fatty acid substrates. Often, a further consequence of compartmental and substrate specificity is to confine each member to a particular metabolic pathway, and for example, LACS1 has been shown to be necessary for pollen development and male fertility in tomato, while in rapeseed (Brassica napus), LCAS2 regulates oil content by fatty acid and lipid synthesis and glycolysis in seeds.
Six acyl-CoA binding proteins have been characterized in Arabidopsis that vary in their subcellular distribution, tissue-specificity, stress-responsiveness and ligand selectivity, and they have been studied intensively in oil seed crops. Acyl-CoA binding proteins contribute to the transport of fatty acids as acyl-CoAs from the chloroplasts to the ER for synthesis of complex lipids, or from the cytosol to the peroxisomes for fatty acid β‑oxidation, and so they are essential for lipid homeostasis. They provide the acetyl-CoA necessary for seed germination and for all stages of plant development and reproduction. One class of ACBPs transports very-long-chain acyl-CoAs only from the ER to and across the plasma membrane for the biosynthesis of surface lipids such as wax and cutin. As regulators of the synthesis of various signalling lipids that include phosphatidic acid, sterols, oxylipins and sphingolipids, and not simply transporters, they are involved in the responses to abiotic and biotic stresses in plants.
3. Acetyl-CoA
Acetyl-CoA is a central metabolite in innumerable metabolic pathways, both catabolic and anabolic, and especially for biosynthesis of essential cellular macromolecules and such lipids as fatty acids, sterols, coenzyme Q and dolichols. It is derived from many different metabolic reactions, such as the catabolism of glucose, fatty acids and amino acids. In the citric acid cycle and the catabolism of carbohydrates in the cytosol, CoA is a key molecule with acetyl-CoA as a major end-product with succinyl-CoA as an intermediate. ATP citrate lyase is the primary enzyme responsible for this synthesis, and it catalyses the ATP-dependent and CoA-dependent cleavage of citrate produced in mitochondria to yield acetyl-CoA, oxaloacetate, ADP and orthophosphate. The enzyme forms homo-tetramers through the C-terminus to promote binding to CoA and thence acetyl-CoA production.
 |
| Figure 2. Biosynthesis of acetyl-CoA. |
Acetyl-CoA can be produced from intracellular acetate by the action of acetyl-CoA synthetases of which there are two main forms in humans, ACSS2 in the cytosol and ACSS1 in mitochondria, with both stimulated by de-acetylation of lysine residues, ACSS2 by Sirtuin 1 and ACSS1 by Sirtuin 3; the acetyl-CoA produced in mitochondria is utilized mainly in the tricarboxylic acid cycle. A third form ACSS3 prefers propionate, but otherwise it is poorly understood. Acetyl-CoA derived from ATP citrate lyase or from ACSS2 in the cytosol is the primary precursor for lipogenesis, especially fatty acid synthesis (see our web page on saturated fatty acids) and cholesterogenesis in animals, and for ketone bodies, which are water-soluble and more readily transported between tissues in plasma for energy and other purposes. In mammalian cells, ACSS2 produces acetyl-CoA for DNA acetylation and post-translational modification of proteins, including histones, and as it is highly expressed in a variety of tumours, it is seen as a drug target. In plants, production of acetyl-CoA by ATP citrate lyase is less relevant to fatty acid biosynthesis as acetyl-CoA cannot cross into plastids, but it is vital for the biosynthesis of innumerable other lipid molecules, including sphingolipids, waxes, sterols and many isoprenoids.
The short-chain acyl-CoAs, acetyl-CoA and malonyl-CoA, and free CoA are well known regulators of metabolic flux, with the ratio of acetyl-CoA to free CoA tightly regulating glycolysis and fatty acid oxidation. As well as its participation in fatty acid synthesis, malonyl-CoA reduces fatty acid oxidation by inhibiting the transport of acyl-CoA into mitochondria, but that derived from the acetyl-CoA synthetase in mitochondria is destined for oxidation. In addition to their role in lipid biosynthesis and catabolism, CoA esters have been shown to regulate a variety of enzymes, including acetyl-CoA carboxylase, a key enzyme for fatty acid synthesis. Many genes and enzymes are regulated by deacylation and acylation reactions via various short-chain acyl-CoAs, such as acetyl- and succinyl-CoA.
4. Catabolism
At high concentrations, acyl-CoAs are inhibitors of innumerable enzyme systems, and the excess must be removed from cells in part as their acyl-carnitine derivatives. There is a super-family of acyl-CoA thioesterases (ACOTs) of two main types (12 in total in humans), which are located in most cells and cellular compartments in plants and animals and catalyse the hydrolysis of acyl-CoAs to the free fatty acids and coenzyme A. In animals, Type I acyl-CoA thioesterases have a molecular mass of approximately 40 kDa, while that of the Type II enzymes is approximately 110–150 kDa, with no sequence homology nor common structural features between them. Of these, the best characterized is ACOT8, which is located in peroxisomes in humans, mice and rats, and is utilized for the catabolism of long-chain and branched-chain fatty acids. These enzymes may supply ligands for nuclear receptors, regulate and terminate fatty acid oxidation in mitochondria (β‑oxidation) and peroxisomes, and control the supply of acetate and of coenzyme A. Two of the type II enzymes, ACOT11 and ACOT12, contain steroidogenic acute regulatory related lipid transfer domains, which are lipid transport or regulatory domains.
CoA per se is degraded by intra- and extracellular pathways via a sequence of reactions that include dephosphorylations and the removal of the nucleotide moiety. Pantetheinase enzymes in peroxisomes hydrolyse the pantetheine moiety to 4'‑phosphopantetheine, eventually releasing pantothenate into the bloodstream, where it is available for cellular uptake and re-synthesis of CoA.
Impaired catabolism can lead to accumulation of CoA and acyl-CoAs within cells and trigger a sequence of reactions that give rise to chronic illness. This is a factor in some neurodegenerative diseases, and for example, the ACOT7 gene in the brain encoding the cytosolic acyl CoA thioester hydrolase is critical for preventing neuronal lipotoxicity and can be a problem in cancer, myopathies and infectious diseases. Pantethine, which can be converted in tissues to pantothenic acid and cysteamine, is used pharmacologically for the treatment of hyperlipidaemia.
5. Acyl Carrier Protein
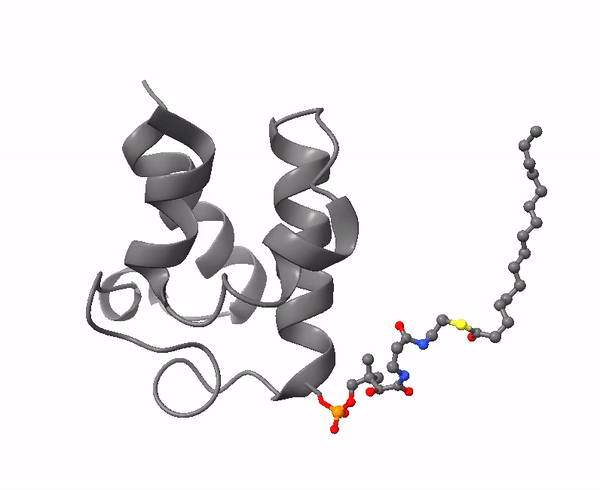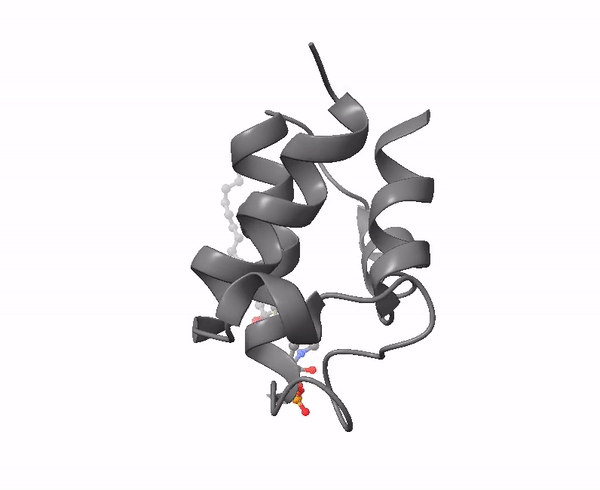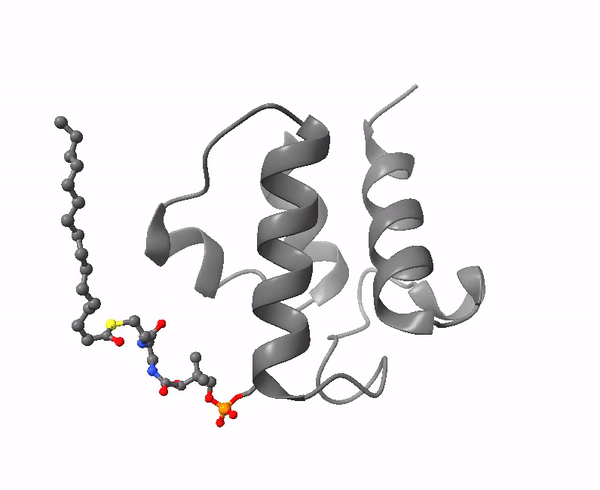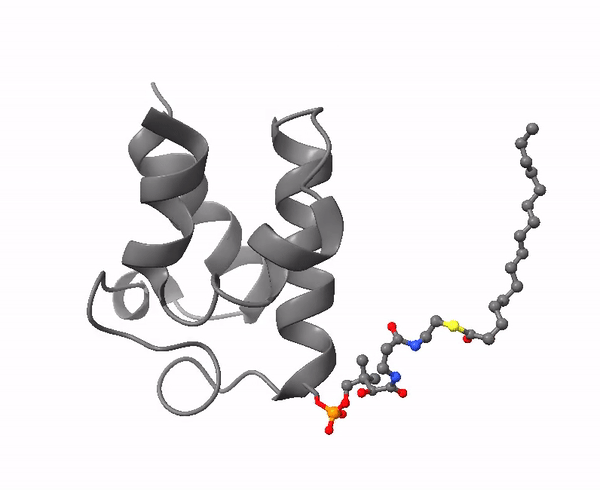 |
| Acyl carrier protein. Illustration by Matthew Conroy, LipidMaps. |
The 4-phosphopantetheine moiety, linked via its phosphate group to the hydroxyl group of a serine residue of a soluble acidic protein, is the critical component of another important molecule in lipid metabolism, Acyl Carrier Protein (ACP). This is a small (8.8 kDa) and negatively charged molecule that exists as a four-helical bundle and is required to shuttle substrates in the correct order between appropriate enzymes in metabolic pathways. It sequesters fatty acyl moieties differing in chain length in such a manner within the four-helical bundle that partner enzymes can distinguish allosterically between chain lengths via protein-protein interactions. A key feature is its flexibility in terms of its structure, substrate and enzyme partners.
ACP is a ubiquitous and highly conserved carrier of acyl groups between catalytic sites in both polyketide and fatty acid synthases (see the web page on synthesis of saturated fatty acids). Thus, in animals, the ACPs are tethered covalently to the type I mega-synthase by flexible linkers in the peptide chain, which permit the intermediates to remain in an energy-rich linkage with access to spatially distinct enzyme-active sites in a manner that resembles an assembly line. The final step in fatty acid synthesis with type I synthases is transfer of the fatty acyl group from ACP to CoA, after which the pool of ACP is regenerated for further fatty acid biosynthesis. By sequestration within a hydrophobic cleft, ACPs protect their cargo of reactive intermediates in the cytosol and prevent premature hydrolysis of the thioester linkage and other side reactions.
In contrast, in type II fatty acid synthases in prokaryotes and plants, ACP-intermediates are diffusible discrete proteins. The Arabidopsis genome encodes eight ACP isoforms, five in plastids and three in mitochondria, which deliver intermediates to the independent catalytic partners of the synthase in a concerted manner. In the chloroplast, the chain elongation process is terminated by acyl-ACP–dependent acyltransferases, which provide acyl groups for lipid biosynthesis, or by thioesterase reactions, which release a non-esterified fatty acid from the ACP for export to other cellular organelles. Many bacterial species produce their own ACP variants, and in Escherichia coli, ACP is now termed AcpP to distinguish it from enzymes utilized in its metabolism.
Biosynthesis of ACP starts with post-translational transfer of a 4'‑phosphopantetheinyl group from CoA to a conserved serine on apo-ACP, catalysed by a 4'‑phosphopantetheinyl transferase (PPTase), to form the functioning holo-ACP (with 3',5'-bisphosphoadenosine as a by-product). Acyl-acyl carrier protein synthetases or acyl-AMP ligases (FAALs) act upon ACPs instead of coenzyme A, installing fatty acids onto the 4'‑phosphopantetheine arm of holo-ACP with hydrolysis of ATP; they are distinct from the acyl-CoA ligases. Families of thioesterases are responsible for hydrolysis of acyl-ACPs in plants and animals.
 |
| Figure 3. Biosynthesis of acyl-ACP. |
ACPs can be used for production of other cellular constituents, such as the octanoate moiety of lipoic acid and other biosynthetic products of acyl transfer, including rhamnolipids, for example, while molecules related structurally to ACP are utilized in non-ribosomal peptide and depsipeptide biosynthesis.
6. Alternative Fatty Acid Activation Reactions in Bacteria
Many bacterial species, both Gram-negative and Gram-positive, produce long-chain acyl-CoA esters for lipid synthesis, and this enables them to make efficient use of exogenous fatty acids. In E. coli, there are two inducible acyl-CoA synthetases, and fatty acid transport and activation are directly coupled to transcriptional control of the genes for various metabolic pathways for fatty acids; in this way, exogenous fatty acids repress synthesis de novo. Alternative methods are utilized to produce CoA esters of the primer molecules for synthesis of iso- and anteiso-methyl branched chain fatty acids.
Acyl-CoA molecules form a separate pool from endogenously synthesized acyl-ACP, and they can be used for phospholipid synthesis or broken down by β‑oxidation but cannot be used for lipopolysaccharide synthesis. Some species, including E. coli, use both acyl-CoA esters and acyl-ACPs for synthesis of phosphatidic acid de novo, but other bacteria do not make use of CoA but only use acyl carrier protein (ACP) linked to newly synthesised acyl groups (and even those of exogenous origin), i.e., the form produced by the type II fatty acid synthases in bacteria, parasites, algae and higher plants.
Acyl-phosphates: It has become apparent that most bacteria, including such human Gram-positive pathogens as Streptococcus pneumoniae, Bacillus subtilis and Staphylococcus aureus, lack the glycerophosphate acyl transferase enzymes that make use of CoA. Instead, a fatty acyl-phosphate is the acyl donor and can be produced by two routes both from fatty acids synthesised de novo and those of exogenous origin. In the first mechanism, the acyl-phosphate is synthesised by reaction of acyl-ACP with phosphate catalysed by an acyl-ACP:phosphate acyltransferase designated PlsX in the cell membrane, and the product then requires an acyl-transferase, designated PlsY, so that it can be utilized for the synthesis of lysophosphatidic acid by acylation of position sn-1 of glycerol-3-phosphate. Acyl-ACP is then the donor for esterification of position sn-2 via an enzyme designated PlsC to generate phosphatidic acid as the precursor for complex glycerolipids. In these species, PlsX is regulatory enzyme that synchronizes fatty acid synthesis with that of phospholipids, and it interacts with PlsY in the membrane to channel the substrate towards glycerolipid synthesis. Although acyl-phosphates are less stable than thiol esters in vitro, this is obviously not a problem in vivo.
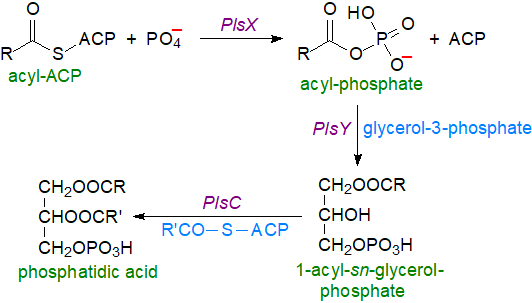 |
| Figure 4. Biosynthesis of acyl phosphates and phosphatidic acid in bacteria. |
A second route to acyl-phosphates, prior to incorporation of the acyl moieties into membrane phospholipids, is the only one to operate in S. aureus. In this instance, a fatty acid kinase (FakA) acting in concert with a fatty acid binding protein (FakB) uses ATP to phosphorylate unesterified fatty acids. There are two such binding proteins, FakB1 and FakB2, the first of which uses saturated fatty acids that are synthesised endogenously, while the second uses exogenous unsaturated fatty acids, such as oleic acid, derived from the host organism. Acyl-phosphates can be converted to acyl-ACPs via PlsX. The fatty acid kinase may control the production of virulence factors in S. aureus.
 |
| Figure 5. Biosynthesis of acyl phosphates in bacteria by a second route. |
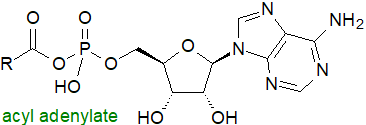 Acyl-adenylates: In many species of bacteria, including Mycobacterium tuberculosis, fatty acyl-adenylates
are produced by fatty acyl-AMP ligases, which are similar in sequence to fatty acyl-coenzyme A ligases.
While the latter perform a two-step catalytic reaction, AMP ligation followed by CoA ligation using ATP and CoA as cofactors
as described above, fatty acyl-AMP ligases produce only the acyl adenylates and do not continue to the second step.
Fatty acids activated as the acyl adenylates are then transferred to the acyl carrier protein domain of polyketide synthases, which, for example,
produce the mycolic acids and other unusual fatty acids of mycobacterial lipids.
They are also utilized by non-ribosomal peptide synthetases for the biosynthesis of various antibiotics, lipopeptides, and potentially virulent
complex lipids.
In cyanobacteria, unesterified fatty acids are linked to AMP by an acyl-ACP synthetase before the same enzyme transfers them to ACP for lipid
synthesis, while a fatty acid AMP ligase catalyses both reactions for production of secondary metabolites.
One explanation of this loss of CoA reactivity and gain of ACP transfer function in FAALs in bacteria is that it aids cells to divert fatty acids
toward different metabolic fates, even in the presence of high CoA concentrations.
Acyl-adenylates: In many species of bacteria, including Mycobacterium tuberculosis, fatty acyl-adenylates
are produced by fatty acyl-AMP ligases, which are similar in sequence to fatty acyl-coenzyme A ligases.
While the latter perform a two-step catalytic reaction, AMP ligation followed by CoA ligation using ATP and CoA as cofactors
as described above, fatty acyl-AMP ligases produce only the acyl adenylates and do not continue to the second step.
Fatty acids activated as the acyl adenylates are then transferred to the acyl carrier protein domain of polyketide synthases, which, for example,
produce the mycolic acids and other unusual fatty acids of mycobacterial lipids.
They are also utilized by non-ribosomal peptide synthetases for the biosynthesis of various antibiotics, lipopeptides, and potentially virulent
complex lipids.
In cyanobacteria, unesterified fatty acids are linked to AMP by an acyl-ACP synthetase before the same enzyme transfers them to ACP for lipid
synthesis, while a fatty acid AMP ligase catalyses both reactions for production of secondary metabolites.
One explanation of this loss of CoA reactivity and gain of ACP transfer function in FAALs in bacteria is that it aids cells to divert fatty acids
toward different metabolic fates, even in the presence of high CoA concentrations.
7. Analysis of CoA and ACP Esters
The profile of CoA esters in tissues can be an indicator of metabolic status, but because of their strongly amphipathic character, analysis is fraught with technical difficulties, which have limited this methodology in clinical settings. The procedures cited in the reading list are typical of those in use (but we have no personal experience). Extraction from tissues presents problems, and it may even be necessary to add a CoA binding protein to ensure quantitative recoveries. Having obtained an appropriate extract, methods are available to separate short- and longer-chain fractions, and individual components can then be resolved by reversed-phase high-performance liquid chromatography. Quantification can be a further problem, as it is not a straightforward matter to produce true solutions of pure CoA esters as standards. Electrospray-ionization tandem mass spectrometry coupled to HPLC is now widely used for analysis. Similarly, the analysis of those acyl moieties attached to acyl-carrier proteins has been a challenging problem, but an LC-MS method is now available.
Recommended Reading
- Bai, L., Yang, P.J., Han, B.T. and Kong, L.H. Progress of the acyl-Coenzyme A thioester hydrolase family in cancer. Front. Oncol., 14, 1374094 (2024); DOI.
- Barritt, S.A., DuBois-Coyne, S.E. and Dibble, C.C. Coenzyme A biosynthesis: mechanisms of regulation, function and disease. Nature Metab., 6, 1008-1023 (2024); DOI.
- Brett, C. and Gout, I. The two faces of coenzyme A in cellular biology. Free Rad. Biol. Med., 233, 162-173 (2025); DOI.
- Buyachuihan, L., Stegemann, F. and Grininger, M. How acyl carrier proteins (ACPs) direct fatty acid and polyketide biosynthesis. Angew. Chem.-Int. Ed., 63, e202312476 (2024); DOI.
- Cronan, J.E. The acyl carrier proteins of lipid synthesis are busy having other affairs. Biochem. J., 480, 855-873 (2023); DOI.
- Dong, H.J., Zou, Q. and Cronan, J.E. Defining the Enterococcus faecalis fatty acid kinase system of exogeneous fatty acid utilization. Mol. Microbiol., in press (2025); DOI.
- Lin, J.H., Lai, Y.F., Lu, F.J. and Wang, W.M. Targeting ACSLs to modulate ferroptosis and cancer immunity. Trends Endocrinol. Metab., 36, 677-690 (2025); DOI.
- Luo, Q., Das, A., Oldoni, F., Wu, P.Y., Wang, J.G., Luo, F. and Fang, Z.F. Role of ACSL5 in fatty acid metabolism. Heliyon, 9, e13316 (2023); DOI.
- Mondal, S., Pal, B. and Sankaranarayanan, R. Mechanistic understanding of bacterial FAALs and the role of their homologs in eukaryotes. Proteins Struct. Funct. Bioinf., 93, 26-37 (2025); DOI.
- Naquet, P., Kerr, E.W., Vickers, S.D. and Leonardi, R. Regulation of coenzyme A levels by degradation: the ‘Ins and Outs’. Prog. Lipid Res., 78, 101028 (2020); DOI.
- Nam, J.-W., Jenkins, L.M., Li, J., Evans, B.S., Jaworski, J.G. and Allen, D.K. A general method for quantification and discovery of acyl groups attached to acyl carrier proteins in fatty acid metabolism using LC-MS/MS. Plant Cell, 32, 820-832 (2020); DOI.
- Qiu, S. and Zeng, B. Advances in understanding the acyl-CoA-binding protein in plants, mammals, yeast, and filamentous fungi. J. Fungi (Basel), 6, 34 (2020); DOI.
- Singh, M., Elfrink, H.L., Harms, A.C. and Hankemeier, T. Recent developments in the analytical approaches of acyl-CoAs to assess their role in mitochondrial fatty acid oxidation disorders. Mol. Gen. Metab., 140, 107711 (2023); DOI.
- Wang, Y.L., Yang, H., Geerts, C., Furtos, A., Waters, P., Cyr, D., Wang, S.P. and Mitchell, G.A. The multiple facets of acetyl-CoA metabolism: Energetics, biosynthesis, regulation, acylation and inborn errors. Mol. Gen. Metab., 138, 106966 (2023); DOI.
- Wundersitz, A., Hoffmann, K.M.V. and van Dongen, J.T. Acyl-CoA-binding proteins: bridging long-chain acyl-CoA metabolism to gene regulation. New Phytol., 246, 1960-1966 (2025); DOI.
- Xie, J.M., Chen, X.Y., Zheng, M., Zhu, J.Z. and Mao, H. The metabolism of coenzyme A and its derivatives plays a crucial role in diseases. Front. Biosci. Landmark, 29, 143 (2024); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: November 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
