Ceramide Phosphoinositol and Related Complex Glycophosphosphingolipids
Ceramide phosphoinositol is an important phospholipid in the membranes of plants and fungi and it is the base unit for complex phytoglycosphingolipids, which were long ignored because of difficulties in isolation and analysis. As a result of advances in analytical methodology, especially mass spectrometry, the latter are now known to be the most abundant sphingolipid class in higher plants, although there is still much to learn. Like the glycosylphosphatidylinositol(GPI)-anchors in animals, there are ceramide phosphoinositol-glycans that can anchor proteins in the membranes of plants and fungi, where they affect innumerable processes at the plasma membrane and cell wall. In fungi, ceramide phosphoinositol is the precursor for mannosylinositolphosphoceramides
1. Ceramide Phosphoinositol
Ceramide phosphoinositol or myo-inositol-(1-O)-phospho-(O-1)-ceramide, the sphingolipid analogue of phosphatidylinositol, is an anionic component of the sphingolipids in many eukaryotes, and while it does not occur in mammals, it has been detected in some marine invertebrates (echinoderms), such as starfish, where it is the precursor of more complex lipids with ganglioside-like properties. In higher plants and fungi, ceramide phosphoinositol and glycosylated forms of this are substantial components of the membranes. Some parasitic organisms, such as Leishmania sp. (in some stages of its growth), contain ceramide phosphoinositol, and it is present in many filamentous fungi and mushrooms, usually together with glycosylated forms with mannose as the most common extra hexose. In addition to ceramide phosphoinositol, the protozoan parasite Trypanosoma brucei contains sphingomyelin and ceramide phosphoethanolamine (the only eukaryote known to synthesise all three).
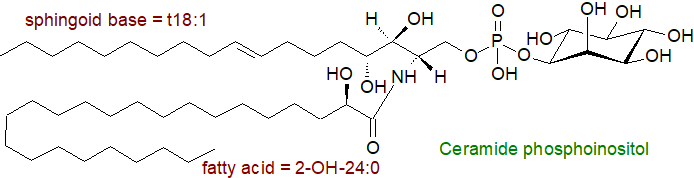
Such lipids are only rarely reported from bacteria, but the genus Sphingobacterium contains ceramide phosphoryl-myo-inositols and ceramide phosphorylmannose, and the genus Bacteroides from the microbiome of the human gut contains ceramide phosphoinositol, which may contribute to sphingolipid metabolism in intestinal tissue. Bacteroides ovatus has a highly complex lipidome, which includes ceramide phosphoinositol with a second ceramide phosphate attached.
The lipid constituents of the ceramide phosphoinositol of the few plants to have been studied are mainly saturated, with primarily phytosphingosine as the long-chain base and tetracosanoic acid (24:0) as the fatty acid, i.e., with the ceramide component produced by class II ceramide synthases. In Leishmania major, the main ceramide components are hexadecasphing-4-enine and sphingosine linked to stearic acid. The main long-chain base in ceramide phosphoinositol in Saccharomyces cerevisiae and filamentous fungi is phytosphingosine, and this is linked to a C26 hydroxy fatty acid (though C18 to C26 hydroxy and non-hydroxy acids are found in other species). In some fungi, such as the yeast Pichia pastoris, it is intriguing that the glycosylinositol phosphoceramides contain sphinganine as the main long-chain base, not (4E,8E)-9-methylsphinga-4,8-dienine as in the glucosylceramides, suggesting that distinct ceramide synthases and separate pools of ceramide are used in the biosynthesis of each of these lipids (see below). The ceramide phosphoinositol in the Gram-negative anaerobic Tannerella forsythia, a periodontal pathogen, contains saturated long-chain bases linked to 3‑hydroxy fatty acids, and it requires an external source of inositol for biosynthesis.
In yeast, there may be a parallel with the animal sphingophospholipid sphingomyelin in that ceramide phosphoinositol occurs in membrane raft domains together with the sterol, ergosterol, where both interact with membrane proteins with signalling functions. An analogous phenomenon is seen in higher plants.
Biosynthesis: In fungi, a very-long-chain fatty acid is linked by an amide bond to phytosphingosine in the endoplasmic reticulum by the ceramide synthase to form phytoceramide, a step that is required for fungal viability and hyphal morphogenesis. Phytoceramide is transported to the outer leaflet of the Golgi membrane by both vesicle-dependent and independent mechanisms and is then flipped to the Golgi inner membrane, where a ceramide phosphoinositol synthase (AUR1) and a closely associated regulatory subunit Kei1 catalyse the transfer of myo-inositol-1-phosphate from phosphatidylinositol to the C1 hydroxyl of phytoceramide, a mechanism analogous to that of the biosynthesis of sphingomyelin via phosphatidylcholine in animals (with sn-1,2-diacylglycerols as the other product). A functionally similar enzyme has been characterized from the phagocytic amoeba Dictyostelium discoideum.
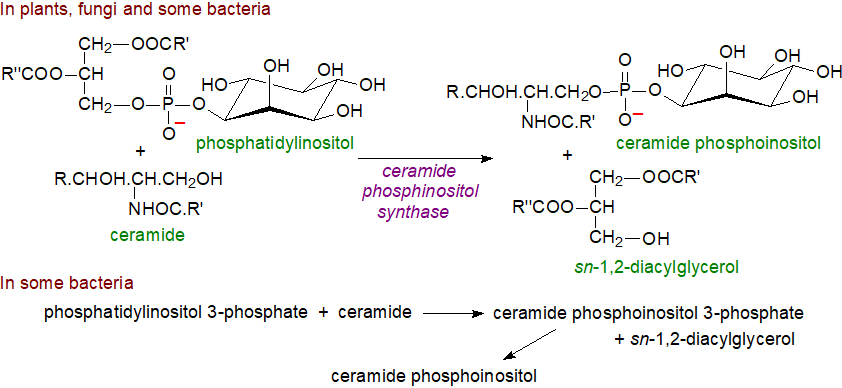 |
| Figure 1. Biosynthesis of ceramide phosphoinositol |
In plants, ceramide is transported to the Golgi by an unidentified mechanism for reaction with the inositolphosphorylceramide synthases of which there are three isoforms (IPCS1-3), which perhaps surprisingly are more closely related structurally to the protozoal than the fungal enzymes, and in rice, they may regulate plant height by affecting cell growth. An analogous mechanism can operate in some bacteria, but in others the precursor is phosphatidylinositol 3-phosphate to generate ceramide inositol-3-phosphate, which is subsequently dephosphorylated; sphinganine is the usual sphingoid base.
In addition to providing a membrane component and the precursor for further complex glycosphingophospholipids (see below), this reaction reduces the pool of ceramide and so inhibits the process of programmed cell death (apoptosis). Ceramide phosphoinositol is a key factor in the virulence of pathogenic fungi by activating the enzyme protein kinase C and other proteins of pathological relevance in infected mammalian cells, so the synthase is seen as a target for pharmaceutical agents to counter fungal infections. This is also true for pathogenic protozoa such as T. brucei, which has four paralogous genes encoding sphingolipid synthases (TbSLS1-4), with TbSLS1 a dedicated IPC synthase, TbSLS2 a dedicated ethanolamine phosphorylceramide synthase, and TbSLS3/4 bifunctional sphingomyelin/ethanolamine phosphorylceramide synthases. The 1,2-diacyl-sn-glycerol formed as a by-product of the biosynthesis of glycosylinositol phosphoceramides is probably re-used for the synthesis of other glycerolipids as it is not a signalling molecule in plants or fungi.
Catabolism: In yeasts, hydrolysis of ceramide phosphoinositol and the other the complex glycosphingophospholipids to ceramide is catalysed by an inositol phosphosphingolipid-phospholipase C. The ceramides produced by this enzyme together with further metabolites, such as long-chain bases, are signalling molecules in these organisms. In radishes, a non-specific phospholipase C3 has been identified that hydrolyses ceramide phosphoinositol to produce (phyto)ceramide-1-phosphate, which may be a mediator of plant growth.
2. Glycosylinositol Phosphoceramides (‘Phytoglycosphingolipids’) in Plants
It is now evident that the complex ceramide-containing glycosylinositol phosphoceramides (GIPCs), formerly termed ‘phytoglycosphingolipids’, are the most abundant sphingolipids in plants (and even on earth!), although they are not found in animals. Unfortunately, they are not easily extracted by conventional methodologies and analysis is technically daunting, so they have often been missed by analysts. After the pioneering papers by H.E. Carter and colleagues in the 1960s, little progress was made for nearly 40 years until modern mass spectrometric methodology was applied to the problem, fuelled by an increasing interest in sphingolipids in general. A corollary of the new findings is that the glycerophospholipids of plant membranes may be relatively less abundant than has been considered hitherto. Thus, the plasma membrane in plants has until recently been estimated to contain roughly 10% of glucosylceramide, 40% sterols and 50% phospholipids, while the glycosylinositol phosphoceramides were ignored. In contrast, when the last are taken into account, sphingolipids can account for as much as 50% of the total lipids (GIPCs up to 40%) and phospholipids only 25% in this membrane. In leaves of Arabidopsis thaliana, one estimate is that is that GIPCs constitute 50% of the lipids of the outer leaflet of the plasma membrane.
It is now well established that higher plants, yeasts and fungi (and some protozoa) contain a number of complex glycosylinositol phosphoceramides with ceramide phosphoinositol as the backbone and with carbohydrate moieties linked to inositol. More than twenty molecular forms of these anionic lipids were identified initially, though only a few of these were fully characterized until relatively recently. It is evident that the nature of the carbohydrate moiety is dependent on species and can be highly complex, with up to seven monosaccharide units (occasionally more) such as glucuronic acid, glucosamine (and its N-acetyl derivative) and many others. In these complex sphingolipids, the oligosaccharide chains are usually linked at position 2 and/or position 6 of the inositol moiety, as with the analogous glycerophospholipids, leading to both linear and branched chains of hexose units. As more plants are studied, it has become evident that the overall structures can be very variable. Glycosylinositol phosphoceramides in algae differ from those in mosses, gymnosperms and monocots, while dicots contain the greatest complexity.
Different classes of organism have different structural building blocks, which can be considered simplistically as –
| Higher plants | Hexose–Glucuronic acid–Ins–P–Cer | |
| Most yeasts and fungi | Man–Ins–P–Cer | |
| Protozoa | Man–GlcNH2–Ins–P–Cer |
In higher plants, the hexose linked to glucuronic acid depends on plant species and cell tissues, and can be D-glucose, D-galactose, or D-mannose, but the stereochemistry and anomeric configuration have only rarely been determined. The first to be fully characterized is N-acetylglucosamine-glucuronic acid-inositolphosphoceramide, which is the most abundant sphingolipid in the membranes of leaves of tomato and soybean at roughly twice the concentration of glucosylceramide. In cabbage, the hexose linked to glucuronic acid via an α-glycosidic bond is D-mannose, and this is probably true for Arabidopsis, which has t18:1/h24:0 as the main ceramide component.
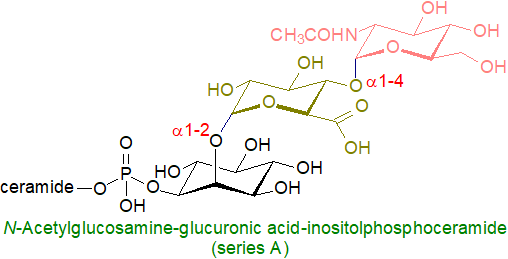
Green and red algae contain inositol-phosphoceramides linked to three or four hexuronic acid moieties, but higher plants contain more complex lipids of this type with up to six hexose units attached to the glucuronic acid residue and present in varying proportions. These have been grouped into six series, which are apparently specific to particular plant families, by Buré et al. as listed in Table 1.
Table 1. Structures of the glycosylinositol phosphoceramides from higher plants. |
|
| Series A: | Glc-GlcA-IPC : Glc-GlcA-IPC : GlcN-GlcA-IPC : GlcNAc-GlcA-IPC |
| Series B: | Hex-Glc-GlcA-IPC : Hex-GlcN-GlcA-IPC : Hex-GlcNAc-GlcA-IPC |
| Series C: | Ara-Hex-Glc-GlcA-IPC : Ara-Hex-GlcN-GlcA-IPC : Ara-Hex-GlcNAc-GlcA-IPC |
| Series D: | (Ara)2-Hex-Glc-GlcA-IPC : Ara-(Hex)2-GlcN-GlcA-IPC : Ara-(Hex)2-GlcNAc-GlcA-IPC |
| Series E: | (Ara)3-Hex-Glc-GlcA-IPC : Ara-(Hex)3 -GlcN-GlcA-IPC : Ara-(Hex)3-GlcNAc-GlcA-IPC |
| Series F: | (Ara)4-Hex-Glc-GlcA-IPC : (Ara)2-(Hex)3-GlcN-GlcA-IPC : (Ara)2-(Hex)3-GlcNAc-GlcA-IPC |
| Abbreviations: IPC, inositol phosphoceramide; Glc, glucose; GlcA, glucuronic acid;
Ins, inositol; Cer, ceramide; GlcN, glucosamine, GlcNAc, N-acetylglucosamine; Hex, hexose; Ara, arabinose. From - Buré, C. et al. Fast screening of highly glycosylated plant sphingolipids by tandem mass spectrometry. Rapid Commun. Mass Spectrom., 25, 3131-3145 (2011); DOI. |
|
In Arabidopsis, the GIPC from series A, Man-GlcA-IPC and GlcN(Ac)-GlcA-IPC, are the main forms in leaves with the latter relatively abundant in seeds also, while GIPC containing GlcNAc from groups D and E are absent from leaf tissue, although they are present in pollen; long-chain bases with Δ4 double bonds are only found in pollen. Series B GIPC are major components of monocots, but further work is necessary to fully elucidate the core structures. Some plants contain GIPCs with branched carbohydrate chains, and from series B onwards variants have been identified with glycan branching from the inositol phosphate group.
The composition of the long-chain bases differs between sphingolipid classes and between species and tissues, but in general the more complex lipids tend to have a much higher proportion of trihydroxy bases (phytosphingosine) than do the glucosylceramides, and this is especially true for fungi. As well as t18:0 and t18:1(8Z and 8E), often the main sphingoid base, d18:0, d18:1(8Z and 8E), d18:2 (4E/8Z and 4E/8E) have been detected in ceramide phosphoinositides of plants. The fatty acid components range in chain-length from C14 to C26, and they usually have a 2-hydroxyl substituent.
 In studies
with tobacco plants, it has been demonstrated that a large part of the lipid component of the outer leaflet (apoplastic side) of the plasma membrane
comprises bulky GIPCs together with sterols (free and glycosylated), while the inner leaflet (cytoplasmic side) contains all the
digalactosyldiacylglycerols, phosphatidylserine and phosphatidylinositol 4,5‑bisphosphate, among other lipids.
In this location, GIPCs have the potential to act as receptors or as mediators of cellular responses to environmental stimuli.
Raft-like microdomains are thought to exist in the plasma membrane, as
studies with model membrane systems in vitro suggest that GIPCs can interact with plant sterols, such as sitosterol
(but not stigmasterol), to form a condensed phase, although it is not yet possible to confirm the effect in vivo.
However, it should not be forgotten that the membrane is in fact a protein-lipid composite, where transmembrane proteins dominate the structure.
The high concentration of GIPCs in the apoplastic leaflet is presumed to present a physical barrier
involved in the maintenance of thermal tolerance, cell integrity and protection from pathogens.
There is evidence that GIPCs in the outer leaflet can be linked covalently via a boron bridge to rhamnogalacturonan II,
a complex acidic polysaccharide of the primary cell wall.
In studies
with tobacco plants, it has been demonstrated that a large part of the lipid component of the outer leaflet (apoplastic side) of the plasma membrane
comprises bulky GIPCs together with sterols (free and glycosylated), while the inner leaflet (cytoplasmic side) contains all the
digalactosyldiacylglycerols, phosphatidylserine and phosphatidylinositol 4,5‑bisphosphate, among other lipids.
In this location, GIPCs have the potential to act as receptors or as mediators of cellular responses to environmental stimuli.
Raft-like microdomains are thought to exist in the plasma membrane, as
studies with model membrane systems in vitro suggest that GIPCs can interact with plant sterols, such as sitosterol
(but not stigmasterol), to form a condensed phase, although it is not yet possible to confirm the effect in vivo.
However, it should not be forgotten that the membrane is in fact a protein-lipid composite, where transmembrane proteins dominate the structure.
The high concentration of GIPCs in the apoplastic leaflet is presumed to present a physical barrier
involved in the maintenance of thermal tolerance, cell integrity and protection from pathogens.
There is evidence that GIPCs in the outer leaflet can be linked covalently via a boron bridge to rhamnogalacturonan II,
a complex acidic polysaccharide of the primary cell wall.
Although much remains to be learned of the biosynthesis and function of the glycosylinositol phosphoceramides in higher plants, this is now an active area of research and some consider them to be the functional equivalents of gangliosides in animals. The inositol phosphorylceramide synthase 2 (IPCS2) at the trans-Golgi network is involved in the production of the ceramide phosphorylinositol for biosynthesis of complex sphingolipids. It is known that an IPC core in the Golgi, synthesised as described above, can be glycosylated by various glycosyltransferases to produce mature GIPCs, and for example, a single inositol phosphoceramide α-glucuronosyltransferase (IPUT1), which adds a glucuronic acid (GlcA) moiety via an α(1,4)-linkage, has been characterized from Arabidopsis. This is the first enzyme in the GIPC glycosylation pathway and has been shown to be essential for normal growth. Further glycosylation of the GlcA-IPC head group is mediated by GIPC mannosyl transferase1 (GMT1), which produces mannose- or hexose-mannose-linked GIPCs, or glucosamine inositol phosphorylceramide transferase 1 (GINT1), which transfers glucosamine (GlcN) or N-acetyl-glucosamine (GlcNAc) mainly to the GlcA-IPCs. Both requires the Golgi-localized nucleotide sugar transporters GONST1 and GONST2 or UDP‑N‑acetyl-D-glucosamine transporter 1 (UGNT1). GINT1 is responsible for the glycosylation of a subgroup of GIPCs found in seeds and pollen that contain GlcN(Ac); loss of this enzyme was found to be fatal to seedlings in rice but not in Arabidopsis.
Evidence is emerging that GIPCs participate in several important processes, including symbiosis, pollen development, and membrane organization and trafficking. Biophysical analysis has shown that GIPCs increase the thickness and electronegativity of model membranes, interact in different ways with each phytosterol, and regulate the gel-to-fluid phase transitions during variations in temperature. It has been demonstrated that GIPCs can act as a salt-sensing mechanism to bind Na+ ions to polarize the cell surface potential and gate the Ca2+ influx channels, leading to the suggestion that these plasma-membrane lipids are associated with adaptation to environmental salt levels. While little is known of the functions of individual members of the GIPC family, improved analytical methodology is enabling progress. In barley grain, there is upregulation of the B- and C-series with increasing maturity, and application of heat stress induces significant remodelling of GIPC profiles, especially that of the B-series.
In general, it is evident that GIPC have critical roles in membrane dynamics, stress adaptation and pathogen resistance. They have been implicated in salicylic acid-dependent signalling and the hypersensitive defence response against pathogens, especially fungi, and it has been determined that they are receptors via the terminal hexose for toxins or cytolysins (necrosis and ethylene-inducing peptide 1-like protein (NLP)) produced by plant pathogens, which attack broad-leafed plants only. These include the toxin responsible for potato blight (Phytophthora infestans) and the Botrytis fungus, which can ruin fruit and vegetable crops. One relevant factor is that GIPCs in broad-leafed plants (eudicots) tend to have only two hexoses in the chain, and the toxin can bind and causes cell lysis. Monocotyledons usually have three or more, hexoses in the chain, and although the cytolysin can connect with the longer receptor in wheat or barley, it cannot reach the cell membrane to exert its deadly effects.
Relatively little is known of the normal catabolism of lipids containing ceramide phosphoinositols in plants, although there is evidence that the complex glycosylinositol phosphoceramides turn over much more rapidly, with generation of ceramides, than do the glucosylceramides. GIPC in membranes of plants attacked by the necrotrophic fungus Botrytis cinerea are hydrolysed by an enzyme of the phospholipase C type with release of inositol phosphate glycans.
3. Mannosylinositolphosphoceramides in Fungi
The glycan moieties of fungal GIPCs are very diverse and complex, varying among species. The more common forms contain glucosamine and mannosyl residues linked to the inositol group of the IPC to give three carbohydrate "cores”, i.e., glucosamine-α-1,2-IPC, mannose-α-1,6-IPC and mannose-α-1,2-IPC, which form the basis for a series of related lipids attached to further mannose or other monosaccharides such as fucose, xylose and galactose, or even to choline-phosphate. For example, the following have been found in the mycelium of the saprophytic filamentous fungus and opportunistic human pathogen Aspergillus fumigatus.
| α-Man-(1,3)-α-Man-(1,6)-α-GlcN-(1,2)-Ins-P-cer |
| α-Man-(1,3)-α-Man-(1,2)-Ins-P-cer |
| α-Man-(1,2)-α-Man-(1,3)-α-Man-(1,2)-Ins-P-cer |
| α-Man-(1,3)-[β-Galf-(1,6)]-α-Man-(1,2)-Ins-P-cer |
| α-Man-(1,2)-α-Man-(1,3)-[β-Galf-(1,6)]-α-Man-(1,2)-Ins-P-cer |
| β-Galf-(1,2)-α-Man-(1,3)-α-Man-(1,2)-Ins-P-cer |
| β-Galf-(1,2)-α-Man-(1,3)-[α-Man-(1,6)]-α-Man-(1,2)-Ins-P-cer |
| β-Galf-(1,2)-α-Man-(1,3)-[β-Galf-(1,6)]-α-Man-(1,2)-Ins-P-cer |
| Choline-P-6-β-Galf-(1,2)-α-Man-(1,3)-α-Man-(1,2)-Ins-P-cer |
In the budding yeast, S. cerevisiae, which does not produce glucosylceramide, ceramide phosphoinositol is accompanied by two further inositol-containing sphingophospholipids with a Man-(α1,2)-Ins core, i.e., mannosylinositolphosphoceramide (Cer-P-Ins-Man) and mannosyldiinositolphosphoceramide (Cer-P-Ins-Man-P-Ins). The last of these is most abundant with phytosphingosine linked to 2‑hydroxy-26:0 as the main ceramide component.
In yeasts, the inositolphosphorylceramide synthase (IPCS) adds an inositolphosphate group to ceramide to produce an IPC molecule in the Golgi as described above. Mannosylinositolphosphoceramide is then synthesised in S. cerevisiae by transfer of a mannose unit from guanosine diphosphate (GDP)-mannose via an β1-2 linkage to ceramide phosphoinositol in the Golgi lumen by means of mannose inositolphosphoceramide synthase complex (MIPCS), which consists of a catalytic subunit Sur1 or Csg1 and a regulatory subunit Csg2; GDP-mannose is provided to this enzyme by the Golgi nucleotide sugar transporters 1 and 2. In a further reaction, another inositolphosphoryl unit can be added to this by transfer from phosphatidylinositol via an α1-2 linkage to the C6 position of mannose, catalysed by an inositolphosphotransferase (IPT1), to form mannosyldiinositolphosphoceramide. Both mannosylinositol lipids are then transferred to the plasma membrane and cell wall, while non-glycosylated inositolphosphoceramide is transported to a vacuole.
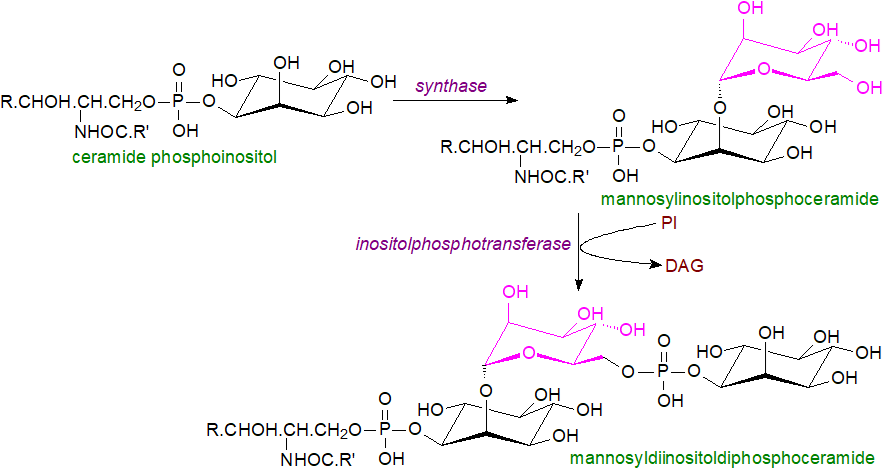 |
| Figure 2. Biosynthesis of complex phosphosphingolipids in S. cerevisiae. |
The very different long-chain base composition of the mannosylinositolphosphoceramides (mainly phytosphingosine) in comparison to the monoglycosylceramides (mainly (4E,8E)-9-methyl-4,8-sphingadienine) suggests a dichotomy in the biosynthetic pathways for fungal neutral and acidic glycosphingolipids, and it has been determined that the dihydroceramide precursors are generated by enzymes encoded by different genes. The fatty acid components also tend to differ, i.e., C16 to C18 in neutral and C18 to C26 in acidic glycosphingolipids, the latter often containing additional hydroxyl groups.
In Neurospora crassa, deletions in this biosynthetic pathway are lethal, and after synthesis, mannosylinositol-phosphoceramide is modified by GSL-5, an inositol-phosphoceramide-B C26 hydroxylase, which adds a hydroxyl group to the penultimate carbon atom in the amide-linked fatty acid. While this is not required for viability, it gives a clear mutant phenotype that affects all stages of the life cycle.
In Candida albicans, mannosylinositolphosphoceramide is first phosphomannosylated (rather than linked to phosphorylinositol) before it is further β‑1,2‑mannosylated by at least two mannosyltransferases, the first of which adds the first and probably the second β-mannose, while the second mannosyltransferase adds a third β‑mannose and elongates the chain; the resulting glycosphingolipid is termed a phospholipomannan. This extensive mannosylation is essential for the transfer of the phosphoglycolipid from the plasma membrane to the cell wall.
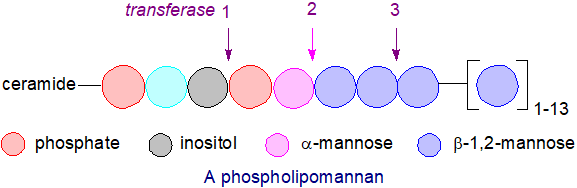
A. fumigatus produces a galactomannan of this type that can be used as a circulating biomarker for the detection of invasive aspergillosis. It is composed of a main chain of α-mannoside and short side chains of galactofuranose residues, and so far, it is unique among fungi in that it occurs in three forms: free and released into the culture medium, covalently bound to cell wall β-1,3-glucans, and membrane bound through a glycosylphosphatidylinositol anchor. The galactomannan polysaccharide is linked to the fourth mannose residue of the core structure below though a glycosidic linkage.
α-Man-(1,2)-α-Man-(1,2)-α-Man-(1,2)-α-Man-(1,6)-α-GlcN-(1,4)-Ins-P-cer
In general, mannosylinositolphosphoceramides in fungi are necessary for replication and growth, and they enable the organisms to evade the immune system of their hosts. The loss of specific subtypes causes impairment of various cellular functions, indicating the physiological importance of this structural diversity. With C. albicans, the mannosylinositolphosphoceramides do not promote virulence directly, but depending on the immune status of the host, they can do so indirectly by activation of the host signalling mechanism for initial recognition of fungi to cause immune system disorder and persistent fungal disease.
4. Ceramide Phosphoinositol-Glycan Anchors for Proteins - Plants and Fungi
Lipid phosphoinositol-glycan anchors for proteins occur in plants in which both phosphatidylinositol and ceramide phosphoinositol are the lipid components for oligosaccharide-linked proteins in an analogous way to the glycosylphosphatidylinositol(GPI)-anchors in animals (see this web page for more detailed discussion), and together with the glycosylinositol phosphoceramides (GIPCs), they are the most abundant sphingolipids in plants. As in animals, these contain a highly conserved core unit –
Man-(α1,4)-Man-(α1,4)-Man-(α1,4)-GlcN-(α1,6)-Ins–1–P–Cer/DAG
Surprisingly, only one of these ceramide-linked lipophosphoglycans has been fully characterized (from a Pyrus communis (pear) cell suspension), and this was devoid of phosphoethanolamine side chains but had a β-linked galactose side chain on oxygen 4 of the first mannose in some molecular species. The proteins can remain tethered to the cell wall in this way, or they can be released by action of a phospholipase. Gene studies suggest that over 200 different proteins occur in membranes in this form in A. thaliana. They are major components of the plasmodesmata, the narrow passages through the cell walls of adjacent cells that allow communication between them, where the membranes form raft-like domains enriched in sterols and sphingolipids containing predominantly very-long-chain fatty acids. At the interface of the plasma membrane and cell wall, GPI-anchored proteins influence several processes, which include signalling, cell wall metabolism and polymer cross-linking. They have the potential to transfer signals into the protoplast and thus activate signalling pathways.
Yeasts contain highly complex lipids of this type, based mainly on a ceramide core, which serves to anchor proteins to cell surfaces. In some of these, it has been established that addition of a glycosylphosphatidylinositol precursor to proteins occurs first, before the ceramide moiety is incorporated by an exchange reaction, i.e., ceramide phosphoinositol per se is not the precursor. A similar process probably occurs in higher plants, but this has still to be confirmed experimentally. On the other hand, in A. fumigatus, structural differences between the glycosylphosphatidylinositol and ceramide phosphoinositol anchors indicate that they may utilize different α‑N‑acetylglucosaminyl-transferases.
5. Other Ceramide Phosphoinositides and Glycophosphosphingolipids
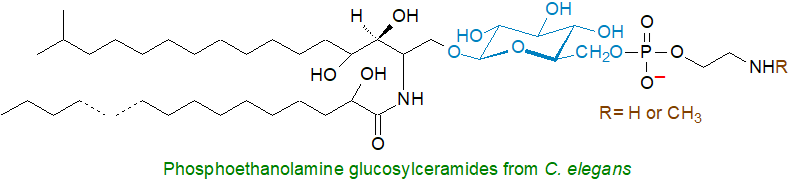
The nematode and primitive animal model Caenorhabditis elegans contains phosphoethanolamine glucosylceramides and monomethyl-phosphoethanolamine glucosylceramides. While this may be the only record of a lipid of this kind in animals, some comparable phosphonoglycolipids have been found in marine animals.
The extracellular parasitic protozoan Trichomonas vaginalis, which is implicated in sexually transmitted diseases in humans, contains a surface lipophosphoglycan with a ceramide phosphoinositol-glycan core, and this complex glycophospholipid is responsible for the immuno-inflammatory response of the host to the organism. The Caribbean sponge Svenzea zeai contains zeamide, a ceramide phosphoinositide with arabinose linked to inositol, i.e., with a 6‑O‑β‑D-arabinopyranosyl-myo-inositol (D-Arap(1β→6)Ins) core motif that may be unique among natural glycoconjugates. It is composed of very-long-chain sphingoid bases (C24 to C28) in combination with a high proportion of branched saturated fatty acids. However, it is not clear whether this lipid originates biosynthetically in the sponge or in symbiotic microorganisms.
Suggested Reading
- Cassim, A.M., Gouguet, P., Gronnier, J., Laurent, N., Germain, V., Grison, M., Boutté, Y., Gerbeau-Pissot, P., Simon-Plas, F. and Mongrand, S. Plant lipids: Key players of plasma membrane organization and function. Prog. Lipid Res., 73, 1-27 (2019); DOI.
- Cassim, A.M. and 18 others. Biophysical analysis of the plant-specific GIPC sphingolipids reveals multiple modes of membrane regulation. J. Biol. Chem., 296, 100602 (2021); DOI.
- Fougère, L., Mongrand, S. and Boutté, Y. The function of sphingolipids in membrane trafficking and cell signaling in plants, in comparison with yeast and animal cells. Biochim. Biophys. Acta, Lipids, 1869, 159463 (2024); DOI.
- Fradin, C., Bernardes, E.S. and Jouault, T. Candida albicans phospholipomannan: a sweet spot for controlling host response/inflammation. Seminars Immunopathol., 37, 123-130 (2015); DOI.
- Haslam, T.M. and Feussner, I. Diversity in sphingolipid metabolism across land plants. J. Exp. Botany, 73, 2785-2798 (2022); DOI.
- Heaver, S.L. and 10 others. Characterization of inositol lipid metabolism in gut-associated Bacteroidetes. Nature Microbiol., 7, 986-1000 (2022); DOI.
- Heise, N., Koeller, C.M., Sharif, M. and Bangs, J.D. DStage-specific function of sphingolipid synthases in African trypanosomes. mBio, in press (2024); DOI.
- Luttgeharm, K.D., Kimberlin, A.N. and Cahoon, E.B. Plant sphingolipid metabolism and function. In: Lipids in Plant and Algae Development. pp. 249-286 (Edited by Y. Nakamura and Y. Li-Beisson, Springer International Publishing, Switzerland) (2016); DOI.
- Pühringer, M. and others. Automated mass spectrometry-based profiling of multi-glycosylated glycosyl inositol phospho ceramides (GIPC) reveals specific series GIPC rearrangements during barley grain development and heat stress response. Plant J., 122, e70279 (2025); DOI.
- Tani, M. Biological importance of complex sphingolipids and their structural diversity in budding yeast Saccharomyces cerevisiae. Int. J. Mol. Sci., 25, 12242 (2024); DOI.
- Usmani, S.A., Kumar, M., Arya, K., Ali, B., Bhardwaj, N., Gaur, N.A., Prasad, R. and Singh, A. Beyond membrane components: uncovering the intriguing world of fungal sphingolipid synthesis and regulation. Res. Microbiol., 174, 104087 (2023); DOI.
- Yeats, T.H., Bacic, A. and Johnson, K.L. Plant glycosylphosphatidylinositol anchored proteins at the plasma membrane-cell wall nexus. J. Integr. Plant Biol., 60, 649-669 (2018); DOI.
- Zhang, Y., Wang, S., Wang, L., Chang, X., Fan, Y., He, M. and Yan, D. Sphingolipids at plasmodesmata: structural components and functional modulators. Int. J. Mol. Sci., 23, 5677 (2022); DOI.
- Zhu, X.M., Li, L., Bao, J.D., Wang, J.Y., Daskalov, A., Liu, X.H., Del Poeta, M. and Lin, F.C. The biological functions of sphingolipids in plant pathogenic fungi. PLOS Pathogens, 19, e1011733 (2024); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: August 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
