Oxidized Phospholipids
1. Introduction
During conditions of oxidative stress and redox imbalance, reactive oxygen species (ROS), such as the superoxide anion (O2•-), hydroxyl radical (OH•), nitric oxide (NO•) and peroxyl radicals (LOO•), can be generated in cells from such external sources as UV radiation and other environmental stresses or internally as by-products of mitochondrial processes, and they can then attack lipid-bound unsaturated fatty acids by non-enzymatic mechanisms (autoxidation). To these can be added non-radical oxidizing agents such as hydrogen peroxide (H2O2), hypochlorous acid (HOCl), ozone (O3) and singlet oxygen (1O2). In particular, the phospholipids in lipid membranes provide a readily accessible platform where the effects of ROS are amplified at every step through a cascade of chemical reactions: the higher the number of double bonds in the fatty acid components, the greater the susceptibility to such processes. Therefore, phosphatidylethanolamine, phosphatidylserine and phosphatidylinositol are often most affected, while a high content of ether lipids can be a further negative factor.
The result is the creation initially of many different lipid-bound fatty acyl hydroperoxides, the primary peroxidation products, which can react further to generate innumerable secondary oxygenated metabolites with hydroxyl, epoxy or oxo groups, often as short-chain fragments with reactive electrophilic carbonyl moieties that can affect tissue metabolism. In addition, a peroxyl radical can react intramolecularly to yield an endoperoxide in which the molecule retains an unpaired electron, and this can rearrange to give 5-membered ring structures such as the cyclopentenone rings in isoprostanes. Oxidized lipids in the circulation and in cell membranes can be both beneficial and harmful to the human body. Excessive amounts of oxidized phospholipids have been linked to the pathogenesis of various cardiopulmonary disorders, such as atherosclerosis and thrombosis, acute lung injury and neurodegenerative processes, while benefits include the induction of anti-inflammatory mediators. Accumulation of lipid hydroperoxides is central to a type of cell death known as ferroptosis.
 |
| Figure 1. Oxidized structures in polyunsaturated fatty acids. |
Oxidized lipids from enzymatic reactions add further complexity. The chemistry, biochemistry, pharmacology, and molecular biology of those oxidized fatty acids from enzyme activity and covered by the generic term oxylipin, including eicosanoids, docosanoids and plant oxylipins, are substantial topics that are described in many web pages in our section on Fatty Acids and Oxylipins. While much work has been concerned with unesterified oxylipins, it is increasingly being recognized that intact oxidized lipids, i.e., phospholipids, cholesterol esters and others containing such oxylipins, need not simply be inert storage molecules but can profoundly affect tissue metabolism in both animals and plants. Often the concentrations of esters are much greater than those of the unesterified oxylipins. Cellular systems must maintain a balance between pro-oxidants and antioxidant defences if oxidative stress is to be avoided, but the varied nature of the oxidized lipids from the two types of reaction mean that their impacts are highly complex and often apparently contradictory. Only a general flavour of such a complex topic is possible here.
While phospholipids containing intact fatty acids with oxygen moieties in the chain have great relevance to this topic, technical difficulties in analysis and the complex nature of the potential products (as many as 20,000 molecular species in animals according to one calculation) may explain why research has lagged behind that of the metabolites from cleavage of hydroperoxides, i.e., volatile aldehydes, which are relevant to any discussion of oxidized lipids and have their own web page. The isoprostanes from non-enzymatic oxidation reactions of esterified fatty acids must be considered in the same context, as do the enzymatic formation and properties of esterified hydroperoxy- and hydroxy-eicosatetraenoic acids (HETE) found in many different lipid classes, including oxidized sterols and cholesterol esters, triacylglycerols and plant glycolipids. Nitrated phospholipids are likewise discussed in a separate web page, as are oxidized lipoproteins.
2. Autoxidation
All polyunsaturated fatty acids can undergo autoxidation by free radical chain-reaction mechanisms as discussed at greater length in several web pages on this website, including those dealing with tocopherols and coenzyme Q as antioxidants, as these are key elements of the defence against oxidative damage to cells. Such reactions occur almost exclusively with polyunsaturated fatty acids esterified in intact lipids as opposed to unesterified.
Radical Reactions: To summarize, autoxidation consists of three main steps: initiation, propagation and termination. The initiation step begins with abstraction of a hydrogen atom by an ROS from the bis-allylic carbon of one of the 1,4‑cis,cis‑pentadiene moieties of a polyunsaturated fatty acid with creation of a carbon-centred radical, which tends to be stabilized through resonance by a molecular rearrangement as a conjugated diene (usually trans-cis). The peroxidation rate is very low in the absence of catalysts but is greatly enhanced by transition metals and by iron (Fe). Normally, the levels of free Fe‑ions are strictly controlled in cells by binding to specific proteins, but when there is an Fe-overload or poorly controlled Fe-protein complexation, aberrant non-enzymatic lipid peroxidation can accelerate rapidly as in ferroptosis (see below).
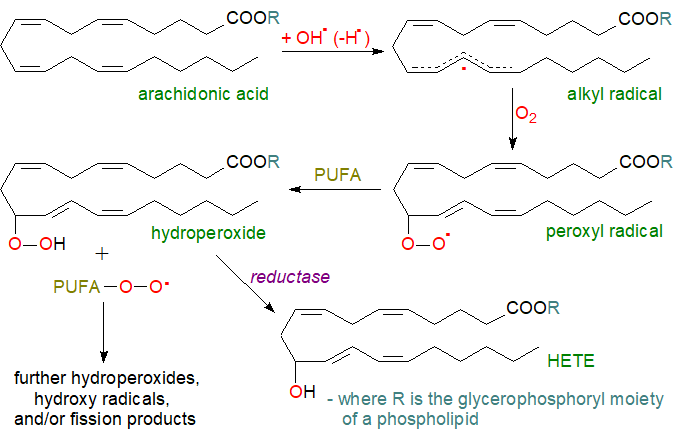 |
| Figure 2. Autoxidation of a lipid-bound unsaturated fatty acid such as arachidonate. |
The initiation step is followed by the propagation (and rate determining) step in which the unstable fatty acid radical reacts with molecular oxygen to generate a peroxyl radical, which propagates the chain reaction by abstracting a hydrogen atom from another polyunsaturated fatty acid to yield a lipid hydroperoxide and a second lipid peroxyl radical. This last radical is the chain carrier of lipid autoxidation (and is the source of the volatile aldehydes). As an alternative propagation reaction, peroxyl radical addition to a double bond can yield more hydroperoxides and epoxides, and thence to aldehydes and truncated glycerolipids following cleavage; when both fatty acids in a phospholipid are unsaturated, an intermolecular propagation reaction of this kind is greatly favoured. Such reactions have limited positional and no stereo specificity, and it is illustrated here for one only of the possible reactions of arachidonate.
Termination of the process occurs when two radicals interact and dimerize, but this is a relatively rare occurrence, and it is much more usual for a radical-trapping antioxidant, such as α-tocopherol, to break the chain reaction and prevent further oxidation of polyunsaturated fatty acids. As a final detoxification step, reduction to the chemically less reactive lipid hydroxide occurs, catalysed by a reductase such as glutathione peroxidase 4 (GPX4), which acts preferentially upon lipid hydroperoxides and is one of a family of selenium-containing enzymes that utilize glutathione as a substrate; this enzyme is of special importance in ferroptosis. In the example illustrated, the product is a hydroxy-eicosatetraenoic acid (HETE) esterified to the lipid.
Many such mono-hydroperoxy isomers of arachidonic acid have been detected in intact lipids, and similar reactions occur during the creation of ring-containing isoprostanes, i.e., with cyclopentenone or endoperoxide structures, which can be cleaved to give isolevuglandins. If the comparable autoxidation reactions of linoleate, linolenate, eicosapentaenoate (EPA) and docosahexaenoate (DHA) by such mechanisms are taken into consideration, together with the lack of stereochemical control and the probable formation of dihydroperoxides, the number of possible hydroperoxy and other oxygenated metabolites is incalculable.
 Although most emphasis has been on oxidation of the unsaturated
fatty acid components of phospholipids, the polar head groups too can be subjected to oxidation, for example by deamination of
phosphatidylethanolamine, probably through radical action on the alpha carbon near the amine group and resulting in a
terminal aldehyde as illustrated.
Comparable reactions occur with phosphatidylserine, but phosphatidylcholine is not affected in this way.
Although most emphasis has been on oxidation of the unsaturated
fatty acid components of phospholipids, the polar head groups too can be subjected to oxidation, for example by deamination of
phosphatidylethanolamine, probably through radical action on the alpha carbon near the amine group and resulting in a
terminal aldehyde as illustrated.
Comparable reactions occur with phosphatidylserine, but phosphatidylcholine is not affected in this way.
Singlet oxygen: Ground-state or triplet molecular oxygen (O2) is a paramagnetic biradical bearing two valence electrons with parallel spins, and this prevents spontaneous reaction with non-radical molecules at ambient temperatures. On the other hand, electronically excited or singlet oxygen (1O2 or O=O), in which the spin of one of the unpaired electrons is changed to yield two electrons with opposite spins, can oxidize non-radical organic molecules. With double bonds, singlet oxygen acts via an ene-reaction to yield fatty acyl hydroperoxides, which can react further as described above, and it can undergo Diels-Alder cycloadditions with conjugated dienes to further add to the complexity. Such reactions are not possible with triplet oxygen.
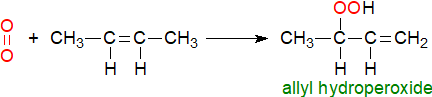 |
| Figure 3. Ene reaction of singlet oxygen. |
Singlet oxygen can be produced endogenously in various ways, including by self-reaction of lipid peroxyl radicals during the chain termination step of autoxidation. Alternatively, it can be generated through photosensitization, i.e., the interaction of UV or visible light with natural chromophores present normally in cells, e.g., riboflavins, porphyrins, bilirubin or melanin, which act to donate energy to convert molecular oxygen to singlet oxygen. This is then able to attack DNA bases, proteins and double bonds in polyunsaturated fatty acids, while the head groups of phospho- and glycolipids are also susceptible to reaction, and photooxidation of phosphatidylethanolamine can lead to phosphatidic acid production.
The reaction with singlet oxygen is utilized for the benefit of patients with cancer, macular degeneration and microbial infections by means of photodynamic therapy in which singlet oxygen is generated in a controlled manner by the action of light on exogenous photosensitizers that have been delivered to target cells. A directed light dose generates sufficient hydroperoxides to destroy these cells with a minimum of peripheral damage.
In plants, singlet oxygen is a highly potent ROS that readily oxidizes cellular molecules. It has long been known to regulate their responses to high-light stress, but it may have a defensive role in plant interactions with insects, bacteria and fungi, when generated by phototoxins in the epidermis. By accumulating in response to diverse stresses that perturb chloroplast metabolism, it engages in retrograde signalling through the EXECUTER1 sensor and generation of carotenoid metabolites to reprogramme nuclear gene expression and modulate hormone signalling and apoptosis.
3. Phospholipids containing Oxylipins from Enzyme Reactions
Fatty acids are oxidized to hydroperoxy fatty acids in animal and plant tissues by several different lipoxygenases and cytochrome P450 enzymes, which give metabolites with a high degree of positional and stereospecificity. These reactions in animals are discussed in our web page dealing with hydroxyeicosatetraenes (HETE), and while the substrates are generally unesterified polyunsaturated fatty acids such as arachidonate, most of the oxylipins can be esterified subsequently by the enzymes of the Lands cycle. However, 15- and 12/15 lipoxygenases (LOX) can oxidize phospholipid-bound fatty acids directly. One recent study with cells overexpressing 15‑lipoxgenase-2 found that 97% of the resulting oxylipins were esterified to phospholipids and triacylglycerols in a ratio of 3:1 (DOI = 10.1016/j.jlr.2025.100841). The nature of the products depends on the substrate polyunsaturated fatty acid and are bound to glycerophospholipids in a specific manner: 15S‑hydroxyeicosatetraenoic acid (15S-HETE) and 15S-hydroxyeicosapentaenoic acid (15S-HEPE) were found in phosphatidylinositol/phosphatidylserine, whereas 17S-hydroxydocosahexaenoic acid (17S-HDHA) was in phosphatidylethanolamine and 13S-hydroxyoctadecadienoic acid (13S-HODE) in phosphatidylcholine. The result is a relatively restricted range of oxidized lipids, which are formed through controlled pathways in appreciable amounts in innate immune cells. Even so, at least 100 unique oxidized phospholipids of this type have been identified, mainly of phosphatidylethanolamine.
Hydrolysis from this pool may be as important a source of lipid mediators for signalling as the process driven de novo from polyunsaturated fatty acid precursors with the nature of the phospholipid as a factor that may contribute to the biological effects. The small proportion (1-2%) of di-arachidonoyl/adrenoyl-phosphatidylethanolamine in tissues in particular is highly susceptible to oxidation by 15-lipoxygenase with a downstream influence on ferroptosis (see below).

Circulating innate immune cells and platelets generate phospholipids containing oxylipins by coordinated signalling pathways mediated by receptor-dependent calcium mobilization. Two main mechanisms have been described in these circumstances. In the first, membrane phospholipids, mainly phosphatidylcholine and phosphatidylethanolamine, are hydrolysed by a phospholipase A2 (PLA2) to release arachidonic acid from position sn-2 to be acted upon by cyclooxygenase (COX) or LOX enzymes to result in oxidized molecules. These can be activated by an acyl-coenzyme A synthase (ACS) and esterified to position sn-2 of a lysophospholipid with a saturated fatty acid in position sn-1 by an sn-2 acyltransferase, and for example, PGE2 and PGD2 synthesised from PGH2 in stimulated platelets by the action of COX-1 are rapidly esterified to position sn-2 of 1-lysophosphatidylethanolamines, while the diepoxide 8,9‑11,12‑diepoxy-13-hydroxyeicosadienoic acid is found in position sn-2 of phosphatidylethanolamine in which position sn-1 is occupied by a 16:0, 18:0 or 18:1 vinyl ether or an 18:0 fatty acid (see our webpage on prostaglandins).
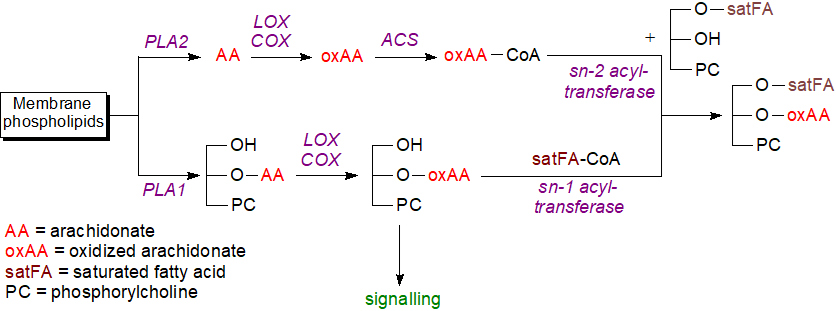 |
| Figure 4. Biosynthesis of oxidized phospholipids, |
In the second pathway, the fatty acid in position sn-1 of a phospholipid is removed by a phospholipase (iPLA2γ, the main phospholipase in mitochondria), which is selective for the fatty acid in position sn-1, to leave a lysophospholipid containing arachidonic acid in position sn-2. Further, cytochrome c is a plasmalogenase and may generate 2-arachidonoyl-lysophospholipids from plasmalogens under conditions of oxidative stress. The products of both reactions can act as substrates for oxidizing enzymes such as human platelet-type 12‑lipoxygenase (12‑LOX) or the 15‑lipoxygenase. The lysophospholipids 12S‑HETE‑lysophosphatidylcholine and 12S‑HETE‑lysophosphatidylethanolamine are lipid mediators per se, but they can be selectively esterified by a saturated fatty acid in position sn‑1 by means of an sn-1 acyltransferase. It has been established that the second pathway is activated markedly by calcium ionophore (A23187) or thrombin treatment of murine platelets to generate oxidized lysophospholipids or phosphatidylcholine. Under conditions of oxidative stress, 15-lipoxygenase and secreted phospholipase A2 act synergistically to form various oxidized lysophospholipids, including hydroxy, hydroperoxy and keto metabolites of 2‑arachidonoyl-lysophosphatidylinositol, which are endogenous agonists for Toll-like receptor 4 (TLR4), to adversely affect patients with rheumatoid arthritis and gout.
On cellular activation, approximately 30% of the 12-HETE generated by human platelets is esterified into these lipids at pico- to nanomole levels at the same rate as for the synthesis of free 12-HETE. Some result from by direct oxidation of intact phospholipids, for example by the action of 15-LOX in human monocytes (or murine 12/15-LOX), but most comes from subsequent esterification of free oxylipins. This has led to the suggestion that the various biosynthetic enzymes are located together to work cooperatively, and it is certain that there is great selectivity towards the substrates and the oxylipins that are generated.
Some of these esterified oxylipins in phospholipids may remain within the membranes (non-raft regions) for storage to be released on appropriate stimulation by platelet-activating factor-acetylhydrolase (type II), possibly into other cellular compartments with different roles from those from a more direct route. In plasma, the same enzyme is termed lipoprotein-associated phospholipase A2 (Lp-PLA2) and is associated with low- and high-density lipoproteins, where it acts on the outside of the phospholipid monolayer that coats the particles to hydrolyse circulating oxidized phospholipids, i.e., those with short acyl-chains, oxidized acyl-chains and F2‑isoprostanes esterified at the sn-2 position. This enzyme has been detected in atherosclerotic lesions and is present in the necrotic core and apoptotic macrophages in human coronary plaques as part of the inflammatory and apoptotic processes leading to plaque rupture, and epidemiological studies suggest that it is a cardiovascular risk factor. On the other hand, circulating oxidized phospholipids can be detoxified through enzymatic degradation by Lp-PLA2 with formation of lysophospholipids and oxidized non-esterified fatty acids. The balance between pro- and anti-inflammatory effects is obviously critical for health.
4. Oxidatively Truncated Phospholipids
Instead of the final reductive step in autoxidation, fission of hydroperoxides can occur with aldehyde generation by oxidative cleavage by a variety of mainly non-enzymatic mechanisms via epoxy and dioxetane intermediates to give many different aldehydes from each hydroperoxide, as discussed at greater length in our web page on aldehydes. A corollary of this process is that a fragment of the oxidized molecule remains esterified to the original phospholipid, i.e., as an oxidized phospholipid, which can originate from both enzymic and non-enzymic precursors. These are sometimes termed 'core aldehydes' or 'oxidatively truncated' phospholipids, and they are discussed briefly in our web page dealing with platelet-activating factor, as this lipid and oxidatively truncated phosphatidylcholine have some metabolic properties in common. The truncated moiety of such oxidized phospholipids can be metabolized subsequently by enzymes of the aldoketoreductase family to yield terminal alcohols or by the aldehyde dehydrogenase family to generate terminal carboxylic acids, resulting in further product diversity and metabolic effects. Other comparable oxidized phospholipids are derived from cardiolipin, phosphatidylserine and phosphatidylethanolamine in cells.
It is now evident that the lipid-bound fragments resulting from oxidative cleavage can trigger profound responses on inflammation, infection and immunity in animal tissues. Phosphatidylcholine is the most abundant phospholipid in most animal cells, and it is not recognized by any pattern-recognition receptors in native low-density lipoproteins (LDL) or on the surface of cells. In contrast, an oxidized lipid such as 1‑palmitoyl-2-(5‑oxovaleroyl)-sn-glycero-3-phosphocholine in oxidized LDL and on apoptotic cells is a ligand that mediates the binding of oxidized LDL to the receptor CD36 and scavenger receptor class B type I (SR‑B1), and this is a factor in the formation of foam cells. A related molecule with a 4'-oxo-butanoyl moiety in position sn-2 is an agonist for the platelet-activating factor receptor, while one with a 9′-oxononanoyl or azelaoyl chain, generated from the oxidation of linoleic acid, triggers apoptosis by interacting with mitochondrial membranes and permeabilizing cytochrome C. Such oxidized phospholipids inhibit uptake of cholesterol esters by SR-BI on liver cells and impair reverse cholesterol transport by this means.

Such oxidized lipids can cause membrane disruption immediately with increased permeabilization, while covalent adducts with membrane proteins can further damage membrane integrity. C9 truncated phospholipid aldehydes and derived nonionized carboxyls do not induce membrane curvature, but they do increase permeability toward larger molecules, while those species with C9 truncated carboxylic acid moieties induce permeabilization and membrane curvature in a pH-dependent manner because of the ionization-dependent exposure of the carboxyl group to the water-bilayer interface. As truncated phospholipids are more mobile and can escape the bilayer to accumulate at interfaces, they have the potential to act on neighbouring cells.
When there is a double bond in the truncated remnant, i.e., in an γ‑oxoalkenal phospholipid, the molecule is highly reactive, and for example, it can selectively interact to cross-link apolipoprotein A1 in high-density lipoproteins (HDL) in plasma (Michael addition). This impairs the cholesterol efflux mediated by apoA1 and may contribute to the loss of atheroprotection by HDL in vivo. Inflammatory pathways can be inhibited by stimulation of the peroxisome proliferator-activated receptor (PPARγ) for which the alkyl phospholipid sn-1-hexadecyl-2-azelaoyl-phosphatidylcholine is a specific and high-affinity ligand.
It should be noted that hundreds of such oxidized lipids are formed in tissues of all kinds under innumerable physiological conditions, but the biology of only a handful of model compounds have been studied in any detail, mainly those with two acyl groups with a few plasmalogen analogues. On the other hand, fewer molecular species are often observed in vivo than might be expected from experiments in vitro. Oxidized lipids of this kind tend to accumulate in tissues of the elderly, often in the lung, where they can potentiate the induction of damaging inflammatory agents.
Oxidatively truncated phospholipids can be degraded by the platelet-activating factor-acetylhydrolase (type II), lysosomal phospholipase A2, lipoprotein-associated PLA2 (Lp-PLA2 also known as Group VII PLA2), and Group VIA calcium-independent PLA2, and the aldehydo-acid component released in this way can participate in other tissue reactions.
5. Oxidized Phospholipids and Tissue Metabolism
Those oxidized phospholipids generated non-enzymatically by free radical mechanisms tend to be very different from those from enzymic oxidation and result in a much wider range of isomers, and they are generally if not universally considered harmful in tissues because they contribute to autoimmune and inflammatory diseases and cell death. On the other hand, oxidized phospholipids generated enzymatically can often have beneficial properties. The complex nature of the products from different lipids and proteins often means that it is not easy to delineate the detailed mechanisms, or whether these are due to molecules containing intact oxidized fatty acids or those that are oxidatively truncated. In any discussion, it is advisable to be aware of which oxidized lipids are present in each situation.
Some properties of oxidized phospholipids are discussed in relation to particular phospholipid classes elsewhere on this website; oxidized phosphatidylserine is a crucial aspect of the mechanism of apoptosis (programmed cell death), while oxidized cardiolipin acts as a required signal for the execution of the intrinsic apoptotic programme (mitophagy) in mitochondria. In brief, upon platelet activation, enzymatically oxidized phospholipids are externalized to the outer leaflet to work in concert with phosphatidylserine to support the coagulation cascade. Aspirin modulates generation of procoagulant phospholipids in cardiovascular disease by regulating the lysophosphatidylcholine acyltransferase 3 (LPCAT3) and preventing the production of diacyl-oxidized phospholipids derived from the action of 12‑lipoxygenase.
Membranes: Many effects of oxidized phospholipids may be exerted through the perturbation of the structures of cellular membranes as they tend to introduce deformation by increasing the membrane surface area and decreasing the membrane thickness. This can result in impairment of barrier function, increased membrane permeability leading to pore formation, and an elevated flip-flop rate, although, in general, membrane integrity is not compromised unless excessive hydroperoxidation occurs as in ferroptosis (next section). As oxidatively modified acyl chains probably protrude into the aqueous medium, they are more accessible for participation in signalling events including macrophage recognition. The electrophilic characteristics of some of these molecules, which can include hydroperoxides, hydroxides, keto groups and epoxides, can promote adduct creation with membrane proteins leading to further disruption to membranes as well as direct acts upon enzymes. It is noteworthy that some exchange of peroxidized lipids in membranes in different cellular organelles may occur. The polar head groups of phospholipids can be affected, and oxidative deamination of phosphatidylserine can yield glycero-3-phosphoacetic acid with subsequent impacts upon membrane structure and perhaps signalling. The presence of polar groups increases the water penetration of membrane, and in extreme conditions this can introduce continuous pores spanning both leaflets of the membrane.
Inflammation: A body of evidence has accumulated to indicate that oxidized phospholipids can promote various acute and chronic inflammatory diseases, including atherosclerosis, microbial infections, lung injury and neurodegenerative diseases, but as some other studies suggest that can be are anti-inflammatory, it is apparent that a complex range of mechanisms is involved. During oxidative stress in vivo, low-density lipoproteins (LDL) are enriched in oxidized phospholipids, and these can react with the apoproteins to increase the atherogenic potential of LDL with a deleterious impact upon cardiovascular disease (see our web page on lipoproteins). Oxidized phospholipids in excess in the circulation are a feature of atherosclerosis, and oxidized phosphatidylcholine bound to lipoproteins containing apoB‑100 is reported to be a biomarker for calcific aortic valve stenosis, stroke and coronary events. Lipoprotein(a) (Lp(a)) is an established risk factor for cardiovascular diseases, and it can carry an appreciable load of oxidized phospholipids, which clinical studies suggest increase the risk of heart failure.
 However, oxidized
phospholipids are not merely markers of pathological conditions but play a causative role in the initiation and progression of diseases.
In inflamed tissue, oxidized phospholipids act as endogenous pain-inducing metabolites, which excite sensory nociceptive neurons by
stimulating transient receptor potential ion channels, namely TRPA1 and TRPV1.
They have been implicated in the response to traumatic brain injury, Alzheimer disease and Parkinson’s disease, and as they have been detected
in brain and nervous tissue in multiple sclerosis, frontotemporal lobe dementia, spinal cord injury and amyotrophic lateral sclerosis,
they are potent neurotoxins and biomarkers of oxidative stress, which must be neutralized by microglia.
In the eye, they are a factor in age-related macular degeneration.
Further, they may promote some diseases of the liver and kidney, diabetes, autoimmune disease and possibly cancer (via immunosuppression).
However, oxidized
phospholipids are not merely markers of pathological conditions but play a causative role in the initiation and progression of diseases.
In inflamed tissue, oxidized phospholipids act as endogenous pain-inducing metabolites, which excite sensory nociceptive neurons by
stimulating transient receptor potential ion channels, namely TRPA1 and TRPV1.
They have been implicated in the response to traumatic brain injury, Alzheimer disease and Parkinson’s disease, and as they have been detected
in brain and nervous tissue in multiple sclerosis, frontotemporal lobe dementia, spinal cord injury and amyotrophic lateral sclerosis,
they are potent neurotoxins and biomarkers of oxidative stress, which must be neutralized by microglia.
In the eye, they are a factor in age-related macular degeneration.
Further, they may promote some diseases of the liver and kidney, diabetes, autoimmune disease and possibly cancer (via immunosuppression).
As a protective measure, oxidized phospholipids are targets of natural antibodies that recognize them as native molecules or covalently bound to proteins to facilitate clearance and neutralization of their pro-inflammatory properties, and in addition, a number of plasma proteins (non-immunoglobulin) have been identified that can bind oxidized phospholipids either covalently or non-covalently to reduce their potential for harm.
Some effects of oxidized phospholipids may be mediated via receptors, and a concept has been developed of damage (or danger)-associated molecular patterns (DAMPs) that arise from the controlled oxidation of lipids and lipoproteins. Those DAMPs derived by oxidation share common structural motifs with microbial pathogen-associated molecular patterns and so interact with the same pattern-recognition receptors that are present on the surface of macrophages and of immune and vascular cells. They then initiate many different inflammatory signalling processes that regulate immune responses by induction of chemokines and proinflammatory cell adhesion molecules to alter innate immune cells and thence the adaptive immune response and so contribute to the pathogenesis of disease. For example, mono-acyl phospholipids such as 2-12S-HETE-lysophospholipids, like non-esterified 12S-HETE, are potent lipid mediators that induce THP‑1 human monocytic cells to generate tumour necrosis factor α (TNFα) and interleukin 8 (IL8) at nanomolar concentrations and thence promote inflammatory cascades. Phosphatidylcholine containing epoxyisoprostane residues is proinflammatory by interacting with the prostanoid receptors EP2 and DP.
An antibody termed E06 has been developed in transgenic mice that blocks the influence of oxidized phosphatidylcholine in experimental models of acute and chronic inflammation including atherosclerosis, ischemia-reperfusion injury and steatohepatitis. As oxidized phospholipids enable binding of endothelial cells to monocytes, this triggers vascular inflammation and formation of atherosclerotic plaques with activation of inflammatory mediators such as the transcription factor nuclear factor (erythrocyte-derived 2)-like 2 (Nrf2).
Infections: Oxidized phospholipids need not always be deleterious, as they can inhibit acute inflammation by promoting anti-inflammatory pathways to attenuate potential damage to tissues. It has been suggested that oxidized lipids at lower concentrations may prime the receptors that respond to bacterial infection to activate dendritic and T cells resulting in enhanced protection. After acute stimulation by bacteria or bacterial toxins, neutrophils generate 5‑HETE-containing phospholipids by 5‑lipoxygenase (5-LOX) reaction, while macrophages and monocytes generate similar phospholipids by means of 15‑LOX and 12/15‑LOX, and 12- and 15-keto-eicosanoic acids attached to phosphatidylethanolamine are known to activate PPARγ.
Binding of such oxidized phospholipids to the receptors that recognize bacterial toxins can result in complete inhibition of the proinflammatory action of lipopolysaccharides during infections, thereby inhibiting TLR4-mediated inflammatory signalling and the expression of cytokines. Oxidized phospholipids of this kind generated in the outer leaflet of the plasma membrane in platelets and leukocytes can stimulate clotting factors and promote blood coagulation following tissue injury to limit bleeding while simultaneously inhibiting infection (in an analogous manner to phosphatidylserine). The phospholipids from 15-LOX reaction are significant factors during the resolution stage of inflammation.
Oxidized phosphatidylcholines have been detected in the lungs of patients infected with influenza virus and Covid, and these can hyperactivate lung macrophages to worsen acute lung injury and drive increased foam cell production and lung fibrosis. Oxidatively truncated phospholipids cause acute endothelial barrier disruption and potentiate inflammation, and they are intimately involved in the progression of chronic obstructive pulmonary disease (COPD), a common lung condition characterized by an increased chronic inflammatory response that leads to persistent airflow limitation. In contrast, oxidized 1‑palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine causes sustained enhancement to the barrier properties of lung endothelial cells and provides protection against vascular permeability induced by many different potentially harmful agents from pathogens to inflammatory cytokines. Increased levels of such oxidized metabolites in the lung improve recovery by facilitating the generation of anti-inflammatory oxylipins.
6. Ferroptosis
Ferroptosis is an often-pathological type of cell death that is genetically, morphologically and biochemically distinct from apoptosis per se in that it is not induced by caspases and pore-forming or functionally related proteins. Rather, it is dependent on iron (and not other metal ions) and is characterized by intracellular iron accumulation and an unrestricted build-up of lipid peroxides derived from polyunsaturated fatty acids together with depletion of antioxidant defences to such an extent that cellular membranes, including the plasma membrane, are disrupted to trigger lethal ion imbalances, membrane permeabilization and ultimately cell rupture. The oxidized phospholipids that induce ferroptosis may be generated both by enzymic and non-enzymic autoxidation, but the latter is the main driver of the propagation of lipid peroxidation in membranes. Several enzymes, including NADPH oxidases, NADPH-cytochrome P450 reductase and NADH-cytochrome b5 reductase (CYB5R1), transfer electrons from NAD(P)H to oxygen to generate hydrogen peroxide, which can react with iron to generate reactive hydroxyl radicals. To add to this, hydroperoxy-derivatives of arachidonoyl- or adrenoyl-phosphatidylethanolamines (20:4(n−6) and 22:4(n−6)) generated by 15‑lipoxygenase are reported to participate in ferroptosis in some contexts.
The process has been described as a failure of the quality control system in cells that removes aberrant molecules arising from errors during biosynthesis, damage by environmental insults, or imbalances in enzymatic and metabolic activity - in this instance oxidative damage. On the other hand, there is evidence to support a theory that an ability to selectively and controllably induce or resist ferroptosis is essential for normal development and cell function.
Phospholipids containing polyunsaturated fatty acids are highly vulnerable to peroxidation and must be present for ferroptosis to occur, and those with two polyunsaturated fatty acyl moieties are most reactive, especially in mitochondria. While several factors regulate ferroptosis, the ability of cells and tissues to control their lipid composition is crucial for their ability to either resist or be sensitized to ferroptosis. Diets that enhance the uptake, synthesis or incorporation of monounsaturated fatty acids into membrane phospholipids in place of polyunsaturates can reduce sensitivity to this process. Although most research has focussed on oxidized phospholipids, sterols in membranes and those with two double bonds in conjugation especially can be relevant.
The nature and concentrations of ferric ions (Fe3+) and ferrous ions (Fe2+) in cellular compartments are crucial. Within the endosomal compartments of the cell, ferric ions are reduced to ferrous ions and released into a labile iron pool in the cytoplasm via the divalent metal transporter 1 (DMT1), where it may be stored in complexation with the major iron storage protein ferritin. Disruption of iron homeostasis by removal of ferritin by the autophagy machinery ('ferritinophagy') leads to release of stored iron, and this has the potential to generate singlet oxygen or highly reactive hydroxyl radicals through the Fenton reaction (see our web page on isoprostanes). These radicals can oxidize the polyunsaturated fatty acids in the phospholipids of membranes to generate hydroperoxides and induce ferroptosis when insufficient levels of antioxidants or antioxidant enzymes are available. Further ferroptosis inducers include erastin, P53, Ras-selective lethal 3 (RSL3) and activating transcription factor 4 (ATF4).
 Many different antioxidant systems and membrane repair pathways work together in the various cellular compartments to limit this membrane damage.
By converting lipid hydroperoxides into non-toxic lipid alcohols, the selenoprotein glutathione peroxidase 4 (GPX4) in three isoforms with
differing subcellular locations prevents ferroptosis with assistance from the ferroptosis suppressor protein 1 (FSP1), α‑tocopherol,
reduced coenzyme Q, and 7‑dehydrocholesterol.
Glutathione (GSH) deficiency and/or inhibition of GPX4 can lead excess lipid peroxidation and to the induction of ferroptosis.
The protein Fas-associated factor 1 (FAF1) protects cells from ferroptosis by sequestering free polyunsaturated fatty acids into a hydrophobic
core and prevents their peroxidation by limiting access to iron.
In that it controls the expression of antioxidant or cytoprotective genes that act against oxidative stresses, the transcription factor
Nrf2 is essential for the regulation of the antioxidant response in animal cells.
Many different antioxidant systems and membrane repair pathways work together in the various cellular compartments to limit this membrane damage.
By converting lipid hydroperoxides into non-toxic lipid alcohols, the selenoprotein glutathione peroxidase 4 (GPX4) in three isoforms with
differing subcellular locations prevents ferroptosis with assistance from the ferroptosis suppressor protein 1 (FSP1), α‑tocopherol,
reduced coenzyme Q, and 7‑dehydrocholesterol.
Glutathione (GSH) deficiency and/or inhibition of GPX4 can lead excess lipid peroxidation and to the induction of ferroptosis.
The protein Fas-associated factor 1 (FAF1) protects cells from ferroptosis by sequestering free polyunsaturated fatty acids into a hydrophobic
core and prevents their peroxidation by limiting access to iron.
In that it controls the expression of antioxidant or cytoprotective genes that act against oxidative stresses, the transcription factor
Nrf2 is essential for the regulation of the antioxidant response in animal cells.
Ferroptosis shifts the balance between oxidants and antioxidants in favour of oxidative damage to cell membranes, and it manifests itself as necrotic changes that include cell enlargement, swelling of some organelles and membrane rupture. Mitochondria and lysosomes make an appreciable contribution to the disruptive nature of the process, and mitochondria participate in the early stages of the process especially. Various mechanisms are involved in the disruption of membranes with oxidized lipid tails undergoing reorientation to protrude into the aqueous phase, leading to a reduction in membrane thickness, assembly of micelles and eventually pores with cell rupture. Accumulation of autoxidation-derived truncated lipids, rather than lipid hydroperoxides, is critical because of their shorter and highly mobile tails.
While ferroptosis can be a necessary mechanism for maintaining cellular homeostasis, dysfunctioning of the process can be a cause of disease and pathological conditions. As cellular disruption induces a rapid and massive influx of Ca2+ into the cytosol, it can promote inflammatory diseases because of the release of endogenous DAMPs, resulting in the recruitment and activation of immune cells. Ferroptosis is currently a subject of intensive study in relation to cell death associated with Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, vascular dementia, kidney degradation, liver disorders and myocardial ischemia-reperfusion injury, and it can assist the invasion of host cells by bacterial and viral pathogens.
On the other hand, ferroptosis can prevent tumour progression in cancer and enhances the effects of chemotherapy, radiotherapy and immunotherapy, so harnessing ferroptosis is seen as a potential strategy to overcome the pitfalls of traditional cancer treatments. Ferroptosis inducers and inhibitors of glutathione peroxidase 4 are currently being tested against various cancers.
It has become evident that the biosynthesis of ether-linked phospholipids is a key element of ferroptosis, and this seems to explain the apparent paradox of why saturated fatty acids have been implicated in the process, as they are reduced by FAR1 to the alcohol precursor of plasmanyl lipids that exacerbate ferroptosis. Another factor is the action of the desaturase TMEM189 to generate the vinyl ether bond of plasmalogens. Peroxidation of the plasmalogen species of polyunsaturated phosphatidylethanolamines drives ferroptosis under some conditions, with the ratio of oxidized phosphatidylethanolamine to other oxidized phospholipids greatly increased in the inner plasma membrane, demonstrating that the oxidative process does not occur randomly. Further, phosphatidylethanolamine-binding protein 1 (PEBP1) promotes 15-lipoxygenase activity, resulting in the selective accumulation of this oxidized lipid at the onset of ferroptosis. As plasmalogens can act as antioxidants, the plasmenyl lipids in membranes may be protective against ferroptosis in other conditions with implications for some disease states, including cancer.
Cells can initiate mechanisms to repair or to isolate and remove damaged membranes, and stimulation of the ESCRT-III (endosomal sorting complex required for transport-III) machinery leads to membrane repair by shedding damaged parts of the cell membrane and so prevents various types of lytic cell death, including ferroptosis. Lipid droplets in cells are protective against ferroptosis in that they sequester polyunsaturated fatty acids so they are less prone to oxidation.
Cuproptosis is triggered by excessive copper ions (Cu2+) binding to lipoylated proteins in the tricarboxylic acid cycle, leading to the assembly of insoluble cytotoxic aggregates under prolonged copper stress. It does not involve lipid peroxidation so differs from the mechanism of ferroptosis.
7. Oxidized Glycerolipids in plants
Intact phospholipids and glycosyldiacylglycerols containing oxidized lipids are synthesised in plants by similar mechanisms to those in animal tissues, and plants have developed appropriate antioxidant defences. As in animals, ROS are generated naturally during normal metabolism in plant cells, but especially in the apoplast, mitochondria, chloroplasts and peroxisomes, and these can react with DNA, lipids and proteins to at worst cause cellular death. Under normal circumstances, a steady state is reached between generation and elimination of ROS, but this balance can be disturbed by biotic and abiotic stresses of all kinds with light intensity, UV-B radiation and temperature extremes as major factors. Although ferroptosis was first observed in plants suffering from heat stress, much the same mechanisms for regulated cell death have been observed as in mammalian cells, i.e., iron-dependent ROS accumulation (triggered by the small-molecule inducer erastin), lipid peroxidation and GSH depletion, and ferroptosis can be prevented by using the same inhibitors.
 Free radical oxidation by all the reactants described above can occur in plants, but superoxide anions (O2•-)
are of special importance as they can be produced during photosynthetic electron transport in chloroplasts, and singlet oxidation is generated
during photosynthesis by both photosystems (PSI and PSII).
Aldehydes, which have vital biological functions in signalling are is discussed
in another web page.
These rather than intact lipid peroxides have been the focus of most research, although the latter can accumulate under conditions
of abiotic stress, and for example, salt-induced oxidative damage to membrane lipids can be used as an indicator of tolerance
to salt stress in barley roots.
The same processes, including ferroptosis, occur in cyanobacteria.
Free radical oxidation by all the reactants described above can occur in plants, but superoxide anions (O2•-)
are of special importance as they can be produced during photosynthetic electron transport in chloroplasts, and singlet oxidation is generated
during photosynthesis by both photosystems (PSI and PSII).
Aldehydes, which have vital biological functions in signalling are is discussed
in another web page.
These rather than intact lipid peroxides have been the focus of most research, although the latter can accumulate under conditions
of abiotic stress, and for example, salt-induced oxidative damage to membrane lipids can be used as an indicator of tolerance
to salt stress in barley roots.
The same processes, including ferroptosis, occur in cyanobacteria.
Cellular compartments such as mitochondria, chloroplasts and cytosol act in coordination to respond to changing oxidative challenges to regulate the redox status. All oxidized lipids are countered by antioxidative systems, including endogenous antioxidants such as ascorbic acid, glutathione, α‑tocopherol, carotenoids, flavonoids and plastoquinone, together with enzymatic systems, such as superoxide dismutase, catalase, ascorbate peroxidase and other peroxidases, and various reductases.
Those oxidized lipids from enzymatic reactions are of increasing interest, and they are described in our web page on plant oxylipins. The best known of these lipids are the arabidopsides, i.e., glycosyldiacylglycerols containing jasmonate precursors, which are generated rapidly in the membranes of leaves under stress, and it seems probable that they serve as a reservoir of oxylipins to be called upon when required. In leaves of potato plants infected by bacteria, fungi or viruses, colneleic and colnelenic acids are synthesised quickly via the action of 9-lipoxygenase and have been found esterified at the sn-2 position of phospholipids, i.e., as a pool that is available immediately in response to challenge by pathogens such as potato blight.
8. Analysis
Oxidized phospholipids are now more easily analysed by modern lipidomics methodology (liquid chromatography coupled to mass spectrometry) than was possible earlier, and this is leading to increasing research on the topic. For example, truncated phospholipids containing aldehyde residues can be analysed after oxime ligation. Nonetheless, it is still a challenging task because of the chemical and thermal instability of oxidized lipids as well as the potential for further propagation of oxidation, and particular attention must be given to antioxidants, extraction and concentration methods, and derivatization approaches. A lack of suitable internal standards compounds the problems. Analysis of adducts between oxidized lipids and proteins in intact tissues remains a considerable analytical challenge but may be surmountable
Suggested Reading
- Choi, J.H. and Kagan, J.C. Oxidized phospholipid damage signals as modulators of immunity. Open Biol., 15, 240391 (2025); DOI.
- Cruciani, G., Domingues, P., Fedorova, M., Galli, F. and Spickett, C.M. Redox lipidomics and adductomics - Advanced analytical strategies to study oxidized lipids and lipid-protein adducts. Free Rad. Biol. Med., 144, 1-5 (2019); DOI - and many more relevant articles in this special review volume.
- Foret, M.K., Lincoln, R., Do Carmo, S., Cuello, A.C. and Cosa, G. Connecting the 'dots': from free radical lipid autoxidation to cell pathology and disease. Chem. Rev., 120, 12757-12787 (2020); DOI.
- Gao, D.T., Ashraf, M.Z., Zhang, L.F., Kar, N., Byzova, T.V. and Podrez, E.A. Cross-linking modifications of HDL apoproteins by oxidized phospholipids: structural characterization, in vivo detection, and functional implications. J. Biol. Chem., 295, 1973-1984 (2020); DOI.
- Goggin, F.L. and Fischer, H.D. Singlet oxygen signalling and its potential roles in plant biotic interactions. Plant Cell Environ., 47, 957-1970 (2024); DOI.
- Juan, C.A., de la Lastra, J.M.P., Plou, F.J. and Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci., 22, 4642 (2021); DOI.
- Jwa, N.S. and Hwang, B.K. Ferroptosis in plant immunity. Plant Commun., 6, 101299 (2025); DOI.
- Kagan, V.E and 16 others. Redox epiphospholipidome in programmed cell death signaling: catalytic mechanisms and regulation. Front. Endocrin., 11, 628079 (2021); DOI - and other relevant articles in this special review volume.
- Lancaster, G.I. and Murphy, A.J. Do physiological changes in fatty acid composition alter cellular ferroptosis susceptibility and influence cell function? J. Lipid Res., 66, 100765 (2025); DOI.
- Lee, J. and Roh, J.L. Lipid metabolism in ferroptosis: Unraveling key mechanisms and therapeutic potential in cancer. Biochim. Biophys. Acta, Rev. Cancer., 1880, 189258 (2025); DOI.
- Pérez-Sala, D. and Domingues, R. Lipoxidation targets: From basic mechanisms to pathophysiology. Redox Biol., 23, 101208 (2019); DOI - and many other articles in this special review volume.
- Protty, M.B.B., Jenkins, P.V., Collins, P.W.W. and O'Donnell, V.B. The role of procoagulant phospholipids on the surface of circulating blood cells in thrombosis and haemostasis. Open Biol., 12, 2103181 (2022); DOI.
- Santos, M., Melo, T., Maurício, T., Ferreira, H., Domingues, P. and Domingues, R. The non-enzymatic oxidation of phosphatidylethanolamine and phosphatidylserine and their intriguing roles in inflammation dynamics and diseases. FEBS Letts, 598, 2174-2189 (2024); DOI.
- Saraev, D.D. and Pratt, D.A. Reactions of lipid hydroperoxides and how they may contribute to ferroptosis sensitivity. Curr. Opinion Chem. Biol., 81, 102478 (2024); DOI.
- Tsimikas, S. and Witztum, J.L. Oxidized phospholipids in cardiovascular disease. Nature Rev. Cardiol., 21, 170-191 (2024); DOI.
- Villasenor, A., Godzien, J., Barker-Tejeda, T.C., Gonzalez-Riano, C., Lopez-Lopez, A., Dudzik, D., Gradillas, A. and Barbas, C. Analytical approaches for studying oxygenated lipids in the search of potential biomarkers by LC-MS. Trends Anal. Chem., 143, 116367 (2021); DOI.
- Xie, M. and others. Structure and pH dependence of membranolytic mechanisms by truncated oxidized phospholipids. J. Am. Chem. Soc., 147, 9175-9189 (2025); DOI.
- Yi, Y.Q., Jia, P., Xie, P.P., Peng, X.R., Zhu, X., Yin, S.T., Luo, Y.F., Deng, Y. and Wan, L.F. Beyond oxidative stress: Ferroptosis as a novel orchestrator in neurodegenerative disorders. Front. Immun., 16 1683876 (2025); DOI.
- Zheng, J.S. and Conrad, M. Ferroptosis: when metabolism meets cell death. Physiol. Rev., 105 651-706 (2025); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: January 2026 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
