Lipid Matters - Archive of Older Blogs - 2018
This Blog is an occasional series of notes on publications or other items dealing with lipid science that seem to be of particular interest to the editor Bill Christie. Inevitably, the selection is highly personal and subjective. In this web page, the blogs for 2018 are archived, while those for other years can be accessed from the foot of the current blog page.
December 19th, 2018
 The
concept of lipid rafts in membranes is an important one in that
it governs many of the functions of sphingolipids in membranes.
Although there are some dissenting voices, there appears to be a large area of agreement among experts in the field
of their existence and relevance.
I can recommend a new and relatively brief review as an introduction to the topic
(Goñi, F.M. ‘Rafts’: A nickname for putative transient nanodomains. Chem. Phys. Lipids, 218,
34-39 (2019); DOI).
Among a number of key conclusions, the author deplores the continued use of cold extraction with Triton X-100 as a standard procedure
for raft isolation, as it is a source of artefacts.
Also, the author obviously does not like the term 'rafts', and I sympathize in that it does appear trivial with little meaning from
a scientific standpoint. Instead, he suggests the term '(transient) nanodomain'.
While I agree with his idea in principle, his suggestion seems to me to be too general in that it could also apply to other membranes regions
such as those enriched in polyunsaturated fatty acids, which may form when sphingolipids segregate.
As sphingolipids are the defining constituents of rafts, can I suggest 'sphingoid nanodomains' as an alternative name?
The
concept of lipid rafts in membranes is an important one in that
it governs many of the functions of sphingolipids in membranes.
Although there are some dissenting voices, there appears to be a large area of agreement among experts in the field
of their existence and relevance.
I can recommend a new and relatively brief review as an introduction to the topic
(Goñi, F.M. ‘Rafts’: A nickname for putative transient nanodomains. Chem. Phys. Lipids, 218,
34-39 (2019); DOI).
Among a number of key conclusions, the author deplores the continued use of cold extraction with Triton X-100 as a standard procedure
for raft isolation, as it is a source of artefacts.
Also, the author obviously does not like the term 'rafts', and I sympathize in that it does appear trivial with little meaning from
a scientific standpoint. Instead, he suggests the term '(transient) nanodomain'.
While I agree with his idea in principle, his suggestion seems to me to be too general in that it could also apply to other membranes regions
such as those enriched in polyunsaturated fatty acids, which may form when sphingolipids segregate.
As sphingolipids are the defining constituents of rafts, can I suggest 'sphingoid nanodomains' as an alternative name?
Phytoprostanes and phytofurans are formed in plants via non-enzymatic, free radical-catalysed pathways similar to isoprostane synthesis in animals. They have similar molecular structures to animal oxylipins so may influence animal biology when they are absorbed from plant foods in the diet with potential benefits to human health. A new review summarizes the available evidence (Medina, S. et al. Structural/functional matches and divergences of phytoprostanes and phytofurans with bioactive human oxylipins. Antioxidants, 7, 165 (2018); DOI - open access).
I wish all my readers a happy Christmas and a prosperous lipidic New Year.
December 12th, 2018
The membranes in photosynthetic cells are essential to all life on earth, so the more we understand them and their lipid components the better. The chloroplast inner and outer membranes and the thylakoid membranes within the chloroplast are obviously vital in this process, but the endoplasmic reticulum is also of great importance and mitochondria cannot be neglected. Monogalactosyldiacylglycerols with distinctive fatty acid compositions are key products, and they are essential for the integrity and function of the photosynthetic apparatus. Fatty acid synthesis and desaturation and synthesis of glyceride precursors occur in different parts of the cell so there is need for extensive lipid trafficking between membranes and organelles. A number of lipid transporters have now been identified, and there is evidence for lipid transport at contact sites between organelles. In addition, there is some evidence for vesicular transport. All of these are discussed in a new review on the topic (LaBrant, E. et al. Lipid transport required to make lipids of photosynthetic membranes. Photosynth. Res., 138, 345-360 (2018); DOI), which happily is open access.
Of course, the plasma membrane of plant cells is just as important as that in animals in that it protects the cell, regulates nutrient exchanges and acts as a platform to receive and send signals. Again, the lipid component are of central importance, but a major shift in our understanding of how the membrane functions has come with the recognition that sphingolipids, i.e., glycosyl inositol phosphoryl ceramides, are major constituents. Until recently, we knew little of them because they are relatively large, polar and complex molecules, which were easily overlooked with the available analytical methodology. The new era of lipidomics has shown that they are one of the most abundant constituents and has enabled comparisons to be drawn with the functions of gangliosides in animal membranes. For example, they are essential for the organization of the plasma membrane and they act as toxin receptors. Again, I am grateful for a new review for bringing me up to speed on the topic (Cassim, A.M. et al. Plant lipids: key players of plasma membrane organization and function. Prog. Lipid Res., 73, 1-27 (2019); DOI).
December 5th, 2018
In my early career at a Dairy Research Institute, we were concerned with the relative lack of essential fatty acids and excess of trans fatty acids in meat and dairy products because of microbial biohydrogenation in the rumen. I am not fully conversant with current research in this area but I guess that it continues. However, there does appear to be great interest in the byproducts of the reaction, i.e., conjugated linoleic and linolenic acids, from a quite different perspective. These have perceived benefits to human health, while the complex mixtures of isomers that are easily obtained from linoleate by chemical isomerization do not always have the same effects when administered in the diet. A number of linoleate isomerases have now been identified from bacteria, some of which occur as multi-enzyme systems. As many of the organisms occur in the human gut, they may affect human metabolism in vivo. A new review discusses the topic (Salsinha, A.S. et al. Microbial production of conjugated linoleic acid and conjugated linolenic acid relies on a multienzymatic system. Microbiol. Mol. Biol. Rev., 82, e00019 (2018); DOI).
New Scientist has just published a leader article with the provocative title "Time to break academic publishing's stranglehold on research". They comment on how little support there has been from funding agencies internationally for open access proposals. To quote further "The scientists who oppose it have real concerns, but are letting the perfect be the enemy of the good". I am sure that not all publishers are making 40% profits, as the leader suggests, but the big three (Elsevier, Springer, Wiley) are doing pretty well as subscription costs continue to rise. One unintended consequence of this is that academic libraries are no longer a place to browse for general information but have become much more specialized. For as long as I can remember, the libraries with which I have been associated have been faced each year with a necessity to cut the numbers of journal subscriptions as their budgets have been constrained. To compensate, we have greater access online to many journals from the big three publishers, but less to smaller publishers, including those linked to many academic societies (such as ACS, Biochemical Society, and University Presses). For example, my former Institute has just dropped its subscription to the Journal of Biological Chemistry as an economic measure in order to retain journals more in line with its limited remit. Is there also a "stranglehold on research", as the title of the leader suggests? You may be better qualified than I to answer. By the way, I would like to see more open access articles in New Scientist itself.
November 28th, 2018
 When
it comes to any opinions on fats in the diet and nutrition in general, I live in a fog of confusion.
I enjoy fish, so I am content to believe that n-3 fatty acids are good for me, and I also enjoy dairy foods with their 'bad' fats
so I may die happy at least. According to a recent paper in Science, most nutritional experts are confused also
(Ludwig, D.S. et al. Dietary fat: From foe to friend? Science, 362, 764-770 (2018);
DOI - open access). A series of questions that require further research are posed,
most relating to the amount and type of fat relative to carbohydrate in the diet.
There has been a substantial reduction in the proportion of fat in the diet in the U.S.A. and U.K. following well-publicized
dietary recommendations in the last century, yet life expectancy is declining.
I looked in vain for the words 'alcohol' and 'exercise' in the paper and these surely must be important confounding factors
in Western nations.
Although I do not suggest that we should give up on research on the topic,
it seems to me that there is little chance of defining an optimum diet for populations at large.
Perhaps we should simply reduce our expectations for nutritional research and concentrate on life-style factors.
When
it comes to any opinions on fats in the diet and nutrition in general, I live in a fog of confusion.
I enjoy fish, so I am content to believe that n-3 fatty acids are good for me, and I also enjoy dairy foods with their 'bad' fats
so I may die happy at least. According to a recent paper in Science, most nutritional experts are confused also
(Ludwig, D.S. et al. Dietary fat: From foe to friend? Science, 362, 764-770 (2018);
DOI - open access). A series of questions that require further research are posed,
most relating to the amount and type of fat relative to carbohydrate in the diet.
There has been a substantial reduction in the proportion of fat in the diet in the U.S.A. and U.K. following well-publicized
dietary recommendations in the last century, yet life expectancy is declining.
I looked in vain for the words 'alcohol' and 'exercise' in the paper and these surely must be important confounding factors
in Western nations.
Although I do not suggest that we should give up on research on the topic,
it seems to me that there is little chance of defining an optimum diet for populations at large.
Perhaps we should simply reduce our expectations for nutritional research and concentrate on life-style factors.
 Scientific anniversaries are useful in that they remind us
of the work of the pioneers in lipid science, often in areas away from our daily research concerns.
For example, a new review reminds us that the most abundant protein in the bacterium E. coli is a triacylated
proteolipid,
termed 'Braun's lipoprotein' after its primary discoverer of 50 years ago (Asmar, A.T. and Collet, J.F.
Lpp, the Braun lipoprotein, turns 50 - major achievements and remaining issues.
FEMS Microbiol. Letts, 365, fny199 (2018); DOI - open access).
This has a molecular weight of only 5.8 kDa and folds into a trimeric helical structure.
Uniquely, much of it is covalently attached by the ε-amino group of the C-terminal lysine to the carboxyl group of
a meso-diaminopimelic acid residue in the peptidoglycan of the cell wall to
provide the only covalent connection between the inner and outer membranes.
This was the first of innumerable such proteins to be discovered that among a host of biological functions
greatly influence the virulence of bacteria.
Scientific anniversaries are useful in that they remind us
of the work of the pioneers in lipid science, often in areas away from our daily research concerns.
For example, a new review reminds us that the most abundant protein in the bacterium E. coli is a triacylated
proteolipid,
termed 'Braun's lipoprotein' after its primary discoverer of 50 years ago (Asmar, A.T. and Collet, J.F.
Lpp, the Braun lipoprotein, turns 50 - major achievements and remaining issues.
FEMS Microbiol. Letts, 365, fny199 (2018); DOI - open access).
This has a molecular weight of only 5.8 kDa and folds into a trimeric helical structure.
Uniquely, much of it is covalently attached by the ε-amino group of the C-terminal lysine to the carboxyl group of
a meso-diaminopimelic acid residue in the peptidoglycan of the cell wall to
provide the only covalent connection between the inner and outer membranes.
This was the first of innumerable such proteins to be discovered that among a host of biological functions
greatly influence the virulence of bacteria.
2-Hydroxyoleic acid has remarkable biological activities in that it suppresses the growth and induces autophagy in certain cancer cells. It therefore has appreciable potential as a non-toxic anticancer drug, and the European Medicines Agency has designated it as an orphan drug for the treatment of glioma. The mechanism for its action is uncertain but a new publication suggests that it may involve effects upon phosphatidylcholine metabolism but not upon activation of sphingomyelin synthesis as originally thought (Lou, B. et al. 2-Hydroxy-oleic acid does not activate sphingomyelin synthase activity. J. Biol. Chem., 293, 18328-18336 (2018); DOI). Intriguingly, it is the 'unnatural' S-enantiomer that produces this effect.
November 21st, 2018
Synaptamide, which I discussed in my last blog, takes its name from its occurrence and function in the central nervous system. Now, two new papers describe its occurrence in human breast milk, together with a suite of other bioactive amides, suggesting that it may be required for the development of the newborn infant (DOI-1 and DOI-2). Related endocannabinoids are produced in parasitic worms (helmiths) and a new publication reports that during infection of a host animal the intestines are stimulated to produce their own endocannabinoids that promote the host immune responses and reduce parasite burden while also reducing pain and inflammation. However, there are also benefits to the parasite. The hope is that this knowledge may lead to new treatments for such infections. I won't have access to the original publication for 6 months, but there is a popular report in Science Daily.
Early in my research career, I was involved in the use of pyrrolidides for structural analysis of fatty acids by mass spectrometry, but I could not make full use of this methodology until I obtained my own instrument in the 1980s, by which time 3-pyridylcarbinol ('picolinyl') esters and dimethyloxazoline derivatives had been described. New alternatives are reported from time to time, but there is now such a body of information on these three that they are likely to remain the derivatives of choice for locating double bonds, ring structures, etc for the foreseeable future (see my Mass Spectrometry web pages). Of course, I had access to electron impact ionization only, so I was intrigued to read a new paper in which Orbitrap mass spectrometry was used with 3-pyridylcarbinol esters to determine the structures of fatty acids with cyclopropane rings (Merlier, F. et al. A gas chromatography full scan high resolution Orbitrap mass spectrometry method for separation and characterization of 3-hydroxymethyl pyridine ester of fatty acids at low levels. J. Chromatogr. A, 1575, 72-79 (2018); DOI). The spectra are clear and informative, although they do differ from the analogous electron-impact spectra in significant ways. I will look forward to seeing further publications on this methodology.
GC-MS was also crucial to demonstrate the introduction of a double bond surprisingly and possibly uniquely into the terminal region of trans-vaccenic acid by a methyl-end desaturase in rats (Garcia, C. et al. Conversion of dietary trans-vaccenic acid to trans11,cis13-conjugated linoleic acid in the rat lactating mammary gland by Fatty Acid Desaturase 3-catalyzed methyl-end Δ13-desaturation. Biochem. Biophys. Res. Commun., 505, 385-391 (2018); DOI).
Nearly five years ago, I first mentioned the next item in my blog, but as I have many new readers, this is how to teach lipid science - http://m.youtube.com/watch?v=6lrG65DdBl8 - with thanks to Michael Eskin!
November 14th, 2018
Two substantial journal issues dealing with aspects of lipid biochemistry and analysis have now been published, although I have to confess that I have been enjoying the sunshine and beaches of Gran Canaria for the last week so I have not had time to do other than browse through them briefly, i.e., "Dietary fatty acids, lipid mediators, cell function and human health" - edited by Philip Calder (Molecular Aspects of Medicine, 64, Pages 1-182 (December 2018) ) - and "Lipidomics" - edited by James Ntambi and Sarah Spiegel (Biochem. Biophys. Res. Commun., 504, Issue 3, Pages 553-628 (7 October 2018)).
I did, however, note a number of interesting reviews dealing with bioactive amides, both endocannabinoids and others, and one especially dealing with N-docosahexaenoylethanolamine (synaptamide) (Kim, H.Y. and Spector, A.A. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol. Aspects Med., 64, 34-44 (2018); DOI). This does not interact with the endocannabinoid receptors, but has its own target (GPR110 or ADGRF1), a G-protein coupled receptor that is highly expressed in the central nervous system and promotes neurogenesis in developing neurons at nanomolar concentrations. It invokes a signalling pathway that leads to the expression of neurogenic genes while suppressing the expression of proinflammatory genes. It hardly needs saying that this is yet another indication of the importance of omega-3 fatty acids for our health and well being.
A separate review elsewhere deals with the how various bioactive amides in the gut influence the microbial biome and vice versa, with eventual impacts on the brain (Russo, R. et al. Gut-brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr. Med. Chem., 25, 3930-3952 (2018); DOI). I need to spend more time with both of these.
October 31st, 2018
 During
embryonic development in many mammals, including the rat, the eyelids migrate over the cornea and fuse in order
to protect the eyes during birth and early postnatal development.
It has now been established that a key factor in this process is the lipid
sphingosine-1-phosphate, which promotes the activation of proteins involved
in cell migration and stimulates signalling by the epidermal growth factor receptor (EGFR) (Bian, G. et al.
Sphingosine 1-phosphate stimulates eyelid closure in the developing rat by stimulating EGFR signaling.
Sci. Signal., 11, eaat147023 (2018); DOI).
In the early part of my career, we looked on sphingolipids as interesting curiosities that simply had ill-defined roles in membranes,
so the all-pervasive nature of their signalling properties has come as a surprise - see the latest review
(Pulli, I. et al. Sphingolipid-mediated calcium signaling and its pathological effects.
Biochim. Biophys. Acta, Mol. Cell Res., 1865, 1668-1677 (2018);
DOI).
During
embryonic development in many mammals, including the rat, the eyelids migrate over the cornea and fuse in order
to protect the eyes during birth and early postnatal development.
It has now been established that a key factor in this process is the lipid
sphingosine-1-phosphate, which promotes the activation of proteins involved
in cell migration and stimulates signalling by the epidermal growth factor receptor (EGFR) (Bian, G. et al.
Sphingosine 1-phosphate stimulates eyelid closure in the developing rat by stimulating EGFR signaling.
Sci. Signal., 11, eaat147023 (2018); DOI).
In the early part of my career, we looked on sphingolipids as interesting curiosities that simply had ill-defined roles in membranes,
so the all-pervasive nature of their signalling properties has come as a surprise - see the latest review
(Pulli, I. et al. Sphingolipid-mediated calcium signaling and its pathological effects.
Biochim. Biophys. Acta, Mol. Cell Res., 1865, 1668-1677 (2018);
DOI).
I was stimulated myself in writing the above to check the spelling of 'signalling' (ll) or 'signaling' (l); apparently the former is British English and the second American English. "We have really everything in common with America nowadays except, of course, language" - Oscar Wilde (The Canterville Ghost, 1887), not George Bernard Shaw to whom it is often attributed.
A fascinating paper in JBC describes how the acyl-coA synthetase 1 can direct fatty acids to many different functions in different membranes in the liver (Young, P.A. et al. Long-chain acyl-CoA synthetase 1 interacts with key proteins that activate and direct fatty acids into niche hepatic pathways. J. Biol. Chem., 293, 16724-17740 (2018); DOI). It is reported that the enzyme can interact with a number of different proteins at the outer mitochondrial membrane and the endoplasmic reticulum to determine whether the fatty acids are directed to oxidation or esterification or to other purposes. Incidentally, my former Institute can no longer afford a subscription to JBC, but the journal permits access to early versions of the paper even after formal publication and this is sufficient for my purposes. How I wish others were equally enlightened, especially ACS publications.
October 24th, 2018
I have a few open access review bargains for you this week of which my favourite and that most useful in updating my web pages in the Lipid Essentials section of the LipidWeb deals with sphingolipids (Harrison, P.J. et al. Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep., 35, 921-954 (2018); DOI). In fact the title is something of a misnomer in that it does not treat sphingolipids as a whole but with the first steps in the biosynthesis of sphingoid bases, i.e., before the involvement of ceramide synthesis, and then with their catabolism via sphingosine-1-phosphate.
An even more substantial review deals with glycolipids from marine organisms (Cheng-Sanchez, I. and Sarabia, F. Chemistry and biology of bioactive glycolipids of marine origin. Marine Drugs, 16, 294 (2018); DOI). This is perhaps one for the specialist, but it encompasses an astonishing array of glycosphingolipids, together with glycosyldiacylglycerols and others that seem to defy simple classifications. Marine organisms can produce polyunsaturated fatty acids in many different ways, and for example marine bacteria can synthesise such fatty acids both by aerobic and anaerobic mechanisms. In particular, marine invertebrates contain many diverse enzymes involved in the introduction of double bonds into fatty acids including both "front-end" and "omega" desaturases, and they are even able to produce what for most other animals are essential fatty acids. This is the subject of my third review of the week (Monroig, O. and Kabeya, N. Desaturases and elongases involved in polyunsaturated fatty acid biosynthesis in aquatic invertebrates: a comprehensive review. Fisheries Sci., 84, 911-928 (2018); DOI). This is not simply an esoteric exercise as such enzymes may have appreciable biotechnological potential.
A month ago, I discussed the discovery of a cholesterol metabolite in a 600 million year old fossil, confirming that it was of an animal. Now the molecular fossil record for animals has been pushed back another 35 million years with the discovery in pre-Cambrian rocks of sterane structures that are made exclusively by demosponges (see the report in Science Daily).
October 17th, 2018
The journal Biochimie has a special issue devoted to "Current trends in oxysterols and related sterols", edited by Gérard Lizard, Giulio Muccioli and Luigi Iuliano (Volume 153, Pages 1-238 (October 2018)). So far I have only had time for a brief overview, but I was especially interested in a multi-author inter-laboratory comparison of methods of oxysterol analysis (Lutjohann, D. et al. International descriptive and interventional survey for oxycholesterol determination by gas- and liquid-chromatographic methods. Biochimie, 153, 26-32 (2018); DOI). Some surprising discrepancies among the various laboratories were noted, highlighting a need for standardized methods, and especially for the use of appropriate deuterated standards. Also, as I suggested some weeks ago to deafening silence, it would also be useful to have ready access to standard mass spectra of sterol derivatives for those new to the subject or with limited access to mass spectral libraries, ideally in a dedicated website - the next task for this multi-author panel? Those working with plant oxysterols have the same or perhaps greater problems because of the wide range of different sterols and triterpenoid alcohols in plant species. Similar aims to standardize methodologies are discussed in a multi-author study of plasma lipidomics (Burla, B. et al. MS‑based lipidomics of human blood plasma: a community-initiated position paper to develop accepted guidelines. J. Lipid Res., 59, 2001-2017 (2018); DOI).
Oxidized lipids were the theme of a number of papers in this week's literature search, headed by a rather substantial review in an ACS journal for those of you who have access (I don't) (Parvez, S. et al. Redox signaling by reactive electrophiles and oxidants. Chem. Rev., 118, 8798-8888 (2018); DOI). On the other hand, I have been able to read and can recommend an open access review on the signalling properties of oxidized phospholipids as opposed to the unesterified oxylipins (Tyurina, Y.Y. et al. "Only a life lived for others is worth living": redox signaling by oxygenated phospholipids in cell fate decisions. Antiox. Redox Signal., 29, 1333-1358 (2018); DOI). Both enzymic and non-enzymic routes to such species are known, and oxidized cardiolipin is crucial to apoptosis and phagocytosis of mitochondria. This has been recognized for some time, but the role of oxygenated phosphatidylethanolamines as pro-ferroptotic signals has emerged relatively recently.
October 10th, 2018
I was intrigued by the title of a recent paper (Zaidi, A. et al. Forgotten fatty acids - Surface properties supply conclusive evidence for including carotenoic acids. Chem. Phys. Lipids, 216, 48-53 (2018); DOI), and I read through it rapidly to see whether I should add anything on these lipids to my Lipid Essential pages on the LipidWeb. I decided against, but was prompted to consider more deeply by a correspondent. I did not change my mind. Yes they are fatty and yes they are acids, but they do not occur naturally with the exception of retinoic acid, which I discuss appropriately in my web page on retinoids. LipidMaps® appear to agree with me as they list it in their isoprenoid group. Similarly, the acidic (carboxy) derivatives of tocopherols, which I mentioned in my blog of two weeks ago (September 26th), are technically fatty acids but to my mind are best discussed with the tocopherols. What about triterpenoids such as oleanolic and betulinic acids? They are fatty and acidic, but do we really need to call them fatty acids? I suppose another question raised by the publication might be whether surface active properties are of sufficient value in determining whether a lipid should be classified as a fatty acid. Retinoic acid, for example, is bound in tissues to specific transporter-carrier proteins, so I doubt whether it ever reaches an effective concentration in vivo where its surface active properties are relevant. If it does, my guess is that this would be a signal for oxidation, glucuronidation and elimination. Should any isoprenoids be classified primarily as fatty acids? In my personal view, phytanic acid should be because of its occurrence in esterified form in main-stream lipids in animals. Of course, all classification systems are simply academic exercises and they all boil down to personal opinions, so the authors of the paper are just as entitled to their view as I am to mine.
The journal Cancer and Metastasis Reviews has published a special issue (Volume 37, Issue 2-3, pp. 199-572 September 2018) that deals with the topic of "Bioactive Lipids" (Issue Editors: Dipak Panigrahy and Allison Gartung).
October 3rd, 2018
Two reviews dealing with mass spectrometry of lipids have caught my eye this week, both by eminent practitioners of the subject. The first by Robert Murphy deals with mass spectrometry of neutral lipids, especially triacylglycerols and cholesterol esters, both by shotgun techniques and as part of chromatographic separations (Murphy, R.C. Challenges in mass spectrometry-based lipidomics of neutral lipids. Trends Anal. Chem., 107, 91-98 (2018); DOI). The large number of molecular species present in triacylglycerols of natural origin, even those with relatively simple compositions, will always cause problems, especially when positional distributions are taken into account, and the author stresses the need for careful calibrations in quantitative analyses with internal standards labelled with stable isotopes. The second review by Fong-Fu Hsu deals with shotgun lipidomics (Hsu, F.F. Mass spectrometry-based shotgun lipidomics - a critical review from the technical point of view. Anal. Bioanal. Chem., 410, 6387-6409 (2018); DOI). As an armchair scientist these days, I often get the impression that life is so much easier for the current generation with such powerful instrumentation at their disposal. This and the previous review clearly demonstrate that there is just as much need for care and attention to detail in experimentation as there ever was. As an example of what can be achieved by such methodologies, a new multi-author paper describes the lipidomics and proteomics of every cell type in the human lung (Kyle, J.E. et al. Cell type-resolved human lung lipidome reveals cellular cooperation in lung function. Sci. Rep., 8, 13455 (2018); DOI - open access). The authors claim that this is the first such lipidome map of any organ.
While strongly supporting the open access movement for science publication, I have expressed concern in past contributions to this blog that some of the new journals may not operate to the same standards as the older pay-for-view journals. A correspondent has drawn to my attention a report in sciencemag.org about the open access journal Nutrients. The Editor-in-Chief and all of his senior editors have resigned "alleging that the publisher, the Multidisciplinary Digital Publishing Institute (MDPI), pressured them to accept manuscripts of mediocre quality and importance." The presumed intention of the publisher is to increase the profitability of the journal, but the editors believe that this will harm the journal's impact factor and lead to a drop in submissions in the long term. I have been assured by my correspondent that this journal has had a good reputation until now.
The journal Biological Chemistry (Volume 399, Issue 10, October 2018) is devoted to the topic of "Sphingolipids in Infectious Biology and Immunology".
September 26th, 2018
 I
have to confess that I was entirely unaware of the importance of the nature and function of extracellular vesicles
(exosomes and microvesicles) in lipid metabolism until the thematic series on this topic was announced in the
Journal of Lipid Research (with the start of formal publication in the August issue).
It seems that I was not alone in this regard as the subject is barely mentioned in two recent text books on lipid biochemistry,
which I consulted.
Although extracellular vesicles were first described as a means of selective elimination of proteins, lipids and RNA from cells,
they are now considered to be a new mode of intercellular communication.
I have a lot of catching up to do via the continuing JLR series, together with two recent reviews in other journals
(DOI-1 and DOI-2).
I
have to confess that I was entirely unaware of the importance of the nature and function of extracellular vesicles
(exosomes and microvesicles) in lipid metabolism until the thematic series on this topic was announced in the
Journal of Lipid Research (with the start of formal publication in the August issue).
It seems that I was not alone in this regard as the subject is barely mentioned in two recent text books on lipid biochemistry,
which I consulted.
Although extracellular vesicles were first described as a means of selective elimination of proteins, lipids and RNA from cells,
they are now considered to be a new mode of intercellular communication.
I have a lot of catching up to do via the continuing JLR series, together with two recent reviews in other journals
(DOI-1 and DOI-2).
The mechanism behind the biological effects of tocopherols other than their role as antioxidants has proved elusive, although evidence has been accumulating that the 13'-carboxy metabolites may be of importance. It has now been established that the 13'-carboxy metabolite of α-tocopherol (α-T-13'-COOH) is a potent inhibitor of 5-lipoxygenase, a key enzyme in the biosynthesis of the inflammatory leukotrienes (Pein, H. et al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nature Commun., 9, 3834 (2018); DOI - open access). α-T-13'-COOH accumulates in immune cells and inflamed exudates both in vitro and in vivo in mice, and the authors suggest that the immune regulatory and anti-inflammatory functions of α-tocopherol depend on this endogenous metabolite.
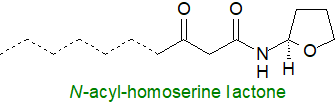
This week's lipid oddity is an N-acylhomoserine lactone produced by bacteria in the human gut (Landman, C. et al. Inter-kingdom effect on epithelial cells of the N-acyl homoserine lactone 3-oxo-C12:2, a major quorum-sensing molecule from gut microbiota. PLOS One, 13, e0202587 (2018); DOI - open access). The full structure of this particular molecule has still to be established, but it has been determined that it has a C12 acyl chain with a 3-oxo group and two double bonds (positions and geometry unknown). Such lipids function normally in a form of intercellular signalling termed 'quorum sensing', which controls gene expression in response to the population density of the species, resulting in coordinated regulation of a range of group-level behaviours, including production of secondary metabolites and virulence factors, bioluminescence and biofilm formation, i.e., when these signal molecules reach a threshold concentration in a particular environment, they bind to their intracellular receptor/activator proteins to induce the expression of relevant genes. The importance of these new molecules is that they also interact with the host intestinal epithelial cells as anti-inflammatory agents with the potential to affect human metabolism including diseases of the gut.
When we are all dead and gone, it appears that our cholesterol lingers on as in the fossil of a 600 million year old animal (pre-Cambrian) (Bobrovskiy, I. et al. Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science, 361, 1246-1249 (2018); DOI).
September 19th, 2018
A new publication examines a range of N-acylethanolamide derivatives to determine whether they are endocannabinoids as defined by an interaction with CB1 and/or CB2 receptors (Alharthi, N. et al. n-3 polyunsaturated N-acylethanolamines are CB2 cannabinoid receptor-preferring endocannabinoids. Biochim. Biophys. Acta, 1863, 1433-1440 (2018); DOI). Saturated and monoenoic N-acylethanolamides are not endocannabinoids, but those derived from other members of the n-6 family of polyunsaturated fatty acids (docosatetraenoic and docosapentaenoic acids in addition to arachidonic) activate both CB1 and CB2 receptors, as well as TRPV1 channels, so these should be considered true endocannabinoids (and 'endovanilloids'). Similarly, N-acylethanolamides derived from the n-3 family of polyunsaturated fatty acids (eicosapentaenoic, docosapentaenoic and docosahexaenoic acids) activate CB2 receptors, and of these, the C22 derivatives also activate TRPV1 channels but not the CB1 receptor. The authors suggest that the preferential activation of CB2 receptors by N-acylethanolamides of the n-3 family of polyunsaturated fatty acids contribute in part to the broad anti-inflammatory profile of the latter.
The journal Current Opinion in Cell Biology has a special issue (open access) dealing with the topic of "Membrane Trafficking" (edited by Satyajit Mayor and Anne Spang): Volume 53, Pages A1-A4, 1-110 (August 2018).
A correspondent has drawn to my attention an article in the Guardian Newspaper that is relevant to my comment on open access publication in last week's blog. The author suggests that scientific publication is a rip-off and has no qualms about using the Sci-Hub web site. I have looked at this web site in the past, although my service provider does not now permit access. My own feeling is that while I don't mind bending the copyright law from time to time, I would feel guilty about breaking it in a more comprehensive manner. Also, I would worry about the security of my computer if I were to download material from this source.
September 12th, 2018
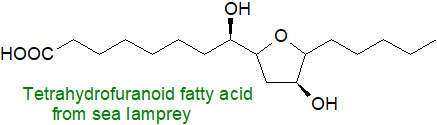
Another new fatty acid caught my eye this week that is novel in terms of both structure and function, i.e., one containing a tetrahydrofuran ring, i.e., (+)-(2S, 3S, 5R)-tetrahydro-3-hydroxy-5-[(1R)-1-hydroxyhexyl]-2-furanoctanoic acid, which is shown to be a secreted pheromone that controls the migratory behaviour of a fish species, the sea lamprey. This is secreted by the fish larvae and draws the mature fish towards the spawning grounds. (Li, K. et al. Fatty-acid derivative acts as a sea lamprey migratory pheromone. Proc. Natl. Acad. Sci. USA, 115, 8603-8608 (2018); DOI - open access). The compound is potentially useful for both control and conservation of sea lamprey populations. As far as I am aware, this is the first natural fatty acid to have been found with a tetrahydrofuran ring, i.e., produced by enzyme systems, although isofurans with a ring structure of this kind are formed adventitiously together with other isoprostanes by autoxidative processes in animal tissues. Fatty acids with a furan ring are common minor components of fish oils, although they are presumed to come from algae and phytoplankton in the diet.
A news item in Nature reports that a number of funding organizations are moving towards a policy of free access to all publications for work that they have supported. I am happy to see an increase in the number of open access publications, but I have the one caveat as to how is it to be decided which open access publications are reputable in the light of innumerable reports that there are a host of frankly fraudulent publications on line. Incidentally, the Nature article has some interesting statistics on the growth of open access publishing. Between 2012 and 2016, the proportion of publications in subscription-only journals fell from 49.2 to 37.7%, though those in fully open access publications only rose by 3%, and there was virtually no change in the number of papers published in journals that permit open access after a fixed period.
It barely touches upon lipids, but who could resist this title (Cao-Pham, A.H. et al. Nudge-nudge, WNK-WNK (kinases), say no more? New Phytologist, 220, 35-48 (2018); DOI).
September 5th, 2018
It is rare to see an announcement of the discovery of novel fatty acids in the popular science news websites, but both Sci-News and Science-Daily have picked up on nebraskanic (illustrated) and wuhanic acids (as the previous but with an additional double bond in position 22) from a seed oil from a Chinese plant. Both reports carry an interview with Prof. Edgar Cahoon, who describes the scientific interest in that biosynthesis involves a break in the cycle of two-carbon additions involved in the assembly of the acyl chain in a manner usually seen only in the synthesis of bacterial fatty acids. Also, the fatty acids seem to form estolide linkages to each other as well as being esterified to glycerol. From a practical standpoint, the oil may have value as a lubricant of natural origin. The names are of course derived from the institutions of the lead authors. This is a long and honorable tradition, as I recall from my days as a post-doc at the Hormel Institute in the 1960s that Helmut Mangold gave the name 'hormelic acid' to a new cyclopentenyl fatty acid. The 60s and 70s were a golden age in the discovery of novel fatty acids, when the Northern Regional Laboratory of the USDA in Peoria, especially, had a major research programme the aim of which was to discover new seed oils of potential industrial value.

Coincidentally, a tweet to LIPID MAPS® alerted me to a report of the presence in plant tissues of other estolide-linked fatty acids, i.e., fatty acid esters of hydroxy fatty acids, which are proving to have some surprising biological properties in animal tissues (Zhu, Q.-F. et al. Comprehensive screening and identification of fatty acid esters of hydroxy fatty acids in plant tissues by chemical isotope labeling-assisted liquid chromatography–mass spectrometry. Anal. Chem., 90, 10056-10063 (2018); DOI).
If I had to pick the most neglected of all lipid classes, I would suggest the non-acidic glycosyldiacylglycerols of animal tissues for which I have to depend on a review from 1987 in my account of the topic in the LipidWeb. I suspect that one reason is that they may suffer degradation in some methods for the isolation of the oligoglycoceramides with which they have similar physical and chromatographic properties. Even the acidic seminolipid or sulfogalactosyldiacylglycerol does not rate a mention in many lipid text books, although it is essential for male reproduction. Hopefully, a new review will rekindle interest in the the latter lipid at least (Tanphaichitr, N. et al. Properties, metabolism and roles of sulfogalactosylglycerolipid in male reproduction. Prog. Lipid Res., 72, 18-41 (2018); DOI.).
August 29th, 2018
 A
new review (open access) covers the topic of glycosylphosphatidylinositol anchored proteins
in plants (Yeats, T.H. et al. Plant glycosylphosphatidylinositol anchored proteins at the plasma membrane-cell
wall nexus. J. Integr. Plant Biol., 60, 649-669 (2018);
DOI).
As might be expected, these are vital for a host of functions in plant development and signalling especially at the interface
of the plasma membrane and cell wall.
However, although the synthesis and structure of GPI anchors is believed to be conserved across eukaryotes,
this appears to be based on a number of assumptions as far as plants are concerned, as it seems that the O-glycan component of only
one species has been determined to date, i.e., that of Pyrus communis (pear), in a publication from 1999.
In this instance, the O-glycan was relatively simple with no phosphoethanolamine side chains and sometimes a β-linked galactose
side chain on the first mannose.
The lipid component was a ceramide, as that in many fungi, rather than a diacylglycerol.
A
new review (open access) covers the topic of glycosylphosphatidylinositol anchored proteins
in plants (Yeats, T.H. et al. Plant glycosylphosphatidylinositol anchored proteins at the plasma membrane-cell
wall nexus. J. Integr. Plant Biol., 60, 649-669 (2018);
DOI).
As might be expected, these are vital for a host of functions in plant development and signalling especially at the interface
of the plasma membrane and cell wall.
However, although the synthesis and structure of GPI anchors is believed to be conserved across eukaryotes,
this appears to be based on a number of assumptions as far as plants are concerned, as it seems that the O-glycan component of only
one species has been determined to date, i.e., that of Pyrus communis (pear), in a publication from 1999.
In this instance, the O-glycan was relatively simple with no phosphoethanolamine side chains and sometimes a β-linked galactose
side chain on the first mannose.
The lipid component was a ceramide, as that in many fungi, rather than a diacylglycerol.
The microbial lipopeptides are fascinating molecules in that they often contain unique fatty acids together with distinctive amino acids, sometimes with the "wrong" stereochemistry. They are usually powerful surfactants, often with anti-bacterial and antifungal properties. Importantly, they are also considered one of the best hopes in the search for novel antibiotics that may replace those to which pathogenic bacteria are becoming resistant. They may be of equal value against plant pathogens. The polymixins are already licensed for topical use against Gram-negative bacterial infections, but they can only be used internally as a last resort because of toxicity problems. A new review discusses the progress that is being made in the synthesis of polymixin analogues that are better tolerated and hopefully will have a greater range of activities (Vaara, M. New polymyxin derivatives that display improved efficacy in animal infection models as compared to polymyxin B and colistin. Med. Res. Rev., 38, 1661-1673 (2018); DOI). It seems that we are no nearer to finding a magic bullet, although individual compounds are showing promise, especially as they may act in synergy with existing antibiotics. Similarly, there is hope for novel lipopeptides produced by Pseudomonas species as also discussed in another new review (open access) (Geudens, N. and Martins, J.C. Cyclic lipodepsipeptides from Pseudomonas spp. - biological Swiss-army knives. Front. Microbiol., 9, 1867 (2018); DOI). Some of these have anticancer activities in vitro in cancer cell lines.
August 22nd, 2018
It seems a highly ambitious undertaking to use lipidomics to assess how the lipidome has changed during evolution even in mammalian species. From the internet - "According to Mammal Species of the World, 3rd Edition (Wilson and Reeder 2005), the most recent authoritative published checklist of modern mammal species, there are 5,416 different species of mammals". A beginning can be made at least to such a study, and a new publication reports data from six tissues of 32 species of mammals representative of primates, rodents, and bats (Khrameeva, E. et al. Lipidome evolution in mammalian tissues. Mol. Biol. Evolution, 35, 1947-1957 (2018); DOI). It appears from this selection that the lipidome has not evolved appreciably except in humans, where many of the unique features were found in the brain cortex, suggesting that there has been an accelerated lipidome evolution in the human brain. The paper is open access.
Another pedantic rant: It is now more than 50 years since IUPAC-IUB published their first set of nomenclature recommendations for lipids in which the stereospecific numbering (sn) system was introduced for triacylglycerol positional distributions. My recollection from that time was that this part of the proposal was approved universally, as a sensible and practical way to characterize the chirality of glycerolipids instead of the R/S or D/L nomenclatures, which could cause confusion especially when applied to more complex lipids. It was fondly assumed that the term 'triglyceride' would fall into disuse, although I can understand why it continues to be used in industry and perhaps more surprisingly in medicine (Sigma-Aldrich sell "Serum Triglyceride Determination Kits"). As I may have mentioned before, my pet hate is the hybrid term "triacylglyceride", which still gets passed by referees and editors of reputable journals. Google Scholar tells me that it has been used in more than 2000 publications since 2017. I don't suppose that many read the original IUPAC-IUB publications nowadays (although you can find links here), but all the text books in my library use triacylglycerol. By all means continue to use 'triglycerides' if you wish, but please not 'triacylglycerides'.
My open access bargain of the week is a 30 page review on protein S-acylation (Zaballa, M.E. and van der Goot, F.G. The molecular era of protein S-acylation: spotlight on structure, mechanisms, and dynamics. Crit. Rev. Biochem. Mol. Biol., 53, 420-451 (2018); DOI).
August 15th, 2018
Although the immune system is essential to protect the body from infection, some immune responses can harm tissues. The eye is especially sensitive to immune reactivity, and it has now been determined that cholesterol sulfate is a key protective factor (Sakurai, T. et al. Cholesterol sulfate is a DOCK2 inhibitor that mediates tissue-specific immune evasion in the eye. Science Sign., 11, eaao4874 (2018); DOI). This is produced by the Harderian gland, which secretes the lipids that form a protective layer in the tear film that covers the eye. Experiments with mice in vitro demonstrated that cholesterol sulfate selectively inhibits the guanine nucleotide exchange factor DOCK2 and by this means suppresses the migration of neutrophils and T cells. When the sulfotransferases responsible for the synthesis of this lipid were inhibited, inflammation occurred that could be cured by administering eye drops containing cholesterol sulfate. As it is produced by most animal cells and circulates in plasma, it seems to me that the next important question is whether cholesterol sulfate might be an endogenous factor that suppresses the immune system in other circumstances, and possibly in a less benign manner in tumours, for example.
The journal Nitric Oxide continues its series of review articles on the chemistry and biochemistry of the potent anti-inflammatory nitro fatty acids in volumes 78 and 79. A separate publication describes the anticancer effects of nitro fatty acids and proposes a mechanism (Kühn, B. et al. Anti-inflammatory nitro-fatty acids suppress tumor growth by triggering mitochondrial dysfunction and activation of the intrinsic apoptotic pathway in colorectal cancer cells. Biochem. Pharm., 155, 48-60 (2018); DOI). The authors suggest that "these naturally occurring lipid mediators are a new class of well tolerated chemotherapeutic drug candidates for treatment of colorectal cancer or potentially other inflammation-driven cancer types." Good news indeed!
I am always interested in lipid oddities, and it is hard to think of anything more unusual than a triacylglycerol with acetate in position sn-2, as in the seed oils from Polygala species. This was first reported briefly in 1977 but has now been confirmed by mass spectrometry (Smith, M.A. et al. 2-Acetyl-1,3-diacyl-sn-glycerols with unusual acyl composition in seed oils of the genus Polygala. Eur. J. Lipid Sci. Technol., 120, 1800069 (2018); DOI). Curiosity aside, if any species from the genus can be developed as a commercial crop, the oil may have potential as a low-viscosity biofuel/lubricant or reduced calorie food ingredient.
August 8th, 2018
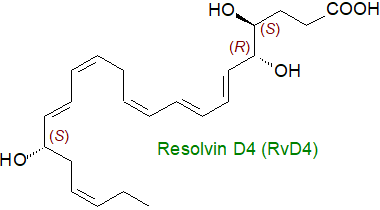
The uncontrolled inflammatory response that is seen in sepsis is now recognized to be a major cause of death in the UK and I am sure elsewhere. One of the best hopes for novel therapeutic responses lies with the specialized pro-resolving mediators - resolvins, protectins and maresins, but how are such highly stereospecific structures to be produced on a scale that permits clinical testing? A new total synthesis of resolvin D4 (RvD4), which has three chiral hydroxyl groups and three cis- and three trans-double bonds, has just been published that has the potential to be developed on a commercial scale (Winkler, J.W. et al. Structural insights into Resolvin D4 actions and further metabolites via a new total organic synthesis and validation. J. Leukocyte Biol., 103, 995-1010 (2018); DOI). The product was tested successfully against ischemia models in mice, and in so-doing the importance of the correct stereochemistry was emphasized. An editorial in the same issue of the journal provides a further perspective on the topic.
Every Saturday morning, I scan rapidly through the titles of around 800 publications dealing with lipid science to pick out a relative few that are useful to me for my web endeavours, and which are subsequently listed in my Literature survey pages. Inevitably, I miss many that are not picked out by the algorithm I use, or whose significance I do not recognize at first glance. One that I greatly regret missing when it first appeared deals with how lipids are distributed in membranes (Murate, M. and Kobayashi, T. Revisiting transbilayer distribution of lipids in the plasma membrane. Chem. Phys. Lipids, 194, 58-71 (2016); DOI). I am now using it to update my web pages. When I was a young scientist, the work of van Deenen and colleagues in the Netherlands in which specific lipases were used to determine the sidedness of membranes attracted great interest. However, by today's standards, these methods seem relatively crude and new procedures involving immunoelectron microscopy are providing much greater selectivity and precision. Perhaps surprisingly, the one lipid for which we still lack reliable data is cholesterol, and it seems that new cholesterol-specific probes are required before it will be possible to reliably determine its transbilayer distribution.
August 1st, 2018
I have belatedly come across two fascinating and important papers on the subject of 12,13-dihydroxy-9Z-octadecenoic acid or 12,13-diHOME. This is a further example of a fatty acid with important biological functions that is not an eicosanoid or a docosanoid but an octadecanoid, derived in this instance from linoleic acid via the action of a CYP epoxygenase followed by an epoxide hydrolase. Last year, this was reported to promote fatty acid transport into brown adipose tissue during cold exposure, while the more recent publication suggests that it is a "novel exercise-stimulated circulating factor that may contribute to the metabolic changes that occur with physical exercise" both in humans and laboratory animals (Stanford, K.I. et al. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metabolism, 27, 1111-1120.e3 (2018); DOI). While these effects seem beneficial, there are earlier reports of adverse properties, for example that such oxidized linoleate metabolites may be atherogenic through the induction of pro-inflammatory cytokines and by formation of foam cells from macrophages by PPAR activation. Life is complicated!
Excuse a moment of pedantry, but I have often complained about the excessive use of abbreviations in publications, and the reason I missed these articles in my weekly searches was because of the use of the abbreviated name of the lipid in the title; this was not recognized by the search algorithm I use. The authors did use the word "lipokine" in the title and I could add this to my search algorithm, but a quick search in the Web of Science suggests that this term has only been used 14 times in the last five years and then mainly for palmitoleic acid for which it was originally coined. Should it be used more?
July 25th, 2018
 While
animals have eicosanoids and docosanoids and plants have jasmonates and other oxylipins as lipid mediators of innumerable biological reactions,
nematodes, including a number of human parasites, have ascarosides.
These are glycolipids that consist of the mono-saccharide α-L-3,6-dideoxymannose or ascarylose, which occurs in few
other organisms, linked glycosidically to the hydroxyl group of a 2-hydroxy alcohol or of an (ω-1)-hydroxy fatty acid.
The nature of the alkyl moiety can vary appreciably and the free hydroxyl and carboxyl groups can be derivatized in various ways.
For example, more than 200 ascarosides have been characterized from the model nematode species Caenorhabditis elegans
with presumably many different functions.
While
animals have eicosanoids and docosanoids and plants have jasmonates and other oxylipins as lipid mediators of innumerable biological reactions,
nematodes, including a number of human parasites, have ascarosides.
These are glycolipids that consist of the mono-saccharide α-L-3,6-dideoxymannose or ascarylose, which occurs in few
other organisms, linked glycosidically to the hydroxyl group of a 2-hydroxy alcohol or of an (ω-1)-hydroxy fatty acid.
The nature of the alkyl moiety can vary appreciably and the free hydroxyl and carboxyl groups can be derivatized in various ways.
For example, more than 200 ascarosides have been characterized from the model nematode species Caenorhabditis elegans
with presumably many different functions.
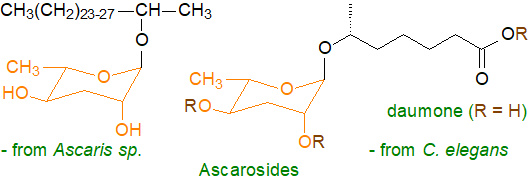
Some of these are structural and provide an impermeability to the shell that protects eggs of certain nematode species from the harsh conditions in the intestines of host animals. Others function as pheromones as well as signalling molecules that regulate development and behaviour. For example, they control the entry and exit of nematodes from a dormant or 'dauer' stage. A new review (open access) describes the properties of these fascinating molecules (von Reuss, S.H. Exploring modular glycolipids involved in nematode chemical communication. Chimia, 72, 297-303 (2018); DOI).
In my blog last week, I cited a review claiming benefits towards heart disease from eating fish (and presumably their fish oils), and since then two other reviews have appeared one claiming no such benefits and the other the opposite including increased longevity. Is it any wonder that I am confused by nutritional advice, even though the Fats of Life newsletter does its best to enlighten me. Of course fish oils have the potential to help with many more inflammatory conditions other than heart disease. Whether or not it will give me any health benefit, I will enjoy my smoked salmon sandwich at lunchtime.
July 18th, 2018
In my notes on proteolipids in the LipidWeb, I had quoted from a paper suggesting that there were approximately 300 myristoylated proteins in humans and a similar number in Arabidopsis. This figure has now been revised to more than 600 in each (Castrec, B. et al. Structural and genomic decoding of human and plant myristoylomes reveals a definitive recognition pattern. Nature Chem. Biol., 14, 671-679 (2018); DOI). The results came after the crystal structure of the N-myristoyltransferase-1 was determined. This showed that the enzyme has a characteristic binding cleft that is involved in the recognition of potential substrates for myristoylation (with some overlap with targets for N-acetylation); it also revealed potential sites for further S-palmitoylation, allowing recognition of sequences exhibiting both acylations.
I tend to pay little heed to dietary recommendations in terms of fats and oils as opinions seem to change with the seasons. On the other hand, when the American Heart Association publishes its recommendations, I feel that I must take note at least (Rimm, E.B. et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation, 138, E35-E47 (2018); DOI - open access). The last line of the abstract succinctly states the AHA position with which I can happily live - "We conclude that 1 to 2 seafood meals per week be included to reduce the risk of congestive heart failure, coronary heart disease, ischemic stroke, and sudden cardiac death, especially when seafood replaces the intake of less healthy foods." Following dietary recommendations that appeal to your taste buds may not be the best policy, but I am sure there are worse.
Aficionados of sphingolipids in general and gangliosides in particular will no doubt appreciate a new book on the topic (Ronald L. Schnaar and Pablo H.H. Lopez (editors) Gangliosides In Health And Disease. Progress in Molecular Biology and Translational Science, Volume 156, Pages 1-462 (2018) available from Science Direct). I have not seen it myself.
July 11th, 2018
It has become commonplace to see new reports of the biochemistry of oxylipins derived from the C20 and C22 polyunsaturated fatty acids, and it is easy to forget that there are some important metabolites of the more simple C18 fatty acids. In particular, I am thinking of the nitro fatty acids derived from oleate and linoleate, which have attracted increasing interest since the turn of the century. It seems that the story starts with the discovery in the 1990s that NO• inhibited the oxidation of membranes and plasma lipoproteins more potently than α-tocopherol and in general had anti-inflammatory, antioxidant and tissue-protective effects. Subsequently, it became apparent that nitro fatty acids had a role in mediating these reactions largely because the nitro-alkene moiety has potent electron-withdrawing properties that favour reversible nitroalkylation reactions (Michael reaction) with proteins. A new brief review provides a fascinating introduction to the subject (Freeman, B.A. et al. The discovery of nitro-fatty acids as products of metabolic and inflammatory reactions and mediators of adaptive cell signaling. Nitric Oxide Biol. Chem., 77, 106-111 (2018); DOI). The next issue/volume of this journal has a number of review articles on this general topic.
I tend to stay clear of medical and nutritional matters and leave the debate to those better qualified than I in these subjects. Nonetheless, I enjoy reading a provocative article from time to time such as the following, which is open access (Tsoupras, A. et al. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients, 10, 604 (2018); DOI). The authors suggest that cholesterol has been demonized but that platelet-activating factor, i.e., 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine or PAF, is the true culprit (apart from its main thesis, the paper is a comprehensive review of PAF activities). While I do not feel qualified to endorse the proposal, I have often wondered if the concentration on cholesterol by clinical scientists is in part due to its ease of analysis as one of the most abundant metabolites in molar terms in plasma (only surpassed by glucose). In contrast, PAF occurs in cells and exerts its effects at concentrations as low as 10-14M, and its analysis is technically daunting. It seems that we may need to employ more lipid analysts skilled in advanced mass spectrometry in future clinical studies.
An analytical development that has truly astonished me is the use of a special knife during surgery for ovarian cancer that enables differentiation of cancerous from borderline tumours in real time from differences in their lipid components by analysing aerosolized tissue by a mass spectrometric technique during electrosurgical dissection (Phelps, D.L. et al. The surgical intelligent knife distinguishes normal, borderline and malignant gynaecological tissues using rapid evaporative ionisation mass spectrometry (REIMS). Brit. J. Cancer, 118, 1349-1358 (2018); DOI). The publication is open access.
July 4th, 2018
The hedgehog proteolipids are fascinating molecules, not least because they require both palmitate and cholesterol in covalent linkage for their essential functions, for example in limb development. Within the cell, they are produced in the endoplasmic reticulum and Golgi but then must be transferred to the exterior leaflet of the plasma membrane. From there, the fully lipidated proteins must travel a distance as much as 15 cell diameters until they encounter their signalling receptors, but exactly how this is accomplished has yet to be determined. Several proteins that are involved in extraction from the membrane and subsequent transport have been characterized, and at least three model systems for this transport have been proposed, although it appears that none is entirely satisfactory. A new review (open access) discusses the alternatives (Manikowski, D. et al. Taking the Occam's razor approach to hedgehog lipidation and its role in development. J. Dev. Biol., 6, 3 (2018); DOI).
It often surprises me how relatively small changes in enzyme structure can alter the nature of their products, e.g. to switch between desaturation and hydroxylation. Animal tissues contain six ceramide synthases with very different specificities for fatty acid substrates and different tissue locations, and they appear to produce distinct molecular species of ceramides for particular functions. They are membrane bound enzymes with six membrane spanning regions. Now, they have been shown to differ primarily in only an 11-residue sequence in a loop between the last two putative transmembrane domains (Tidhar, R. et al. Eleven residues determine the acyl chain specificity of ceramide synthases. J. Biol. Chem., 293, 9912-9921 (2018); DOI). As an editors' choice, the paper is open access.
Incidentally, the authors cite the LIPID MAPS® Lipidomics Gateway to the effect that ~40,000 different lipids have been identified to date, ~4000 of which are sphingolipids. I suspect that in the long term many more will be added to the totals, especially as more lipidomic analyses of plant lipids are undertaken. Whatever the true figure, I am sure that lipid scientists are going to be gainfully employed for many years to come - and I am looking forward to recording and celebrating their efforts.
June 27th, 2018
 I encounter
publications dealing with new lipidomics studies of animal tissues in all my weekly literature searches,
and as these often contain comparisons with human different disease states, it is important to take note of them.
On the other hand, lipidomics studies of plants appear relatively infrequently, although it is vital that we understand what keeps plants healthy,
especially when phosphate is limiting or when they are under salt stress.
In the long term, this knowledge may also be essential to human health and nutrition.
Analysis is daunting technically, as in addition to the common phospholipid classes,
plants contain a wide range of distinctive lipids not encountered in animals.
These include many different classes of glycosylmono- and diacylglycerols, glycosylinositol phosphoceramides
and several sterols and sterol glycosides.
This complexity is apparent in a new study in which 600 lipid species from 23 lipid classes were identified from a barley root extracts.
These included 142 species of glycosyl inositol phosphorylceramides alone (Yu, D.Y. et al. A high-resolution HPLC-QqTOF
platform using parallel reaction monitoring for in-depth lipid discovery and rapid profiling. Anal. Chim. Acta,
1026, 87-100 (2018); DOI).
I encounter
publications dealing with new lipidomics studies of animal tissues in all my weekly literature searches,
and as these often contain comparisons with human different disease states, it is important to take note of them.
On the other hand, lipidomics studies of plants appear relatively infrequently, although it is vital that we understand what keeps plants healthy,
especially when phosphate is limiting or when they are under salt stress.
In the long term, this knowledge may also be essential to human health and nutrition.
Analysis is daunting technically, as in addition to the common phospholipid classes,
plants contain a wide range of distinctive lipids not encountered in animals.
These include many different classes of glycosylmono- and diacylglycerols, glycosylinositol phosphoceramides
and several sterols and sterol glycosides.
This complexity is apparent in a new study in which 600 lipid species from 23 lipid classes were identified from a barley root extracts.
These included 142 species of glycosyl inositol phosphorylceramides alone (Yu, D.Y. et al. A high-resolution HPLC-QqTOF
platform using parallel reaction monitoring for in-depth lipid discovery and rapid profiling. Anal. Chim. Acta,
1026, 87-100 (2018); DOI).
For similar reasons, it is important that we understand the biosynthesis, metabolism, and action of plant oxylipins, especially the jasmonates, which are so essential to the development of healthy plants as well as their response to stresses, and I can recommend a new review that gives a comprehensive account of this topic (Wasternack, C. and Feussner, I. The oxylipin pathways: biochemistry and function. Annu. Rev. Plant Biol., 69, 363-386 (2018); DOI).
I have never paid any attention to Twitter, as I had conceived the idea that it was simply a vanity platform for would-be celebrities or a font for trivia. Now, I have had to reconsider this view as the virtues of the Twitter link on the LIPID MAPS® website have been pointed out to me. I have not had the courage to send a tweet myself yet, but you never know. Incidentally, the LIPID MAPS® Lipidomics Gateway has had a substantial revamp and is certainly much more eye-catching.
June 20th, 2018
An interesting review publication suggests that long-chain polyunsaturated fatty acids, as opposed to linoleic and linolenic acids, are the true essential fatty acids (Anez-Bustillos, L. et al. Redefining essential fatty acids in the era of novel intravenous lipid emulsions. Clin. Nutr., 37, 784-789 (2018); DOI). Mice fed arachidonic and docosahexaenoic acids exclusively for five generations grew and reproduced normally, and these fatty acids are certainly vital for eicosanoid and docosanoid production and for innumerable other purposes when esterified to lipids in tissues. There is no doubt that we must have adequate amounts to ensure health. On the other hand, linoleic acid is required for skin ceramides and cardiolipin in heart mitochondria, for example. If the skin barrier integrity and energy production were less than optimal (if adequate for life) in the experimental animals, would this have been noticed? The authors suggest that linoleate could be supplied for other functions by retro-conversion of arachidonic acid, but this seems to me a circular argument - linoleate produces arachidonate produces linoleate - the chicken versus the egg. The debate is important in that alternative injectable lipid emulsions low in the C18 precursors are apparently being considered for clinical use. It seems to me that a sensible compromise would be to ensure that there are adequate amounts of all fatty acids that may have essential functions in any artificial feeding regime.
In my last blog, I urged other senior lipid experts to consider keeping active in or near retirement by writing for the web. My web career was initiated by a desire to see that the large repository of mass spectrometric information (electron impact) on fatty acids and other simple lipids, which I had accumulated, was preserved. There are now more than 2,100 spectra available in the LipidWeb. On the other hand, my former colleagues recently asked me to advise on an analytical problem involving plant sterols. As I did not have access to the Wiley Library and had only a few representative spectra of my own, this proved to be a time-consuming and rather tedious task to search the literature. Is there anyone out there who would consider producing a website akin to mine dealing with electron-impact mass spectra of sterols and their derivatives? You would do the lipid community a great service. Again, I would be happy to advise.
Although we are probably stuck with it, I don't particularly like the term "endocannabinoid", as to use yet another cliché - it is putting the cart before the horse. For example, anandamide does not mimic cannabinol, but rather cannabinol mimics anandamide. Whatever we call them, there is no doubt that endocannabinoids have profound biological effects in humans, and drugs that influence their metabolism are undergoing clinical trials. Therefore, it would not be surprising if cannabinoids per se have medicinal properties, although there is currently some controversy in the UK about such applications. It in no way endorses the use of cannabis for recreational purposes if we accept that drugs derived from it may have a legitimate place in pharmacopoeias. Few politicians appear to understand the difference between the two.
June 13th, 2018
 I enjoy eating fish, and I am not going to be deterred by the findings that the
arseno-hydrocarbons, which they contain albeit at very low levels, are highly toxic.
From experiments with human cell lines in vitro, a new publication reports that arsenic-containing hydrocarbons influence
gene expression and DNA methylation with the nature and magnitude of the effects dependent on the chain-length of the hydrocarbon
(Müller, S.M. et al. Arsenic-containing hydrocarbons: effects on gene expression, epigenetics,
and biotransformation in HepG2 cells. Arch. Toxicol., 92, 1751-1765 (2018);
DOI).
One surprise was that high proportions of the starting compounds were transformed into thioxo analogues,
i.e., with the oxygen atom replaced by sulfur, with trace levels as arseno-fatty acids and alcohols.
Thioxo-arseno lipids might be expected to be more lipophilic than the parent compounds,
but it is not yet known whether this transformation results in an increase in toxicity.
I enjoy eating fish, and I am not going to be deterred by the findings that the
arseno-hydrocarbons, which they contain albeit at very low levels, are highly toxic.
From experiments with human cell lines in vitro, a new publication reports that arsenic-containing hydrocarbons influence
gene expression and DNA methylation with the nature and magnitude of the effects dependent on the chain-length of the hydrocarbon
(Müller, S.M. et al. Arsenic-containing hydrocarbons: effects on gene expression, epigenetics,
and biotransformation in HepG2 cells. Arch. Toxicol., 92, 1751-1765 (2018);
DOI).
One surprise was that high proportions of the starting compounds were transformed into thioxo analogues,
i.e., with the oxygen atom replaced by sulfur, with trace levels as arseno-fatty acids and alcohols.
Thioxo-arseno lipids might be expected to be more lipophilic than the parent compounds,
but it is not yet known whether this transformation results in an increase in toxicity.
When I have what my wife calls "a senior moment", it seems that the fault may lie with my lipids and in particular my leukotrienes. Experiments with mice engineered genetically to have excess tau proteins, the second-most important lesion in the brain in patients with Alzheimer's disease, showed that they developed learning and memory problems as they aged. However, the effects were reversed by a drug that inhibits leukotriene formation by blocking the 5-lipoxygenase enzyme. There is a popular account of the work in Science Daily.
One of the main virtues of writing for the web is its immediacy. Not only do you see the results of your efforts at once, but you also have the opportunities to update anything you write whenever new information becomes available. For example, the figures and comments in this and last weeks' blogs were prepared not for the blog per se but initially for the essays on the appropriate topics in the Lipid Essentials section of this website. I make changes to one or other of these web pages nearly every day - sometimes simply to add or replace a reference and occasionally I regret to say to correct an error. Sometimes, I merely find a better way of explaining a point. If I had intended to use these figures in a review on one of these topics for a print publication, it might be a year before it appeared in a journal - not the same day - and then there would be no opportunities for correction or updating. There are hundreds of senior scientists out there with a wealth of knowledge on lipid science who I am sure would find some fulfillment by setting up their own web sites and writing for the web. It is so easy to do - why not give it a go? I will be happy to offer advice to anyone who wants to try.
June 6th, 2018
 I have been enjoying the sunshine of Gran Canaria for the last week, and lipid science has not been at the forefront
of my thoughts.
However, it took only a preliminary look at the literature on my return, to see that I had missed an important paper.
The mechanism for the cleavage of the vinyl ether bond in plasmalogens has now been revealed
as the result of a master class in elegant mass spectrometric
experiments involving the use of stable isotopes (Jenkins, C.M. et al. Cytochrome c is an oxidative
stress–activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage
J. Biol. Chem., 293, 8693-8709 (2018); DOI
- open access as an editors' pick, as is an additional useful commentary by Howard Goldfine).
Perhaps surprisingly, the key enzyme is cytochrome c, best known for its role in the respiratory chain of mitochondria.
This must first be activated to produce peroxidase activity by an interaction with cardiolipin.
After a complex series of reactions, the products are a lysophospholipid and an α-hydroxyaldehyde.
The carbonyl oxygen is derived from water while that of the
α-hydroxyl group comes from molecular oxygen (or possibly from oxidized cardiolipin).
As the resulting lysophospholipid is usually enriched in arachidonic acid, this may have interesting implications for eicosanoid production.
The findings are also relevant to Alzheimer's disease,
as it has long been known that α-hydroxyaldehydes accumulate in the brains of affected patients.
I have been enjoying the sunshine of Gran Canaria for the last week, and lipid science has not been at the forefront
of my thoughts.
However, it took only a preliminary look at the literature on my return, to see that I had missed an important paper.
The mechanism for the cleavage of the vinyl ether bond in plasmalogens has now been revealed
as the result of a master class in elegant mass spectrometric
experiments involving the use of stable isotopes (Jenkins, C.M. et al. Cytochrome c is an oxidative
stress–activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage
J. Biol. Chem., 293, 8693-8709 (2018); DOI
- open access as an editors' pick, as is an additional useful commentary by Howard Goldfine).
Perhaps surprisingly, the key enzyme is cytochrome c, best known for its role in the respiratory chain of mitochondria.
This must first be activated to produce peroxidase activity by an interaction with cardiolipin.
After a complex series of reactions, the products are a lysophospholipid and an α-hydroxyaldehyde.
The carbonyl oxygen is derived from water while that of the
α-hydroxyl group comes from molecular oxygen (or possibly from oxidized cardiolipin).
As the resulting lysophospholipid is usually enriched in arachidonic acid, this may have interesting implications for eicosanoid production.
The findings are also relevant to Alzheimer's disease,
as it has long been known that α-hydroxyaldehydes accumulate in the brains of affected patients.
Incidentally, a further new publication is relevant to the suggestion that oxidized cardiolipin may be involved in the reaction (Vähäheikkilä, M. et al. How cardiolipin peroxidation alters the properties of the inner mitochondrial membrane? Chem. Phys. Lipids, 214, 15-23 (2018); DOI).
May 23rd, 2018
 It
is very rare to see a statue raised to commemorate a scientist,
but I was pleased to see that Stephen Hawking was honoured at his death by being interred in Westminster Abbey.
I only know of one lipid scientist who has been commemorated by a statue, and that is the great French chemist Michel Chevreul of whom
there is a bronze statue in the Jardin des Plantes d'Angers in Paris.
Of course that great stalwart of lipid research, the laboratory mouse, is commemorated by a bronze statue in a park in front
of the Institute of Cytology and Genetics of the Russian Academy of Sciences in the city of Novosibirsk in Siberia, Russia.
He/she is depicted knitting DNA (see the Wikipedia entry).
In the main city square here in Dundee, we have a statue of Desperate Dan,
a character from children's comics and a superhero of my own childhood. Our priorities must be different.
It
is very rare to see a statue raised to commemorate a scientist,
but I was pleased to see that Stephen Hawking was honoured at his death by being interred in Westminster Abbey.
I only know of one lipid scientist who has been commemorated by a statue, and that is the great French chemist Michel Chevreul of whom
there is a bronze statue in the Jardin des Plantes d'Angers in Paris.
Of course that great stalwart of lipid research, the laboratory mouse, is commemorated by a bronze statue in a park in front
of the Institute of Cytology and Genetics of the Russian Academy of Sciences in the city of Novosibirsk in Siberia, Russia.
He/she is depicted knitting DNA (see the Wikipedia entry).
In the main city square here in Dundee, we have a statue of Desperate Dan,
a character from children's comics and a superhero of my own childhood. Our priorities must be different.
A candidate for the most unusual new lipid of the year is 1-phosphatidyl-2-acyl-glycero-3-phosphoethanolamine from a Gram-negative bacterial species; the structure has been tentatively identified by tandem mass spectrometric analysis (Luo, Y. et al. Nutrient depletion-induced production of tri-acylated glycerophospholipids in Acinetobacter radioresistens. Sci. Rep., 8, 7470 (2018); DOI - open access). It is produced together with cardiolipin and lysocardiolipin, presumably from a common intermediate, only in the stationary phase of growth of the organism.
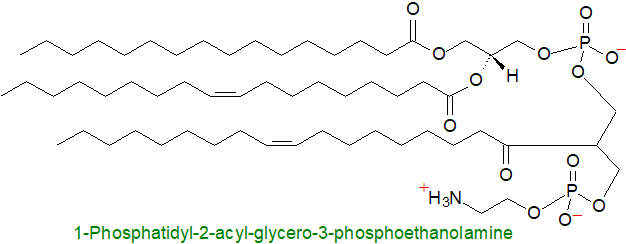
Issue 8 (Volume 592, April 2018) of FEBS Letters contains a number of review articles on the theme of "Focus on… Yeast Lipid Biochemistry", all of which are open access.
May 16th, 2018
α-D-Galactosylceramides, i.e., cerebrosides with an α-D- rather than the usual β-D-linkage between galactose and ceramide, are present in trace amounts only in human tissues but they have profound biological effects. For example, studies with animal models have suggested that treatment with α-D-galactosylceramides may be effective against lung and colorectal cancers, melanomas and leukemia. Now, a phase I trial with high-risk melanoma patients has given promising preliminary results (Gasser, O. and 19 others. A phase I vaccination study with dendritic cells loaded with NY-ESO-1 and α-galactosylceramide: induction of polyfunctional T cells in high-risk melanoma patients. Cancer Immunology, Immunotherapy, 67, 285-298 (2018); DOI). It is always pleasing to see that the potential of lipids in therapy is being realized. Unfortunately, not all glycosphingolipids are beneficial and a new short review of the influence of glycosphingolipids on cancer has been published (Zhuo, D.H. et al. Biological roles of aberrantly expressed glycosphingolipids and related enzymes in human cancer development and progression. Front. Physiol., 9, 466 (2018); DOI - open access).
The bargain of the week is an open access review of triacylglycerol metabolism (Alves-Bezerra, M. and Cohen, D.E. Triglyceride metabolism in the liver. Comprehensive Physiology, 8, 1-22 (2018); DOI). There are nearly 300 references, it is very well illustrated and it should be especially useful for teaching purposes. My only caveat is the use of the term 'triglyceride' instead of 'triacylglycerol', which has been recommended by IUPAC-IUB for more than 50 years. Two generations of biochemists have been taught the recommended nomenclature, so I am surprised to find the old used here. At least the authors did not use the hybrid term 'triacylglycerides', which I find much too often in the lipid literature. Am I being pedantic?
May 9th, 2018
In my blog of March 14th, I discussed a paper describing the synthesis of linoleic acid in primitive invertebrates, including insects, nematodes and snails. Hot on its heels, a new paper has just been published demonstrating that a large number of aquatic invertebrates possess the gene for a Δ15-desaturase and so can synthesise α-linolenic acid and polyunsaturated fatty acids of the omega-3 family (Kabeya, N. et al. Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Science Advances, 4, eaar684902 (2018); DOI - open access). It was pleasing to see that some of the authors were from the University of Stirling in Scotland. Until now, it had been believed that microorganisms were the main producers of polyunsaturated fatty acids of omega-3 fatty acids in the marine food web, but now it appears that animal species may make a significant contribution. In addition to adding the new information, I have had to make a small but important change to my web page on polyunsaturated fatty acids, by changing phrases such as "animals cannot produce essential fatty acids" to "higher animals cannot, etc".
It is 40 years since, the discovery of glycosylphosphatidylinositol (GPI)-anchored proteins, and thirty since the first complete structure was determined for the parasitic organism Trypanosoma brucei, and it since then it has become evident how important these are for so many aspects of metabolism in Eukaryotes. In their functional site on the outer leaflet of the plasma membrane, the flexible carbohydrate linkage provides GPI-proteins with a much higher degree of rotational freedom than is available to most other membrane proteins, facilitating their functions as signal receptors and host-recognition molecules. They have essential functions in the interaction of cells with their external environment by enabling the receipt of signals and the response to challenges as well as mediating adhesion of extracellular compounds to the cell surface. In parasitic protozoa, yeasts and fungi, GPI-proteins also participate in the structural integrity of the cell wall and with other complex glycans provide a layer of protection to the organisms. A new review is a valuable guide to the latter (Komath, S.S. et al. Generating anchors only to lose them: the unusual story of glycosylphosphatidylinositol anchor biosynthesis and remodeling in yeast and fungi. IUBMB Life, 70, 355-383 (2018); DOI).
May 2nd, 2018
There has been no shortage of publications dealing with the molecular species of mitochondrial cardiolipin in recent years, but a new publication suggests that most of them suffer from a flaw in that they do not allow sufficiently for overlap with isobaric species (Oemer, G. et al. Molecular structural diversity of mitochondrial cardiolipins. Proc. Natl. Acad. Sci. USA, 115, 4158-4163 (2018); DOI). I have been too long from the bench to fully comprehend the arguments, but the authors use a combination of HPLC-mass spectral data and a mathematical structural modeling approach to overcome the problems. The data are presented elegantly in graphical form for many different organisms and tissues, but I wish the authors had used the opportunities offered by having appendices to tabulate data at least for the major species, as they have done for fatty acid compositions. I would love to be able to list tabulated data from a modern paper in my web page on this lipid class to replace that from a 25 year old publication.
Do we now fully comprehend the structures of natural cardiolipins? Unfortunately, the answer is no because we know little or nothing about the positional distributions of fatty acids in the molecule. Cardiolipin has two chiral centres, one in each outer glycerol group, and this means that the four positions to which fatty acids are esterified are each metabolically distinct and can have different fatty acid compositions. As far as I am aware, no one has attempted to tackle the problem, which is unlikely to be solved by mass spectrometry. Analysts may have to resurrect enzymatic hydrolysis methods, which are stereo-selective but have been sadly neglected.
An interesting new paper (though I have only seen the abstract) suggests a close relationship between plasmalogen and cardiolipin biosynthesis (Kimura, T. et al. Substantial decrease in plasmalogen in the heart associated with tafazzin deficiency. Biochemistry, 57, 2162-2175 (2018); DOI)). The authors establish that plasmenylcholine, which is abundant in linoleoyl species in heart mitochondria, is a substrate for tafazzin and may be important for the remodelling of cardiolipin. This may be especially relevant to the debilitating genetic disease Barth syndrome.
April 25th, 2018
 New
biological functions for lipids and new lipids per se continue to be found,
and I have just caught up on one concerning the model nematode Caenorhabditis elegans.
This contains a novel glucosylceramide with phosphoethanolamine or its monomethylated form attached to carbon 6 of the glucose moiety
(Boland, S. et al. Phosphorylated glycosphingolipids essential for cholesterol mobilization
in Caenorhabditis elegans. Nature Chem. Biol., 13, 647-654 (2017);
DOI)
The ceramide moiety contained an iso-branched C17 sphingoid base of the phytosphinganine type
(i.e., with a 4-hydroxyl group - normally considered a plant product)
and amide-linked 2-hydroxy long-chain fatty acids with variable chain lengths (C22, C23 and C24).
This lipid is shown to be essential for the development of C. elegans through its regulation of sterol mobilization
(the organism requires an exogenous source of cholesterol).
It is able to rescue larval arrest that has been induced by sterol starvation.
New
biological functions for lipids and new lipids per se continue to be found,
and I have just caught up on one concerning the model nematode Caenorhabditis elegans.
This contains a novel glucosylceramide with phosphoethanolamine or its monomethylated form attached to carbon 6 of the glucose moiety
(Boland, S. et al. Phosphorylated glycosphingolipids essential for cholesterol mobilization
in Caenorhabditis elegans. Nature Chem. Biol., 13, 647-654 (2017);
DOI)
The ceramide moiety contained an iso-branched C17 sphingoid base of the phytosphinganine type
(i.e., with a 4-hydroxyl group - normally considered a plant product)
and amide-linked 2-hydroxy long-chain fatty acids with variable chain lengths (C22, C23 and C24).
This lipid is shown to be essential for the development of C. elegans through its regulation of sterol mobilization
(the organism requires an exogenous source of cholesterol).
It is able to rescue larval arrest that has been induced by sterol starvation.
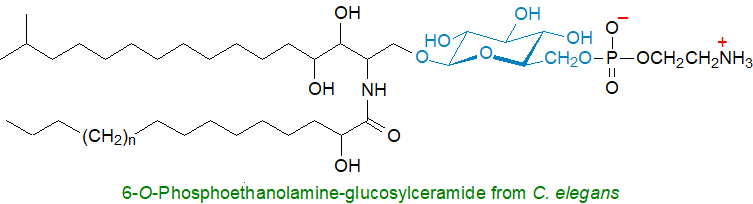
Related lipids had been reported earlier from a species of earth worm and from a marine worm, but with galactose as the carbohydrate moiety and with phosphocholine as the attachment, while phosphono analogues have been recorded from marine invertebrates (see my web page on glycosylceramides). However, the functions of these lipids have not been explored. Note that these lipids should be termed "phosphoglycosphingolipids", not "glycophosphosphingolipids", an important distinction that is explained for the glycerolipid equivalents here...
April 18th, 2018
Concerns over the nutritional effects of fatty acids with trans double bonds have created a need for methodologies that enable analysts to determine the nature and amounts of the trans isomers of polyunsaturated fatty acids that may be generated in the refining process of commercial oils. Two papers have just appeared on the topic. The first looks at geometric isomers of stearidonic acid (18:4(n-3)), which are isolated by silver ion chromatography and then subjected to structural analysis to determine the order of elution by GC (Delmonte, P. et al. Structural determination and occurrence in ahiflower oil of stearidonic acid trans fatty acids. Lipids, 53, 255-266 (2018); DOI). The second is even more daunting technically as it involves docosahexaenoic acid isomers. I don't have access to the original publication, but from the abstract the authors use a very different approach involving epoxide intermediates (Menounou, G. et al. Trans lipid library: synthesis of docosahexaenoic acid (DHA) monotrans isomers and regioisomer identification in DHA-containing supplements. Chem. Res. Toxicol., 31, 191-200 (2018); DOI).
I have seen (and cited in my literature survey pages for 2016) two publications dealing with the use of gas chromatography linked to vacuum ultraviolet spectroscopy as a means of identifying and quantifying trans-fatty acids in samples. Now a new publication describes an application of the technique to a much wider range of fatty acid types including those with branched-chains, cyclopropane rings and hydroxyl groups (Santos, I.C. et al. Analysis of bacterial FAMEs using gas chromatography-vacuum ultraviolet spectroscopy for the identification and discrimination of bacteria. Talanta, 182, 536-543 (2018); DOI). I don't see such equipment supplanting GC-MS, but it may compliment it well.
I have just read a report that the owner of the Web of Science has purchased a software company that has a web-browser extension to simplify the process of finding and legally downloading scholarly publications. This will be incorporated as a tool that offers one-click access to journal articles to which we may have legal access without having to sign into the journal or go through an Institution's account. When it is implemented, it will make my weekly literature searches much easier.
April 11th, 2018
The Journal of Experimental Biology has published a special issue (March, 2018 vol. 221 (Suppl. 1) - open access) on the theme of "The biology of fat" with guest editors Raul K. Suarez and Hans H. Hoppeler. There is an eclectic array of topics, with brown fat, adipose tissue metabolism and the metabolic syndrome well to the fore. However, there are a number of interesting papers dealing with aspects of the subject less often encountered in main-stream publications, for example adipogenesis in fish and energy metabolism in migrating birds. I am always fascinated by novel functions of lipids, so I can recommend a paper on fats in over-wintering insects. Did you know that some insects can tolerate being completely frozen thanks to the presence of triacylglycerols containing acetate that remain liquid well below 0°C, while others contain glycolipids that serve as anti-freeze agents? An older story but one well worth recalling is the observation that dolphins and toothed whales have an organ in the head with mixtures of wax esters and triacylglycerols so arranged that they serve to focus sound during echolocation and hearing.
A cliché that I dislike intensely is "thinking outside the box", so I will refrain from using it when citing a new publication that makes use of clever chemistry in a procedure for isolating sphingoid bases (Gowda, S.G.B. et al. Facile chemoselective strategy toward capturing sphingoid bases by a unique glutaraldehyde-functionalized resin. ACS Omega, 3, 753-759 (2018); DOI - open access). The authors utilize the selective but reversible reaction of glutaraldehyde with the 1,3-diol groups in sphingoid bases. By employing the functional groups bonded to a resin, they were able to develop a relatively simple procedure to concentrate a clean fraction of sphingoid bases for further analysis.
The journal Bioanalysis has devoted an issue (March, 2018) to the topic of "Bioanalytical techniques in lipidomics" (edited by D. Vuckovic). Some of the articles are open access.
Nature News reports that the EU is proposing a change in copyright law that might make life difficult for scientists in general and websites like this in particular. For example, publishers could demand a fee from any publication that quotes them in any way, including listing tabulated data. Although it is unlikely to be enforced, they could even demand a royalty for citing a paper. It would also "compel repositories of research articles to prevent uploads of copyrighted papers and other content" (this direct quotation could require a fee). Fortunately, any new law must be approved by the EU parliament and by member states before it can be enacted (here I have been careful to paraphrase the original). Not surprisingly, publishers are in favour of the proposals.
April 4th, 2018
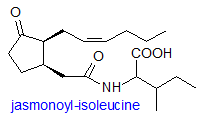 Plant oxylipins have been the subject of intensive study in recent years, and the
jasmonates are especially important as the title of a new review makes clear
(Koo, A.J. Metabolism of the plant hormone jasmonate: a sentinel for tissue damage and master regulator of stress
response. Phytochem. Rev., 17, 51-80 (2018); DOI).
(+)-7-Jasmonoyl-isoleucine appears to be the key molecule as this is the only one known to have a specific receptor,
although it is the precursor of at least 11 known metabolites many of which have biological activities in their own right.
Jasmonic acid per se is a 12-carbon cyclic fatty acid derived from α-linolenic acid and with a cyclopentanone ring
resembling that in mammalian prostaglandins (surely no coincidence) as a key structural feature.
Together with the other plant oxylipins, jasmonates are an essential part of a complex interactive network of phytohormones
that controls all aspects of plant growth and development and the manner in which plants adapt to the environment.
As an example, next time you enjoy a plate of chips (French fries) you may care to recall that the glucopyranosyl derivative of tuberonic acid,
derived from jasmonic acid after hydroxylation at C-12, induces tuber formation in potato plants through its influence
upon gibberellic acid signalling.
Jasmonates even enable plant to talk to each other, and when one is damaged by insect attack, volatile methyl jasmonate is released
to be taken up by neighboring plants to stimulate them to set their own defence mechanisms in action.
Plant oxylipins have been the subject of intensive study in recent years, and the
jasmonates are especially important as the title of a new review makes clear
(Koo, A.J. Metabolism of the plant hormone jasmonate: a sentinel for tissue damage and master regulator of stress
response. Phytochem. Rev., 17, 51-80 (2018); DOI).
(+)-7-Jasmonoyl-isoleucine appears to be the key molecule as this is the only one known to have a specific receptor,
although it is the precursor of at least 11 known metabolites many of which have biological activities in their own right.
Jasmonic acid per se is a 12-carbon cyclic fatty acid derived from α-linolenic acid and with a cyclopentanone ring
resembling that in mammalian prostaglandins (surely no coincidence) as a key structural feature.
Together with the other plant oxylipins, jasmonates are an essential part of a complex interactive network of phytohormones
that controls all aspects of plant growth and development and the manner in which plants adapt to the environment.
As an example, next time you enjoy a plate of chips (French fries) you may care to recall that the glucopyranosyl derivative of tuberonic acid,
derived from jasmonic acid after hydroxylation at C-12, induces tuber formation in potato plants through its influence
upon gibberellic acid signalling.
Jasmonates even enable plant to talk to each other, and when one is damaged by insect attack, volatile methyl jasmonate is released
to be taken up by neighboring plants to stimulate them to set their own defence mechanisms in action.
Seed oil triacylglycerols have been the subject of intensive study because of their importance in commerce. Their biological role to supply energy and structural components to the developing plant embryo has not been neglected, and it has generally been assumed that the small concentrations of triacylglycerols in lipid droplets in vegetative tissues serve a similar purpose. However, a new review suggests that the latter may have many more dynamic functions (Yang, Y. and Benning, C. Functions of triacylglycerols during plant development and stress. Curr. Opinion Biotechn., 49, 191-198 (2018); DOI). The authors discuss how triacylglycerol metabolism is involved in cell division and expansion, stomatal opening, and membrane lipid remodeling, while in reproductive tissues, they are important for organ formation and successful pollination.
In most plants and algae under phosphate deprivation, phosphatidylcholine in membranes is exchanged for digalactosyldiacylglycerols and/or betaine lipids. However, in a model marine diatom, it is replaced in part by a diglycosylceramide, suggesting that sphingolipids may be more important in these organisms than has been believed hitherto (Hunter, J.E. et al. Lipidomics of Thalassiosira pseudonana under phosphorus stress reveal underlying phospholipid substitution dynamics and novel diglycosylceramide substitutes. Appl. Environm. Microbiol., 84, UNSP e02034-17 (2018); DOI).
Staying on a botanical theme, several correspondents have admired the Scottish thistles that adorn these web pages. My intention is merely to provide something appropriate, decorative and not too intrusive to brighten large areas of text and not simply to illuminate my origins. In fact, it does not always do to cast light on your ancestry as a family legend has it that about six generations back, an ancestor of mine was hung for piracy in Cornwall. We are quite proud of having a pirate in the family, but it is worrisome that he may have been an Englishman.
March 28th, 2018
 Early
in my career, an ozone generator was essential equipment in all major chemistry laboratories.
They were bulky and expensive pieces of kit that had to reside in fume hood for safety reasons - partly because of the toxic nature
of ozone and partly because there was a risk of explosions.
The main application in lipid chemistry was for oxidation of double bonds as a means of determining their positions in fatty acyl chains.
This seems to be still an important technique, except that it is now applied to intact lipids prior to analysis by mass spectrometry.
A new publication shows that rather than using a purpose-built generator for the purpose,
it is possible to carry out the reaction simply using a UV lamp and dissolved oxygen (Stinson, C.A. et al.
UV lamp as a facile ozone source for structural analysis of unsaturated lipids via electrospray ionization-mass
spectrometry. J. Am. Soc. Mass Spectrom., 29, 481-489 (2018);
DOI).
I enjoy seeing clever thinking of this kind, although I have never really been convinced of the need for this and related methodologies
other than when sample size is truly limiting.
If this is not the case, it is much simpler and much better resolution is possible when the structures of fatty acids are determined separately
by GC-MS using the methods described in my mass spectrometry pages.
Early
in my career, an ozone generator was essential equipment in all major chemistry laboratories.
They were bulky and expensive pieces of kit that had to reside in fume hood for safety reasons - partly because of the toxic nature
of ozone and partly because there was a risk of explosions.
The main application in lipid chemistry was for oxidation of double bonds as a means of determining their positions in fatty acyl chains.
This seems to be still an important technique, except that it is now applied to intact lipids prior to analysis by mass spectrometry.
A new publication shows that rather than using a purpose-built generator for the purpose,
it is possible to carry out the reaction simply using a UV lamp and dissolved oxygen (Stinson, C.A. et al.
UV lamp as a facile ozone source for structural analysis of unsaturated lipids via electrospray ionization-mass
spectrometry. J. Am. Soc. Mass Spectrom., 29, 481-489 (2018);
DOI).
I enjoy seeing clever thinking of this kind, although I have never really been convinced of the need for this and related methodologies
other than when sample size is truly limiting.
If this is not the case, it is much simpler and much better resolution is possible when the structures of fatty acids are determined separately
by GC-MS using the methods described in my mass spectrometry pages.
Perhaps it is my Scottish Calvinistic heritage, but readers of this blog may have noticed that I enjoy freebies in the form of open access publications. The latest to reach my computer deals with ether lipids (Dean, J.M. and Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell, 9, 196-206 (2018); DOI). In addition to its virtue of being open access, this is a useful and readable account of the subject. Incidentally, I now know that the term "plasmalogens” comes from the discovery back in 1924 that acid staining of tissues released aldehydes in the cytoplasm, although the origin of these was not then known. The same journal issue contains a further open access article dealing with carboxylesterases in lipid metabolism.
March 21st, 2018
In recent years, any number of review publications have appeared on the subject of lipidomics, many dealing with technical aspects, others with applications to mammalian lipids and then often to various disease states. I have cited most of these in my literature survey pages on this website, and I have read them with great interest. However, a new review has caught my eye that is somewhat different from the others (Řezanka, T. et al. Lipidomic analysis: from archaea to mammals. Lipids, 53, 5-25 (2018); DOI). Only one page is devoted to animal lipidomics and the rest to the many fascinating lipids that have been found in archaebacteria, bacteria, yeast, fungi, algae and plants thanks to the new technology. Incidentally, I note that the authors cite my web page on lipid definitions and nomenclature, which you can read here.., as well as my more extensive discussion of what constitutes a lipid - A Lipid Primer. These express my personal opinions, which some may find idiosyncratic.
I am grateful to a friend who made available to me a new review on protein-lipid modifications (Jiang, H. et al. Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem. Rev., 118, 43-112 (2018); DOI). I can't imagine a more substantial review on the subject and it has been a great help in updating my web page on proteolipids. Returning to the subject of lipid nomenclature, I suggest in this web page that that the term 'proteolipid', which was used in the first paper on the topic in 1971, should be used for those molecules in which the two components are linked covalently, while the term 'lipoprotein' should be reserved for the relatively loose protein-lipid complexes of plasma. Unfortunately, the latter term is also used and almost universally for the covalent lipid-protein complexes present in bacteria.
March 14th, 2018
Following on from my discussion of 16:1 isomers in my last blog, a new review of the biological effects of palmitoleic acid (9-16:1), sometimes termed a lipokine, has been published (de Souza, C.O. et al. Is palmitoleic acid a plausible nonpharmacological strategy to prevent or control chronic metabolic and inflammatory disorders? Mol. Nutr. Food Res., 62, 1700504 (2018); DOI). After a thorough review of the evidence, the answer to the question posed in the title seems to be that we do not yet know, although the results with animal studies are promising, and that further human-based research is required. This publication and those for the next two topics are open access.
I also mentioned the essential fatty acids last week, and a new review discusses the evolutionary significance of the biosynthesis of linoleate in primitive invertebrates, including some species of insects, nematodes and pulmonates (air-breathing slugs and snails) (Malcicka, M. et al. An evolutionary perspective on linoleic acid synthesis in animals. Evol. Biol., 45, 15-26 (2018); DOI). It appears that there is no consistent lineage in the occurrence of the ability to produce linoleate, not even within any given family, and that this ability was lost and repeatedly gained during the evolution of distinct invertebrate groups. One key factor may have been the development of bifunctionality in desaturase enzymes (Δ12/Δ15). Much of the information was new to me, and my first thought was for endosymbiont-mediated linoleate synthesis, but it seems that no examples of this have yet been discovered. Of course, much of the discussion is of necessity speculative, but the paper is certainly thought-provoking.
I suppose that few of us have given much thought to the function of butyric acid in animal metabolism. From my years in an animal research institute, I was well aware of its importance in the metabolism of ruminant animals, and of course it is an important component of the triacylglycerols of cow's milk, where it is located specifically in position sn-3. However, it is also produced in significant amounts by microbial fermentation of dietary fibers in the lower intestinal tract of all animals, and a new review describes its many functions within the tissues of the host animal (Liu, H. et al. Butyrate: a double-edged sword for health? Adv. Nutr., 9, 21-29 (2018); DOI).
While I am on the subject of bioactive fatty acids, the first report of the occurrence of resolvins in a non-mammalian tissue (marine diatoms) has just appeared (Rettner, J. et al. Survey of the C20 and C22 oxylipin family in marine diatoms. Tetrahedron Letts., 59, 828-831 (2018); DOI).
March 7th, 2018
The essential nature of linoleic acid in the diet was first reported in 1929 by George and Mildred Burr, although it was many years before this was recognized by the scientific community at large. It was much later in the last century before many other vital functions of specific fatty acids were recognized, for example palmitic and myristic acids as covalent conjugates with proteins to target them to membranes or octanoic acid that is required for grehlin activation. Three 16:1 isomers are known in human tissues with double bonds in the 6, 7 and 9 positions. Of these, 9-16:1 or palmitoleate is best known and has been termed a 'lipokine', i.e., it is an adipose tissue-derived hormone, which amongst other effects stimulates the action of insulin in muscle; it is linked very specifically to a conserved serine residue in the Wtn family of proteins (O-acylated proteolipids) involved in adipose tissue development, and it is essential for their function. 7-16:1 has some biological functions in common with 9-16:1, and it is regarded as an anti-inflammatory molecule, while 6-16:1 or sapienic acid is found mainly in human skin and has biocidal properties. Now all three isomers have been detected in macrophages both from mice and humans (Astudillo, A.M. et al. Occurrence and biological activity of palmitoleic acid isomers in phagocytic cells. J. Lipid Res., 59, 237-249 (2018); DOI)). However, in contrast to the other two isomers, the levels of 6-16:1 were not regulated by the activation state of the cell, and it appears that we do not yet know its function in these cells. Incidentally, it seems that the trivial name 'sapienic' from Homo sapiens is now a misnomer as it is now shown to be present in mice - brings to mind the John Steinbeck novel - "Of Mice and Men" (I may be showing my age again).
I can recommend an authoritative review of sphingolipid biosynthesis and function that has just been published (Hannun, Y.A. and Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nature Rev. Mol. Cell Biol., 19, 175-191 (2018); DOI).
February 28th, 2018
 When I do
my weekly literature search, I have to scan lists of around 400 references to select those that are useful to me or should appear
in my literature survey section, and then transfer them to my data base in an appropriate format.
I can only spare about 20 minutes for the selection phase and I have to rely on the clarity of the titles,
so inevitably I miss some important papers; also, the search algorithm I use is less than perfect.
I am therefore grateful when correspondents point out items that I have missed.
For example, two publications from 2016 dealing with novel methodology for the determination of trans fatty acids
involving gas chromatography-vacuum ultraviolet spectroscopy have just been drawn to my attention,
and they are listed in my current monthly analysis list (and will appear subsequently in that for 2016).
When I do
my weekly literature search, I have to scan lists of around 400 references to select those that are useful to me or should appear
in my literature survey section, and then transfer them to my data base in an appropriate format.
I can only spare about 20 minutes for the selection phase and I have to rely on the clarity of the titles,
so inevitably I miss some important papers; also, the search algorithm I use is less than perfect.
I am therefore grateful when correspondents point out items that I have missed.
For example, two publications from 2016 dealing with novel methodology for the determination of trans fatty acids
involving gas chromatography-vacuum ultraviolet spectroscopy have just been drawn to my attention,
and they are listed in my current monthly analysis list (and will appear subsequently in that for 2016).
The Lipid Maps "Lipid Of the month" for February (see their home page) was another novelty for me, i.e., the elovanoids - C32 and C34 analogues of the protectins, which have been found in retinal cells. I presumably missed these because my weekly search did not contain suitable key words. Again, I have gone back to the Web of Science, to recover the relevant references which are now in my new monthly list for "Lipid essentials". What intrigued me especially, when I read the original papers was the fact that the protectins per se are derived from DHA in position sn-2 of phospholipids, while the precursors of the elovanoids are in position sn-1. This implies that the enzymes involved in the release of the two classes of fatty acid substrates are very different and so must be the stimulatory and regulatory mechanisms. There is a comparable difference for eicosanoid and anandamide biosynthesis; the arachidonic acid for the former comes from position sn-2 of phospholipids, while that for the latter comes from position sn-1.
This brings me back to an old theme of mine that in the world of lipidomics positional distributions are not given sufficient weight in comparison to molecular species analyses. I am sure that positional distributions obtained by mass spectrometry offer nothing near the precision of the older methods using selective lipases, but I would like to see an experimental comparison.
February 21st, 2018
As I have mentioned from time to time in this blog, I am an armchair analyst these days so I am reluctant to endorse what seems novel methodology. I do read and I can comment, however, and others can correct me if they wish. For example, a major problem with HPLC of all polar lipids has always been adsorption on columns. It is usually necessary to add ionic species to the mobile phase to get sharp peaks, and these can cause detection problems or degrade stationary phases. I never attempted to analyse CoA esters in my research days, but I am aware of some of the technical difficulties and I found a new publication had some interesting ideas to eliminate adsorption effects (Abranko et al. Comprehensive quantitative analysis of fatty-acyl-Coenzyme A species in biological samples by ultra-high performance liquid chromatography-tandem mass spectrometry harmonizing hydrophilic interaction and reversed phase chromatography. J. Chromatogr. A, 1534, 111-122 (2018); DOI); the paper is open access. The authors tested various ammonium salts in the mobile phase and found that ammonium bicarbonate (10 mM adjusted to pH 8.5) was much the best over the whole range of chain-lengths, aided appreciably by the incorporation of a 0.1% phosphoric acid wash step between injections. Of course, the nature of the stationary phase is also important, and I have complained in the blog from time to time of the use of the term 'HILIC chromatography', which tells us nothing about the mode of interaction with analytes, adsorption, ion-exchange, etc. In this work, a Waters BEH HILIC column was used in part; it took me an age to find what 'BEH' implied, although I am little further forward in my understanding.
An interesting article by a young scientist was published in the Guardian newspaper last week complaining about the inappropriate use of metrics to evaluate scientific research. During much of my career, the pressures were different and I did not have to pay too much attention to this aspect of publication; my usual philosophy in selecting a particular journal for a new publication was whether it would reach the intended audience. The important objective was to see that any new information I had uncovered was disseminated - not how. It is a different world now and I recognize that young scientists starting on their careers face real difficulties. Many of the best papers I read have multiple authors (often into double figures) because multiple technologies and expertise may be required to provide answers. At the start of their careers, scientists working on their own can only hope to make incremental progress in their chosen fields, and I would hope that this would be recognized by scientific administrators. In my later years of research, my pet hate was projected 'milestones' - if I knew where the results were going to lead 2-3 years ahead, the work was not worth doing.
What really worries me now having just reviewed the above comments, is that I am beginning to sound like my father (no disrespect intended) in his later years - "when I was a boy, etc., etc.!" It is bad enough that I now look like he did. I will have to try to resurrect the youthful inner me.
February 14th, 2018
In plants,glycerolipid biosynthesis in chloroplasts has long been termed the "prokaryotic pathway", and it produces galactosyldiacylglycerols and phosphatidylglycerol with C18 acids in position sn-1 and C16 acids in position sn-2. This pattern is seen in cyanobacteria and it has been assumed that the similarity was a consequence of endosymbiosis during evolution. A new study demonstrates that this is not true (Sato, N. and Awai, K. "Prokaryotic Pathway" is not prokaryotic: noncyanobacterial origin of the chloroplast lipid biosynthetic pathway revealed by comprehensive phylogenomic analysis. Genome Biol. Evolution, 9, 3162-3178 (2017); DOI); the paper is open access. It is now evident that the two steps of acylation in cyanobacteria and chloroplasts utilize enzymes that have no phylogenetic relationship. The structural differences in the diacylglycerol moieties of galactolipids from various species of algae and higher plants originate in fact in compartmentalization of the biosynthetic pathways or precursors in cells, especially between the chloroplasts and endoplasmic reticulum, each compartment having its own distinctive enzymes with characteristic specificities (see my web page on galactosyldiacylglycerols for further discussion).
My prize for the most unusual new lipid that I have encountered this year so far goes to a novel arsenolipid. The unicellular marine alga Dunaliella tertiolecta contains phytyl 5‑dimethylarsinoyl-2-O-methyl-ribofuranoside as 35 to 65% of the total arsenolipids. Apart from its arsenic content, this lipid is unique in containing both an ether-linked phytyl group and a 2‑O‑methylriboside of a type normally found only in RNA. (Glabonjat, R.A. et al. A 2-O-methylriboside unknown outside the RNA world contains arsenic. Angew. Chem.-Int. Ed., 56, 11963–11965 (2017); DOI.
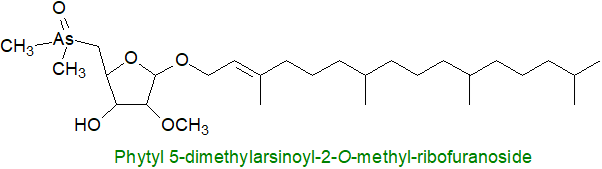
There is an interesting article in Nature on the importance of scientific blogs. Regretfully, they missed this one!
February 7th, 2018
In my blog of two weeks ago, I discussed the therapeutic potential of nitro fatty acids and in particular how subcutaneous injection of nitro-oleic acid suppressed allergic contact dermatitis in mice. Alas, a new publication from the same research group reports that topical applications have the opposite effect (Mathers, A.R. et al. Topical electrophilic nitro-fatty acids potentiate cutaneous inflammation. Free Rad. Biol. Med., 115, 31-42 (2018); DOI). The problem appears to lie in the finding that nitro-conjugated linoleate is formed naturally "in the skin microenvironment as products of cutaneous inflammatory responses and, in high local concentrations, may exacerbate inflammatory skin diseases".
I couldn't have put better the philosophy implied in the title to an editorial contribution to a Nature journal (Marx, V. Fats add structure, they signal, they interact. In the lab, lipids are tough to work with but worth the challenge. Nature Methods, 15, 35-38 (2018); DOI).
I always enjoy reading the personal reminiscences of lipid scientists and that by Dennis E. Vance was no exception, and it has a title that recalls my previous paragraph (From masochistic enzymology to mechanistic physiology and disease. J. Biol. Chem., 292, 17169-17177 (2017); DOI). However, it also served to remind me that a new edition of one of the most valued books in my personal library was now available, but with new editors (Ridgway, N.D. and McLeod, R.S. Biochemistry of Lipids, Lipoproteins and Membranes, 6th Edition. (Elsevier, Amsterdam) (2016) - see Science Direct) - and prompted recourse to Amazon. It covers much the same range of topics as in previous editions, but often with replacement authors, and I expect to make good use of it. As expected with modern textbooks it is very well produced from a technical standpoint. Comparison with the book by Gurr et al. (Lipids: Biochemistry, Biotechnology and Health, 6th Edition), which I reviewed some months ago is probably unfair, as they probably aim at different audiences. My superficial first impression is that the new book goes into the subjects in greater depth but the coverage is not so exhaustive. For example, it was of great help to me in updating my web page on proteolipids, but there is no mention of "endocannabinoids" or "lipid sulfates" in the index.
January 31st, 2018
 It
has been a puzzle to me how the endocannabinoids, anandamide and 2-arachidonoylglycerol,
can have so many different functions in a given tissue while operating through a single G protein receptor.
A new review illustrates how this happens using brain as the model
(Busquets-Garcia, A. et al. CB1 receptor signaling in the brain:
extracting specificity from ubiquity. Neuropsychopharmacology, 43, 4-20 (2018);
DOI).
It seems that endocannabinoid receptors exert their wide variety of different cellular effects by specific interactions
with many other G proteins and that this is dependent on such factors as cell type, subcellular location and cellular functional state.
Within a given tissue, expression of a receptor can vary between different locations.
For example, in brain some types of neuron contain very high levels of CB1 receptor protein, whereas others have much lower levels;
there are even lower levels in some regions of the hypothalamus and astroglial cells.
In turn, variation in the location of CB1-interacting proteins may have a role in the cell-specific modulation
of endocannabinoid signalling.
This paper is part of a special issues on "Cannabinoids and endocannabinoids" and the only one to be open access.
However, most of the other contributions appear to be more suitable for specialists.
It
has been a puzzle to me how the endocannabinoids, anandamide and 2-arachidonoylglycerol,
can have so many different functions in a given tissue while operating through a single G protein receptor.
A new review illustrates how this happens using brain as the model
(Busquets-Garcia, A. et al. CB1 receptor signaling in the brain:
extracting specificity from ubiquity. Neuropsychopharmacology, 43, 4-20 (2018);
DOI).
It seems that endocannabinoid receptors exert their wide variety of different cellular effects by specific interactions
with many other G proteins and that this is dependent on such factors as cell type, subcellular location and cellular functional state.
Within a given tissue, expression of a receptor can vary between different locations.
For example, in brain some types of neuron contain very high levels of CB1 receptor protein, whereas others have much lower levels;
there are even lower levels in some regions of the hypothalamus and astroglial cells.
In turn, variation in the location of CB1-interacting proteins may have a role in the cell-specific modulation
of endocannabinoid signalling.
This paper is part of a special issues on "Cannabinoids and endocannabinoids" and the only one to be open access.
However, most of the other contributions appear to be more suitable for specialists.
The number of different molecular species of lipids in a given cell is astonishing (at least 1,000). Yet it is surprising how often only a single molecular species of a given lipid is required for optimum activity in a specific function. Cardiolipin in heart muscle is a prime example. If it had say 10 different fatty acid components, as is usual in most glycerolipids, together with the four different positions for acylation, there could in theory be 104 different molecular species. Instead, as is well known, linoleic acid is by far the most abundant fatty acid (as much as 80%) and the tetralinoleoyl species amounts to at least half the total. Many have speculated as to why this should be so, but a new publication demonstrates that if linoleate is displaced by docosahexaenoate in mitochondrial cardiolipin in the rat then the enzyme activities of the respiratory complexes are greatly reduced, apparently by preventing the formation of phospholipid domains that regulate enzyme activity (Sullivan, E.M. et al. Docosahexaenoic acid lowers cardiac mitochondrial enzyme activity by replacing linoleic acid in the phospholipidome. J. Biol. Chem., 293, 466-483 (2013); DOI). I wonder what would be the result if they tried to displace linoleate with palmitate as this is the most abundant fatty acid in testis cardiolipin and has a much less mobile conformation than DHA.
January 24th, 2018
Nitro fatty acids are fascinating 21st Century molecules with important anti-inflammatory properties (they were first recognized as natural lipid components in 1999). In tissues, they occur in the free form, bound reversibly to thiol-containing proteins and glutathione, and as esters in triacylglycerols and phospholipids. In human serum in addition to non-covalent binding with albumin, nitro-conjugated linoleic acid has been shown to form covalent adducts at Cys-34 (Michael reaction), suggesting that this may be a means of systemic distribution. The effective concentrations of nitro fatty acids in tissues are reduced by this means, but a mechanism for the reversal of the reaction has been revealed under conditions of oxidative stress in vitro in plants at least. Reactive oxygen and nitrogen species, as represented by hydrogen peroxide and peroxynitrite, respectively, have the ability to oxidize cysteine-adducted nitro fatty acids with the release of free nitroalkenes, which can presumably then exert their anti-inflammatory effects (Padilla, M.N. et al. In vitro nitro-fatty acid release from Cys-NO2-fatty acid adducts under nitro-oxidative conditions. Nitric Oxide, Biol. Chem., 68, 14-22 (2017); DOI). Incidentally, nitro fatty acids are present in olives and virgin olive oil at concentrations that may be significant biologically, and it has been argued that they could be one reason for the beneficial effects of the Mediterranean diet. Their clinical potential is under active investigation, for example to inhibit cutaneous inflammation (Mathers, A.R. et al. Electrophilic nitro-fatty acids suppress allergic contact dermatitis in mice. Allergy, 72, 656-664 (2017); DOI).
The concept of lipid rafts in membranes, i.e., laterally segregated domains usually enriched in sphingolipids and cholesterol that provide platforms for signalling proteins, is now well established in the scientific literature. The terms termed 'membrane rafts', 'nanodomains' and 'microdomains' tend to be used interchangeably, but a new review suggests that in plants at least, different types of domain exist with differing compositions and functions so more precise definitions are required (Ott, T. Membrane nanodomains and microdomains in plant-microbe interactions. Curr. Opinion Plant. Biol., 40, 82-88 (2017); DOI).
Two substantial review articles deal with the methodology of lipidomics (Rustam, Y.H. and Reid, G.E. Analytical challenges and recent advances in mass spectrometry based lipidomics. Anal. Chem., 90, 374-397 (2018); DOI. And - Hu, T. and Zhang, J.L. Mass-spectrometry-based lipidomics. J. Sep. Sci., 41, 351-372 (2018); DOI). The second of these is open access.
January 17th, 2018
In recent years, I have written to several journals, most recently two months ago, to point out major errors in the interpretation of mass spectra in published papers. On most occasions, the journal has replied promptly and has promised to publish a correction - I still have to see one. Usually, the problem lies in computerized identification of methyl esters of fatty acids, as computers don't recognize that they cannot distinguish between positional and geometrical isomers of monoenoic or dienoic fatty acids, but often the authors (and reviewers) have simply not done their homework. One example, was a report of iso-methyl branched fatty acids in higher plants on the basis of a large ion equivalent to m/z = M-43; it has been known since the 1950s that this is common to mass spectra of all straight-chain fatty acids as a result of a complex rearrangement involving expulsion of carbons 2 to 4.
Problems also arise with interpretation of mass spectra of dimethyloxazoline derivatives of fatty acids. The first authors described a simple rule for how to locate double bonds. However, lacking appropriate standards, they were not aware that the rule did not apply when the double bonds were close to either end of the molecule. In addition, 3-isomers isomerize to 2-isomers on derivatization. The answer is to compare with authentic spectra, such as those on this website. Unfortunately, in spite of my best efforts, there are many flawed publications out there.
The ISI Web of Science seems to be just catching up after the holiday, and my weekly literature seach has given me three special review volumes to digest. I will list selected individual papers in my next literature update, but many of you will wish to consult the original journals for the full list. The December issue of Current Opinion in Plant Biology (Volume 40, Pages 1-168 (2017)) is devoted to the topic of "Cell biology: Membrane dynamics - being at the right place at the right time" (edited by Eugenia Russinova and Karin Schumacher) and includes a number of reviews of interest to plant lipid biochemists. The open access journal Antioxidants (Volume 6, issue 4 (2017)) contains three review articles dealing with tocopherols, including their biosynthesis in plants and their metabolism in humans. The journal Prostaglandins & Other Lipid Mediators (Volume 133, November (2017)) is a special issue from the "6th European Workshop on Lipid Mediators" (edited by Bannenberg, G. et al.) with a number of review articles on polyunsaturated fatty acids and oxylipins (eicosanoids and docosanoids).
January 10th, 2018
Although the most important aspect of any new publication is its content, the manner of presentation can make a big difference to how well the message gets across. Text books have lead the way in this regard, but the ability to use colour in diagrams now afforded by many journals has been a considerable step forward, and as more journals go online only I am sure that this facility will be used increasingly. I am also rather envious of those authors who have access to design departments who produce figures and diagrams that are works of art. One new article that meets all these quality criteria deals with phosphoinositides (Choy, C.H. et al. Phosphoinositide diversity, distribution, and effector function: stepping out of the box. Bioessays, 39, 1700121 (2017); DOI). I found this review to be of considerable value in updating my web page here on the topic.
Some years ago, I was rather pleased with myself when my cholesterol level was measured and found to be in the bottom quartile for my age group, but a friend brought me down to earth by telling me that all this meant was that rather than having a heart attack I would probably die of cancer. The editors' selection (and therefore open access) in the latest issue of JBC explains how two isoforms of phospholipase A regulate the nature of the eicosanoids produced during a heart attack and thence the damage done. In non-failing human hearts, one isoform channels arachidonic acid into protective epoxyeicosatrienoic acids (EETs), whereas in failing hearts, activation of a second isoform channels arachidonic acid into toxic hydroxyeicosatetraenoic acids (HETEs) (Moon, S.H. et al. Heart failure-induced activation of phospholipase iPLA2γ generates hydroxyeicosatetraenoic acids opening the mitochondrial permeability transition pore. J. Biol. Chem., 293, 115-129 (2018); DOI). One way or another your lipids probably get you in the end!
January 3rd, 2018
As a New Year gift to lipid analysts, I draw your attention to a 72 page open access publication reviewing NMR spectroscopy (1H, 13C and 31P) of lipids (Alexandri, E. et al. High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules, 22, 1663 (2017); DOI). It is a large file at 34Mb, so you may need a fast broadband connection.
The lipid A (endotoxin) component of bacterial lipopolysaccharides is a fascinating complex molecule that serves to protect the organism from attack from external agencies, including antibiotics, but is a major reason for the virulence of pathogenic bacteria. Many factors are involved, including the number and nature of the fatty acid constituents, but the general mechanism of the immune response is usually considered to be a binding to a large hydrophobic pocket in a receptor such as toll-like receptor 4 (TLR4) via the lipid chains, while the phosphate groups can interact directly with the receptor leading to formation of a heterodimer complex that is active in immune signalling. However, a new publication demonstrates that the TLR4 receptor does not recognize the endotoxin of a rather nasty pathogen, Francisella novicida, which is thus able to evade the host innate immune system. Instead, this stimulates the cyclooxygenase-2-dependent inflammatory pathway and is responsible for the lethality of such infections through overproduction of proinflammatory effectors such as prostaglandin E2 (Scott, A.J. et al. Host-based lipid inflammation drives pathogenesis in Francisella infection. PNAS, 114, 12596-12601 (2017); DOI).
The regulation of cholesterol levels in animal tissues is a complicated topic involving innumerable factors, and I struggle to come to grips with it. One novel feature that has just come to light is that the first 100 amino acid in a key enzyme in cholesterol biosynthesis, i.e., squalene monooxygenase, is a proteasomal degradation signal or 'degron'. This sequence attaches reversibly to the ER membrane, and in the presence of excessive cholesterol levels, it is ejected and unravels to expose a hydrophobic patch, which then says "eat me" (Chua, N.K. et al. A conserved degron containing an amphipathic helix regulates the cholesterol-mediated turnover of human squalene monooxygenase, a rate-limiting enzyme in cholesterol synthesis. J. Biol. Chem., 292, 19959-19973 (2017); DOI).
Blogs for the previous year (2017) can be located here..
| © Bill Christie |  |
|
| Updated: January 11th, 2023 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
