Arsenolipids
A wide range of organic compounds that contain atoms of arsenic have been found in fish and other marine organisms, and they include lipids, although these are usually present at low levels. With improvements in analytical methodology, more of these arsenolipids are being isolated, characterized and quantified. Methylation of inorganic arsenic may be a de-toxification method in some organisms that yields products that can be incorporated into organic compounds including lipids with fish at the end of the marine food chain, but the biosynthetic mechanisms for arsenolipids have not been determined. While most research has been concerned with marine organisms, arsenolipids can be produced in freshwater algae (e.g. Chlamydomonas reinhardtii) grown in a medium containing arsenate. While the potential toxicity of arsenolipids to human consumers of fish products requires further evaluation and monitoring, there appears to be little cause for concern at present.
Some authors take a rather wide view of what constitutes an arsenolipid and thus define compounds such as trimethylarsine and its metabolites as lipids, simply based on their solubility in organic solvents. I do not consider this an appropriate definition (see my web page on nomenclature), and only those lipids, both simple and complex, which have been characterized sufficiently to be certain that they contain long aliphatic chains and arsenic atoms somewhere in the molecule are considered here.
1. Hydrocarbons and Fatty Acids containing Arsenic Atoms
Long-chain hydrocarbons with a terminal dimethylarsinoyl moiety were first isolated and characterized from capelin, Mallotus villosus, as recently as 2008, and they have since been detected in other fish species and in brown algae (seaweeds). The main isomer is 1‑dimethylarsinoylpentadecane, found together with C17 and C19 analogues as well as one thought to be 1-dimethylarsinoyl all-cis-4,7,10,13,16,19-docosahexaene, if a biosynthetic relationship to docosahexaenoic acid is assumed. In one organism, traces of even-numbered forms were found, and further molecules with one to five double bonds have now been identified. Indeed, an arsenohydrocarbon with seven double bonds has been detected in oils from cod and blue whiting (C35H56AsO). Other molecular forms include some with multiple double bonds in conjugation.

2. Fatty Acids (Free and Esterified) and Alcohols containing Arsenic Atoms
A series of saturated fatty acids with a terminal dimethylarsinoyl moiety and with 15 to 19 carbon atoms was detected initially in cod-liver oil, i.e., the dimethylarsinoyl group replaces the terminal methyl group of conventional even-numbered fatty acids. In addition, unsaturated fatty acids, which are related structurally to oleic, docosapentaenoic and docosahexaenoic fatty acids, were detected, although only one fatty acid with an even-number of carbon atoms was found, i.e., C24H38AsO3. From further studies, an even wider range of such fatty acids are now known including some with hydroxyl substituents, and more will no doubt be reported in future. The two fatty acids illustrated below are presumably related biosynthetically to hexadecanoic and docosapentaenoic acids. All fish oils examined to date contain a comparable range of hydrocarbons and fatty acids of this type, with the proportions varying somewhat among species and at overall concentrations of 4.3 to 10.5 mg per kg.

In many fish and plants, arsinoyl fatty acids are linked to the complex lipids, mainly phosphatidylcholine and lysophosphatidylcholine, and for example, in herring roe, mass spectrometry established that highly unsaturated fatty acids of this type are esterified in phosphatidylcholine, while in salmon roe, an analogous C30 fatty acid with eight double bonds was identified in phosphatidylethanolamine. Evidence has been obtained for their presence in triacylglycerols from blue whiting, and many different lipid classes, and in the green alga Coccomyxa (Trebouxiophyceae, Chlorophyta) cultivated in a medium containing arsenic, various phospholipids (phosphatidylethanolamine, phosphatidylinositol, phosphatidylglycerol and possibly ceramides), have esterified arseno-fatty acids.
One further type of arsenolipid with a long alkyl chain to have been discovered consists of cationic trimethylarseno-fatty alcohols of which two homologues have so far been detected in fish oils. Other molecular forms have several hydroxyl groups.

The uptake of arsenate in algae probaby occurs by the transport processes intended for the essential nutrient phosphate. Although the mechanism for the biosynthesis of arsenolipids is a matter for speculation at present, one suggestion is that dimethylarsinoylpropionic acid might be the primer molecule for a fatty acid synthase. While it is not known where these compounds arise in the marine food-chain, brown algae are one potential source.
3. Glycerolipids containing Arsenic Atoms in the Polar Head Group
 The presence of arsenic-containing complex lipids has been inferred from degradative studies
of marine lipids in which arsenical compounds have been isolated from hydrolysates.
These include analogues of phosphatidylcholine and sphingomyelin, which are present at such low levels that it has not been possible to isolate
them in pure form for definitive characterization.
In the first studies, based simply on the isolation and identification of glycerophosphorylarsenocholine after alkaline hydrolysis
of lipid extracts, phosphatidylarsenocholine was identified as a minor component of the lipids of mullet, lobster
and other marine animals.
Mass spectrometry, both as inductively coupled plasma (ICPMS) and liquid chromatography coupled by an electrospray source,
has now enabled greatly improved analysis in marine foods, including tentative identification of a phosphatidylethanolamine analogue.
The presence of arsenic-containing complex lipids has been inferred from degradative studies
of marine lipids in which arsenical compounds have been isolated from hydrolysates.
These include analogues of phosphatidylcholine and sphingomyelin, which are present at such low levels that it has not been possible to isolate
them in pure form for definitive characterization.
In the first studies, based simply on the isolation and identification of glycerophosphorylarsenocholine after alkaline hydrolysis
of lipid extracts, phosphatidylarsenocholine was identified as a minor component of the lipids of mullet, lobster
and other marine animals.
Mass spectrometry, both as inductively coupled plasma (ICPMS) and liquid chromatography coupled by an electrospray source,
has now enabled greatly improved analysis in marine foods, including tentative identification of a phosphatidylethanolamine analogue.
From studies of lipid hydrolysates, it has been known for some time that a complex arsenic-containing glycophospholipid is present in fish and other marine organisms, i.e., diacylglycerophospho-2-hydroxypropyl-5-deoxy-5-(dimethylarsinoyl)-β-ribofuranoside, and this has now been characterized definitively by modern mass spectrometric methods from several multicellular brown and red algae (seaweeds), some of which are harvested for human consumption.

In the brown macroalga Saccharina latissima and related organisms, the concentrations of the lipid vary in different parts of the organism. The main fatty acid components are saturated (C15 to C20) with 16:0 predominating and small amounts of unsaturated fatty acids (16:1 to 18:3); much of the palmitic acid is in position sn-2 of the glycerol moiety. The more conventional phospholipids in the organism have very different fatty acid compositions. In dulse (red alga) samples, the main molecular species was found to be 18:1/16:1. A mono-acyl (lyso) form of the lipid has been found in the brown alga Saccharina japonica.
The freshwater cyanobacterium Synechocystis sp. PCC 6803 contains this lipid, and it requires exogenous trimethylarsine for biosynthesis of the presumed arsenosugar precursor. In contrast, Nostoc sp. PCC 7120, a typical filamentous cyanobacterium ubiquitous in aquatic systems, is able both to methylate inorganic arsenic and to produce arsenosugars and thence the arsenosugar phospholipid with C17 saturated and unsaturated fatty acid components; it does not produce arsenohydrocarbons. The unicellular alga Dunaliella tertiolecta methylates arsenate to produce dimethylarsinate and thence the arsenosugar and the complex arsenolipid. While this has been detected in the developing brain of pilot whales together with arseno-hydrocarbons, presumably after crossing the blood-brain barrier, it is presumed to come from their diet of squid.
4. Other Arsenolipids
The unicellular marine alga D. tertiolecta contains phytyl 5-dimethylarsinoyl-2-O‑methyl-ribofuranoside as 35 to 65% of the total arsenolipids together with an arsenic-containing hydrocarbon. As well as the arsinoyl moiety, the lipid is unique in containing both a phytyl group and a 2‑O‑methylriboside of a type normally found only in RNA, with the two linked by an ether bond.
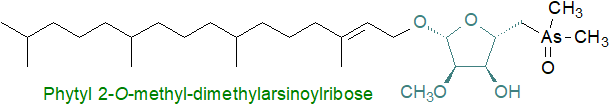
A range of related lipids have been found in sediments from a hypersaline lake, where they may have originated in detritus from phytoplankton.
5. Toxicity
An initial conclusion that the presence of most of these lipids in fish oils did not raise toxicity concerns when consumed by mammals is no longer strictly tenable, although the current view is that this is not a practical problem. When arsenolipids are absorbed from the gastrointestinal tract of mice and humans, much of the arsenic is excreted rapidly in the form of organic water-soluble metabolites, mainly compounds containing dimethylarsine oxide moieties and arsenobetaine, and including 4‑dimethylarseno-butanoic and propanoic acids (and thio analogues), which have been detected in human urine after consumption of cod liver. Similarly, after human consumption of seaweed products, arsenosugars and their metabolites including trace levels of thio-dimethyl arsenate were detected in urine. Some of these may be products of metabolism by gut microorganisms.
Arsenic-containing hydrocarbons are now known to be highly toxic from tests with cultured cells from human bladder and liver in vitro, and from studies with fruit flies (Drosophila melanogaster), nematodes (Caenorhabditis elegans) and the brain of infantile male rats. In addition, arsenic-containing hydrocarbons and arsenite exerted significant toxic effects at µM concentrations in human liver cells. In experiments with a model intestinal barrier system, both arsenic-containing hydrocarbons and fatty acids were reportedly able to diffuse passively into the intestinal cells, although the arsenic-containing fatty acids were largely metabolized before crossing into the blood stream. As they can cross the blood-brain barrier, they are neurotoxins that might even facilitate the transfer of other toxicants into the brain. They exert a strong toxic effect in the hippocampus of the infantile male rat in vitro, and have the potential to be harmful towards development, learning and memory. On the other hand, arsenic-containing fatty acids and triacylglycerols had no significant toxicity affects upon C. elegans. It is noteworthy that arsenic-containing hydrocarbons and fatty acids have both been detected in human milk albeit at very low levels, and they can then pass to the nursing infant.
 In
experiments with human cell lines in vitro, arsenic-containing hydrocarbons were found to influence gene expression
and DNA methylation with the nature and magnitude of the effects dependent on the chain-length of the hydrocarbon.
A high proportion of the starting compounds were transformed into thioxo analogues, i.e., with the oxygen atom replaced by sulfur,
with trace levels as arseno-fatty acid and alcohol derivatives.
This transformation can occur through the action of the gut microbiota in humans and during cooking, and thioxoarsenates have been detected in
ancient marine sediments, where they may be of diagenetic origin.
Thioxo-arseno lipids might be expected to be more lipophilic than the parent compounds, but it is not yet known whether this transformation
results in an increase in toxicity, although thio-dimethyl arsenate is known to be toxic.
In
experiments with human cell lines in vitro, arsenic-containing hydrocarbons were found to influence gene expression
and DNA methylation with the nature and magnitude of the effects dependent on the chain-length of the hydrocarbon.
A high proportion of the starting compounds were transformed into thioxo analogues, i.e., with the oxygen atom replaced by sulfur,
with trace levels as arseno-fatty acid and alcohol derivatives.
This transformation can occur through the action of the gut microbiota in humans and during cooking, and thioxoarsenates have been detected in
ancient marine sediments, where they may be of diagenetic origin.
Thioxo-arseno lipids might be expected to be more lipophilic than the parent compounds, but it is not yet known whether this transformation
results in an increase in toxicity, although thio-dimethyl arsenate is known to be toxic.
However, in Japan where fish is an important component of the diet, the average daily intake of the two most toxic arseno-hydrocarbons was calculated to be approximately 3000 and 360 ng As/person/day, and this is well below the level at which they might pose a health risk.
6. Analysis
The main difficulties in the analysis of arsenolipids are the low levels at which they occur naturally and a lack of commercially available standards other than one reference material. The dimethylarsinoyl group imparts additional polarity to arsenolipids, but a procedure in which the extracts are reacted with hydrogen sulfide to convert oxo-arsenolipids to thio compounds is reported to permit sharper peaks on chromatography with improvements in resolution and quantification by mass spectrometry. Modern mass spectrometric methods of high sensitivity coupled to HPLC are essential for analysis of the more complex arsenolipids, and it is possible to detect arsenic atoms derived from their precursor lipids as monatomic cations, As+.
Recommended Reading
- Al Amin, M.H., Xiong, C., Francesconi, K.A., Itahashi, Y., Yoneda, M. and Yoshinaga, J. Variation in arsenolipid concentrations in seafood consumed in Japan. Chemosphere, 239, 124781 (2020); DOI.
 Bornhorst, J.,
Ebert, F., Meyer, S., Ziemann, V., Xiong, C., Guttenberger, N., Raab, A., Baesler, J., Aschner, M., Feldmann, J.,
Francesconi, K., Raberc, G. and Schwerdtle, T. Toxicity of three types of arsenolipids: species-specific effects
in Caenorhabditis elegans. Metallomics, 12, 794-798 (2020);
DOI.
Bornhorst, J.,
Ebert, F., Meyer, S., Ziemann, V., Xiong, C., Guttenberger, N., Raab, A., Baesler, J., Aschner, M., Feldmann, J.,
Francesconi, K., Raberc, G. and Schwerdtle, T. Toxicity of three types of arsenolipids: species-specific effects
in Caenorhabditis elegans. Metallomics, 12, 794-798 (2020);
DOI. - Chávez-Capilla, T. The need to unravel arsenolipid transformations in humans. DNA Cell Biol., 41, 64-70 (2022); DOI.
- Coniglio, D., Ventura, G., Calvano, C.D., Losito, I. and Cataldi, T.R.I. Strategies for the analysis of arsenolipids in marine foods: A review. J. Pharm. Biomed. Anal., 235, 115628 (2023); DOI.
- Dembitsky, V.M. and Levitsky, D.O. Arsenolipids. Prog. Lipid Res., 43, 403-448 (2004); DOI.
- El-Ghiaty, M.A. and El-Kadi, A.O.S. The duality of arsenic metabolism: impact on human health. Annu. Rev. Pharm. Toxic., 63, 341-358 (2023); DOI.
- García-Salgado, S., Raber, G., Raml, R., Magnes, C. and Francesconi, K.A. Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae. Environm. Chem., 9, 63-66 (2012); DOI.
- Glabonjat, R.A., Raber, G., Jensen, K.B., Ehgartner, J. and Francesconi, K.A. Quantification of arsenolipids in the certified reference material NMIJ 7405-a (Hijiki) using HPLC/mass spectrometry after chemical derivatization. Anal. Chem., 86, 10282-10287 (2014); DOI.
- Glabonjat, R.A., Raber, G., Jensen, K.B., Guttenberger, N., Zangger K. and Francesconi, K.A. A 2-O-methylriboside unknown outside the RNA world contains arsenic. Angew. Chem.-Int. Ed., 56, 11963-11965 (2017); DOI.
- Li, C. and others. Speciation analysis and toxicity evaluation of arsenolipids - an overview focusing on sea food. Arch. Toxicol., 98, 1-16 (2023); DOI.
- Liu, X.L. Streamlined arsenolipid identification via direct arsenic detection using RPLC-ESI-QTOF-MS with collision-induced dissociation. J. Am. Soc. Mass Spectrom., 35, 300-306 (2023); DOI.
- Raab, A., Newcombe, C., Pitton, D., Ebel, R. and Feldmann, J. Comprehensive analysis of lipophilic arsenic species in a brown Alga (Saccharina latissima). Anal. Chem., 85, 2817-2824 (2013); DOI.
- Stiboller, M., Espinoza, A.C., Scholz, S., Raber, G. and Schwerdtle, T. Isolation and purification of arsenolipids from natural marine sources for use in speciation and toxicological studies. Environm. Chem., 20, 31-43 (2023); DOI.
- Taylor, V.F., Li, Z.G., Sayarath, V., Palys, T.J., Morse, K.R., Scholz-Bright, R.A. and Karagas, M.R. Distinct arsenic metabolites following seaweed consumption in humans. Sci. Rep., 7, 3920 (2017); DOI.
- Viczek, S.A., Jensen, K.B. and Francesconi, K.A. Arsenic-containing phosphatidylcholines: a new group of arsenolipids discovered in herring caviar. Angew. Chem.-Int. Ed., 55, 5259-5262 (2016); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: August 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
