Nitro Fatty Acids
 Nitric oxide is a
signalling molecule that takes part in regulation of many metabolic pathways in animals that include vasodilation, and inflammatory cell
and neuronal function, and many related Reactive Nitrogen Species (RNS) are produced non-enzymatically.
While the involvement of free radical-catalysed addition of nitric oxide radicals (NO•) and secondary metabolites of nitrite
anions like nitrogen dioxide radicals (NO2•) to unsaturated and hydroperoxy fatty acids in vitro has been
known for many years, it was only in 1999 that the first paper appeared to show that nitro fatty acids were present in the membrane
phospholipids of human tissues in vivo and at concentrations that had the potential to exert physiological effects.
Since then, the nature and biology of these fascinating lipids have attracted increasing research interest.
RNS are produced continuously under normal physiological conditions, and they are present in foods and the environment so can generate
nitro-lipids in healthy populations, but especially during stress conditions such as inflammation and disease when RNS can be in excess.
Nitric oxide is a
signalling molecule that takes part in regulation of many metabolic pathways in animals that include vasodilation, and inflammatory cell
and neuronal function, and many related Reactive Nitrogen Species (RNS) are produced non-enzymatically.
While the involvement of free radical-catalysed addition of nitric oxide radicals (NO•) and secondary metabolites of nitrite
anions like nitrogen dioxide radicals (NO2•) to unsaturated and hydroperoxy fatty acids in vitro has been
known for many years, it was only in 1999 that the first paper appeared to show that nitro fatty acids were present in the membrane
phospholipids of human tissues in vivo and at concentrations that had the potential to exert physiological effects.
Since then, the nature and biology of these fascinating lipids have attracted increasing research interest.
RNS are produced continuously under normal physiological conditions, and they are present in foods and the environment so can generate
nitro-lipids in healthy populations, but especially during stress conditions such as inflammation and disease when RNS can be in excess.
These nitro metabolites are classified as electrophilic fatty acids, together with related lipids with α,β‑unsaturated carbonyl and epoxide substituents, which have a propensity to undergo reversible Michael addition reactions with cellular nucleophiles such as cysteine and histidine-containing peptides and proteins. In part through these reactions, they have a role in diverse signalling events, which including inducing peroxisome proliferator-activated receptor (PPAR)-dependent gene expression, inhibiting oxidative stress, increasing endothelial nitric oxide synthesis and suppressing inflammation induced by cytokines. In these reactions, it has been demonstrated that they afford protection from inflammatory injury in several experimental models and have therapeutic potential, which is under investigation in clinical trials.
Most research on the topic relates to animal biochemistry, but it is now recognized that nitro fatty acids may be important in plant metabolism. While the main emphasis has been on nitro fatty acids per se, it is increasingly being realized that they can function in tissues in the intact lipids in which they are formed.
1. Occurrence in Animal Tissues
It was initially thought that the main nitrated species of fatty acids in animal tissues were derived from oleic acid, i.e., 9- and 10-nitro-9Z-octadecenoic acids, and these are still the most studied because of their relative availability by chemical synthesis. Note that under the official IUPAC rules of nomenclature these should strictly speaking be designated as trans/E isomers to reflect the orientation of the nitro group relative to the alkyl substituent on the adjacent carbon atom, but those working on this research topic prefer the more familiar usage based upon the precursors.

It is now apparent that 'conjugated linoleic acid' (9Z,11E-octadecadienoic acid or CLA) may be the primary endogenous substrate for fatty acid nitration in vitro and in vivo, yielding up to 105 more nitration products (mainly 9- and 12-nitro-octadeca-9,11-dienoic acids) than linoleic acid per se, presumably because of resonance stabilization of the radical intermediates during biosynthesis (see below). These have been detected in plasma and urine of healthy humans with and without CLA supplementation, and they are generated during digestion, metabolic stress and inflammation, and indeed may often be the only isomers detectable. Red meat and dairy foods are the main dietary source of CLA, although some comes from isomerization of linoleate by microorganisms in the intestines and some by desaturation of vaccenic acid (11E‑18:1) in the liver. Cows' milk contains 5 μM nitro-CLA.

Analogous compounds reportedly derived from linoleate were detected at significant concentrations in some studies, but not in others, and it is possible that these were misidentified with CLA as the true origin. Similarly, nitrated derivatives of palmitoleic, linolenic, arachidonic and eicosapentaenoic acids have been detected at trace levels in human plasma and urine by sensitive analytical mass spectrometric methods. In general, there is some selectivity in terms of which of the various isomers are found in tissues, and for example, the nitro-eicosatetraenoic acids have the NO2 groups in positions 9, 12, 14 and 15 mainly. Comparable metabolites have been described from other fatty acids, including eicosapentaenoic and docosahexaenoic acids (n-3 family), some with two nitro groups. Such findings are controversial, and they were contradicted in one recent study, which reported that nitro derivatives of conjugated linoleate and linoleate were the only isomers detectable in urine.
In plasma, nitro fatty acids occur in the free state and esterified in cholesterol esters and triacylglycerols, while in adipose tissue they have been detected in triacylglycerols and membrane phospholipids, but they are also present bound reversibly to thiol-containing proteins and glutathione in tissues. Free and esterified concentrations of nitro fatty acids in plasma and red blood cells were originally greatly overestimated, and subsequent studies with stable isotope-dilution methodology suggests that the true basal level of the unesterified state in plasma of healthy humans is 3 to 12 nM, while a figure of 9 nM for total nitro-isomers has been quoted recently for urine. Other reports suggest that even these may be overestimates, although concentrations do increase significantly under inflammatory conditions such as vascular injury and myocardial ischemia and reperfusion. In any case, cellular nitro fatty acids would be expected to have a short half-life because of the ease with which they undergo non-enzymatic Michael addition reactions with thiol-containing compounds (see below). Inevitably, these reactions compound the analytical difficulties and can lead to underestimates of the rates of formation of such fatty acids, since as much as 99% may be covalently bound to cysteine in proteins.
Nitro fatty acids can be precursors of further nitrated and nitroxidized species such as nitroso, dinitroso, nitronitroso, and di- and trinitro species, or they can be oxidized to generate various nitroxidized structures such as nitro-hydroxy, nitro-hydroperoxy, nitro-epoxy and nitro-keto species in the test tube at least; representative structures of nitro-hydroxy derivatives of oleate and linoleate are illustrated. Although nitro-eicosatetraenoic, α,β‑nitrohydroxy-eicosatrienoic and trans-arachidonic acids, derived from arachidonic acid via such reactions, have been characterized both in vitro and in vivo, little is yet known of their biological properties.

In addition, simple (non-nitrated) geometrical (trans/E) isomers of unsaturated fatty acids can originate as a by-product of a nitration reaction, and those derived from arachidonate are of special relevance.
For historical reasons, most metabolic studies to date have concentrated on nitro-oleate isomers, which are easily characterized and are available from chemical synthesis, but the evidence to date is that nitro-CLA isomers are metabolized in the same way. When 10-nitro-oleic acid was administered orally to dogs, it was efficiently absorbed and esterified to position sn-2 mainly of triacylglycerols more rapidly than its metabolite 10‑nitro-stearic acid and incorporated into lipoproteins (chylomicrons) for distribution in plasma to other tissues. Esterification into triacylglycerols and other lipids may shield the electrophilic nature of these mediators from deactivation in plasma and the liver and so enables efficient distribution to target organs, where they can be released by lipases and thence transported into cells and incorporated into the cellular lipids. Whole body studies of rats treated with radio-labelled 10‑nitro-oleic acid demonstrated rapid incorporation into kidney, liver, lungs and heart. Adipocytes may act as an inert store since over a longer term (two weeks), with unsaturated nitro-fatty acids incorporated mainly into mono- and diacylglycerols, while analogous saturated metabolites were enriched in triacylglycerols. As nitro fatty acid conjugates with coenzyme A have been detected in tissues, they can presumably be re-esterified after hydrolysis from a lipid-bound state.
2. Synthesis of Nitro Fatty Acids in Tissues
The nitric oxide radical is produced endogenously in animal tissues from L-arginine and oxygen by various nitric oxide synthases, but formation of nitro fatty acids occurs in tissues through the non-enzymatic reactions of many reactive nitrogen species (RNS), i.e., free radicals and their precursors, such as nitrite, nitric oxide (NO•) and NO•‑derived oxides of nitrogen, (nitrogen dioxide (NO2•), peroxynitrite (ONOO•), nitrosoperoxocarbonate (ONOOCO2) and dinitrogen trioxide (N2O3)), which can give rise to different types of reaction, including nitration, nitrosation and nitro-oxidation. Of these, the NO2• radical is the most important intermediate, and it can arise spontaneously from various endogenous and exogenous sources in humans. Meat and other foods may contain appreciable quantities of nitrite (NO2-), which may be added as a preservative, and nitrate can be reduced to nitrite by aerobic bacteria in the mouth. In the stomach, dietary nitrite decomposes rapidly in the acidic environment to NO• and NO2• and other nitrogen oxides, probably via N2O3 as an intermediate, and these are absorbed from the intestines and thence enter the circulation.
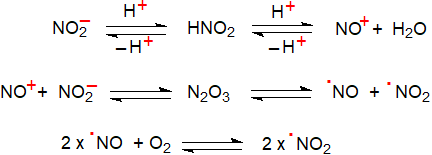 |
| Figure 1. Formation of the nitrogen dioxide radical. |
These radicals operate in conjunction with oxygen-derived inflammatory mediators such as superoxide (O2•), hydrogen peroxide (H2O2) and lipid peroxyl radicals (LOO•) in tissues. There are many different mechanisms for the production of secondary radicals and their subsequent reactions, which are controlled by such factors as the concentration of the NO2• radicals, tissue/organelle, oxygen tension, and the concentrations and membrane environment of the target molecules and of many catalysts and antioxidants. In addition, immune responses to inflammatory stimuli induce nitric oxide synthase in certain cells that form NO•, which is then oxidized to NO2•. The latter is a common air pollutant and can be absorbed via the lungs. Although NO• per se does not participate as a direct nitrating species, its presence in tissues may be required for nitration to occur. Reactions would then be expected to take place mainly in the membranes of cells because partitioning of NO•/NO2• into this cellular component occurs preferentially.
Mechanistic studies of nitro fatty acid formation in human and other animal tissues support biosynthetic mechanisms proposed first from chemical studies in vitro, though whether all of these occur to a significant extent in vivo is doubtful. The NO2• radicals can react with unsaturated lipids and lipid radicals to make all the types of products found in tissues, and at low oxygen tensions, homolytic attack to the double bond yields nitroalkyl radicals, which combine with other NO2• radicals to form nitro-nitrito intermediates. Loss of nitrous acid (HNO2) from these intermediates results in nitroalkenes mainly, with isomerization of a double bond from the cis (Z) to the trans (E) configuration, while hydrolysis leads to nitro-alcohols. The nitro-conjugated linoleate isomers illustrated above, the most abundant metabolites in vivo, come from this mechanism. In an alternative reaction, abstraction of a hydrogen atom from the nitroalkyl radicals leads to nitro-allyl derivatives. As these reactions are non-enzymatic with intact lipids rather than the free acids, they have much in common with isoprostane formation.
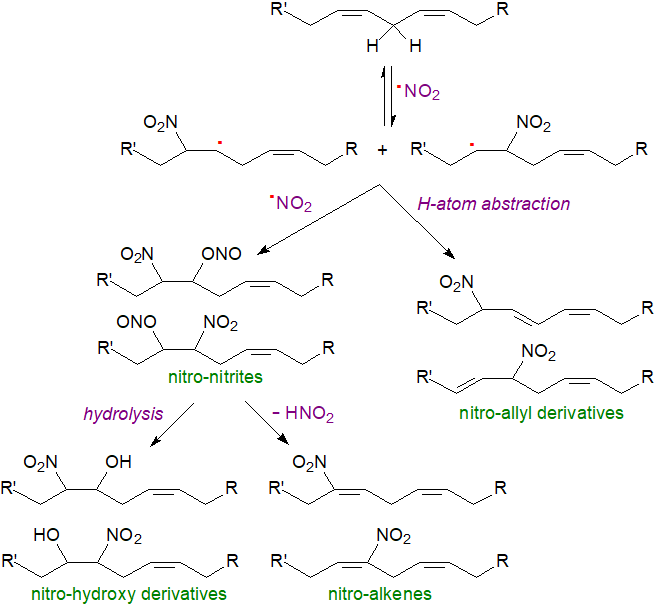 |
| Figure 2. Nitro fatty acid formation by free radical reactions. |
Under the acid conditions of the stomach, it has been established that both unesterified fatty acids and those in triacylglycerols can be rapidly nitrated, with conjugated linoleic acid from microbial fermentation or in foods as a major reactant, and the resulting nitro fatty acids are taken up by the intestinal tissue and incorporated into chylomicrons for transport in plasma to the liver and other tissues. In the rat stomach, it has been determined that nitration with conjugated linoleic acid proceeds by generation of nitro-nitrate intermediates (NO2‑ONO2‑FA) via reactions dependent upon oxygen and nitrite, and they represent ~70% of all nitrated lipids in the stomach. These metabolites decay in vitro at neutral or basic pH by the loss of the nitrate ester group (-ONO2) from the carbon backbone to yield an electrophilic fatty acid nitroalkene (NO2‑FA) together with nitrate, nitrite and nitrosative species. Alternatively, decay can proceed via a mono-nitro radical intermediate.
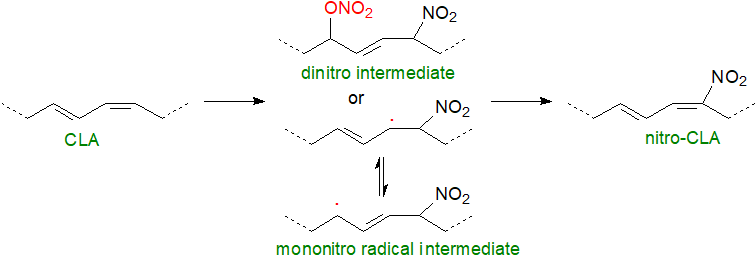 |
| Figure 3. Nitration of conjugated linoleate. |
As an NO2• radical can initiate lipid oxidation reactions, yields of nitration versus oxidation products will depend on the concentration of oxygen. At elevated oxygen levels, the NO2• radical can interact with an unsaturated fatty acid to form a carbon-centred radical, which can react with oxygen to give a lipid hydroperoxide. Unstable alkyl peroxynitrite intermediates can result from the reactions of a lipid peroxyl radical (LOO•) and NO•, of peroxynitrite radicals, and of a lipid hydroperoxide reaction with N2O4 or with HNO2, the last leading to nitro-epoxy fatty acids, and under these conditions, nitro-hydroxy and nitro-oxo oleate derivatives can be formed from conjugated linoleate. On the other hand, nitro fatty acid radicals may be produced that lose HNO2 to regenerate the unsaturated fatty acid but with one of the double bonds isomerized from the cis/Z to the trans/E configuration.
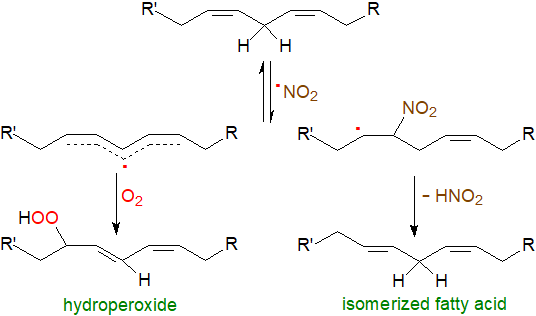 |
| Figure 4. Nitration reactions under high oxygen tension. |
A further mechanism for nitroalkene formation is addition of a nitronium ion (NO2+) from reaction of a transition metal with peroxynitrite by electrophilic substitution at the double bond.
 |
| Figure 5. Nitro fatty acid formation by electrophilic substitution at the double bond. |
Catabolism: Nitrogen oxide radicals react very specifically with γ-tocopherol in vivo to yield a 5-nitro-metabolite and not with α‑tocopherol. Deactivation of nitro fatty acids is achieved mainly by reduction to non-electrophilic nitroalkanes by prostaglandin reductase-1 in the liver, and for example, 9-nitro-oleate infused into mice and 10‑nitro‑oleate given orally to dogs were hydrogenated in part to nitro-stearates, which are not electrophilic, although some of the 9-isomer was desaturated to a nitro-octadecadienoic acid. In addition, 10‑nitro-oleate was isomerized to 10‑nitro-8E-octadecenoate, possibly because of reversible formation of Michael adducts (see below), i.e., deprotonation of the α‑carbon followed by β‑elimination of the thiolate group.
A proportion of the 9-nitro-oleate can be subjected to β-oxidation to yield nitro-7Z-hexadecenoic, nitro-5Z-tetradecenoic and nitro-3Z-dodecenoic acids, and their corresponding coenzyme A derivatives. 10‑Nitro‑oleate was found to be subjected to ω- and β‑oxidation to generate several oxidized metabolites with 4‑nitro-octanedioic acid as a major urinary product, and these were excreted in part as N‑acetylcysteine, taurine and sulfo-conjugates. In phosphate buffers and presumably in the cytoplasm of cells, nitroalkenoic fatty acids decay rapidly because of solvation reactions with the release of nitric oxide radicals, and while various reaction mechanisms have been proposed, their relevance in vivo is uncertain.
3. Physiological Impact of Nitro Fatty Acids
It has long been known that nitric oxide per se is a factor in innumerable metabolic processes in tissues, and in contrast to the prevailing dogma, experimental evidence was obtained in the 1990s that NO• inhibited the oxidation of membranes and plasma lipoproteins more potently than α‑tocopherol and in general had anti-inflammatory, antioxidant and tissue-protective effects. Subsequently, the role of nitro fatty acids in mediating these reactions has become apparent. It is now well established from experiments both in vitro and in vivo that nitro fatty acids elicit metabolic responses that are generally beneficial, and they are anti-inflammatory mediators with appreciable therapeutic potential. They are known to modulate the expression of more than 300 genes crucial for cytoprotective, metabolic and anti-inflammatory purposes in addition to acting directly upon enzymes. On inflammatory stimulation, they may be generated in situ or released from membrane or adipose tissue stores.
While it is evident that they can act physiologically on their own, nitro fatty acids can act by releasing NO•, for which there are at least two hypothetical mechanisms although confirmation of the exact one is required. This is an important aspect of their metabolism and may be a means of delivering NO• to remote tissues.
Reaction with thiols: The main mechanisms and signalling actions of nitro fatty acids are mediated by post-translational modification of proteins by covalent adduction. Nitrated unsaturated fatty acids are powerful electrophiles, i.e., the nitro-alkene moiety has strong electron-withdrawing properties that render the β‑carbon electron deficient and thus susceptible to attack by nucleophiles. This favours reversible nitroalkylation (Michael reaction) with deprotonated thiolate anions, such as the thiol groups of glutathione and thio-amino acids, as well as the imidazole moiety of histidine and the ε-amino group of lysine residues of proteins, thereby regulating the structure and function of the latter. The kinetically rapid and reversible nature of these reactions differentiates nitro fatty acids from other endogenous signalling electrophiles such as the cyclopentanone prostaglandins and aldehydes derived from lipid oxidation, e.g., 4‑hydroxy-2-nonenal, that react more slowly and often irreversibly with nucleophiles. Although such post-translational modifications of proteins are non-enzymic in nature, and 184 potential protein targets for nitro-alkylation have been identified in macrophages alone, these interactions may be more selective than might be expected. Transmembrane proteins in the endoplasmic reticulum and nuclear membranes are most susceptible, and then intact lipids containing nitro fatty acids are the main reactants rather than those in an unesterified state.
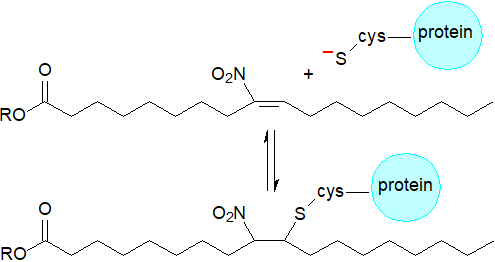 |
| Figure 6. Michael addition reaction of nitro fatty acids. |
Again, 10-nitro-oleate has been used in most studies, although nitro adducts of conjugated linoleate isomers are particularly potent electrophiles, and they are the main nitro fatty acids in serum. With these, mechanistic and kinetic studies have demonstrated that reactions with thiols occur rapidly to yield adducts both β and δ to the nitro group, although the δ-adducts are formed nearly 10 times as quickly as β-adducts. Indeed, the cysteine-δ-adducts have been detected in human urine. In human serum in addition to non-covalent binding with albumin, nitro-CLA has been shown to add covalently at Cys-34, suggesting that this may be a means of systemic distribution to influence signalling and metabolic responses. The effective concentrations of nitro fatty acids in plasma (and other tissues) are reduced by this means, and they may constitute a temporary circulating reservoir. Glutathione-nitroalkene adducts are eventually removed from cells by the multidrug resistance protein-1 transporter.
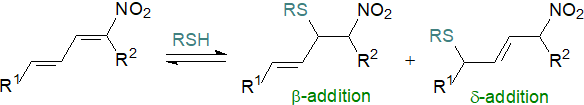 |
| Figure 7. Reaction of nitro-CLA with thiols. |
Under conditions of oxidative stress in vitro in plants at least, reactive oxygen and nitrogen species, as represented by hydrogen peroxide and peroxynitrite, respectively, can oxidize cysteine-adducted nitro fatty acids with the release of free nitroalkenes.
Anti-inflammatory reactions: Fatty acid nitro-alkenes are potent anti-inflammatory agents, and there is abundant evidence that nitro lipids promote cyto-protective and anti-inflammatory responses by a variety of mechanisms that include acting directly upon certain enzymes. Nitroalkylation can result in changes in protein structure, function and subcellular distribution, and this is now considered to be a significant post-translational modification. As both nitric oxide formation and lipoxygenase-mediated pathways utilize highly reactive free-radical species and act on signalling cascades in relation to inflammation and the immune response especially, interactions between the two are to be expected. Electrophilic nitro fatty acids can be anti-inflammatory by interacting with nucleophilic amino acids in lipoxygenases, and an irreversible inhibition of ALOX5 has been observed. 10‑Nitro-oleic acid is an inhibitor of the epoxyhydrolase, i.e., the enzyme that hydrolyses epoxyeicosatrienoic acid, which is protective against hypertension, and it is an irreversible inhibitor of the enzyme xanthine oxidoreductase, which generates pro-inflammatory oxidants and secondary nitrating species. In this instance, it has been established that the carboxyl group, nitration at the 9 or 10 olefinic carbons and the double bond are all required for this action, and comparable mechanisms operate with prostaglandin endoperoxide H synthase and NADPH-oxidase. One further example is covalent addition of nitro fatty acids to an N‑terminal cysteine residue of SIRT6, an enzyme that is critical for both glucose and lipid homeostasis.
 Nitro-CLA
isomers in red cells and plasma may constitute the single largest pool of oxides of nitrogen in the vasculature, where they bring about
vasorelaxation.
In neutrophils and platelets, nitro fatty acids induce cAMP-dependent protein kinase signalling pathways, which have an anti-inflammatory
role in cells, while both nitro-oleate and nitro-linoleate have been shown to inhibit the lipopolysaccharide-induced secretion of pro-inflammatory
cytokines in macrophages, actions that are independent of nitric oxide formation or of activation of PPARs, for example during pulmonary injury.
They complement the pro-resolving docosanoids such as the resolvins, protectins and maresins in this way.
While studies are still at an early stage, it would not be surprising if there were significant influences upon the eicosanoid cascades
through redirection of arachidonic acid metabolism and signalling.
Arachidonate isomeric by-products of nitration reactions are emerging as biomarkers that target various metabolic systems,
such as inhibition of prostaglandin endoperoxide H synthases (COX) 1 and 2 and the synthesis of thromboxanes.
Nitro-arachidonic acid inhibits superoxide production by NADPH oxidase by a reversible covalent addition to critical cysteines on a
supportive enzyme protein disulfide isomerase.
On the other hand, the discovery of the potent effects of nitro-conjugated octadecadienoic acids
as signalling mediators may mean that the relative importance of many isomers in vivo may have to be re-evaluated.
Nitro-CLA
isomers in red cells and plasma may constitute the single largest pool of oxides of nitrogen in the vasculature, where they bring about
vasorelaxation.
In neutrophils and platelets, nitro fatty acids induce cAMP-dependent protein kinase signalling pathways, which have an anti-inflammatory
role in cells, while both nitro-oleate and nitro-linoleate have been shown to inhibit the lipopolysaccharide-induced secretion of pro-inflammatory
cytokines in macrophages, actions that are independent of nitric oxide formation or of activation of PPARs, for example during pulmonary injury.
They complement the pro-resolving docosanoids such as the resolvins, protectins and maresins in this way.
While studies are still at an early stage, it would not be surprising if there were significant influences upon the eicosanoid cascades
through redirection of arachidonic acid metabolism and signalling.
Arachidonate isomeric by-products of nitration reactions are emerging as biomarkers that target various metabolic systems,
such as inhibition of prostaglandin endoperoxide H synthases (COX) 1 and 2 and the synthesis of thromboxanes.
Nitro-arachidonic acid inhibits superoxide production by NADPH oxidase by a reversible covalent addition to critical cysteines on a
supportive enzyme protein disulfide isomerase.
On the other hand, the discovery of the potent effects of nitro-conjugated octadecadienoic acids
as signalling mediators may mean that the relative importance of many isomers in vivo may have to be re-evaluated.
Nitro fatty acids can inhibit pro-inflammatory enzymes and the expression of pro-inflammatory genes, and for this reason, protein targets such as PPARγ and nuclear factor kappa B (NF-κB) have received most attention. Nitro fatty acids can bind to all three PPAR isotypes with high affinities, and as signalling mediators, they have the potential to regulate the expression of multiple target genes in the immune system or relevant to inflammatory diseases. 12‑Nitro-linoleate is a particularly potent agonist for PPARγ and binds covalently with high specificity to Cys 285 via Michael addition. By this means, it can induce the expression and activity of endothelial nitrous oxide synthase. Nitro fatty acids form covalent adducts with cysteine residues of Kelch-like ECH-associated protein (Keap-1) with the result that stimulation of nuclear factor erythroid 2‑related factor 2 (Nrf2) occurs to in turn affect gene expression of anti-inflammatory enzymes. By nitroalkylating specific cysteines in calcineurin, a protein phosphatase that activates the T cells of the immune system, it exerts further anti-inflammatory effects. Taken together with the finding of significant levels of protein cysteine adducts of nitro-oleic acid and the free acid in fresh olives, there are interesting implications both for plant biology and human nutrition.
Intact lipids: Although it is the unesterified statess that have attracted most attention, nitro fatty acids are generated while esterified to lipids, and they have been detected in all glycerolipid classes. Dietary 10‑nitro-octadec-9-enoic acid is rapidly absorbed by rats and is esterified into triacylglycerols, which are then incorporated into chylomicrons and transported via the lymphatic system, so bypassing initial hepatic metabolism. In the process, they reduce lymph flow and chylomicron secretion significantly, impacting upon the lymphatic triacylglycerol profile and transit, and they reduce absorption of dietary fat by up to 75%.
Intact phospholipids containing such fatty acids have the potential to be disruptive to membranes, and they induce physiological responses as well, as has become increasingly evident for oxidized phospholipids; improved analytical methodologies with mass spectrometry are now facilitating study of complex lipid metabolites of this kind. Phosphatidylcholine containing nitro-oleate has been shown to induce cellular changes such as cytoskeletal rearrangement and cell shrinkage with eventually loss of cell adhesion or impaired cell attachment in various cell types in culture, effects not seen with the free nitro fatty acid, while nitrated phosphatidylcholine and phosphatidylethanolamine have been identified in cardiac mitochondria from the heart of an animal model of type 1 diabetes mellitus. Similarly, nitrosylated cardiolipins have been characterized and might be expected to interfere with mitochondrial metabolism. In models of inflammation in vitro, other nitrated phospholipids have been identified and shown to be antioxidant and anti-inflammatory agents, and such studies are in progress in living systems.
4. Pharmaceutical Properties of Nitro Fatty Acids
There is considerable therapeutic potential for the use of nitro-fatty acids against the inflammation that is symptomatic of cardiovascular and kidney diseases and inflammatory bowel disease. Nitro-oleate isomers are the preferred drug candidates as they are relatively easy to synthesise and have greater stability with similar signalling actions and reactivity toward nucleophiles. In human trials, they are tolerated well when administered orally, and phase II clinical trials of 10-nitro-oleic acid are underway for the treatment of focal segmental glomerulosclerosis, pulmonary arterial hypertension and asthma in obese patients. Delivery systems utilizing lipid nanoparticles are under development that can carry their protective payloads to target tissues.
Nitro-fatty acids have been shown to inhibit hepatic triacylglycerol accumulation and protect against fibrosis during the development of non-alcoholic fatty liver disease in mice; glucose intolerance and insulin resistance were inhibited without increasing weight gain. Administration of this nitro fatty acid reportedly preserves the integrity of the blood-brain barrier and confers neurovascular protection in ischemic brain damage, and there is a preliminary report that it may be neuroprotective in neurological diseases. Also in mice, subcutaneous injections of nitro-oleic acid have been shown to inhibit allergic contact dermatitis by inducing immunosuppressive responses. On the other hand, although a substantial increase in the concentration of nitro-conjugated linoleic acid was found to occur naturally in lesional skin in inflammatory skin diseases and oral administration showed appreciable anti-inflammatory and anti-immune effects in psoriasis, topical applications of nitro-oleic acid were found to exacerbate the problem.
In relation to cardiovascular disease, promising results have been observed in vitro and in vivo with rodent models with respect to arterial hypertension, atherosclerosis, platelet aggregation and ischemic heart disease. Nitro-fatty acids transported in low-density lipoproteins (LDL) are cardioprotective by anti-inflammatory and antioxidant mechanisms that include adduct formation and thence critical signalling inhibition of the redox-sensitive transcription nuclear factor kappa B (NF-κB), a potential therapeutic target. They reduce lipid accumulation and promote plaque stability in atherosclerosis and are beneficial towards myocardial infarction. In a mouse model, nitro-oleate ameliorates diastolic dysfunction and heart failure symptoms, and it showed benefits towards neurodegeneration in oxygen-induced retinopathy.
9-Nitro-oleate been shown to be anti-tumorigenic in rodent models of colorectal cancer in cell culture in vitro and in a murine xenograft model of the human disease. In contrast to their well-known anti-oxidative properties, the nitro fatty acid reduced tumour growth by mediating the generation of mitochondrial oxidative stress in the cancer cells to trigger mitochondrial dysfunction and induction of the intrinsic apoptotic pathway while suppressing the transcription of harmful genes. 10-Nitro-oleic acid in combination with antineoplastic DNA-damaging agents may have benefits against triple-negative breast cancer. Preclinical studies have demonstrated their potential as cancer therapeutics with favourable safety and tumour-selective profiles.
5. Nitro Fatty Acids in Plants
Nitro fatty acids derived from conjugated linoleate, a minor component, and oleate in the free state and as protein cysteine adducts were first detected in olives and virgin olive oil, and it has been suggested that they may constitute one of the benefits of the Mediterranean diet, but they have since been found in many other plants. Low levels only are found unesterified with most stored, and probably synthesised, as esters in phytosterol esters, triacylglycerols and phospholipids (or as protein conjugates), from which they can presumably be released on appropriate stimulation although bioactivity as esters may be possible as in animals.
It has now been determined that these and other nitro fatty acids are signalling mediators in plants such as Arabidopsis (seeds, seedlings and leaves) with nitro-linolenic acid being a key metabolite. As a single peak is seen on GC analysis, there seems to be only one isomer in vivo, but the precise structure does not appear to have been determined. Although research is still at an early stage, it has been shown that this nitro fatty acid acts as a signalling molecule during seed and plant development, and it takes part in plant defence responses against different abiotic-stress conditions, mainly by inducing heat shock proteins. It reacts to oxidative stress conditions by inducing high levels of the antioxidant protein ascorbate peroxidase, the concentration of which increases after wounding or exposure to salinity, cadmium ions and low temperatures.
As nitro-linolenic acid can release nitric oxide with its manifold signalling properties in aqueous media, it may act as a signalling molecule indirectly as well as directly, for example by transferring nitric oxide to glutathione to form S-nitrosoglutathione, which may be a major mobile reservoir for nitric oxide and signal transduction. It modulates S-nitrosothiol content through S-nitrosylation of S-nitrosoglutathione reductase1 (GSNOR1) with an impact on the onset of germination. With tomato cell suspensions, exogenous application of nitro-oleate was found to induce ROS via stimulation of NADPH oxidases, requiring calcium entry from the extracellular compartment and protein kinase activation.
6. Analysis
Aside from the multiplicity of different metabolites that can be present at low levels in tissues, a major difficulty in the analysis of nitrated lipids is that they are easily generated artefactually via adventitious nitrite anions during sample work-up and chromatographic analysis under acidic conditions, so acidic pHs must be avoided at all critical phases of lipid extraction. It is therefore necessary to include extensive control experiments to preclude spurious by-products by adding unsaturated fatty acids labelled with stable isotopes as internal standards, while inclusion of 15NO2− during tissue handling and extractions will demonstrate the formation of 15NO2‑fatty acids if there is unwanted nitration during processing. It should be noted that nitrated lipids are sensitive to light and are thermally unstable. Thereafter, modern mass spectrometric techniques, HPLC with electrospray ionization MS or GC-MS of pentafluorobenzyl esters with electron-capture negative-ion chemical ionization, provide the enhanced sensitivity and resolution required for analysis of nitro fatty acids. As fewer derivatization steps are required, the former may be preferable. Analysis of intact nitrated lipid classes or protein adducts adds to the challenge.
Recommended Reading
- Colussi, N., Chang, F. and Schopfer, F.J. The specificity of endogenous fatty acid nitration: only conjugated substrates support the in vivo formation of nitro-fatty acids. Redox Biochem. Chem., 9, 100037 (2024); DOI.
- Duarte, S., Melo, T., Domingues, R., Alché, J.D. and Pérez-Sala, D. Insight into the cellular effects of nitrated phospholipids: Evidence for pleiotropic mechanisms of action. Free Rad. Biol. Med., 144, 192-202 (2019); DOI.
- Elshoura, Y., Herz, M., Gad, M.Z. and Hanafi, R. Nitro fatty acids: A comprehensive review on analytical methods and levels in health and disease. Anal. Biochem., 694, 115624 (2024); DOI.
- Fang, M.-Y., Huang, K.-H., Tu, W.-J., Chen, Y.-T., Pan, P.-Y., Hsiao, W.-C., Ke, Y.-Y., Tsou, L.K. and Zhang, M.M. Chemoproteomic profiling reveals cellular targets of nitro-fatty acids. Redox Biol., 46, 102126 (2021); DOI.
- Fazzari, M., Vitturi, D.A., Woodcock, S.R., Salvatore, S.R., Freeman, B.A. and Schopfer, F.J. Electrophilic fatty acid nitroalkenes are systemically transported and distributed upon esterification to complex lipids. J. Lipid Res., 60, 388-399 (2019); DOI.
- Freeman, B.A., O'Donnell, V.B. and Schopfer, F.J. The discovery of nitro-fatty acids as products of metabolic and inflammatory reactions and mediators of adaptive cell signaling. Nitric Oxide, 77, 106-111 (2018); DOI - many more related reviews are published together in the next two volumes of this journal.
- Grippo, V., Mojovic, M., Pavicevic, A., Kabelac, M., Hubatka, F., Turanek, J., Zatloukalova, M., Freeman, B.A. and Vacek, J. Electrophilic characteristics and aqueous behavior of fatty acid nitroalkenes. Redox Biol., 38, 101756 (2021); DOI.
- Koutoulogenis, G. and Kokotos, G. Nitro fatty acids (NO2-FAs): an emerging class of bioactive fatty acids. Molecules, 26, 7536 (2021); DOI.
- Mata-Pérez, C. and others. Nitro-fatty acids modulate germination onset through S-nitrosothiol metabolism. Plant Physiol., 197, kiaf038 (2025); DOI.
- Neves, B., Pérez-Sala, D., Ferreira, H.B., Guerra, I.M.S., Moreira, A.S.P., Domingues, P., Domingues, R. and Melo, T. Understanding the nitrolipidome: From chemistry to mass spectrometry and biological significance of modified complex lipids. Prog. Lipid Res., 87, 101176 (2022); DOI.
- Ni, H., Tan, X., Du, J. and Wang, Y. Nitro-fatty acids: mechanisms of action, roles in metabolic diseases, and therapeutics. Curr. Med., 3, 3 (2024); DOI.
- Rodríguez, L., Fernandez-Rojas, M., Morales-Malvarez, L., Fuentes, E. and Trostchansky, A. Nitrated fatty acids: Dietary origins, metabolic pathways, and therapeutic potential in cardiometabolic health. Food Biosci., 75, 108175 (2026); DOI.
- Roos, J., Manolikakes, G., Schlomann, U., Klinke, A., Schopfer, F.J., Neumann, C.A. and Maier, T.J. Nitro-fatty acids: promising agents for the development of new cancer therapeutics. Trends Pharm. Sci., 45, 1061-1080 (2024); DOI.
- Schopfer, F.J., Teng, L.H., Sekandari, A., Ekhator, E.S., Kohan, A.B., Freeman, B.A. and Fazzari, M. Fatty acid nitroalkenes regulate intestinal lipid absorption. J. Lipid Res, 66, 100855 (2025); DOI.
- Wilkinson, M.L. and Gow, A.J. Effects of fatty acid nitroalkanes on signal transduction pathways and airway macrophage activation. Innate Immun., 27, 175342592110153 (2021); DOI.
- Wood, I., Trostchansky, A., Xu, Y., Qian, S., Radi, R. and Rubbo, H. Free radical-dependent inhibition of prostaglandin endoperoxide H synthase-2 by nitro-arachidonic acid. Free Rad. Biol. Med., 144, 176-182 (2019); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: January 2026 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
