Lipid Matters
Or "Lipids Matter". An occasional series of notes on publications or other items dealing with lipid science that are moderated by Bill Christie. All good things must come to an end, and regretfully, I have decided that I am no longer able to continue with this blog as age takes its toll. Thank you for your interest over the years, and for the many kind comments I have received. Older entries are archived by year -
| 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 |
The blog lives on in other hands, and you can find it at Lipid Matters.
June 7th, 2023
 There have been numerous studies
focused on the generation and metabolism of lipid droplets.
Despite these, our understanding of how these droplets develop from the endoplasmic reticulum (ER) has been incomplete.
For example, triacylglycerol and cholesterol esters are two of the most abundant neutral lipids in these structures but they are very
different molecules with very different physical properties.
This is highlighted by the fact that triacylglycerols melt at -4°C while cholesterol esters melt at a much lower temperature of -44°C.
A recent article from Abdou Rachid Thiam’s laboratory, and in collaboration with Ilp Vattulainen and Elina Ikonen, data are presented that
indicate cholesterol esters can form supercooled lipid droplets in the presence of triacylglycerols (Nat. Commun., 14,
915 (2023); DOI).
These authors demonstrate that cholesterol esters form supercooled lipid droplets above 20 mol% with respect to triacylglycerol levels,
and liquid-crystalline phases when the level increases to above 90 mol% at 37°C.
They further show that at physiological temperatures, seipin-mediated triacylglycerol clusters catalyze the nucleation of cholesterol
esters in the ER bilayer to initiate the formation of lipid droplets.
Their data are particularly interesting given, as suggested by their melting temperatures, cholesterol esters would be expected to form a
crystalline phase at physiological temperatures, but in the presence of triacylglycerols these lipids are condensed into nascent lipid droplets.
Their data not only provides insights into the formation of lipid droplets, it suggests how macrophages generate cholesterol ester-rich
lipid droplets leading to foam cells, as well as how spatially distinct other lipid droplets can form for other physiological processes
such as steroid hormone synthesis.
There have been numerous studies
focused on the generation and metabolism of lipid droplets.
Despite these, our understanding of how these droplets develop from the endoplasmic reticulum (ER) has been incomplete.
For example, triacylglycerol and cholesterol esters are two of the most abundant neutral lipids in these structures but they are very
different molecules with very different physical properties.
This is highlighted by the fact that triacylglycerols melt at -4°C while cholesterol esters melt at a much lower temperature of -44°C.
A recent article from Abdou Rachid Thiam’s laboratory, and in collaboration with Ilp Vattulainen and Elina Ikonen, data are presented that
indicate cholesterol esters can form supercooled lipid droplets in the presence of triacylglycerols (Nat. Commun., 14,
915 (2023); DOI).
These authors demonstrate that cholesterol esters form supercooled lipid droplets above 20 mol% with respect to triacylglycerol levels,
and liquid-crystalline phases when the level increases to above 90 mol% at 37°C.
They further show that at physiological temperatures, seipin-mediated triacylglycerol clusters catalyze the nucleation of cholesterol
esters in the ER bilayer to initiate the formation of lipid droplets.
Their data are particularly interesting given, as suggested by their melting temperatures, cholesterol esters would be expected to form a
crystalline phase at physiological temperatures, but in the presence of triacylglycerols these lipids are condensed into nascent lipid droplets.
Their data not only provides insights into the formation of lipid droplets, it suggests how macrophages generate cholesterol ester-rich
lipid droplets leading to foam cells, as well as how spatially distinct other lipid droplets can form for other physiological processes
such as steroid hormone synthesis.
Daniel M. Raben,
The Johns Hopkins University School of Medicine, Baltimore, MD, USA
May 24th, 2023
Each animal cell can contain up to 1000 distinct molecular species, with each lipid class containing multiple combinations of the fatty acid components. It seems likely that a high proportion of these are simply present to provide the correct blend of physical properties required for the structural function of a lipid in membranes, but it is surprising how many individual molecular forms have been recognized as having unique biological roles within tissues. One good example of this is 1-palmitoyl-2-oleoyl-phosphatidyl-sn-glycerol of lung surfactant, which attenuates inflammation by antagonizing the cognate ligand activation of the toll-like receptors (TLR2/1, TLR3, TLR4, and TLR2/6), while it disrupts the binding of virus particles to the plasma membrane receptors required for viral uptake in host cells, including influenza and SARS-CoV-2 viruses (Numata, M. et al. The anti-inflammatory and antiviral properties of anionic pulmonary surfactant phospholipids. Immun. Rev., in press (2023); DOI).
It has been recognised for some time that the 18:0-18:1 species of phosphatidylserine has a distinctive role in membranes probably through physical interaction with sphingolipids (Skotland, T. and Sandvig, K. The role of PS 18:0/18:1 in membrane function. Nature Commun., 10, 2752 (2019); DOI). More surprising is a recent publication demonstrating that a bacterial species from human gut, produces a phosphatidylethanolamine species with two different branched chain components (anteiso-15:0 and iso-15:0) that has remarkable specificity for immune signalling in its host via a toll-like receptor TLR2-TLR1 heterodimer; no other combination of acyl groups works (Bae, M. et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature, 608, 168-173 (2022); DOI).
May 17th, 2023
Some biochemical terms can have needlessly complex or obscure meanings, so it is always pleasing to find terms that are immediately understandable and useful, such as ‘flippases’ and ‘scramblases’ for proteins that mediate the movement of phospholipids between the leaflets of membrane bilayers. Flippases direct phosphatidylethanolamine and phosphatidylserine to the cytoplasmic leaflet (floppases work in the opposite direction), while scramblases as the name suggests randomly scramble phospholipids between leaflets across the membrane and collapse the membrane asymmetry. In particular, the latter can transfer phosphatidylserine to the outer leaflet where its exposure on the cell surface is an ‘eat-me’ signal to macrophages, another memorable term (we Scots would call them ‘couthy’). A new review on the topic is worth a read (Sakuragi, T. and Nagata, S. Regulation of phospholipid distribution in the lipid bilayer by flippases and scramblases. Nature Rev. Mol. Cell Biol., in press (2023); DOI).
Pick up any newspaper and you will see that artificial intelligence (AI) is giving concern for any number of reasons. I understand that one such programme passed a US bar exam with flying colours, and universities world-wide are concerned with their use to cheat in essays. There are also worries that they are being used to create bogus scientific publications, and I have seen so many review articles on ferroptosis especially lately – I will say no more! There are several commercial programmes available that purport to improve the standard of written English - a worthy objective, and I am sure that they could be of legitimate value especially for those who have difficulty with the language. Are they too open to abuse?
May 10th, 2023
 I have commented
often here in favour of the open access movement for journals.
From a personal point of view, I have an honorary position with a plant research institute, the James Hutton Institute in Dundee,
which gives me access to a good range of biological journals from the larger publishing companies but poorer access to the chemistry literature.
The more publications that are freely available the better for me.
The growth of new academic publishing companies which are entirely open access has been helpful, although the quality of some may not be
the same as with older established journals.
I recall the board of one such journal resigning en masse because of a failure to properly referee submissions.
I have commented
often here in favour of the open access movement for journals.
From a personal point of view, I have an honorary position with a plant research institute, the James Hutton Institute in Dundee,
which gives me access to a good range of biological journals from the larger publishing companies but poorer access to the chemistry literature.
The more publications that are freely available the better for me.
The growth of new academic publishing companies which are entirely open access has been helpful, although the quality of some may not be
the same as with older established journals.
I recall the board of one such journal resigning en masse because of a failure to properly referee submissions.
Perhaps I should have been more aware that what is free for me to read may not be free for you to publish, and I did not realise how expensive this can be. An article in the Guardian newspaper was an eye opener. The claim is that academic publishers, and Elsevier specifically, are over-charging to the extent that their profits are excessive and indeed greater than those of the big tech companies. The journal Neuroimage is not on my reading list, but the editorial board has resigned in protest over publishing charges. A headline such as "Authors/editors go on strike” may not capture much popular attention, but I hope it will make publishers re-think. Unfortunately, I fear that they will simply use their own editorial staff with minimal scientific input to keep the journal going - am I too cynical?
April 26th, 2023
Lipid trends blog: Lipid metabolizing enzymes are critical components of many signaling systems. It’s no surprise, therefore, that there has been considerable attention given to the lipid and protein mediators of these enzymes. What has received less attention is the role of membrane architecture in the regulation of these enzymes. Indeed, there is considerable evidence suggesting that the alterations in membrane curvature are a major component involved in regulating the accumulation of enzymatically generated lipid products. Similarly, the resulting products may influence membrane architecture. While it’s not clear why there hasn’t been much recognition of the roles of membrane architecture on these enzymes, a major factor is likely be due to the complexity of the topic and difficulty in approaching the issue experimentally. In this, there are two reviews that may help familiarize interested readers. The first is a review by Fanani et al. that addresses the impact of membrane curvature on phospholipases (Membranes (Basel), 13, 190 (2023); DOI), discusses the effects of membrane composition on phospholipases, and the effects of the products of these enzymes on architecture. Similarly, a 2022 review by Bozeli et al. (Cancers (Basel), 14, 5259 (2022); DOI) discuss how a particular species of diacylglycerol and phosphatidic acid, the substrate and product respectively of diacylglycerol kinase alpha, is influenced by membrane morphology. Given the role of this enzyme in cancers and immunology, they suggest DGK-α may be a viable target for some cancers.
Daniel M. Raben,
The Johns Hopkins University School of Medicine, Baltimore, MD, USA
April 19th, 2023
 I have enjoyed keeping up with
the story on nitro fatty acids since they were first reported as an interesting natural
curiosity until the present, where they are seen as having great therapeutic potential, all within the 21st century.
They have been detected in most if not all eukaryotes and have an important distinguishing characteristic in that they are electrophiles.
This promotes a reversible Michael addition with reactive nucleophilic cysteines on target proteins, and in turn, this can modify gene expression.
A relatively simple molecule such as nitro-oleic acid (10-nitro-octadec-9-enoic acid) has been shown to confer benefits in many animal models
of inflammatory diseases and is now undergoing preclinical and clinical safety studies.
A new publication described the potential for protective effects in Parkinson’s disease for which there are as yet no treatment
(Di Maio, R. et al. Neuroprotective actions of a fatty acid nitroalkene in Parkinson’s disease.
npj Parkinson's Disease, 9, 55 (2023);
DOI).
I have enjoyed keeping up with
the story on nitro fatty acids since they were first reported as an interesting natural
curiosity until the present, where they are seen as having great therapeutic potential, all within the 21st century.
They have been detected in most if not all eukaryotes and have an important distinguishing characteristic in that they are electrophiles.
This promotes a reversible Michael addition with reactive nucleophilic cysteines on target proteins, and in turn, this can modify gene expression.
A relatively simple molecule such as nitro-oleic acid (10-nitro-octadec-9-enoic acid) has been shown to confer benefits in many animal models
of inflammatory diseases and is now undergoing preclinical and clinical safety studies.
A new publication described the potential for protective effects in Parkinson’s disease for which there are as yet no treatment
(Di Maio, R. et al. Neuroprotective actions of a fatty acid nitroalkene in Parkinson’s disease.
npj Parkinson's Disease, 9, 55 (2023);
DOI).
April 12th, 2023
I have just become aware that a large family of fatty acid metabolites from cyanobacteria had entirely escaped my attention. It appears that these organisms have distinctive lipid synthetic and oxidative mechanisms, and for example, unesterified fatty acids are linked to AMP by an acyl-ACP synthetase before the same enzyme transfers them to ACP for lipid synthesis, while a fatty acid AMP ligase catalyses both reactions for production of secondary metabolites. Cyanobacteria appear to lack a β-oxidation pathway such as that in E. coli, and they may remove any excess by producing a range of unusual metabolites in which fatty acids or their alkyl chains are incorporated. Some species can insert cyclopropane rings, halogen atoms and other functional groups into fatty acid chains. The range of unique lipids include simple molecules, such as alkenes to more complex esterified forms linked to benzene rings or lipopeptides. The secondary metabolites are likewise distinctive, and some have triple or allenic bonds, others have aromatic structures and yet more have heterocyclic rings (nitrogen and/or sulfur). How these fit into the LipidMaps categories is thankfully not my problem. You can read about these in an accessible new review (Leão, P.N. et al. Incorporation and modification of fatty acids in cyanobacterial natural products biosynthesis. Chem. Commun., in press (2023); DOI).
April 5th, 2023
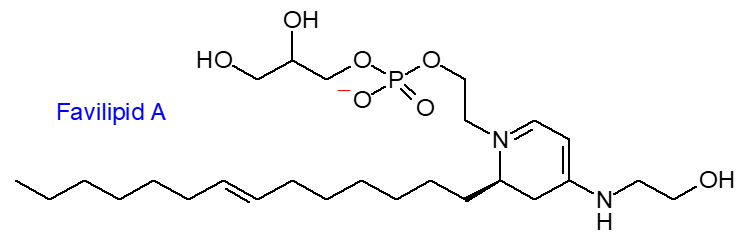
The most unusual new lipids to be described so far this year are phospholipids from a marine source, which the authors term ‘favilipids’ (Scarpato, S. et al. Molecular networking revealed unique uv-absorbing phospholipids: favilipids from the marine sponge Clathria faviformis. Marine Drugs, 21, 58 (2023); DOI). Identification required an elegant combination of mass spectrometry, NMR and other techniques. Favilipid A is illustrated below while a second component has two glycerophosphate units. How do we classify them in the LipidMaps system – they are undoubtedly glycerophospholipids, but no fatty acids!
Another novel lipid may not seem so very different from known lipids, i.e., it is a digalactosyldiacylglycerol from plants but with an alpha rather than a beta linkage between the two galactopyranosyl units (Bar, F.M.A. et al. Galactolipids from Launaea capitata (Spreng.) Dandy with in vitro anti-inflammatory and neuroprotective activities. Separations, 10, 83 (2023); DOI). The difference may appear small, but there are potential therapeutic applications. I would not be surprised if this structural feature is more common, but how often does anyone look for it?
There is nothing new about erucic acid (13-22:1), other than a review suggesting that a rethink may be necessary (Galanty, A. et al. Erucic acid-both sides of the story: a concise review on its beneficial and toxic properties. Molecules, 28, 1924 (2023); DOI). Studies with laboratory rats in the 1970s showed that it could adversely affect the metabolism of the heart, but there have apparently been no studies to confirm the toxicity of erucic acid in humans, and it is now argued that the rat studies were flawed. Meantime, erucic acid has been used as a major component of 'Lorenzo's oil' to treat adrenoleukodystrophy without ill effects.
March 29th, 2023
Lipid trends blog - cyclic phospholipids: Something seems to have gone under the radar for many lipid researchers.
Well, at least it went under my radar.
It turns out, as reported by Mathew Cordes’ group, that there are phospholipase D (PLD) enzymes in some bacterial, fungal and spider toxins
that catalyze the hydrolysis of sphingomyelin and lysophosphatidycholine to generate a cyclic phosphate instead of the expected monoester phosphate
(see [1]). This was determined via 31P NMR and mass spectrometry that showed the enzymes catalyze a transphosphatidylation reaction,
rather than hydrolysis, leading to the generation of the cyclic phosphate products cyclic ceramide phosphate and cyclic lysophosphatidic acid.
It is proposed that substrate hydroxyl group appears to serve as an internal nucleophile in this mechanism although it is not clear this is the
case for all of these enzymes.
Not surprisingly, primary sequence of these PLDs from these organisms appears to differ from their mammalian counterparts in that they don’t
possess the usual "HKD” motifs that is characteristic of the mammalian PLDs.
Indeed, this difference, and the fact that the catalytic chemistry appears to be different, justifies the description of these enzymes as
sphingomyelinases D (SMase D).
While these activities probably contribute to the toxicity of these toxins, the precise role(s) they play, their precise chemistry of all of
the enzyme, and if and how they are regulated remain mysterious.
This looks like fertile ground for some interesting future research.
1. Lajoie, D.M. and Cordes, M.H. Spider, bacterial and fungal phospholipase D toxins make cyclic phosphate
products. Toxicon, 108, 176-180 (2015);
DOI).
Daniel M. Raben,
The Johns Hopkins University School of Medicine, Baltimore, MD, USA
March 15th, 2023
 Readers
may have noted that I enjoy learning of the history of lipid science.
I have just come across a fascinating review of the seminal discovery of how thermogenesis in brown adipose is regulated (Nicholls, D.G.
Fifty years on: How we uncovered the unique bioenergetics of brown adipose tissue. Acta Physiol., in press (2023);
DOI).
50 years ago, David Nicholls was a post-doc in the laboratory of Olov Lindberg in Stockholm measuring fatty acid oxidation by mitochondria
from brown adipose tissue. He noticed "a curious nonlinearity in the respiration rate”, which many experiments and 15 years later (much of it
here at the University of Dundee) lead to the discovery of an uncoupling protein, UCP1, activated by fatty acid, now recognized as
the mechanism behind non-shivering thermogenesis.
Readers
may have noted that I enjoy learning of the history of lipid science.
I have just come across a fascinating review of the seminal discovery of how thermogenesis in brown adipose is regulated (Nicholls, D.G.
Fifty years on: How we uncovered the unique bioenergetics of brown adipose tissue. Acta Physiol., in press (2023);
DOI).
50 years ago, David Nicholls was a post-doc in the laboratory of Olov Lindberg in Stockholm measuring fatty acid oxidation by mitochondria
from brown adipose tissue. He noticed "a curious nonlinearity in the respiration rate”, which many experiments and 15 years later (much of it
here at the University of Dundee) lead to the discovery of an uncoupling protein, UCP1, activated by fatty acid, now recognized as
the mechanism behind non-shivering thermogenesis.
Review articles appear every month that cover some aspect of the biology of lipid droplets - endocrine function, stress, cancer, infection, ceramides, lipophagy, eicosanoid production, proteomics, fat-soluble vitamins, and so forth. This week, I rather enjoyed one that dealt simply with the fatty acid components, especially the polyunsaturated fatty acids, and the importance of the precise regulation of their release into metabolic and signalling pathways (Danielli. M. et al. Lipid droplets and polyunsaturated fatty acid trafficking: Balancing life and death. Front. Cell Developm. Biol., 11, 1104725 (2023); DOI).
February 22nd, 2023
Older readers may remember a western movie with the title "The Good, the Bad and the Ugly", and this could be applied to lipids, although the Good and the Bad are not always easily distinguished. Cholesterol in membranes or as a hormone precursor is good, but in the wrong lipoproteins it is bad, and further comparisons could be made for any number of other lipids. Arsenolipids must be among the Uglies. They are produced in marine organisms and enter fish and then us via the food chain. So far, the consensus appears to be that only hydrocarbons containing arsenic are really toxic, and they occur in fish at levels that are too low to be a problem. However, we must remain vigilant.
On the other hand, the cyanolipids appear to have been designed by nature with a 'license to kill'. They occur in seeds in some plants from the family Sapindaceae, for example as fatty acid esters of 1-cyano-2-hydroxymethylprop-2-en-1-ol, and on consumption by insect or other predators, they decompose spontaneously and release hydrogen cyanide. Some bacterial species produce alkyl nitriles, such as saturated or unsaturated with an omega-7 double bond, such as (Z)-11‑octadecenenitrile, sometimes with methyl branch points, and sesquiterpenes containing nitrile or isonitrile moieties have been identified in marine invertebrates. These have potent antibiotic activities, but whether they are anti-human does not appear to be known.
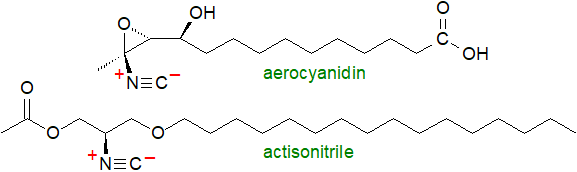
An alkyl isonitrile (actisonitrile) is known from one animal species, the nudibranch mollusc Actinocyclus papillatus, and is based on a 1,3-propanediol ether skeleton; it has cytotoxic properties. On the other hand, many pathogenic bacteria, but especially Actinobacteria (including Streptomyces sp.) and Mycobacteria, produce lipopeptides in the form of diacyl dipeptides linked to fatty acids with isonitrile substituents. In M. tuberculosis, for example, these have been termed 'kupyaphores' and contain C12 fatty acids with isonitrile substituents in position 3 and linked to a dipeptide core of ornithine and phenylalaninol. By binding to metal ions, they are toxic to their hosts.
February 15th, 2023
When I do my weekly literature search, I tend to spend most time on review articles rather than the primary literature as the former usually give me a better perspective on new developments. Sometimes this is hard work, but this week I found one, which aside from being instructive, is as entertaining as any novel (Mechoulam, R. A delightful trip along the pathway of cannabinoid and endocannabinoid chemistry and pharmacology. Annu. Rev. Pharm. Toxic., 63, 1-13 (2023); DOI). Prof. Raphael Mechoulam describes his early life in a pro-German country hiding from anti-Semitic factions, where he was spared because allied victories in North Africa and Stalingrad lead to a change in the policies of the Bulgarian government (he attributes this in part to Alan Turing!). Subsequently, he moved to Israel to a University degree and ultimately a career on the chemistry and pharmacology of natural products. His best known work was on plant cannabinoids – not an obvious candidate as the raw material was not the easiest to obtain by legal means. Later, his search for natural animal mimetics of cannabinoids in brain, lead to the discovery of the endocannabinoids, anandamide and 2-arachidonoylglycerol, as described in seminal papers in the early 1990s.
It is a while since I have complained here about the publishing industry and their access policies, largely because they have improved greatly in general in recent years. There are still outliers who appear to have been resisting the trend, and the Annual Review series is one of them. However, the article cited above is in an open access issue, and is accompanied by several further lipid reviews, most of which deal with fatty acids and oxylipins although there is one that may be relevant to those with a ‘taste’ for arsenolipids.
February 1st, 2023
As I mentioned in my last blog, I enjoy a healthy debate on scientific matters. However, it takes two to tango or tangle. Last year, a highly contentious article on the science of what have been termed specialized proresolving lipid mediators was published (DOI = 10.3389/fphar.2022.838782) by a multi-author/multi-national group, and I drew attention to it in this blog. There were several criticism of which the most damning was that the analytical methodology that had been used to identify and quantify these lipids in tissues at natural levels was not adequate, was not validated and therefore could not substantiate the claims. A new opinion piece re-draws attention to the defects (DOI = 10.1093/function/zqac067). Two substantial new publications in Seminars in Immunology have now been published, and while they appear to answer some of the criticisms (e.g., with regard to the receptors), neither cites the critical article nor attempts to refute it. I have used the Citation Index to check, but I was sorry to find nothing that does so. I am disappointed as I have nothing but admiration for the work on the structural/stereochemical characterization of these oxylipins and on the clinical trials. Again, I should add that I am merely an observer with no investment in either side of the argument.
January 25th, 2023
 I have written here before
on the debate concerning the nutritional value of saturated fatty acids.
As long as I have had any interest in the subject (more than 50 years), public health policy in most developed countries has been determined
by the belief that saturated fat causes cardiovascular disease by raising serum cholesterol.
However, this hypothesis is increasingly being questioned as re-examination of the clinical data has apparently failed to establish a causal link.
A newly published commentary (or polemic) on the subject makes a strong case that the current guide lines should be changed and makes some
interesting and contentious suggestions as to why this has not happened already (Teicholz, N. A short history of saturated fat:
the making and unmaking of a scientific consensus. Curr. Opinion Endocrin. Diab. Obes., 30, 65-71 (2023);
DOI - open access).
I have written here before
on the debate concerning the nutritional value of saturated fatty acids.
As long as I have had any interest in the subject (more than 50 years), public health policy in most developed countries has been determined
by the belief that saturated fat causes cardiovascular disease by raising serum cholesterol.
However, this hypothesis is increasingly being questioned as re-examination of the clinical data has apparently failed to establish a causal link.
A newly published commentary (or polemic) on the subject makes a strong case that the current guide lines should be changed and makes some
interesting and contentious suggestions as to why this has not happened already (Teicholz, N. A short history of saturated fat:
the making and unmaking of a scientific consensus. Curr. Opinion Endocrin. Diab. Obes., 30, 65-71 (2023);
DOI - open access).
I have no axe to grind, and I am happy to sit on the side lines (my preferred margarine contains both olive oil and butter fat) and enjoy a robust debate between others who are better qualified in nutrition and the clinical consequences. It may be worth pointing out that saturated fatty acids can be negative factors in cancer and inflammatory disease not just cardiovascular disease. In contrast, palmitic acid has many important essential properties, for example as precursors of sphingoid bases and S-acyl proteins (I love to cite the hedgehog proteins which contain both covalently linked cholesterol and palmitate). Meantime, is anyone doing any clinical/epidemiological studies on Asian populations who rely on palm oil as a relatively inexpensive source of calories?
January 11th, 2023
Of all the organisms that I have discussed in the writing of these web pages, the most distinctive in terms of their lipid components are the Mycobacteria and related genera. For example, the mycolic acids are β-hydroxy-α-alkyl branched structures of high molecular weight with 60 to 90 carbons, large alkyl branches and numerous other functional groups, and these are often linked to novel lipids. In addition, there are phosphatidylinositol mannosides, lipomannans, phenolphthiocerol and phthiocerol dimycocerosates, trehalose esters, which include di- to penta-acyltrehaloses and sulfatides, glucose monomycolates, mannosyl-phosphomycoketide, gentiobiosyl-diacylglycerols, (glyco)-peptidolipids, 6-O-methylglucose-containing lipopolysaccharides and not to forget more conventional glycerolipids. You can find links to all of these from my web page on mycolic acids. The more we learn of these unusual lipids from M. tuberculosis the better as this is still a major killer around the world.
Second on my list of organisms with the most unusual lipid classes are nematodes. For example Caenorhabditis elegans, used as a model species for primitive animals in research, synthesises iso-methyl-tetradecanoic and iso-methyl-hexadecanoic acids de novo, and it has been shown to be absolutely dependent on these for its growth and development. It can also synthesise linoleic acid de novo. In addition, they produce a range of distinctive cell wall and signalling lipids termed ascarosides (>300), which consist of the mono-saccharide α-L-3,6-dideoxymannose or ascarylose, which occurs in few other organisms, linked glycosidically to the hydroxyl group of a 2-hydroxy alcohol or of an (ω-1) hydroxy fatty acid (and many other side chains depending on species). These are especially abundant for egg and dauer larvae stages, where they can form a dense protective layer. In at least one species, there is a wax layer covering the dauer larvae that consists largely of a wax ester in which both the acid and alcohol components have C30 chains and cis double bonds in positions 7, 15, 18, 21, 24 and 27. Maradolipids are are the only known trehalose-containing lipids to be isolated from an animal source. To top this off, they produce a number of other unusual lipid mediators, and you can find information on all of these in a new review (Machado, R.A.R. and von Reuss, S.H. Chemical ecology of nematodes. Chimia, 76, 945-953 (2022); DOI).
Everyone with an interest in lipidomics will want to access - Zhixu, N. et al. Guiding the choice of informatics software and tools for lipidomics research applications. Nature Methods, in press (2022); DOI.
January 4th, 2023
It seems that lipid scientists (or their instruments) are boldly going into space once more this time to the moons of Jupiter on board NASA's upcoming Europa Clipper mission. As the spacecraft flies by the moons, it will pass through clouds of ice particles emitted from the surface that can be analysed by an impact ionization mass spectrometer such as the SUrface Dust Analyzer (SUDA) to identify identify fatty acids and other organics and discriminate between their abiotic and biotic origins. Patience (and longevity) is required as unfortunately it will be a while before any results are available; lift-off is not until 2024 and Jupiter will not be reached until 2030. A new study demonstrates the sensitivity of the instrument that will be used (Dannenmann, M. et al. Toward detecting biosignatures of DNA, lipids, and metabolic intermediates from bacteria in ice grains emitted by Enceladus and Europa. Astrobiology, in press (2022); DOI). I must pay tribute to the dedication of scientists who spend years designing an instrument to be carried on a rocket with the potential to be an enormous bomb to travel though space for a significant period of their careers while they keep fingers crossed that it will start up and function properly at the appointed time.
Plants produce a host of oxylipins and other lipid mediators that are distinct from those in animal tissues and have innumerable functions in plants both in their developmental processes and as defence agents against inflammatory pressures from bacteria, fungi, viruses, insect predators and other stresses. While they are different from those in animals, there are some similarities, and for example, jasmonates have a pentacylic ring like that in prostaglandins. It is not surprising therefore that they can have biological effects when administered to animals, including protective and therapeutic properties. A new review provides a comprehensive account of what is known to date and hopefully will stimulate further research (Savchenko, T. et al. Therapeutic potential of plant oxylipins. Int. J. Mol. Sci., 23, 14627 (2022); DOI).
A happy, healthy and prosperous New Year to all my readers!
Earlier entries in this blog (older than 4 months) are archived by year as follows -
| 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 |
| © Bill Christie with Guest Contributors |  |
|
| Updated: August 2nd, 2023 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
