Rhamnolipids, Sophorolipids and Other
Simple Glycolipids
Innumerable simple glycolipids comprising fatty acids or other alkyl groups linked directly to a carbohydrate moiety via O‑glycosidic or ester bonds have been described in nature from animals, plants and microorganisms, and it is not possible to discuss more than a few representative types of such molecules here. They can vary in structure from monosaccharides with one or more fatty acyl substituents to complex carbohydrates, which can in turn be linked to terpenoids, aromatic compounds or nucleosides, as well as having multiple points of attachment to fatty acids and alcohols. Some are integral or structural components of membranes in cell walls and are important antigens in bacterial infections, while others produced by microorganisms are secreted into the growth medium for various biological purposes.
 Because of their
amphipathic nature, simple glycolipids are natural surfactants, which are biodegradable and have low ecotoxicity, and can even function
under extreme conditions of temperatures, pH and salinity.
Some are reputed to have valuable pharmaceutical properties as antibiotic, anti-fungal or even anticancer agents.
As major products of certain microorganisms, they are relatively accessible through fermentation technologies,
although high costs still restrict their commercial use.
Because of their
amphipathic nature, simple glycolipids are natural surfactants, which are biodegradable and have low ecotoxicity, and can even function
under extreme conditions of temperatures, pH and salinity.
Some are reputed to have valuable pharmaceutical properties as antibiotic, anti-fungal or even anticancer agents.
As major products of certain microorganisms, they are relatively accessible through fermentation technologies,
although high costs still restrict their commercial use.
The biosynthetic mechanisms for only a few of these glycolipids have been investigated and can be discussed below, but in general glycosyltransferases or acyltransferases are presumed to be required. The former catalyse the transfer of the sugar moiety from an activated glycosyl donor such as a sugar-nucleotide to a lipid, while the latter catalyse the transfer of the lipid moiety from an acyl donor, usually acyl-CoA or acyl-ACP, to a glycosyl acceptor. Catabolism must involve glycoside hydrolases or lipases.
Polymeric compounds of high molecular weight, e.g., polysaccharides, proteins or lipoproteins/lipopolysaccharides, are not described here, although these may be described on other web pages on this site as are more complex glycero- and sphingo-glycolipids. While substantial amounts of simple fatty acyl derivatives of sugars, e.g., sucrose esters, are manufactured in industry by chemical means, the discussion here is restricted to natural glycolipids.
1. Some Simple Carbohydrate-Fatty Acid/Alcohol Conjugates
Simple conjugates of mono- and disaccharides with fatty acids via glycosidic or ester bonds (alkyl or acyl glycosides) are common in nature, but especially in marine organisms and in plants, and a few examples only are described below. Little or nothing is known of the functions or biosynthesis of most. A glucopyranosyl derivative of tuberonic acid (a jasmonic acid metabolite) is known to induce tuber formation in potatoes (see our web page on plant oxylipins).
Leukotoxin glucuronides: Linoleic acid is oxidized in the human liver by a cytochrome P450 mono-oxygenase to a mixture of 9,10- and 12,13-epoxides, which are converted to the corresponding diols, known as leukotoxin and isoleukotoxin, by an epoxide hydrolase (note that the term 'leukotoxin' is sometimes used in a wider sense for many non-lipid compounds toxic to leukocytes - see our web page on hydroxy-eicosatetraenoic acids (HETE)). Specific enantiomers of each of the four possible hydroxyl groups can then be converted to glucuronides by the action of a UDP-glucuronosyltransferase. The products from 9,10‑dihydroxyoctadec-12-enoate are illustrated.
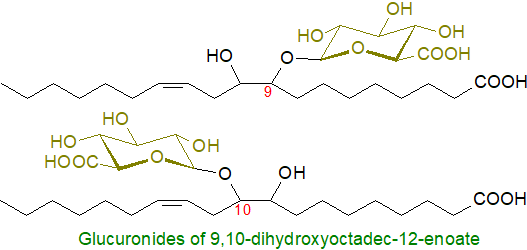
As the precursor monoepoxides of linoleic acid are formed at high levels during acute inflammation and in patients with adult respiratory distress syndrome or suffering from severe burns, glucuronidation may be a detoxification mechanism to facilitate excretion. Alternative suggestions are that some fatty acyl glucuronides, such as those of phytanic and docosahexaenoic acids, may be ligands for hormone receptors in the nucleus or act in signalling.
Trichome sugar esters: Plants in the Solanaceae and many other families have glandular trichomes, i.e., secretory organs often associated with hairs on the leaves and other external surfaces. These secrete mixtures of sugar esters, which vary in composition according to plant species, onto the plant aerial surfaces where they may act as protective agents against insect herbivores, pathogenic bacteria and fungi and may serve in the regulation of plant growth. In the tomato, these metabolites consist of a carbohydrate backbone, usually glucose or sucrose (occasionally inositol), to which two to five fatty acids are esterified, while S. nigrum produces triacyl-inositols and diacyl- and triacyl-glucoses. The aliphatic acyl chains in such sugar esters vary in length from C2 to C12 and are straight-chain or have iso- or anteiso-methyl-branches. In the petunia (S. pennellii), molecular species containing malonic acid have been detected.
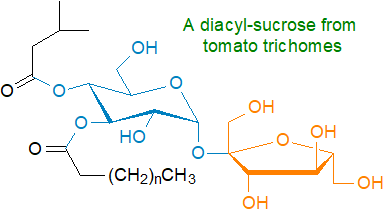
For biosynthesis, there is a series of reactions catalysed by a family of at least four acylsucrose acyltransferases in which fatty acids as the CoA esters are transferred sequentially onto characteristic positions of the sugar core, and plant breeders have generated improved breeding lines with increased acyl-sugar content and trichome density for better insect resistance in crops.
Heterocyst glycolipids: Many cyanobacterial species contain distinctive organelles termed heterocysts, which are capable of fixing nitrogen. The cell walls of these maintain a micro-anaerobic environment to protect the nitrogenase and enable the reaction to occur, and they consist of three extra layers external to the normal cell envelope, the innermost of which is comprised of unusual glycolipids, i.e., very-long-chain fatty alcohols (up to 32 carbons) linked to a carbohydrate moiety, such as the 1‑(O‑α‑D‑glucopyranosyl)-3R,25R-hexacosanediol illustrated.
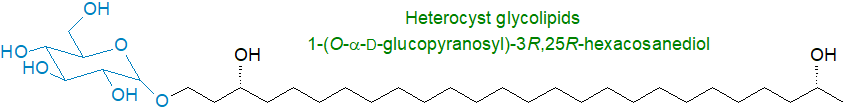
Other forms exist differing in the number of carbon atoms, and the number and position of hydroxyl and/or keto groups, but they usually have C26 or C28 carbon-chains with hydroxyl groups at the C-3, omega-1 or omega-3 positions. Often, keto-ols and keto-diols have their carbonyl moieties mainly in the C-3 position. Marine endosymbiotic cyanobacteria contain related heterocyst glycolipids with longer-chain alcohols (C28 to C32) linked to the C5 sugar ribose or 6‑O-methyl-β-D-glucopyranose. Nitrogen-fixing cyanobacteria of the order Stigonematales have 1‑(O‑hexose)-3,29,31-dotriacontanetriol (C32) as the main isomer with a related compound with a 29-keto group.
Ascarosides: Nematodes, including some human parasites, contain unusual glycolipids termed ascarosides mainly in the eggs and ovaries. These consist of the mono-saccharide α-L-3,6-dideoxymannose or ascarylose, which occurs in few other organisms, linked glycosidically to the hydroxyl group of a 2-hydroxy alcohol or of an (ω‑1)‑hydroxy fatty acid. The free hydroxyl groups of the ascarylose moiety may be acetylated or linked to amino acids such as tyrosine or tryptophan, or they can have a glucose residue attached to carbon 2' of ascarylose or indole-3-carboxylic acid, hydroxybenzoyl or tigloyl moieties attached to position 4'. Among further modifications, the carboxyl group can be amide-linked to ethanolamine or p-aminobenzoic acid or ester-linked to a carbohydrate or nucleoside; the chain length of the alkyl component can vary from 3 to 33 and can contain further hydroxyl or keto groups or double bonds. The result is innumerable structural variations (>300) with presumably many different functions of which two representative examples are illustrated.
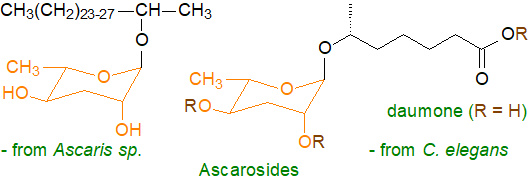
The eggs of Ascaris sp. have a four-layer shell, the innermost layer of which consists of 75% of ascarosides and is responsible for the impermeability of the shell, which protects the contents from the harsh conditions in the intestines of host animals. Some of the 200 or so ascarosides known from the model nematode species Caenorhabditis elegans act as pheromones as well as signalling molecules that regulate many aspects of aging, development and behaviour. Of these, daumone, a short-chain (ω-1)-hydroxy acid linked to ascarylose (and often then to more complex entities), is part of a signalling mechanism that acting mainly through G-protein-coupled receptors controls the entry and exit of nematodes from a 'dauer' stage, in which they enter a form of suspended animation until the external conditions improve. Biosynthesis involves dedicated pathways requiring carboxylesterases in lysosome-related organelles. In human hosts, ascarosides inhibit allergic responses and have pharmaceutical potential. With plants, ascarosides act as extracellular signalling molecules that elicit defences and pathogen resistance by activation of protein kinases or through salicylic acid- and jasmonic acid-mediated defence signalling pathways. In at least one nematode species, there is an additional wax layer covering the ascarosides that consists largely of an unusual wax ester ('nematoil').
Maradolipids: These are the only known trehalose-containing lipids isolated from an animal source. Like daumone, they are present in the dauer larvae of the nematode C. elegans and are structurally similar to the 'cord factor' trehalose lipids from Mycobacteria and described below, i.e., they are 6,6'-di-O-acyltrehaloses linked to various saturated, branched-chain and monoenoic fatty acids, of which the most abundant molecular species is 6-O-(13-methylmyristoyl)-6’-O-oleoyltrehalose (monoacyl-maradolipids are known also).
Other glycolipids: Glycolipids consisting of a repeated xylose-mannose backbone (tetra-, hexa- and octasaccharide subunits) with fatty acyl moieties attached act as an antifreeze in an Alaskan beetle and some species of insects, fish, arthropods, plants, fungi, and bacteria, but the nature of the lipid-polysaccharide linkage has still to be established. Lanceolitols are glycolipids with myo-inositol linked to glucose or xylose and a fatty acid and were isolated from leaves of Solanum lanceolatum. Myxomycetes (slime moulds) produce unusual glycolipids containing glucose, mannose or rhamnose linked to a multimethyl-branched-polyunsaturated fatty acid (quite different from the more conventional fatty acids).
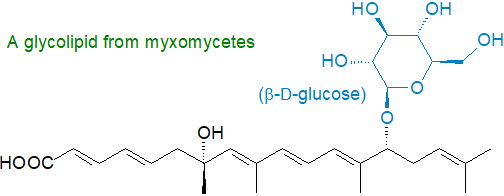
The bacterial species Serratia rubidaea synthesises simple glucose derivatives termed rubiwettins, e.g., β-D-glucopyranosyl 3‑(3'‑hydroxytetradecanoyloxy)decanoate and related molecular species, as well as rhamnolipids (next section). Diacyl maltose derivatives (sporangimicins A-D) are produced by an actinomycete Pseudosporangium sp. RD061809. Pathogenic mycobacteria contain phenolic glycolipids related structurally to the phthiocerol wax esters that are known to enhanced mycobacterial virulence in vivo and suppress the inflammatory responses of host. Mandimycin, obtained by genome mining in bacteria, is a polyene macrolide linked to three deoxy sugars with potent fungicidal activity against fungal pathogens by targeting phospholipids in their cell membranes.
Many different glycolipids of variable complexity have been isolated from marine organisms, and these include agminosides, ancorinosides, bartolosides, caminosides, clathrosides and simplexosides. As an example, glucosyl-tetra(3-hydroxy-acyl)-glycine from the marine bacterium Alcanivorax borkumensis is a surface-active lipid located in the cell wall that aids the organism to solubilize and ultimately degrade alkanes in oil spills.
2. Rhamnolipids
Pseudomonads are rod-shaped gram-negative bacteria and opportunistic human pathogens that are found in soils and secrete extracellular lipids known as rhamnolipids, which are the most intensively studied biosurfactants because they have high surface activities and are made in high yields after relatively short incubation periods by a well-understood microorganism. The name is indicative of the fact that these lipids contain one or two L‑(+)-rhamnose units, linked glycosidically to a 3R-hydroxy acid, thence by an ester bond to a further 3R-hydroxy acid as illustrated; two rhamnose units are joined by an α-1,2-glycosidic linkage, while forms that lack the second fatty acid or have a third occur. Thus, the mono-rhamnolipid from Pseudomonas aeruginosa grown on hydrocarbons is 2‑O‑α‑L-rhamnopyranosyl-α-L-3-hydroxydecanoyl-3-hydroxydecanoic acid. The non-pathogenic species P. chlororaphis produces only mono-rhamnolipids as it lacks a key enzyme. While 3- or β-hydroxydecanoic acid is the most common fatty acid constituent, others may be found depending on the Pseudomonas species or growth conditions, including 12:0, 12:1, 12:2 and 8:2 (each with a 3-hydroxyl group), resulting in many molecular species. Rhamnolipids are essential for the growth of P. aeruginosa on hydrophobic carbon sources.
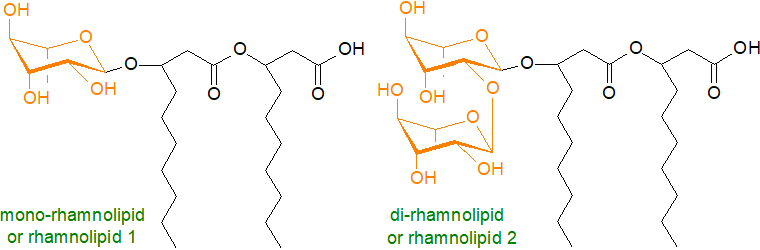
Rhamnolipids are considered one of the most effective biosurfactants, because they reduce the surface tension in water from 72 to <30 mN/m with critical micellar concentration values between 20 and 225 mg/L, depending largely on the nature of the acyl groups. As potent detergents, they are available commercially as soil remediation agents to remove hydrocarbon and heavy metal pollutants and to combat marine oil pollution. Although the exact mechanism is not clear, it is evident that rhamnolipids are able to bind to substrates with low degrees of aqueous solubility including hydrophobic pollutants. The mono- and di-rhamnolipids appear to be used for separate purposes in the swarming motility of the organisms, leading to the formation of biofilms that facilitate colonization of new environmental niches. All these glycolipids are potent fungicides to Fusarium and Phytophthora species, but it has been suggested that claims of antiviral and bactericidal properties are not robust, although they may stimulate plant innate immunity. Although they are anti-cancer agents in vitro, they are one of the virulence factors in human infections by Pseudomonas sp.
Biosynthesis of rhamnolipid in Pseudomonas species occurs during the late exponential and stationary phase of growth under limiting concentrations of iron and nitrogen. There are two sequential glycosyl-transfer reactions catalysed by rhamnosyltransferases, in which the dimeric estolide 3‑hydroxydecanoyl-3-hydroxydecanoate is linked to an activated rhamnose moiety (thymidine diphospho-rhamnose), generated from glucose-1-phosphate, while monoacyl-rhamnolipids are derived from hydrolysis of the diacyl forms, not by release of the partially acylated intermediate.
Rhamnolipids are a source of L-rhamnose for the chemical industry, and some genetically modified Pseudomonas species can generate as much as 100g/L of culture medium under optimum conditions. While the wild organisms are pathogenic and must be cultured in a strictly regulated environment, the recombinant Pseudomonads used in industry are known to be safe.
Some non-pathogenic thermophilic bacteria of the genera Thermus and Meiothermus synthesise rhamnolipids in a wide range of molecular forms, including mono- and di-rhamnolipid homologues containing one or two 3-hydroxy-fatty acids with zero to two double bonds and up to 24 carbon atoms. Similarly, the nitrogen-fixing soil bacterium Burkholderia kururiensis makes appreciable amounts of rhamnolipids, while Serratia species can produce both rhamnolipids and the surfactant lipopeptides termed serrawettins. Two unusual rhamnolipids, designated myxotyrosides A and B, have been isolated from a Myxococcus sp. (gliding bacteria); these have a rhamnose unit linked to tyrosine and thence to a fatty acid such as 15‑methyl-2Z-hexadecenoic and 2Z-hexadecenoic acid. Rhamnolipid-like metabolites from the non-human pathogenic bacterium Pantoea ananatis contain glucose instead of rhamnose residues and termed ananatoside A, a 15‑membered macrodilactone-containing glucolipid (akin to the sophorolipids below), and ananatoside B, its open-chain congener.
3. Sophorolipids
Some yeast species, and in particular the non-pathogenic Candida (Torulopsis or Starmerella) bombicola, secrete extracellular glycolipids known as sophorolipids (or sophorosides) as they contain the sugar sophorose (β-D-Glc-(1→2)-D-Glc). This is linked glycosidically to the hydroxyl group of a 17‑(L)‑hydroxy-C18 saturated or monoenoic (cis-9) fatty acid, the carboxyl group of which is usually linked to the 4’‑hydroxyl group of the second glucose unit to form a lactone though it can remain in free form; one or both 6‑hydroxyl groups on the glucose units are acetylated. With the organism C. bogoriensis, the fatty acid is 13‑hydroxydocosanoate, while in C. batistae it is 18‑hydroxy-stearic acid (and the acidic form is the main one). Up to twenty different molecular forms have been found in various yeast species, differing in the nature and number of acyl constituents, but usually one or two forms predominate in any given species. In so-called 'bolaform surfactants' (dimeric or trimeric), the free acid group is attached to a separate sophorose unit. Although these are present in trace amounts only under normal conditions, they can accumulate in genetically modified organisms. Forms with two or three of the acidic sophorolipids linked to glycerol are also formed in S. bombicola and represent ~17% of the total sophorolipid content. i.e., they are di- and triacylglycerols of acidic sophorolipids.
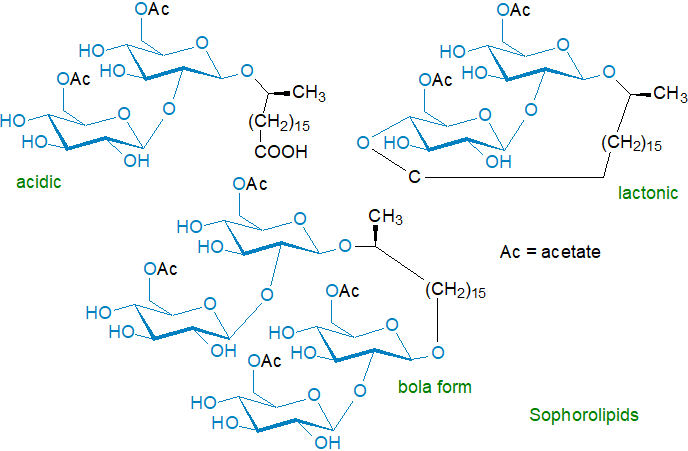
Biosynthesis starts with hydroxylation of a fatty acid by the cytochrome P450 monooxygenase enzyme CYP52M1 in the ω- or (ω‑1)‑position, followed by sequential transfer of activated glucose molecules (UDP-glucose - see our web page on glycosyldiacylglycerols) to the hydroxy acid in reactions catalysed by two different glycosyltransferases, i.e., glucosyltransferase I, which adds the first glucose, followed by linkage of a second glucose to the first via a glucosyl-β-(1,2)-bond by glucosyltransferase II. The next step is formation of the bola form as an intermediate, before the molecule is acetylated by an acetyltransferase followed by lactonization by a lactone esterase (and not from the acidic form, which is an alternative endpoint not an intermediate). The fatty acid constituents can be made de novo from acetate or by modifying alkanes in the growth medium. Biosynthesis of such secondary metabolites occurs under conditions of nitrogen starvation mainly, and the relevant genes may be organized in clusters that facilitate transcription and regulation.
While the physiological role of sophorolipids in yeast species is uncertain, it seems likely that they serve for extracellular carbon storage (reducing the cellular sugar content) and as a defence against competing microorganisms by antimicrobial, anti-biofilm and antifungal activities.
These lipids are obtained on a commercial scale by culturing the organism on substrates containing glucose and a source of alkyl moieties, such as alkanes or seed oils, which influence the nature of the fatty acid constituent; genetic modification of the organism is used to improve yields, which can be as much as 300g/L from organisms in the stationary phase, and to modify the nature of the products. As a generality, sophorolipid lactones have better surface tension lowering and antimicrobial activity, whereas the acidic forms display better foam production and solubility. They are being used increasingly as detergents and 'eco-surfactants', and they are used in cosmetics as deodorant, anti-dandruff and bacteriostatic agents, while the hydroxy acid constituents are in demand for lactonization and thence for use in perfumes. They are known to possess antifungal, antiviral and spermicidal properties, and as they induce apoptosis of cancer cells specifically in vitro, they have some clinical potential.
4. Mannose- and Mannitol-containing Lipids from Yeasts
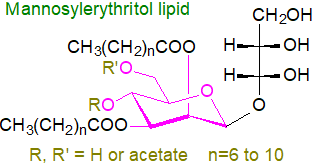 Mannosylerythritol lipids are formed abundantly in yeast strains of the genus
Pseudozyma; they consist of fatty acids linked to either 4-O-β-D-mannopyranosylerythritol
or 1-O-β-D-mannopyranosylerythritol as the hydrophilic head group.
One or two of the hydroxyls (4' and/or 6') on the mannose residue are acetylated, and there are one to three esterified fatty acids,
which are both odd- and even-numbered from C8 to C12 in chain-length (longer in related species).
Biosynthesis of the carbohydrate moiety occurs first, followed by acylation with short- and medium-chain fatty acids at positions C2 and C3
by acyltransferases Mac1 and Mac2, respectively.
In the final step, positions C4 and C6 of the mannosyl moiety are acetylated by acetyl-CoA-dependent acetyltransferase Mat1.
The genes for the relevant enzymes occur as a cluster.
Mannosylerythritol lipids are formed abundantly in yeast strains of the genus
Pseudozyma; they consist of fatty acids linked to either 4-O-β-D-mannopyranosylerythritol
or 1-O-β-D-mannopyranosylerythritol as the hydrophilic head group.
One or two of the hydroxyls (4' and/or 6') on the mannose residue are acetylated, and there are one to three esterified fatty acids,
which are both odd- and even-numbered from C8 to C12 in chain-length (longer in related species).
Biosynthesis of the carbohydrate moiety occurs first, followed by acylation with short- and medium-chain fatty acids at positions C2 and C3
by acyltransferases Mac1 and Mac2, respectively.
In the final step, positions C4 and C6 of the mannosyl moiety are acetylated by acetyl-CoA-dependent acetyltransferase Mat1.
The genes for the relevant enzymes occur as a cluster.
The yeast Pseudozyma (Candida) antarctica secretes an extracellular mannosylerythritol lipid (4-O-(2’,6’-di-O-acyl-β-D-mannopyranosyl)-D-erythritol) in high yield as biosurfactants when grown on a vegetable oil or alkane substrate. When grown on glucose, the same lipid accumulates intra-cellularly as an energy store until it amounts to 10% or more of the dry weight of the cell, and as with sophorolipids, enhanced yields are obtained under conditions of nitrogen deprivation. Several other species of the genus Pseudozyma are now known to synthesise similar lipids in which the nature, number and positions of the acyl groups vary, and for example, P. aphidis produces a tri-acylated molecule in which the third fatty acid chain is attached to the primary alcohol of the erythritol moiety and is comprised mainly of oleic and linoleic acids, which are not found in conventional di-acylated mannosylerythritol lipids. Some of these lipids are finding commercial applications in food, pharmaceuticals and cosmetics.
As with other biosurfactants, these compounds facilitate dissolution of organic hydrophobic compounds so that they can be consumed by the organism. Mannosylerythritol lipids have been shown to have profound biological effects in animals and can induce the differentiation of certain cancer cells in vitro. They are anti-inflammatory in that they inhibit the secretion of inflammatory mediators from mast cells, and they are effective antibiotics against Gram-positive bacteria. Because of their non-toxicity, easy biodegradability and environmental compatibility, they have applications in industry as antimicrobial agents in food and as moisturizers in cosmetics, while in agriculture they are used as wetting agents.
Although Pseudozyma species give the greatest yields of these lipids, they were first found in the fungus Ustilago maydis, which causes smut disease on corn, and termed ustilipids. In this instance, the 2‑hydroxyl group of the mannose residue is esterified with a C2 to C8 fatty acid, while the 3-hydroxyl group is esterified by a C12 to C20 fatty acid.
Other Pseudozyma sp. make related lipids in which the erythritol moiety is replaced by mannitol, arabitol or ribitol. Acremomannolipin A from the filamentous fungus Acremonium strictum has D-mannitol linked to mannose, in which all the hydroxyls of the latter are acylated with saturated fatty acids. Malassezia pachdermatis is an opportunistic skin fungal pathogen and contains a unique glycolipid with an unusual triol-triester architecture comprising an L‑mannitol core and three 10-hydroxystearic acid groups to which mono- or dimannosyl units are attached. This is recognized by the human immune system (Mincle (see below) and Dectin-2).
Liamocins are a class of mannitol-lipids from the black yeast Aureobasidium pullulans, which are used commercially to generate pullulan polysaccharide for breath-freshener films. They have a mannitol head group ester linked to 3,5-dihydroxydecanoate acyl chains, several of which are joined by 1,5‑polyester linkages (up to six). Four such lipids were characterized from Aureobasidium strain NRRL 50380 with the A series containing three acyl groups and the B series containing four; liamocins A1 and B1 are non-acetylated, whereas A2 and B2 each contain a single 3-O-acetyl group. Some forms are showing promise as anticancer agents and others are antibiotics with a specificity for Streptococcus species.
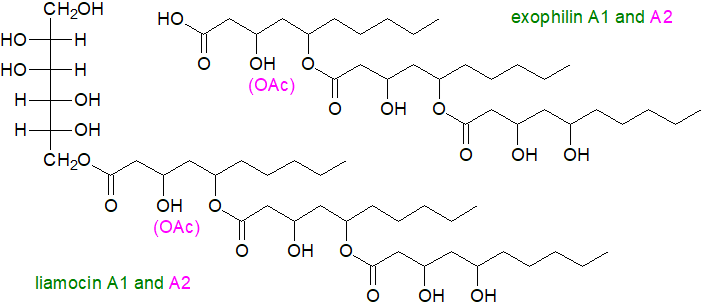
The exophilins are related molecules lacking the mannitol residue, i.e., they are free estolides, which were first found in the marine yeast Exophiala pisciphila but have been detected in Aureobasidium; they have pronounced antibacterial properties. Similar lipids containing fatty acids linked to polyols are present in yeasts belonging to the Rhodotorula genus.
5. Trehalose Lipids from Mycobacteria and Related Organisms
Trehalose is a non-reducing disaccharide in which two glucopyranosyl units are linked in an α,α-1,1-glycosidic linkage that has high thermostability and resistance to acid hydrolysis, while not partaking in the Maillard reaction. In nature, it is considered to be a universal stress-protector molecule with effects upon cryoprotection, growth regulation and osmoregulation in plants. In those bacteria of the Actinomycetales that degrade alkanes, such as Mycobacteria and Corynebacteria, it is a basic component of some cell wall glycolipids.
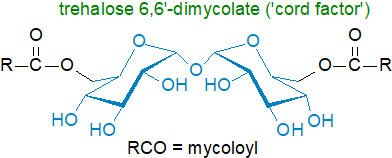 Of
these trehalose lipids, cord factor is the best known
(the name comes from its supposed influence on the arrangement of M. tuberculosis cells into long slender formations).
They are components of the inner and outer membranes of the cell wall of M. tuberculosis and comprise distinctive branched and
very-long-chain mycolic acids esterified to the 6-hydroxyl group of each glucose to give trehalose 6,6’-dimycolate.
In this esterified form, the mycolic acid residues adopt a singular 'W' conformation with their mycolates
oriented toward the cell plasma membrane and their trehalose moiety to the external environment.
Such is the complexity of mycolic acids, which differ in chain length and the presence of cyclopropane rings
and various oxygenated substituents, that 500 molecular species are possible.
Our web page on mycolic acids discusses the cell wall lipids of M. tuberculosis
at greater length.
Of
these trehalose lipids, cord factor is the best known
(the name comes from its supposed influence on the arrangement of M. tuberculosis cells into long slender formations).
They are components of the inner and outer membranes of the cell wall of M. tuberculosis and comprise distinctive branched and
very-long-chain mycolic acids esterified to the 6-hydroxyl group of each glucose to give trehalose 6,6’-dimycolate.
In this esterified form, the mycolic acid residues adopt a singular 'W' conformation with their mycolates
oriented toward the cell plasma membrane and their trehalose moiety to the external environment.
Such is the complexity of mycolic acids, which differ in chain length and the presence of cyclopropane rings
and various oxygenated substituents, that 500 molecular species are possible.
Our web page on mycolic acids discusses the cell wall lipids of M. tuberculosis
at greater length.
Trehalose diesters are major pathogenic components on the external surface of the cell wall, which influence adversely many aspects of the immune system and metabolism in host animals via specific pattern recognition receptors, of which the kinase-linked receptor macrophage inducible C-type lectin receptor (Mincle) is of special importance. This is a transmembrane protein with an extracellular carbohydrate recognition domain, and it is expressed on a variety of bone marrow-derived myeloid cells including monocytes, macrophages, neutrophils and dendritic cells. Mincle can recognize and respond to minute quantities of glycolipids derived from microorganisms by producing chemokines, cytokines and inducible nitric oxide synthase. Trehalose diesters are responsible for the low permeability of the Mycobacterial membranes and confer appreciable antibiotic resistance to the organisms. As tuberculosis is one of the most ancient infectious diseases known to man and is still responsible for over a million deaths world-wide every year, there is an intense interest in the molecular biology of the lipids of the organism with the hope of finding a drug that disrupts cell wall biosynthesis.
During biosynthesis, trehalose is first esterified with mycolic acids in the cytoplasm to form the trehalose monomycolate, which is the precursor to the di- and poly-mycolates at the plasma membrane. The initial step involves transfer of mycolic acid in thioester linkage first to mannopyranosyl-1-phosphoheptaprenol and thence to trehalose 6-phosphate to yield 6‑O‑mycolyl-trehalose-6'-phosphate, which is dephosphorylated by a phosphatase for further acylation. Via the action of a mycolyl transferase, this intermediate can translocate to the periplasm or outer membrane to serve as the donor of mycolic acid residues to the cell wall arabinogalactan to produce the mycolyl-arabinogalactan-peptidoglycan complex.
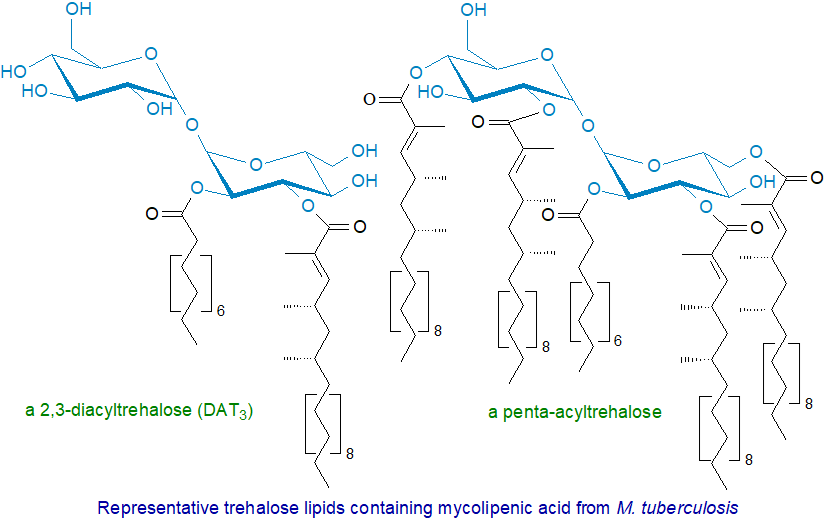
Among the other antigenic glycolipids in the mycobacterial cell wall based upon trehalose, there are acylated trehaloses with various di- and trimethyl fatty acids (mycobacteric acids) attached to the 2‑ and 3‑hydroxyl groups of the same glucose together with tri- and penta-acyltrehaloses, which have structural functions in the cell envelope, while the 2,3‑di‑O-acyltrehalose family (DAT1 to DAT4) are capable of modulating host immune responses and can inhibit the proliferation of murine T cells. The core structure consists of a central trehalose moiety, which is esterified on the 2-position with a saturated straight-chain fatty acid (C10 to C18) with on other positions as many as four multimethyl-branched long-chain fatty acids, including C21‑25 α-methyl-branched fatty acids and C24‑28 α-methyl-branched, β-hydroxy fatty acids, as well as the mycolic acids. These lipids exist in 30 basic structural forms with up to six isomeric forms in each, and they are synthesised sequentially by adjacent enzyme systems. Sulfoglycolipids consisting of 2,3,6,6′-tetraacyl-α-α’-trehalose-2′-sulfates are present in M. tuberculosis (see our web page on sulfolipids).
Trehalose lipids from Corynebacteria and Nocardia have comparable structures but contain the corynomycolic or norcardomycolic acids, respectively, which are related in structure to the mycolic acids. For example, 28 molecular species of trehalose esters have been characterized from C. glutamicum that contain 3 or 4 long-chain fatty acids, some with 2-hydroxyl groups that can be acetylated. Some Mycobacteria, e.g., M. abscessus, synthesise trehalose polyphleates, which contribute to the pathogenicity of the organisms.
Lipooligosaccharides from M. tuberculosis are highly polar lipids that are associated with biofilm formation and motility. They have one trehalose unit with one or two long-chain poly-methylated branched fatty acids, which can be saturated or unsaturated, and they are located in both glucose residues of the trehalose end of a polymer with up to six glucose units, depending on species. They are useful for serotyping mycobacterial isolates and participate in the infection of host macrophages.
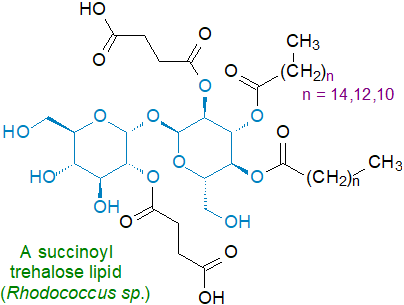
Strains of Rhodococcus sp., which are closely related to Mycobacteria and Corynebacteria, contain many different trehalose-containing lipids, including trehalose-6-monocorynomycolates, trehalose-6,6'-dicorynomycolates and other species containing up to eight acyl groups, which can comprise corynomycolic acids, long-chain dibasic acids and methyl-branched acids, such as 10‑methyloctadecanoic acid. Some of these lipids contain succinic acid, e.g., 2,3,4,2'-di-O-succinoyl-di-O-alkanoyl-α,α-trehalose and 2,3,4-mono-O-succinoyl-di-O-alkanoyl-α,α-trehalose, and a 3,4-di-O-alkanoyl-2-O-succinoyl-α-D-glucopyranosyl-2'-O-succinoyl-α-D-glucopyranoside from Rhodococcus sp. SD-74 is illustrated; they are powerful surfactants. These lipids are located in the cell envelope of Rhodococcus species, and they are produced mainly when the organisms are grown on hydrocarbons. As they are immunomodulatory in vivo, they are attracting some biomedical interest.
Arabinose-mycolate esters of increasing complexity from arabinose monomycolates to penta-arabinose tetramycolates have been identified and induce production of tumour necrosis factor-alpha (TNF-α). In a glucose-rich environment, M. tuberculosis synthesises simple glucose monomycolates in which the mycolic acid is from the organism, and glucose is derived from the host; they are recognized as glycolipid antigens. Phosphatidylinositol mannosides and glycosyl phthiocerol esters from Mycobacteria and related species are discussed in separate web pages.
6. Cellobiose Lipids
As well as the mannose-containing lipid described above, the fungus Ustilago maydis contains cellobiose lipids termed ustilagic acids, consisting of the disaccharide cellobiose linked O‑glycosidically to the ω‑hydroxyl group of the unusual long-chain fatty acids 15,16‑dihydroxyhexadecanoic or 2,15,16‑trihydroxyhexadecanoic acid, which are synthesised by sequential oxidations of palmitic acid by cytochrome P450 enzymes. The remaining hydroxyl groups are esterified either to acetate or a medium-chain 3-hydroxy fatty acid. In this organism, rapid and substantial production of the mannosyl ustilipids (see above) and ustilagic acid occurs under conditions of nitrogen starvation, the yield and ratio of each class depending on the available carbon source. They are biosurfactants with antifungal properties.
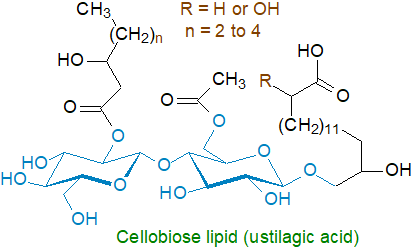
A further cellobiose lipid from the fungal biocontrol agent, Pseudozyma flocculosa, and has been shown to be 2‑(2',4'‑diacetoxy-5'-carboxy-pentanoyl)octadecyl cellobioside (flocculosin), the fungicidal compound in the organism. Various other structurally distinct cellobiose lipids are generated in related species such as Cryptococcus humicola.
7. Polymeric Exolipopolysaccharides
Emulsan is a lipopolysaccharide from Acinetobacter calcoaceticus and the less toxic A. venetianus and consists of a trisaccharide backbone of D‑galactosamine, D-galactosaminouronic acid and a deoxyaminohexose, to which fatty acid groups ranging in chain-length from C10 to C22 are linked via ester and amide bonds. It is variable in composition depending on growth conditions, both in the nature of the fatty acyl constituents and the degree of branching of the carbohydrate backbone, with an approximate molecular weight of 1,000 kDa.
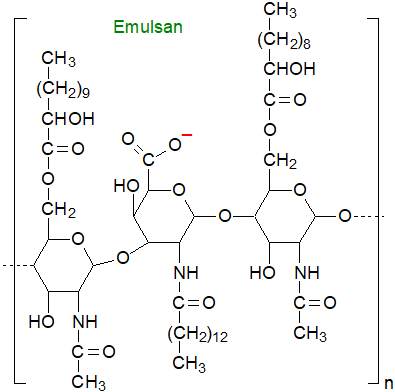
Rather than reducing surface tension, it is a very strong emulsifier, especially for hydrocarbons, even at low concentrations (0.01 to 0.001%), i.e., it is a bioemulsifier not a biosurfactant. It is already in use for commercial applications in oil recovery, transportation and bioremediation, and it is approved for use as an adjuvant to enhance the human immune response.
Suggested Reading
- Abdel-Mawgoud, A.M. and Stephanopoulos, G. Simple glycolipids of microbes: chemistry, biological activity and metabolic engineering. Synth. Syst. Biotechn., 3, 3-19 (2018); DOI.
- Coelho, A.L.S., Feuser, P.E., Carciofi, B.A.M., de Andrade, C.J. and de Oliveira, D. Mannosylerythritol lipids: antimicrobial and biomedical properties. Appl. Microbiol. Biotechn., 104, 2297–2318 (2020); DOI.
- D'Almeida, A.P., de Albuquerque, T.L. and Ponte Rocha, M.V.P. Recent advances in Emulsan production, purification, and application: Exploring bioemulsifiers unique potentials. Int. J. Biol. Macromol., 278, 133672 (2024); DOI.
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 1. Glycosides of fatty acids and alcohols. Lipids, 39, 933-953 (2004); DOI - there are six further reviews by this author in other issues of Lipids that may be relevant.
- Gallego, R.P., von Meijenfeldt, F.A.B., Bale, N.J., Damsté, J.S.S. and Villanueva, L. Emergence and evolution of heterocyte glycolipid biosynthesis enabled specialized nitrogen fixation in cyanobacteria. Proc. Natl. Acad. Sci. USA, 122, e2413972122 (2025); DOI.
- Gong, Y., Wang, J., Li, F. and Zhu, B.D. Polysaccharides and glycolipids of Mycobacterium tuberculosis and their induced immune responses. Scand. J. Immun., 97, e13261 (2023); DOI.
- Khandare, V.C. and Madankar, C.S. An overview on trehalolipids: a promising eco-friendly bio-surfactant. Tenside Surfact. Deterg., 61, 92-104 (2024); DOI.
- Lavanya, M. Rhamnolipids: an insight to the overall characteristics of these extraordinary biomolecules. Green Chem. Letts Rev., 17, 2371012 (2024); DOI.
- Lou, Y.R., Anthony, T.M., Fiesel, P.D., Arking, R.E., Christensen, E.M., Jones, A.D. and Last, R.L. It happened again: Convergent evolution of acylglucose specialized metabolism in black nightshade and wild tomato. Sci. Adv., 7, eabj8726 (2021); DOI.
- Mnif, I., Ellouz-Chaabouni, S. and Ghribi, D. Glycolipid biosurfactants, main classes, functional properties and related potential applications in environmental biotechnology. J. Polymers Environ., 26, 2192-2206 (2018); DOI.
- Münssinger, S., Beck, A., Oraby, A. and Zibek, S. Past, present and future of glycolipids from Ustilaginaceae - A review on cellobiose lipids and mannosylerythritol lipids. J. Surfact. Deterg., 27, 647-689 (2024); DOI.
- Sar, P., Kundu, S., Ghosh, A. and Saha, B. Natural surfactant mediated bioremediation approaches for contaminated soil. RSC Adv., 13, 30586-30605 (2023); DOI.
- Wan, C., Min, L., Qin, F., Liang, S., Pan, Y., Yi, T. and Zhang, Y. Production of liamocins by Aureobasidium spp. with potential applications. Biochem. Engin. J., 188, 108687 (2022); DOI.
- Wang, Y., Chen, J. and Liu, X. A review on antimicrobial activity, anti-biofilm and synergistic effects of sophorolipids since their discovery. Appl. Biochem. Microbiol., 59 580-596 (2023); DOI.
- Witting, M., Schmidt, U. and Knölker, H.J. UHPLC-IM-Q-ToFMS analysis of maradolipids, found exclusively in Caenorhabditis elegans dauer larvae. Anal. Bioanal. Chem., 413, 2091–2102 (2021); DOI.
- Yang, B.Y., Wang, J., Zheng, X. and Wang, X. Nematode pheromones: structures and functions. Molecules., 28, 2409 (2023); DOI.

 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
