Microbial Lipopeptides and Proteolipids
Many bacteria and fungi produce proteolipids (lipoproteins) and lipopeptides (or peptidolipids) that are vital to their survival, and for example, bacterial di- and triacyl proteolipids are cell wall constituents that provide structural integrity and defend pathogenic organisms against the host immune system. Of these, lipopeptides tend to be smaller molecules with relatively few amino acids (usually <10) and one fatty acid component, and they have many different functions of benefit to the producers and are secreted into the natural medium where they are defensive against competing organisms. They can aid the interaction with their environment by creating and sustaining symbiotic relationships in mixed bacterial communities by producing biofilms or by detoxifying heavy metals. A single species can produce many structural variants or isoforms differing in one or more of the amino acids or of the fatty acid component, and a few representatives only can be discussed here. The peptides are synthesised mainly on ribosomes but are then modified post-translationally by acylation with fatty acyl moieties produced by conventional or modified fatty acid synthases or sometimes by polyketide synthases.
As such lipopeptides often have surfactant, antibacterial, antifungal, antiviral, insecticidal or haemolytic properties with a lower risk of toxicity or allergy problems, they have attracted interest from the agricultural, chemical, bioremediation, food and pharmaceutical industries. Those produced by commensal bacteria in the human intestines may have a profound influence on the metabolism of the host. Identification of the genes for lipopeptide synthesis in different organisms ('genome mining') together with an understanding of their organization within the genome holds immense promise for the discovery of new antibiotic lipopeptides of value in human medicine. Hopefully, genetic manipulation will lead to the development of other related molecules for therapeutic purposes, with assistance from enzymatic modification or chemical synthesis to produce structural analogues that may have fewer side effects. Some have promise as bio-preservatives against food-borne pathogens and those that cause deterioration of food during storage. In relation to plants, bacterial lipopeptides may be of value as biocontrol agents to enhance host resilience both by direct inhibition or elimination of phytopathogens and by induction of systemic immune responses that prime the plant for enhanced defence.
Simple fatty acid amino acid conjugates or lipoamino acids, such as the ornithine lipids, and kupyaphores (dipeptides linked to fatty acids with isonitrile moieties) are discussed in separate web pages as are the Eukaryotic proteolipids. Note that the terms ‘lipoprotein’, ‘lipopeptide’ and ‘proteolipid’ are used interchangeably for these compounds in the literature. To avoid confusion, I would prefer to reserve the term ‘lipoprotein’ for the non-covalently linked protein-lipid complexes in plasma, but as the wider usage has been in place for fifty years, it is unlikely to change.
1. Introduction to Bacterial Lipopeptides
 Bacterial lipopeptides are amphiphilic molecules that consist of short linear chains or cyclic structures of amino acids and can
include depsipeptides, i.e., peptides in which one or more of the amide bonds is replaced by an ester bond (or a lactone in cyclic structures).
They are linked via an ester or an amide bond to a fatty acid, which can vary in chain length and in the presence or absence of substituents
of various kinds such as hydroxyl groups, acetylenic bonds or methyl branches, often very different from those in Eukaryotes or even in bacterial
glycerolipids.
The amino acids consist of a mixture of proteinogenic, modified and non-proteinogenic amino acids,
including those of the D- rather than the usual L‑configuration.
Bacterial lipopeptides are amphiphilic molecules that consist of short linear chains or cyclic structures of amino acids and can
include depsipeptides, i.e., peptides in which one or more of the amide bonds is replaced by an ester bond (or a lactone in cyclic structures).
They are linked via an ester or an amide bond to a fatty acid, which can vary in chain length and in the presence or absence of substituents
of various kinds such as hydroxyl groups, acetylenic bonds or methyl branches, often very different from those in Eukaryotes or even in bacterial
glycerolipids.
The amino acids consist of a mixture of proteinogenic, modified and non-proteinogenic amino acids,
including those of the D- rather than the usual L‑configuration.
In many species, biosynthesis is not by the conventional ribosomal route but by large multi-modular, multi-domain protein complexes of linear non-ribosomal peptide synthases or hybrids with polyketide synthases. Cyclic lipopeptides tend to be the more bioactive than linear forms, with macrocyclization occurring during the last stage of synthesis by catalysis with C-terminal thioesterases. Use of non-proteinogenic amino acids and macrocyclization probably confers protection against degradation by exo- and endoproteases. As a generality, Gram-positive bacteria, and especially the genus Bacillus, are rich sources of antimicrobial cyclic lipopeptides, while Gram-negative bacteria are better known for rhamnolipid and glycolipid biosurfactants.
Overuse of broad-spectrum antibiotics to control human and plant pathogens has greatly accelerated the development of antibiotic resistance among bacteria and fungi, and anti-bacterial lipopeptides (bacteriocins) are now of great interest as part of the search for novel antibiotics and a challenge for medicinal chemistry. Many bacteriocins have been shown to have a broad spectrum of activity in that they kill bacteria rapidly and show synergy with established antibiotics, although problems remain in transferring research findings to clinical practice. Of the 3000 or so antimicrobial peptides discovered, only two lipopeptides have been approved to date by the U.S. Food and Drug Administration (FDA) for therapeutic applications with reservations because of toxicity problems, i.e., daptomycin and colistin (polymyxin E), although gramicidin could perhaps be added to the list. Chemical modification of other bacterial peptides, including addition of alternative fatty acyl moieties, is leading to potential new clinical products.
A variety of different mechanisms may be involved in the antibiotic actions of lipopeptides, both linear and cyclic, as they are very diverse amphiphilic agents that not only interact electrostatically with the charged head groups of membrane lipids, but also with the hydrophobic region of lipid bilayers. This can result in electrostatic and mechanical changes, reduction in surface tension, promotion of metal ion sequestration and a disturbance of the structures of lipid bilayers in bacterial and fungal membranes. Often, there is a general tendency to induce pore formation.
In concentrating on the potential of bacterial lipopeptides in therapeutics or in industrial applications, it is easy to forget that they are crucial for the viability of the producing organisms as chemical mediators of ecological interactions. For example, in a natural environment, Bacillus cyclic lipopeptides can induce apoptosis in fungal cells, prevent microbial adhesion to a substrate, and promote the death of phytopathogens by stimulating plant immune responses. By increasing bacterial motility as surfactants, bacteria are enabled to search for more favourable, nutrient-rich environments and colonize new habitats. In this process, they can be broad spectrum antibiotics and inhibit the growth of competing microorganisms, although in other circumstances, they can help to establish relationships with other (micro)organisms for their mutual benefit. Lipopeptides can promote or inhibit biofilm formation by changing the hydrophobic interactions with surfaces so that the producing organisms gain a competitive advantage.
In excess, heavy metals in the environment, such as arsenic, cadmium, mercury, lead and many others, are toxic to the growth of microbial communities, decreasing their diversity and population structure. Bacteria can use lipopeptides as chelating agents for these and indeed can incorporate them into their metabolism as micro-nutrients in redox reactions or as cofactors for enzymes. Thus, surfactin in monomeric form can sequester zinc and copper from soils by an interaction of the glutamic acid and aspartate residues with metal cations.
2. Lipopeptides of Mycobacteria
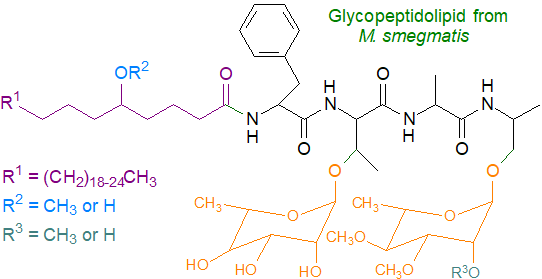
The glycopeptidolipids or ‘C-mycosides’ from non-tuberculosis Mycobacteria are amongst the best known and most studied of the lipopeptides, as they are both species and type specific. They are present in the outer leaflet of the cell wall and consist of a lipopeptidyl core, glycosylated on the peptide unit. The illustration below is of a typical member of the glycopeptidolipids of the Mycobacterium avium complex, a human pathogen that is often associated opportunistically with acquired immunodeficiency syndrome (AIDS).

The fatty acid component is usually 3-hydroxy-octacosanoate (C28), but it can consist of a range of constituents with an average chain-length of C30 and with variable numbers of double bonds; 3-methoxy fatty acids are seen on occasion. The fatty acid is linked to the N‑terminus of a tripeptide of hydrophobic amino acids of the D‑configuration (produced from the L-forms by the action of a racemase) and thence to L‑alaninol and dimethyl-rhamnose; a complex oligosaccharide is linked to the peptide via a disaccharide (deoxy-talose-rhamnose).
Mycobacterial glycopeptidolipids can be classified into two groups, polar and non-polar, differing in the structure of the oligosaccharide attached to the allo-threonine residue, which can carry additional O-acyl moieties at undefined locations. Within the M. avium complex, all have in common an N‑acylated lipopeptide core attached to a rhamnosylated alaninyl C-terminus. In other Mycobacteria, the basic structure of the lipopeptide unit does not vary appreciably, but the nature of the carbohydrate moieties does differ importantly in the degree of substitution of the deoxy-talose and rhamnose units by methyl or acetyl groups. It is the complex and highly variable oligosaccharide component that carries most of the antigenicity and type (serovar) specificity.
In M. smegmatis, the glycolipopeptides consist of C26-C34 fatty acyl chains with rather unusually either hydroxyl or methoxyl groups in position 5 that are linked to the same tetrapeptide as before (Phe-Thr-Ala-alaninol), in which the hydroxyl groups of threonine and the terminal alaninol are glycosylated. M. xenopi produces serine-containing glycopeptidolipids with a C12 fatty acyl group, while those from M. fortuitum have a somewhat different oligosaccharide and peptide structure.

M. tuberculosis produces several lipopeptides termed mycobactins and carboxymycobactins of which dideoxymycobactin-838 (illustrated) has a relatively simple structure and potently stimulates T cells upon binding to the MHC-like antigen-presenting molecule CD1a. The organism uses mycobactins as siderophores to obtain iron (Fe3+) from host immune cells as this is required for growth and other essential processes to sustain its life within the host.
Glyco-peptidolipids and other lipopeptides are variable, distinctive and highly antigenic molecules, which play significant roles in pathogenesis by inducing the host immune response. They are present on the external membrane of the organisms where an assortment of extracellular polysaccharides and lipids are located. The lipid components include phthiocerol dimycocerosates, triacylglycerols and acylated trehaloses, which are common to most Mycobacteria, and the glycopeptidolipids, which are variable in structure depending upon species. In addition to interactions with the host’s immune system, they are required for biofilm formation, aggregation, motility and cell wall integrity. While a number of models have been put forward to describe the associations of these various components within the membrane, one of the more popular situates the lipopeptides in the outermost region of the layer, where they interact via hydrophobic attractions with the mycolic acids, where there is a more extensive discussion of the cell wall structure and composition of M. tuberculosis. The amphiphilic diisocyanolipopeptides termed kupyaphores produced by Mycobacteria are discussed in our web page on cyanolipids.
In bacteria of the order Corynebacteriales, an unusual post-translation O-acyl modification of certain proteins by mycolic acids is one means of targeting them for assembly in the outer membrane (mycomembrane); a short linear amino acid motif for O-acylation of proteins has been revealed that seems to be preserved throughout the kingdoms of life.
3. Lipopeptides from Bacillus and Paenibacillus
Bacteria of the Gram-positive genus Bacillus produce numerous cyclic lipopeptides, many of which have appreciable antibacterial or antifungal properties, although only the more important of these for their pharmaceutical or industrial potential can be discussed here. There is considerable structural diversity in the fatty acid components in chain-length (C6 to C18) and often the presence of hydroxyl groups and/or iso- or anteiso-methyl branches, as well as in the type, number and configuration of the amino acids in the peptide chain. For example, various strains of B. subtilis produce more than twenty different molecules that are antibiotics including many lipopeptides. Related species produce a wide range of polyketide metabolites, some of which are linked to amino acids, but this would take us into a quite separate aspect of lipid chemistry and biochemistry.
While our interest in these lipids is their potential as antibiotic and even antiviral agents, their main purpose in the producing organisms in their natural environment is often suggested to be induction of apoptosis in fungal cells, preventing microbial adhesion to the substrate and promoting the death of some phytopathogens by stimulating plant immune responses, but this may be an over-simplification. At low concentrations, they may act as signalling molecules in the metabolism of the bacteria as part of their adaptive response to stress, perhaps to trigger the differentiation of the population into other specialized cell types, as well as to communicate and cooperate to establish mutually beneficial relationships with other organisms.
Biosynthesis: Bacterial strains in general produce lipopeptides through either ribosomal or nonribosomal pathways. In the former, a ribonucleoprotein complex generates a linear peptide, as directed by mRNA, and this is released from the ribosome into the cytoplasm, where several post-translational modifications, including epimerization and cyclization, can occur to adapt the peptide for its function. In contrast, peptides formed by nonribosomal peptide synthetases can self-modify and incorporate D- or other non-proteinogenic amino acids without a need for prior epimerization, and cyclization takes place as part of this process through macrolactonization or macrolactamization. Lipopeptides synthesised by nonribosomal mechanisms are produced by four families or genera of bacteria mainly, namely Paenibacillaceae, Bacillus, Streptomyces and Pseudomonas, each of which produces at least one group with unique structural motifs.
In Bacillus and Paenibacillus, lipopeptides are synthesised in a ribosome-independent manner by mega-enzyme complexes or nonribosomal peptide synthetases with molecular weights greater than 1.0 MDa in some instances. It has been established that production of these enzymes in an active form requires not only transcriptional induction and translation but also post-translational modification and assembly. They are organized in a systematic modular manner in assembly lines that permit the structural alteration of lipopeptide products by swapping domains or modules to create novel molecular structures. In general, the order of these modules is co-linear with the peptide sequence of the product, and each module contains characteristic domains with different enzyme components. For example, an adenylation domain recognizes a specific amino acid and forms an acyl-adenylate intermediate at the expense of ATP. The adenylated amino acid then binds covalently to a phosphopantetheine carrier or peptidyl carrier protein domain before peptide bond formation of two consecutively bound amino acids is catalysed by a condensation domain. Conversion of L-amino acids to D-isomers is carried out by an epimerization domain on the module that incorporates the latter into the growing peptide.
Bacillus species employ distinct pathways for different families of cyclic lipopeptides: the surfactin and fengycin families, which containing β‑hydroxy fatty acids, are produced by dedicated nonribosomal peptide synthetase systems, while the iturin family, characterized by β-amino fatty acids, is synthesised via a hybrid polyketide synthase/nonribosomal peptide synthetase pathway. In the former, the fatty acid component is synthesised by the normal bacterial fatty acid synthases before the 3-hydroxyl group is introduced by a CYP 450 mono-oxygenase; as the coA ester, it is linked to the first amino acid before further amino acids are added in sequence to form the peptide. In the last step in both families, a termination module, i.e., a C‑terminal thioesterase, accomplishes cyclization by employing the β-hydroxy or β-amino moiety of the fatty acid to form macrolactone and macrolactam rings, respectively, before release of the fully formed lipopeptide. In some bacteria, external tailoring enzymes can further modify the lipopeptide structure.
Lipopeptide products: Surfactin, produced by B. subtilis and B. licheniformis strains, in addition to acting as an antibiotic, is one of the most powerful biosurfactants known; it can lower the surface tension of water from 72 mN/m to 27 mN/m at concentrations as low as 20 µM. The form illustrated is composed of seven different amino acids of both the D- and L-configurations, which are assembled in a cyclic structure incorporating a fatty acid such as 3‑hydroxy-13-methyl-tetradecanoic acid, and it necessary for biofilm formation and root colonization.

Very similar molecules are produced by many other Bacillus species, and various isoforms have been described and given different names, such as bacircine, halo- and isohalobactin, lichenysin, daitocin and pumilacidin. As well as the rare D-amino acids, these can contain unusual β-amino acids and hydroxy- or N-methylated amino acids. Surfactins and lichenysins contain the chiral sequence LLDLLDL. The peptide moiety is linked to a β‑hydroxy fatty acid (C9 to C19) with a linear structure or with iso- or anteiso-methyl branches.
 The
amino acids glutamic acid and asparagine are the main polar components that counterbalance the fatty acyl moiety and give
the molecule its amphiphilic character while explaining its antibiotic activity.
For the latter, various mechanisms have been proposed, all of which depend on the fact that the hydrocarbon tail of the molecule
can insert itself readily into the membranes of both Gram-positive and Gram-negative bacteria,
where it forms associations with the hydrophilic fatty acid chains of the phospholipids.
One suggestion is that the two amino acid residues are arranged spatially so that they can stabilize divalent cations such as Ca2+.
The proximity of this to the polar head group of the phospholipids in the membrane
causes the complex to cross the lipid bilayer via a flip-flop mechanism, delivering the cation into the intracellular medium.
Alternatively, self-association of surfactin molecules on both sides of an uncharged membrane may create a pore through which cations can pass.
A third hypothesis is that such self-association of surfactin molecules
leads to the formation of mixed micelles and ultimately causes disruption of the bilayer.
The last effects are non-specific so do not produce resistant strains of bacteria.
Indeed, at high concentrations, surfactin can disrupt most membranes including those of erythrocytes and so limits pharmaceutical use,
although some modified synthetic analogues are less toxic.
The
amino acids glutamic acid and asparagine are the main polar components that counterbalance the fatty acyl moiety and give
the molecule its amphiphilic character while explaining its antibiotic activity.
For the latter, various mechanisms have been proposed, all of which depend on the fact that the hydrocarbon tail of the molecule
can insert itself readily into the membranes of both Gram-positive and Gram-negative bacteria,
where it forms associations with the hydrophilic fatty acid chains of the phospholipids.
One suggestion is that the two amino acid residues are arranged spatially so that they can stabilize divalent cations such as Ca2+.
The proximity of this to the polar head group of the phospholipids in the membrane
causes the complex to cross the lipid bilayer via a flip-flop mechanism, delivering the cation into the intracellular medium.
Alternatively, self-association of surfactin molecules on both sides of an uncharged membrane may create a pore through which cations can pass.
A third hypothesis is that such self-association of surfactin molecules
leads to the formation of mixed micelles and ultimately causes disruption of the bilayer.
The last effects are non-specific so do not produce resistant strains of bacteria.
Indeed, at high concentrations, surfactin can disrupt most membranes including those of erythrocytes and so limits pharmaceutical use,
although some modified synthetic analogues are less toxic.
A surfactin variant produced by B. amyloliquefaciens, WH1fungin, induces apoptosis in fungal cells by a mitochondria-dependent pathway, while surfactin and related lipopeptides can stimulate defence responses to generate signalling molecules that induce systemic resistance to pathogens in plants. Surfactin is distinctive in that it is antiviral by causing disintegration of enveloped viruses, including both the viral lipid envelope and the capsid, through ion channel formation. However, it only affects cell-free viruses and not those within cells. As a detergent, surfactin has been investigated as a potential bio-remediation agent to assist in the degradation of oil spills and to mop up heavy metals from contaminated soils.
Iturins: B. subtilis produces further related families of lipopeptide antibiotics, which include the iturins (bacillomycins, iturins and mycosubtilins) and fengycins (plipastatins). The iturins are unusual in that they contain long-chain fatty acids (C14 to C17) with an amine group in position 3 (should we classify these as amino acids also?), which form part of a cyclic heptapeptide structure. They differ from other lipopeptides in that they are synthesised by a non-ribosomal peptide synthetase complexed with a polyketide synthase. Iturins are constituents of many Bacillus strains that have been commercialized as biological control agents against fungal plant pathogens and as plant growth promoters. By interacting with sterol components in fungal membrane, iturins create a pore that leads to increased loss of K+ and other cellular constituents and eventually to cell death. They are surfactants with commercial value in food emulsification and cosmetics, and they are reported to have anti-viral and anti-cancer properties, in vitro at least.
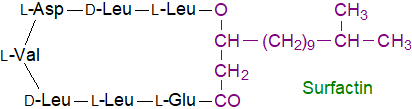
In the related fengycins, a decapeptide ring structure is formed by an ester bond between a tyrosine residue at position 3 in the peptide sequence and the C-terminal residue, and they have a 3-hydroxy fatty acid tail. They inhibit the growth of filamentous fungi on plants by degrading their cell walls and membranes and interfering with intracellular metabolism to induce programmed cell death and autophagy, while triggering defence responses. They have commercial potential in fruit preservation.
Polymyxins:  Various strains of Paenibacillus polymyxa,
which are efficient plant growth promoting rhizobacteria that protect plants from phytopathogenic microorganisms in the soil,
together with others of the Bacillales produce a variety of linear and cyclic lipopeptides.
In particular, Paenibacillus species produce at least four families of basic lipopeptides with potent antibiotic actions
of which the most studied are the polymyxins (15 variants).
These are decapeptides (7‑membered cyclic peptides attached to a linear peptide) linked to a fatty acid such as 6-methyl-octanoic
or 6-methyl-heptanoic acid.
Six of the amino acids in polymyxin B are the uncommon L‑2,4‑diaminobutyric acid (DAB),
which give the molecule a positive charge.
Various strains of Paenibacillus polymyxa,
which are efficient plant growth promoting rhizobacteria that protect plants from phytopathogenic microorganisms in the soil,
together with others of the Bacillales produce a variety of linear and cyclic lipopeptides.
In particular, Paenibacillus species produce at least four families of basic lipopeptides with potent antibiotic actions
of which the most studied are the polymyxins (15 variants).
These are decapeptides (7‑membered cyclic peptides attached to a linear peptide) linked to a fatty acid such as 6-methyl-octanoic
or 6-methyl-heptanoic acid.
Six of the amino acids in polymyxin B are the uncommon L‑2,4‑diaminobutyric acid (DAB),
which give the molecule a positive charge.
Polymixins act as antibiotic agents by binding to the lipid A moiety of the lipopolysaccharides of the anionic outer membrane of Gram-negative bacteria that are responsible for much of the virulence. By adhering to the anionic phosphate and pyrophosphate groups of these molecules to displace the calcium and magnesium bridges that stabilize the outer leaflet of the outer membrane, they cause disruption of the permeability barrier, leakage of intracellular contents and bacterial cell death. With E. coli, metabolic activity is essential for the lethality of polymyxin B, which kills the organism in its exponential-phase but not in its stationary-phase when the antibiotic appears to be tolerated.
Polymixins are used to treat a variety of infections including those caused by pseudomonads, enterobacteria and Acinetobacter in topical applications such as wound creams and eye or ear drops. While they were once considered to be too toxic to be used as systemic antibiotics because of a potential to cause injury to the kidney, the use of polymyxin E ('colistin') is now permitted as a last-line therapy against multi-drug-resistant Gram-negative bacilli following clinical studies to optimise dosage regimes (similar studies are underway for polymixin B). Unfortunately, some strains of Gram-negative bacteria have developed resistance to polymyxins by remodelled their lipid A by addition of palmitate to the R‑2‑hydroxymyristate residue, increasing the hydrophobicity of the outer membrane to hinder the diffusion of the lipopeptide through it, while others add further phosphoethanolamine or 4-amino-arabinose residues to block anionic binding sites. On the other hand, there is an encouraging development in that polymyxin derivatives that are not themselves antibiotics can sensitize bacteria to other antibiotics by damaging and increasing the permeability of their outer membranes. Novel semi-synthetic analogues in which changes have been made to one or more of the amino acids or the fatty acyl group are under development, and some are undergoing clinical testing.
Octapeptins, i.e., naturally occurring truncated polymyxins from B. circulans, together with cationic polypeptins are anti-microbial molecules in which the N‑terminal fatty acyl group varies in chain length from C8 to C10 and can be linear or branched and has a 3R‑hydroxyl group. Octapeptin A is active against Gram-negative bacteria, Gram-positive bacteria and fungi, while battacin (octapeptin B5), isolated from Paenibacillus tianmuensis, attacks multidrug-resistant E. coli and P. aeruginosa and is threefold less toxic than polymyxin B. Thus, octapeptins are considered strong candidates for therapeutic use.
Tridecaptins, isolated from strains of P. polymyxa, are linear cationic tridecapeptides with a combination of L- and D-amino acids that are acylated with β‑hydroxy fatty acids such as a 3-hydroxy-6-methyloctanoyl moiety in tridecaptin A1. They are especially potent against Gram-negative bacteria, exerting their bactericidal effect by binding to the bacterial cell-wall precursor lipid II on the inner membrane to disrupt the proton motive force. By sensitizing the outer membrane, unacylated tridecaptins can act synergistically with established clinically relevant antibiotics.
Fusaricidins are cyclic lipopeptides from Paenibacillus spp. that contain the unusual 15-guanidino-3-hydroxypentadecanoic acid as the fatty acid component linked to six-membered cyclic peptides (some Microbacterium and Nocardia sp. produce lipopeptides containing structurally related fatty acids). As with most lipopeptides, a family of structural variants (more than twenty) is now known to exist. There are three main families, mainly differing in position 3 of the peptide chain, with a fourth having an additional alanine attached to the hydroxyl group of threonine in position 4 via an ester bond. Fusaricidins are effective against many fungal pathogens of plants, but in mammalian cells, they are toxic to mitochondria and induce apoptosis in consequence of their ion channel-forming properties.

Paenibacterin produced by P. thiaminolyticus consists of a cyclic 13-residue peptide with a C15 fatty acyl chain at the N-terminus; it is attracting interest as it binds to negatively charged Gram negative endotoxins in vitro and inhibits drug-resistant P. aeruginosa in vivo. Paenibacillus sp. OSY-N produces cyclic and linear lipopeptides ('paenipeptins'), and both types show antimicrobial activity against Gram-negative and Gram-positive bacteria by binding to lipopolysaccharides and lipoteichoic acid to disrupt the cytoplasmic membrane.
As a representative of a linear lipopeptide, tauramadine from Brevibacillus laterosporus consists of five amino acids linked to iso-methyl-octadecanoic acid (7‑methyloctanoyl-Tyr-Ser-Leu-Trp-Arg). It strongly inhibits pathogenic Enterococcus sp. Others include cerexins and tridecaptins. Kurstakins are lipoheptapeptides that are fungicides and were first characterized as linear molecules, though cyclic forms are now known. Linear lipopeptides are of pharmaceutical interest in that they are more accessible by chemical synthesis, although they tend to have weak proteolytic, heat and/or acid stability, and to date the known cyclic lipopeptides have greater antibacterial potency and greater oral bioavailability.
The mixture of D- and L-amino acids in lipopeptides from Bacillus and Paenibacillus sp. results in enhanced stability to proteolytic enzymes from target organisms as well as to proteases in human plasma. In general terms, these lipopeptides control motility, attachment to surfaces and the concentrations of other microorganisms, although they may have a signalling function to coordinate growth and differentiation. All these peptidolipids and indeed the organisms per se, especially strains of P. polymyxa, are under investigation as agents for the control of plant diseases. Not only do they have the potential to act directly against phytopathogens, including bacteria, fungi and oomycetes, but they stimulate defence mechanisms in the plant hosts and promote plant growth.
Gramicidins, produced by a soil bacterium B. brevis, are not strictly speaking lipopeptides, unless a terminal formyl residue is considered to be a fatty acid, but they are listed here as one of the few approved by the FDA for topical applications in control of Gram-positive infections. They consist of a mixture of at least six linear 15-amino acid polypeptides, of which that termed gramicidin D is the commercial form, and they consist of alternating mainly hydrophobic D- and L-amino acids with no ionizable side chains. The molecule is insoluble in water and is adsorbed strongly at lipid membranes.
4. Lipopeptides from Actinomycetes (Streptomyces)
The Actinomycetes in general and the genus Streptomyces in particular are sources of many antifungal and antibiotic compounds. Streptomyces roseosporus (Actinobacteria), for example, produces daptomycin, which is an acidic cyclic lipopeptide consisting of 13 amino acids and includes three D‑amino acid residues (D‑asparagine, D-alanine and D-serine) linked via the N‑terminal trypsin to decanoic acid; related lipopeptides contain anteiso-undecanoyl, iso-dodecanoyl or anteiso-tridecanoyl residues. The macrocycle contains ten amino acid residues with a terminal kynurenine (unique to these species) connected by an ester bond to the hydroxyl group of threonine to form a macrolactone. Both kynurenine and 3-methylglutamic acid have been shown to be crucial for daptomycin activity. In these and related molecules, the positioning of the D-amino acids is conserved as is the Asp‑X-Asp-Glc motif, which is a Ca2+ binding region. In contrast to other common lipopeptides, daptomycin has a negative net charge, but Ca2+ ions reduce this and stimulate oligomerization. Like the lipopeptides produced by Bacillus sp., daptomycin is synthesised by a non-ribosomal mechanism.

Daptomycin, one of a few calcium-dependent antibiotics, was licensed by the FDA in the United States for use against skin and soft tissue infections by Gram-positive bacteria in 2003, and as a last resort for methicillin-resistant S. aureus (MRSA) infections of the bloodstream in 2006. The mechanism of action in vivo has been somewhat controversial, but there are now known to be two independent antibacterial effects, i.e., targeting of precursor lipids and thence inhibition of cell wall synthesis, and secondly membrane depolarisation, that explains why development of resistance towards daptomycin is slow. Calcium-dependent binding of the lipophilic tail of daptomycin to the bacterial plasma membrane occurs in conjunction with an interaction with phosphatidylglycerol, which must be of the usual stereochemical conformation, and undecaprenyl-coupled cell envelope precursors as a tripartite complex. One report concludes that daptomycin forms a unique complex with calcium ions and phosphatidylglycerol molecules in membranes in the stoichiometric ratio: Dap(2):Ca2+(3):PG(2), while another suggests that the ratio is 1:1:2. The result is interruption of cell wall biosynthesis, followed by delocalization of components of the peptidoglycan biosynthesis machinery and massive membrane rearrangements that cause potassium efflux, triggering of auto-digestive enzymes and eventually cell death.
Overexpression of the synthase for diglucosyldiacylglycerol, the primary precursor for lipoteichoic acids in the cell wall, leads to daptomycin resistance in B. subtilis. Some resistant strains of target organisms synthesise lysyl-phosphatidylglycerol, a cationic metabolite of phosphatidylglycerol, which changes the membrane charge from negative to positive and limits the ability of daptomycin to bind to the membrane in a calcium-dependent manner.
Others of the Streptomyces and Actinomyces contain related antibiotic molecules, including amphomycins, friulimicins and glycinocins (laspartomycins), with macrocycles closed with a lactam rather than a lactone bond, while certain of the amino acids are modified during biosynthesis via enzymatic oxidation and methylation to produce new amino acids not found in proteins. Many of these lipopeptides incorporate piperazic (diazinane-3-carboxylic) and pipecolic (piperidine-2-carboxylic) acids, which are structural units of many other natural products of microbial origin. The lipopeptide enramycin is unique in that it contains the amino acid enduracididine, a cyclic analogue of arginine. The fatty acids are C13 to C16 with iso- or anteiso-methyl branches and a double bond in position 3 (or in position 2 in the case of the glycinocins). Many Actinobacteria produce relatively simple diacyldipeptides containing isonitrile groups and termed kupyaphores, which contribute to the toxicity of the organisms by binding to metal ions. Unusual fatty acids produced by polyketide synthases are discussed below.
Ramoplanin is a glycolipodepsipeptide antibiotic (i.e., with carbohydrate, ester and amide bonds in the molecule) obtained from fermentation of Actinoplanes sp. ATCC 33076 that is active against multi-drug-resistant, Gram-positive pathogens including Enterococcus sp., Staphylococcus aureus (MRSA) and Clostridium difficile. It acts by disrupting bacterial cell walls by sequestering the peptidoglycan intermediate lipid II. While it is unsuitable for intravenous use in humans because of side effects, it is being trialled for oral use against gastrointestinal infections; friulimicin B is also undergoing clinical trials. Again, such lipopeptides are providing biochemists with opportunities for genetic and chemical modifications both to the peptide and fatty acid moieties to produce novel compounds as antibiotics against infections by Gram-positive bacteria but with fewer disadvantages.
5. Lipopeptides from Pseudomonas
The genus Pseudomonas produces many cyclic lipopeptides (lipodepsipeptides) with surfactant, antibacterial and antifungal properties; some have even been reported to be anti-cancer agents. They are based on a similar structural blueprint and consist of an oligopeptide (8 to 25 amino acids), which is N‑terminally acylated with a linear fatty acid (C5 to C16), usually with a β-hydroxyl moiety of the R‑configuration, but occasionally bis-hydroxylated, unsaturated or with a second carboxyl. They have been classified into at least 14 groups within two main families distinguished by the number of amino acids, and these comprise numerous structurally homologous members of which the viscosin, syringomycin, amphisin, putisolvin, tolaasin and syringopeptins are the best known. While it is hoped that some of these lipopeptides will prove to have pharmaceutical use as antibiotics in humans, others have potential against plant pathogens of various kinds, although some of the producing organisms are themselves plant pathogens.
The phytopathogenic bacterium Pseudomonas syringae pv. syringae produces two classes of necrosis-inducing lipodepsipeptide toxins termed the syringomycins and syringopeptins. Syringomycin form SRE is illustrated; it contains nine amino acids of which three are unusual (Dab = 1,4‑diaminobutyric acid; Dhb = 2,3‑dehydroamino-butyric acid; 4(Cl)Thr = C-terminal chlorinated threonine residue), while three are of the D‑form; in general, there is a high content of basic amino acids. The fatty acid component is often 3-hydroxy-decanoic or 3-hydroxy-dodecanoic acid.

The viscosin group, which has antiviral properties, consists of lipopeptides with nine amino acids, whereas members of the amphisin family have eleven. Viscosin has been shown to inhibit metastasis of breast and prostate cancer cell lines without causing toxicity, while pseudofactin II, a cyclic lipopeptide biosurfactant isolated from a strain of Pseudomonas fluorescens, was found to induced apoptosis of melanoma cells by an interaction with the plasma membrane. The tolaasin group are more varied because of differing lengths of the peptide chains (19 to 25 amino acids, including 2,3-dehydro-2-aminobutyric acid and homoserine). 3‑Hydroxydecanoic acid is usually the lipid moiety in these groups. In contrast, the putisolvins have a hexanoic lipid tail and a peptide moiety of 12 amino acids with a different mode of cyclization.
Plusbacins produced by a Pseudomonas sp. are very similar to tripropeptins and empedopeptin found in Gram-negative soil bacteria. They are cyclic lipopeptides differing mainly in the first three amino acids and the nature of the fatty acid component. The last of these binds and de-activates lipid II, a key molecule in the biosynthesis of cell wall peptidoglycans in bacteria, and it may be a strong candidate as an antibiotic in pharmaceutical applications. Pseudomonads produce relatively few linear lipopeptides, treated as the syringafactin and corrugatin groups. The latter contain rare β-hydroxy histidine, which is a bidentate ligand for Fe3+ ions.
While the non-ribosomal mechanism for assembly of lipopeptides in Pseudomonas has much in common with that for Bacillus described above, the two are evolutionarily distinct and there are some differences. The modules have some flexibility in the selection of amino acids, the fatty acyl groups can be modified by polyketide synthases, and they do not rely on external tailoring enzymes to complete the synthesis.
6. Lipopeptides from Cyanobacteria
Many cyanobacteria, but mainly those of marine origin, are now known to produce lipopeptides and glycolipopeptides with novel structures. Several molecular forms of hassillidins have been isolated from Hassallia sp., puwainaphycins have been characterized from Cylindrospermum alatosporum and anabaenolysins from Anabaena sp. In some, the fatty acid component (C12 to C18) contains a hydroxyl group in position 2 and an amine group in position 3 (cf., the iturins above) with the latter forming part of the ring structure. Usually, the fatty acid chain is saturated, but at least one C18 fatty acid has six double bonds (two groups of three in conjugation) while others contain methyl branches and methoxyl groups. Dragomide E from Lyngbya majuscule (marine cyanobacteria) has five amino acids in a linear peptide linked to an acetylenic C8 fatty acid (such acids are common in lipopeptides from the genus Nostoc). Selidamides are cyclic lipopeptides produced by several bacterial phyla, including cyanobacteria and alphaproteobacteria, with fatty acyl units (often 3-hydroxy) attached to (hydroxy)ornithine or lysine side chains by maturases of the GCN5-related N-acetyltransferase superfamily.

Although the pharmacology of these lipopeptides have barely been explored, some are known to have anti-fungal or anti-parasitic actions by cholesterol- and ergosterol-dependent disruption of membranes, or they are cytotoxic towards mammalian cell lines in vitro. During biosynthesis, fatty acyl chains are the first monomers incorporated into the peptidyl backbone via a process known as lipoinitiation, before the lipopeptides are extended through the successive additions of both proteinogenic and non-proteinogenic amino acids by nonribosomal peptide synthetases.
7. Fungal Lipopeptides
More than 30 genera of fungi produce cyclic and linear lipopeptides, which are antibiotics and fungicides. These can have from three to thirteen amino acids, often modified from the usual forms, and many different fatty acid constituents. The best known of these are the echinocandins, which are nonribosomal cyclic hexapeptides that are potent fungicides produced by fungi such as Glarea lozoyensis. There are three main forms, echinocandin, pneumocandin A0 and pneumocandin B0, and these contain two types of hydroxy-L-prolines linked to a fatty acid that can be linoleate or 10R,12S‑dimethyl-tetradecananoate. Semi-synthetic forms, cilofungin and adilulafungin, are first line antimycotics for the treatment of invasive mycosis by inhibiting the 1,3-β-D-glucan synthase in fungal cell walls, but other comparable fungal metabolites inhibit many different enzyme systems.
Other lipopeptides from fungi include the peptaibols, pleofungins, beauvericins and enniatins, and lipopeptaibols are a family of non-ribosomal peptides, 4- to 21-mer long with a high content of the turn/helix forming α-aminoisobutyric acid, that are antibacterial and cytotoxic agents and contain a fatty acyl chain (C8 to C15) at the N‑terminus and a 1,2-amino alcohol at the C‑terminus, e.g., phenylalaninol.
8. Bacterial Triacyl Proteolipids (Lipoproteins)
 All bacteria contain large numbers of proteins with a unique post-translational lipid modification with three
fatty acyl groups, and this must be necessary for their survival and pathogenicity via host-pathogen interactions.
More than 2000 have been identified by proteomic analysis, and in Staphylococcus aureus, for example, there are 63 different proteolipids
of this type.
All bacteria contain large numbers of proteins with a unique post-translational lipid modification with three
fatty acyl groups, and this must be necessary for their survival and pathogenicity via host-pathogen interactions.
More than 2000 have been identified by proteomic analysis, and in Staphylococcus aureus, for example, there are 63 different proteolipids
of this type.
The lipid components consist of N-acyl- and S‑diacylglycerol groups attached to an N‑terminal cysteine, i.e., it contains a thio ether bond. In Mycobacterium bovis, for example, positions sn-1 and 2 of the glycerol moiety are linked to palmitic and tuberculostearic acids, respectively, and either fatty acid can be the N‑acyl moiety. In E. coli, the fatty acid components resemble those in the membrane phospholipids especially phosphatidylglycerol, i.e., they are 16:0, 16:1, 17:1, 18:1, and 19:1, but this is not true of many other bacteria. Lipoproteins from Helicobacter pylori and some others contain mainly 16:0 and 18:0 in both ester and amide linkage, while 19:0 cyclopropane and 14:0 fatty acids are present in the membrane phospholipids.
As with other proteolipids, the lipid moieties act as an anchor to hold the protein tightly to a hydrophobic cellular membrane while permitting it to operate in an aqueous environment in transport, signalling, adhesion, digestion and growth; they have a role in nutrient and ion acquisition, enabling pathogens to survive better in the host. They are crucial constituents of the outer leaflet of the cytoplasmic membrane of Gram-positive bacteria, with their protein components spanning the cell wall, and of the outer leaflet of the cytoplasmic and the inner leaflet of the outer membranes of Gram-negative bacteria (see our web page on Lipid A for a description of the membrane structure). Like the endotoxins (lipopolysaccharides) of Gram-negative bacteria, they are potent stimulants of the human immune system, eliciting pro-inflammatory immune responses as ligands for receptors like the Toll-like receptor 2 (TLR2).
The first of these to be discovered and termed "Braun's lipoprotein" is one of the most abundant membrane proteins in the cell walls of Gram-negative bacteria such as Escherichia coli. It has a molecular weight of only 5.8 kDa and folds into a trimeric helical structure. Uniquely, much of it is covalently attached by the ε-amino group of the C-terminal lysine to the carboxyl group of a meso-diaminopimelic acid residue in the peptidoglycan (murein) of the cell wall to provide the only covalent connection between the inner and outer membranes. It is embedded in the outer membrane by its hydrophobic head and provides a tight link between the two layers, giving structural integrity to the outer membrane while fixing the distance between the two membranes. An endopeptidase has been identified that cleaves the connection to the peptidoglycan polymer and is a potential antimicrobial drug target.
In the main secretory pathway, proteins destined to become lipidated have N-terminal signal peptides containing a motif known as a lipobox with an invariant cysteine residue, which directs them to the lipoprotein biogenesis machinery after transport mainly in an unfolded state. The three fatty acyl groups and the glycerol component responsible for binding to the membrane surface can be derived from the membrane phospholipids, but other origins are possible. Three enzymes are employed in the biosynthetic pathway, which occurs in the cytoplasmic (inner) membrane. In E. coli, the first (Lgt; phosphatidylglycerol:prolipoprotein diacylglyceryl transferase) attaches the diacylglycerol group from phosphatidylglycerol to the thiol of cysteine, the first amino acid in the pro-lipoprotein after an 8- to 36-amino-acid-long signal peptide, which is distinguished by a C-terminal lipobox comprising a conserved three-amino-acid sequence in front of an invariable cysteine.
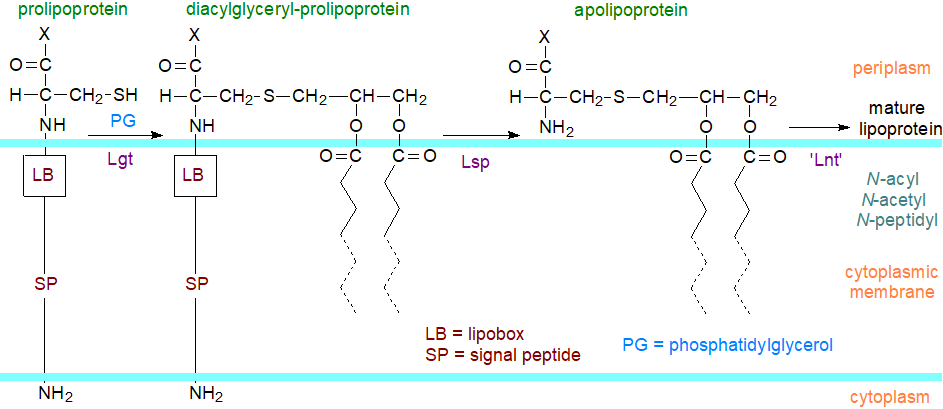 |
| Figure 1. Proteolipid biosynthesis in bacteria. |
A second enzyme (Lsp; prolipoprotein signal peptidase) then removes the signal peptide, leaving the cysteine as the new amino-terminal residue of the protein component. The third enzyme (Lnt; apolipoprotein N-acyltransferase) acylates the N-terminal amine group of the modified cysteine with a fatty acid from position sn-1 of whatever phospholipid is available (usually phosphatidylethanolamine), while the resulting lysophospholipid is flipped back across the membrane and re-esterified. This last step in biosynthesis always occurs in Gram-negative bacteria but is found only rarely in Gram-positive bacteria (Lgt and Lsp are present in all bacteria). Others have related Lnt-like enzymes that can acylate or alternatively acetylate or add a peptide unit to the N-terminal cysteinyl residue. Most of the proteolipids are then transferred to the inner leaflet of the outer membrane by a complex mechanism with five proteins (Lol pathway), which sort and translocate them via signal residues located C-terminally to the diacylglyceryl-cysteine so that the acyl chains are within the membrane. In the outer membrane, proteolipids undergo topological changes that govern the biogenesis and integrity of the membrane. A proportion can be linked covalently to peptidoglycan macromolecules.
In Bacillus subtilis, lipoproteins are N-acetylated to form acetylated lipoproteins after being transported across the membrane with an acetylated heptaprenylglyceryl carrier as the acetyl donor. Enterococcus faecalis, a Gram-positive Firmicute, and some others synthesise a diacylated proteolipid (lyso-form or N‑acyl,S‑monoacylglycerol lipoprotein) by transfer of the fatty acid from position sn-2 of the diacylglycerol moiety to the N‑terminal cysteine residue of the protein by means of an integral membrane enzyme designated Lit (lipoprotein intramolecular transacylase), i.e., leaving a 1‑monoacyl-sn-glycerol attached to the thiol group. This proteolipid can also be formed from the triacyl-lipoprotein by the action of an O‑lipoprotein deacylase.
In Gram-positive bacteria, lipoprotein maturation and processing are not vital to the organism, but they are required for their pathogenicity. It is now evident that the degree of proteolipid acylation and the nature of the fatty acid components has a substantial influence on the immune response by stimulating macrophages, neutrophils, lymphocytes, and endothelial and epithelial cells. Thus, exposure to diacylated proteolipid (lyso-form) induces immune suppression by enabling evasion of immune recognition by the signalling cascades induced by TLR2 and to a lesser extent with TLR1, TLR6 and TLR10, while exposure to triacylated proteolipid induces a much smaller response. Like the lipopolysaccharides, these proteolipids can induce sepsis in human hosts and have the potential to be used in vaccines.
9. Lipopeptides and Proteolipids and Other Bacteria
Lipopeptides: A complex mixture of water-soluble lipodepsipeptides is produced by Gram-negative Lysobacter spec. One of these, designated WAP-8294A2 or lotilibcin, is a dodeca-peptide linked to 3-hydroxy-7-methyl-octanoic acid and is a potent antibacterial agent against Gram-positive bacteria, including antibiotic-resistant strains. It interacts with the phospholipids cardiolipin and phosphatidylglycerol in the bacterial cell membrane and eventually causes cell death.
Serratia marcescens, a Gram-negative, facultative anaerobe, produces three surfactant exolipids designated serrawettins W1 to W3 together with stephensiolide A and rhamnolipids (glycolipid surfactants). Serrawettin W2 is 3-hydroxydecanoyl-D-leucyl-L-seryl-L-threonyl-D-phenylalanyl-L-isoleucyl lactone, while stephensiolide A has a peptide moiety of five amino acids of Thr-Ser-Ser-Val-Val attached to a C8 fatty acid chain. They reduce the surface tension of thin films of water on solid surfaces, assisting with motility, cellular communication and nutrient accession of the bacteria, and they are promising antibiotics towards Gram-positive bacteria.
 A Gram-negative bacterium Myxococcus sp. produces unusual glycopeptidolipids, termed myxotyrosides,
with a normal or an iso-branched fatty acid amide-linked to a tyrosine-derived structure and thence to rhamnose, while
corallorazines are cyclic lipodipeptides produced by the myxobacterium Corallococcus coralloides B035 with N‑methylglycine and
dehydro-alanine cyclized to form a piperazine ring.
In addition, genome mining of Myxobacteria has found many strains that produce lipopeptides termed myxochromides.
Cystobacter fuscus produces lipopeptides (cystomanamides) containing N‑glycosylated 3-amino-9-methyldecanoic acid,
a fatty acid that is rare in nature and was first found in the iturins (see above).
Simple bacterial lipoamino acids, including some dipeptides, are discussed in another web page.
A Gram-negative bacterium Myxococcus sp. produces unusual glycopeptidolipids, termed myxotyrosides,
with a normal or an iso-branched fatty acid amide-linked to a tyrosine-derived structure and thence to rhamnose, while
corallorazines are cyclic lipodipeptides produced by the myxobacterium Corallococcus coralloides B035 with N‑methylglycine and
dehydro-alanine cyclized to form a piperazine ring.
In addition, genome mining of Myxobacteria has found many strains that produce lipopeptides termed myxochromides.
Cystobacter fuscus produces lipopeptides (cystomanamides) containing N‑glycosylated 3-amino-9-methyldecanoic acid,
a fatty acid that is rare in nature and was first found in the iturins (see above).
Simple bacterial lipoamino acids, including some dipeptides, are discussed in another web page.
The chitinopeptins are cyclic lipopeptides produced by Chitinophaga sp. (Bacteroidetes). They exist in various forms with many non-proteinogenic amino acids, such as diaminopropionic acid and N-methyl-valine, and unusual fatty acids that include 2,9-dimethyl-3-amino-, 3‑amino-9-methyl- and 3-hydroxy-9-methyl-decanoic acids. They inhibit Gram-negative and Gram-positive bacteria and bind iron. The Gram-negative Burkholderia genus is likewise known to produce some novel antimicrobial lipopeptides.
A new class of lipopeptide antibiotics, named lipolanthines, contain fatty acids produced by unconventional fatty acid synthases or polyketide synthases and can include those with a terminal N,N'-dimethylguanidyl group, e.g., from Nocardia terpenica, or more complex very-long-chain polyhydroxy forms, e.g., (C31) from a Streptomyces sp. It is noteworthy that these are much more polar than conventional fatty acids, so the parent molecules must be much more hydrophylic than the better known lipopeptides.
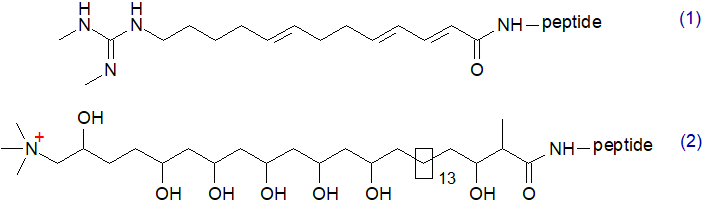 |
| Figure 2. Examples of fatty acids in lipopeptides synthesised by unconventional fatty acid
synthases. (1) From nocavionin; (2) from a goadvionin. |
Proteolipids: Some pathogenic bacteria, including Bordetella pertussis, E. coli and Kingella kingae, produce inert protein protoxins, which must be acylated post translation at the ε-amino groups of two internal conserved lysine residues by acyl transferases specific to each organism before they can exhibit cytotoxicity. The acyl donors are acyl-acyl carrier proteins (ACP), and the fatty acyl groups are either palmitate or myristate, depending on species.
Bacteria of the genus Mycoplasma lack a cell wall and are obligate parasites that must obtain all their lipids from the host. Recently, it has been demonstrated that otherwise cytoplasmic proteins, lacking signal peptides, are tethered to the outer membrane by a link from glutamine near the C‑terminus of the protein to rhamnose and thence to a phospholipid, presumed for the moment to be phosphatidic acid. Whether other bacteria have the same mechanism has yet to be determined.
Recommended Reading
- Asamizu, S. Recent advances in discovery and biosynthesis of ribosomally synthesized and post-translationally modified peptides (RiPP)-derived lipopeptides. Nat. Prod. Rep., 42, 1622-1638 (2025); DOI.
- Balleux, G., Höfte, M., Arguelles-Arias, A., Deleu, M. and Ongena, M. Bacillus lipopeptides as key players in rhizosphere chemical ecology. Trends Microbiol., 33, 80-95 (2025); DOI.
- Bann, S.J., Ballantine, R.D. and Cochrane, S.A. The tridecaptins: non-ribosomal peptides that selectively target Gram-negative bacteria. RSC Med. Chem., 12, 538-551 (2021); DOI.
- Buttress, J.A., Schäfer, A.B., Koh, A., Wheatley, J., Mickiewicz, K., Wenzel, M. and Strahl, H. The last resort antibiotic daptomycin exhibits two independent antibacterial mechanisms of action. Nature Commun., 16, 10320 (2025); DOI.
- Cole, G.B., Bateman, T.J. and Moraes, T.F. The surface lipoproteins of gram-negative bacteria: Protectors and foragers in harsh environments. J. Biol. Chem., 296, 100147 (2021); DOI.
- Dembitsky, V.M. Hydrobiological aspects of fatty acids: unique, rare, and unusual fatty acids incorporated into linear and cyclic lipopeptides and their biological activity. Hydrobiology, 1, 331-432 (2022); DOI.
- Ding, N., Dong, H.S. and Ongena, M. Bacterial cyclic lipopeptides as triggers of plant immunity and systemic resistance against pathogens. Plants-Basel, 14, 2644 (2025); DOI.
- Evidente, A. Bioactive lipodepsipeptides produced by bacteria and fungi. Int. J. Mol. Sci., 23, 8077-8087 (2022); DOI.
- Götze, S. and Stallforth, P. Structure, properties, and biological functions of nonribosomal lipopeptides from pseudomonads. Nat. Prod. Rep., 37, 29-54 (2020); DOI.
- He, Y., Chen, Y., Tao, H., Zhou, X., Liu, J., Liu, Y. and Yang, B. Secondary metabolites from cyanobacteria: source, chemistry, bioactivities, biosynthesis and total synthesis. Phytochem. Rev., 24, 483–525 (2025); DOI.
- Hüttel, W. Echinocandins: Structural diversity, biosynthesis, and development of antimycotics. Appl. Microbiol. Biotechnol., 105, 55-66 (2021); DOI.
- May, K.L. and Grabowicz, M. Outer membrane lipoproteins: late to the party, but the center of attention. J. Bact., 207, e00442-24 (2025); DOI.
- Pang, L., Tian, X.L., Pan, W.H. and Xie, J.P. Structure and function of mycobacterium glycopeptidolipids from comparative genomics perspective. J. Cell. Biochem., 114, 1705-1713 (2013); DOI.
- Petitfils, C. and others. Identification of bacterial lipopeptides as key players in IBS. Gut, 72, 939-950 (2023); DOI.
- Slingerland, C.J. and Martin, N.I. Recent advances in the development of polymyxin antibiotics: 2010-2023. ACS Infect. Dis., 10, 1056-1079 (2024); DOI.
- Sreedharan, S.M., Rishi, N. and Singh, R. Microbial lipopeptides: Properties, mechanics and engineering for novel lipopeptides. Microbiol. Res., 271, 127363 (2023); DOI.
- Théatre, A. and 15 others. The surfactin-like lipopeptides from Bacillus spp.: natural biodiversity and synthetic biology for a broader application range. Front. Bioengin. Biotechn., 9, 623701 (2021); DOI.
- Yaraguppi, D.A., Bagewadi, Z.K., Patil, N.R. and Mantri, N. Iturin: a promising cyclic lipopeptide with diverse applications. Biomolecules, 13, 1515 (2023); DOI.
- Zeng, P., Wang, H.L., Zhang, P.F. and Leung, S.S.Y. Unearthing naturally-occurring cyclic antibacterial peptides and their structural optimization strategies. Biotechn. Adv., 73, 108371 (2024); DOI.

 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: December 2025 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
