Glycero-Phosphoglycolipids and Glycophospholipids
Phosphoglycolipids and glycophospholipids are glycerolipid molecules that contain both phosphate and carbohydrate as integral structural components as the names suggest. There are two main types of glycophospholipid based on a diacylglycerol backbone, which with two exceptions discussed below are exclusively of microbial origin. One type is derived biosynthetically from glycosyldiacylglycerols, in which the sugar moiety is phosphorylated, i.e., in which the carbohydrate moiety is linked to a diacylglycerol - these were termed 'phosphoglycolipids' by Fischer (see his review cited below). The second group comprises more conventional phospholipids, with a phosphate moiety attached to a diacylglycerol unit but with the phosphate further glycosylated, and these were termed 'glycophospholipids'. Although it is not always easy to differentiate the two, the stereochemistry of the glycerophosphate unit can be a distinguishing feature as this is dependent on the biosynthetic origin. Both types are present in the Archaea.
A further distinction is that with glycophospholipids, the phosphoester bond is at the anomeric (glycosyl) bond, while with phosphoglycolipids, the phosphate can be bound to any position on the carbohydrate other than at the anomeric position (although this will include lipids where the carbohydrate and phosphate are not linked to each other), i.e., the difference between the two is whether the phosphate is present at the anomeric position or not.

This phosphoglycolipid/glycophospholipid terminology does not appear to have been widely adopted, but the differences are important, and I have used these terms here for want of better. Analogous sphingo-glycophospholipids and sphingo-phosphoglycolipids are known. While there are some lipids that have features of both groups, most of these occur in relatively small amounts in bacteria, and it has been suggested that they do not have a significant role in membranes, although they may have some metabolic function that has yet to be defined. Innumerable lipids of both types are now known with new structures continuing to be reported, so only a flavour of this complexity can be described below.
1. Phosphoglycolipids
One of the first phosphoglycolipids to have its structure fully elucidated was found in Streptococcus and related bacteria, i.e., 1,2‑diacyl-3-[6’’-(sn-glycero-1-phospho-)-α-D-kojibiosyl]-sn-glycerol. It is derived from a diglucosyldiacylglycerol, with the diglucoside unit equivalent to kojibiose in that it has an α-(1→2) linkage. The other distinctive feature is the stereochemistry of the glycerophosphate moiety attached to position 6 of the second glucose unit; this is linked via position sn-1 of glycerol rather than the sn-3 position as in most other phospholipids. Subsequently, an analogous lipid with a single glucose moiety was characterized from this organism, while a related lipid with a phosphorylated galactofuranosyl residue was found in Bifidobacterium bifidum. In some species, similar triglycosyl lipids or diglycosyl analogues with the sn-glycerol-1-phosphate residue in different positions from that illustrated or with more than one such substituent are known, or there can be an alkenyl ether moiety in position sn-1 of the glycerolipid component rather than a fatty acid.
![S2: Formula of 1,2-diacyl-3-[6'-(sn-glycero-1-phospho)-apha-D-kojibiosyl]-sn-glycerol Formula of 1,2-diacyl-3-[6'-(sn-glycero-1-phospho)-apha-D-kojibiosyl]-sn-glycerol](FigureS2.png)
As in the biosynthesis of other glycosyldiacylglycerols, the first step in the biosynthesis of such lipids in Streptococcus sp. is the sequential reaction of 1,2‑diacyl-sn‑glycerol and UDP-glucose to yield as intermediates first glucosyldiacylglycerol and then kojibiosyldiacylglycerol, both of which are found in the organism. The sn‑1‑glycerophosphate moiety is added last probably by an enzyme-catalysed trans-phosphatidylation reaction with phosphatidylglycerol as the donor molecule by analogy with comprehensive studies of the biosynthesis of phosphatidylglucosyldiacylglycerols discussed next.
The key to the classification of these lipids within this first type of phosphoglycolipid has come from biosynthetic studies, which have shown that they are synthesised by an enzyme-catalysed transphosphatidylation of monoglucosyldiacylglycerols with phosphatidylglycerol as donor of the phosphatidyl group, rather than by a glycosylation reaction.
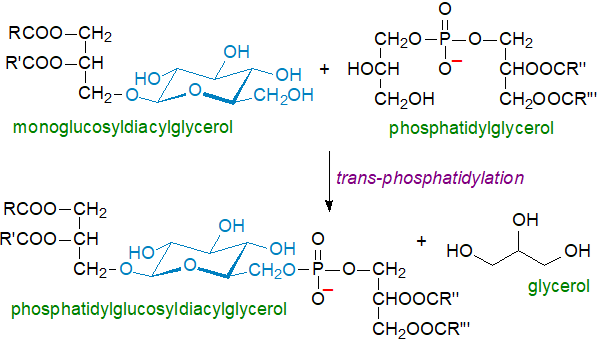 |
| Figure 1. Biosynthesis of phosphatidylglucosyldiacylglycerol |
As both a glycosyldiacylglycerol moiety and a diacylglycerophosphate group are present within a single molecule in this example, they can be classified as either phosphoglycolipids or phosphoglycolipids (or both).
A choline-containing phosphoglycolipid, i.e., 6'-O-phosphocholine-α-glucopyranosyl-(1'→3)-1,2-diacyl-sn-glycerol, from the human pathogenic bacterium Mycoplasma fermentans is unique in many ways. In structural terms, it differs from most of the other phosphoglycolipids described here in that the phosphate moiety on position 6’ of glucose is linked to a choline moiety, rather than to glycerol. In biological terms, it has been suspected of involvement in the pathogenesis of rheumatoid arthritis and of acquired immunodeficiency syndrome (AIDS), as it is a major immunological determinant for the organism in infected tissues. A second phosphoglycolipid with strong antigenicity found in M. fermentans has a related if more complex structure, with position 6’ of glucose linked to phospho-1,3-dihydroxy-2-aminopropane and then to the phosphocholine moiety.

An analogous phosphoethanolamine-containing glycosyldiacylglycerol, phosphoethanolamine-6’-D-GlcNAc-β(1’-3)-diradylglycerol, which can occur in both diacyl and alkenyl-acyl (plasmalogen) forms, and other unusual lipids (including glycerolacetals of plasmenylethanolamine) have been identified in the membranes of several anaerobic Clostridia, including Clostridium tetani, the causative agent of tetanus.
Phosphatidylglucosyldiacylglycerol or 3‑O‑[6’-O-(1’’,2’’-diacyl-3’-phospho-sn-glycerol)-α-D-glucopyranosyl]-1,2-diacyl-sn-glycerol (illustrated), and the analogous diglycosyl phosphatidylkojibiosyldiacylglycerol together with more complex substituted forms have been characterized from Mycoplasma laidlawii, some Lactococcus and Streptococcus species, and Pseudomonas diminuta. Comparable lipids with galactose as the carbohydrate component have been found in Bifidobacterium bifidum var. Pennsylvanicus and in the marine diatom Thalassiosira weissflogii; the fatty acyl chains are polyunsaturated in the latter. Scytonema julianum (cyanobacteria) contains unusual phosphoglycoglycerolipids in which the sugar and phosphate moieties are not linked to each other.

Lipoteichoic acids: Glycosyldiacylglycerols are the lipid unit at the terminal end of the zwitterionic lipoteichoic acids, which are complex phospho-polysaccharides that form part of a thick layer in the cell walls of Gram-positive and some Gram-negative bacteria and protect the susceptible protoplast from lysis and detrimental environmental factors. They are generally classified into five types of which Type I (Bacillus subtilis and Lactobacillus brevis) and Type IV (Streptococcus pneumoniae) have been most studied. The type I lipoteichoic acid from Bacillus subtilis has β‑gentiobiosyldiacylglycerol (diglucosyldiacylglycerol), i.e., with a β(1→6) linkage between two glucose units, as the lipid component attached to an unbranched 1→3 linked glycerolphosphate polymer. The stereochemistry of the polymer is unusual in that it is has sn‑1‑glycerolphosphate units (an average of about 25 in length) attached to gentiobiosyldiacylglycerol. The hydroxyl groups at the C2 position of the repeating units are modified with D-alanyl or glycosyl groups to varying degrees.
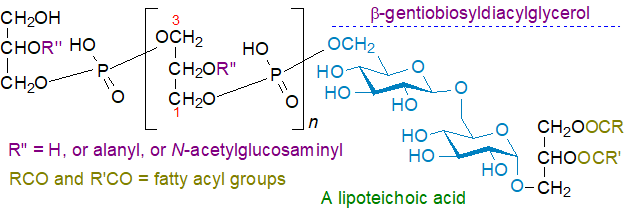
The diacylglycerol unit serves as an anchor to hold the molecule in the outer leaflet of the cytoplasmic membrane by hydrophobic interactions with the glycerolphosphate polymer passing through the thick layer of peptidoglycan and teichoic acid that constitutes the outer portion of the cell wall. While this is the main type of lipoteichoic acid, many exceptions are known to exist that differ in the chemical nature of the substituents decorating the glycerol phosphate subunits, the length of the polymer and the type of carbohydrate unit of the glycolipid anchor in the membrane. The four further lipoteichoic acid classifications recognized have poly(digalactosylglycerophosphate), ribitol, amino acids (e.g., D‑alanyl residues), glycosyl units (often N-acetylglucosamine), or choline phosphate in place of or attached to the glycerophosphate residues. Some of these differ in the nature of the glyco-portion of the lipid-anchor, and in many Bacillus sp. and other Gram-positive bacteria, mono- and diglycosyldiacylglycerols and glycerophospho-diglycosyldiacylglycerol (together with a mono-alanyl form), i.e., the monomeric precursors of lipoteichoic acids, have been detected in the lipidome.
Together with peptidoglycans, the lipoteichoic acids constitute a polyanionic matrix that provides elasticity, porosity and tensile strength to the cell wall and has ion-exchange properties that function in homeostasis of metal cations, in trafficking of ions, nutrients, proteins and antibiotics, in the regulation of proteolytic enzymes, and in the orientation of envelope proteins. The various moieties attached to the glycerol phosphate units can modulate the net anionic charge and determine the cationic binding properties. While the phosphate residues of the repeating unit impart a negative charge to the cell surface, the D-alanine residues, which partly substitute hydroxyl groups in the repeating units, contribute a positive charge. Intriguingly, the polymer parts of the peptidoglycans and wall teichoic acids are superficially similar to that of the lipoteichoic acids but differ in the stereochemistry, i.e., they are based upon sn-3-glycerolphosphate repeating units.
The glycolipid anchor, i.e., the diglycosyldiacylglycerol unit, is produced within the cell by the transfer of two UDP-glucose molecules onto diacylglycerol by a glycosyltransferase YpfP, before this is moved to the outer leaflet of the membrane by the multimembrane spanning protein LtaA. The glycerol-phosphate groups, which are derived from the head group of the membrane lipid phosphatidylglycerol, are then added one by one to the tip of the growing chain by the lipoteichoic acid synthase (LtaS), and the process continues with the addition of D‑alanine residues and glycosyl units outside of the cell by multi-enzyme complexes. For each phosphatidylglycerol molecule utilized in this way, one molecule of diacylglycerol is produced, which re-enters the cell for recycling to phospho- or glycolipids.
In many Gram-positive bacteria, lipoteichoic acids are potent cell wall virulence factors that lead to inflammatory diseases, ranging from minor skin ailments to severe sepsis, by means of an interaction with Toll-like receptor 2 (TLR2) in host animals. This causes initiation of innate immune responses and thence ideally to the development of adaptive immunity, but if an excessive immune response occurs, as with many pathogenic bacteria, sepsis can result. The presence of substituents on the hydroxyls of the repeating units modifies the virulence of the organisms, while the length and abundance of lipoteichoic acids regulate the cellular level and activity of autolytic enzymes, specifically LytE. In Bacillus subtilis, overexpression of the diglucosyldiacylglycerol synthase leads to increased resistance to the lipopeptide antibiotic daptomycin. On the other hand, Lactiplantibacillus plantarum is a probiotic, and its surface lipoteichoic acids adhere strongly to intestinal epithelial cells and alleviate insulin resistance and improve vitamin and amino acid metabolism.
2. Glycophospholipids
Some of the lipids in the second group of glycerol-containing glycophospholipids defined above, i.e., with a phosphatidyl backbone, are discussed in relation to the parent phospholipids in the relevant pages of this website, and these include the glycosyl-phosphatidylinositols, which are ubiquitous lipids that serve to anchor proteins in membranes. Complex mannosyl derivatives of phosphatidylinositol are typical components of the membranes of Actinomycetes and of coryneform bacteria, and they are also discussed separately on this website with related phosphoinositides. The trace levels of glycosylated phosphatidylethanolamine and phosphatidylserine formed by non-enzymatic Maillard reactions are not described here.
Phosphatidylglucoside: This lipid structure was first reported from the bacterium Staphylococcus aureus in 1970, but the finding does not appear to have been confirmed and is now considered doubtful. The first definitive isolation and characterization of phosphatidylglucoside was as recently as 2001, when surprisingly it was found in mammalian cell types rather than in a microorganism.
Thus, the first confirmed report of phosphatidyl-β-D-glucopyranoside (or 1,2-diacyl-sn-glycero-3-phospho-(1'-β-D-glucose)) was in human cord red cells, and while its fatty acid composition and positional distributions were initially reported to be comparable to the other phospholipids, it is now known that this was because the sample was contaminated. Subsequently, it was characterized from rat brain, human neutrophils, several human epithelium cells, an erythroblastic leukaemia cell line, and then from developing astroglial membranes of HL60 cells (together with phosphatidyl-β-D-(6-O-acetyl)-glucopyranoside). Its primary location is in radial glia and nascent astrocytes, but it may be a good cell surface marker for neural stem cells. With the HL60 cells, it was isolated by an improved procedure involving a monoclonal antibody, from which it was shown to exist in the form of a single saturated molecular species with 18:0 at position sn-1 and 20:0 at position sn-2 of the glycerol backbone, a highly un-mammalian-like structure for a phospholipid! Another unusual feature is that it exists in enantiomeric forms, i.e., in fetal rat brain, a proportion (~15%) has the phosphoglucose moiety attached to position sn-1 of an sn‑2,3‑diacylglycerol backbone and can be designated S-phosphatidylglucoside. As this characteristic may be unique to this lipid, it suggests that the biosynthetic pathway and perhaps the evolutionary origin are distinctive.

Disaturated phosphatidylglucoside (and its acetylated form) is located predominately in the outer leaflet of the plasma membrane in HL60 cells, erythrocytes and neutrophils. Although its polar head group might be expected to be similar in physical properties to that of phosphatidylinositol, it is smaller than the latter especially when differences in the degree of hydration are considered; phosphatidylglucoside has a transition temperature that can be 20°C higher and has more extensive lipid-lipid head group interactions. Although it is a minor lipid in quantitative terms, phosphatidylglucoside has special significance in that it forms signalling microdomains in membranes, akin to but not identical to rafts. It has a melting point of 73°C, like that of lactosylceramide, which is also located in separate raft-like domains in the plasma membrane of neutrophils, and the observation that both lipids have saturated lipid backbones may be relevant.
While phosphatidylglucoside per se is reported to have a role in signal transduction, its acetylated form is immunogenic and may participate in extracellular signalling. Lactosylceramide forms lipid microdomains that mediate neutrophil chemotaxis, phagocytosis and superoxide generation, whereas those enriched in phosphatidylglucoside have a different protein complement and mediate neutrophil differentiation and spontaneous apoptosis. There is evidence that the latter is a factor in the differentiation of HL60 to neutrophil-like cells, while in immature neural stem cells, it is involved in their differentiation into astroglia. When administered to mice, phosphatidylglucoside is reported to alleviate atherosclerosis by decreasing serum cholesterol levels and increasing cholesterol efflux by modifying bile acid metabolism, while it reduces cognitive impairment by improvement of neuroinflammation and neurotrophin signalling.
 Much remains to be
learned of how phosphatidylglucoside is synthesised in cells, but it is glucose-dependent and occurs in the luminal membrane of the endoplasmic
reticulum, where uridine diphosphate glucose:glycoprotein glucosyltransferase 2 (UGGT2) converts phosphatidic acid to phosphatidylglucoside.
It is noteworthy that UGGT2 does not accept unsaturated fatty acid-containing phosphatidic acids as substrates.
This reaction may be a means of regulating autophagy and mitigating stress in the endoplasmic reticulum induced by an excess of saturated lipids.
Much remains to be
learned of how phosphatidylglucoside is synthesised in cells, but it is glucose-dependent and occurs in the luminal membrane of the endoplasmic
reticulum, where uridine diphosphate glucose:glycoprotein glucosyltransferase 2 (UGGT2) converts phosphatidic acid to phosphatidylglucoside.
It is noteworthy that UGGT2 does not accept unsaturated fatty acid-containing phosphatidic acids as substrates.
This reaction may be a means of regulating autophagy and mitigating stress in the endoplasmic reticulum induced by an excess of saturated lipids.
Lysophosphatidylglucoside, i.e., with one fatty acid constituent in the primary position, has been detected in brain and has its own activity in guiding the specific location of axons in the developing spinal cord, while acting as an intercellular signalling molecule to mediate glia-neuron communication. It influences a variety of physiological and pathological events, including inflammatory pain and cancer, and it is a chemotactic molecule for human monocytes and macrophages. The mechanism involves a G protein-coupled receptor GPR55, which was first identified as a receptor for lysophosphatidylinositol (and other lipid mediators) but is now determined to have a much higher affinity for lysophosphatidylglucoside. Like the parent diacylphospholipids, two stereochemical forms (R or S) can be formed in vitro at least, and these both react with GPR55 but mediated via different Gα subunits to produce differing biological outcomes.
Phosphatidylglucoside may be more abundant in animal tissues than has been realized, since molecular species have the same mass numbers as for phosphatidylinositol on analysis by electrospray mass spectrometry so might easily be missed.
Bacterial glycophospholipids: Lipids related to phosphatidylglucoside but with cholesterol attached to the glucose moiety, i.e., cholesteryl-6'-O-phosphatidyl-α-glucoside and cholesteryl-6'-O-lysophosphatidyl-α-glucoside, occur in the pathogenic bacterium Helicobacter pylori, together with simple cholesterol-α-glucosides (see or web page on cholesterol derivatives). Lactobacillic acid is esterified to position sn-2 of glycerol. 6‑Phosphatidyltrehalose and 6,6'-diphosphatidyltrehalose are closer analogues and consist of trehalose attached to one or two phosphatidic acid units, respectively, with a long-chain saturated fatty acid in position sn-1 and again with a cyclopropyl fatty acid in position sn-2. These glycophospholipids were isolated from the typhoid fever-causing, Gram-negative bacterium Salmonella typhi, and they have potent immunostimulatory properties.

One of the first glycophospholipids with a phosphatidyl backbone to be discovered was in a Bacillus sp. and was characterized as a glucosaminylphosphatidylglycerol in which the glucosaminyl residue is linked to position 2’ of the sn-glycero-1-phosphate moiety of phosphatidylglycerol. Soon thereafter, an isomeric compound with the galactosamine residue in position 3’ was discovered, and analogous lipids with N‑acetyl-galactosamine and glucose attached to phosphatidylglycerol in a similar manner have been isolated from other bacteria.

With such compounds, it is known that pre-formed phosphatidylglycerol is glycosylated by an enzyme that transfers a glycosyl unit from the appropriate uridine 5-diphosphate(UDP)-hexose as in the glycosylation of many other complex lipids, such as in the biosynthesis of the mono- and digalactosyldiacylglycerols.
A related lipid, cardiolipin, containing an α-D-linked glucopyranose unit in position 2’, was first found as a minor component of certain Streptococci. Subsequently, glucosylcardiolipins with 1-5 glucose units were detected in the thermophilic bacterium Geobacillus stearothermophilus and related species, where it was suggested that they might stabilize the membranes when they are exposed to high temperatures. As further examples, a major phosphoglycolipid from Deinococcus radiodurans, a Gram-positive bacterium, has been shown to be 2'‑O‑(1,2‑diacyl-sn-glycero-3-phospho)-3'-O-(α-galactosyl)-N-D-glyceroyl alkylamine, i.e., in which a phosphatidic acid moiety is linked to glyceric acid and thence to a long-chain amine and to galactose; these now known to be the main lipid components of Bacillota bacteria. Scytonema julianum (cyanobacteria) contains a number of unusual glycophospholipids.
Related lipids have been found in thermophiles of the genera Thermus and Meiothermus. Hydrogenobacter thermophilus, an extremely thermophilic hydrogen bacterium, contains a phospholipid with a 1-amino-pentanetetrol moiety, i.e., 1,2-diacyl-3-O-(phospho-2'-O-(1'-amino)-2',3',4',5'-pentanetetrol)-sn-glycerol. Analogous lipids but with alkyl moieties rather than fatty acids and tertiary and quaternary amine groups are known from the archaeon Methanospirillum hungatei. Many more such glycophospholipids are present in extremophile bacteria, and for example, the gram-negative Chthonomonas calidirosea and Thermus thermophilus contain several glycophospholipids based on a phosphatidylglyceroylalkylamine core unit to which alpha-linked glucosyl, xylosyl, glucosamine or N‑alanylglucosaminyl monosaccharides are attached.
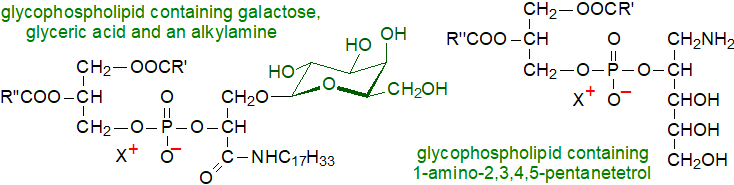 |
| Figure 2. Two examples of complex glycophospholipids from bacteria. |
Lipopolysaccharides: The Enterobacterales, including Escherichia coli produce a glycophospholipid-lipopolysaccharide (distinct from those containing lipid A) in its inner membrane, designated "membrane protein integrase (MPIase)" as it has activity that initially appeared to be that of an enzyme before its true nature was revealed. It has a long glycan chain composed of 9 to 11 repeating trisaccharide units, i.e., the amino sugars GlcNAc, ManNAcA and Fuc4NAc, and an anchor composed of pyrophosphate attached to a diacylglycerol, i.e., it is a glycopyrophospholipid. It alters the physicochemical properties of the membrane and is essential for protein insertion into it while facilitating protein translocation across it. Biosynthesis of the diacylglycerol pyrophosphate component requires two CDP-diacylglycerol synthases, YnbB and CdsA, of which the latter is a universally conserved enzyme in all organisms. MPIase is rapidly upregulated in the cold, as protein export requires a higher level of MPIase at a low temperature.

A comparable molecule in these species termed the 'enterobacterial common antigen' (ECAPG) contains the same repeating trisaccharide unit (as many as 55) linked to diacylglycerol-phosphate through a phosphodiester bond and is located in the outer membrane in the outer leaflet together with lipopolysaccharides. It contributes to the barrier function of the membrane and resistance to toxic molecules. Two related molecular forms exist, one with the repeating unit linked to lipid A and the other having a cyclic structure without a lipid component.
Many bacteria produce capsules consisting of long-chain polysaccharides with repeat-unit structures attached to a conserved reducing terminal glycolipid composed of lysophosphatidylglycerol, i.e., monoacylated, and a poly-3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) linker. In Bacteroides fragilis from the human gut microbiome, the polysaccharide component is zwitterionic and contains more than 100 repeating units of a tetrasaccharide consisting of D‑galactopyranose, 2,4-dideoxy-4-amino-D-FucNAc, D‑N‑acetylgalactosamine and D‑galactofuranose with 4,6‑pyruvate. It is considered to be the model symbiotic immunomodulatory molecule that confers benefits to the host in respect of autoimmune, inflammatory and infectious diseases, although these glycophospholipids can be virulence factors for some pathogens. In Gram-negative bacteria, they are synthesised via ATP-binding cassette (ABC) transporter-dependent processes.

Algae: An unusual glycero-glycophospholipid containing a dimethylarsinoyl moiety linked to ribose occurs in brown algae (see our web page on arsenolipids).
3. Analysis
Analysis of glycophospholipids and phosphoglycolipids is not straightforward as no standards are available, and structural characterization is a task for the specialist as the correct stereochemistry of the glycerol and carbohydrate moieties is required for biological activity. High-performance thin-layer chromatography has been the technique employed most often for isolation purposes, though HPLC in the adsorption and ion-exchange modes is increasingly being used. Modern techniques of nuclear magnetic resonance spectroscopy and of mass spectrometry are indispensable aids, but care is necessary with the mammalian phosphatidylglucoside to avoid confusion with phosphatidylinositol with which it is isobaric.
Suggested Reading
- Fischer, W. Bacterial phosphoglycolipids and lipoteichoic acids. In: Handbook of Lipid Research 6. Glycolipids, Phosphoglycolipids and Sulfoglycolipids, pp. 123-235 (ed. M. Kates, Plenum Press, NY) (1990).
- Greimel, P. Biophysical properties of phosphatidylglucoside and phosphatidylinositol: specific differences in head group interaction. Trends Glycosci. Glycotechn., 30, E1-E13 (2018); DOI.
- Guy, A.T. and Kamiguchi, H. Lipids as new players in axon guidance and circuit development. Curr. Opinion Neurobiol., 66, 22-29 (2021); DOI.
- Han, J., Zhao, X., Xilian Zhao, X., Li, P. and Gu, Q. Insight into the structure, biosynthesis, isolation method and biological function of teichoic acid in different gram-positive microorganisms: A review. Int. J. Biol. Macromol, 253, 126825 (2023); DOI.
- Hanafusa, K., Hotta, T. and Iwabuchi, K. Glycolipids: linchpins in the organization and function of membrane microdomains. Front. Cell Developm. Biol., 8, 589799 (2020); DOI.
- Jeong, G.-J., Khan, F., Tabassum, N., Cho, K.-J. and Kim, Y.M. Controlling biofilm and virulence properties of Gram-positive bacteria by targeting wall teichoic acid and lipoteichoic acid. Int. J. Antimicrobial Agents, 62, 106941 (2023); DOI.
- Li, X.J. and others. Lysophosphatidylglucoside is a GPR55-mediated chemotactic molecule for human monocytes and macrophages. Biochem. Biophys. Res. Commun., 569, 86-92 (2021); DOI.
- Mori, S., Shionyu, M., Shimamoto, K. and Nomura, K. Bacterial glycolipid acting on protein transport across membranes. Chembiochem, 25, e202300808 (2024); DOI.
- Osawa, T., Fujikawa, K. and Shimamoto, K. Structures, functions, and syntheses of glycero-glycophospholipids. Front. Chem., 12, 1353688 (2024); DOI.
- Pieringer, R.A. Biosynthesis of non-terpenoid lipids. In: Microbial Lipids. Volume 2, pp. 51-114 (ed. C. Ratledge and S.G. Wilkinson, Academic Press, London) (1989).
- Pinheiro, L., Freitas, M. and Branco, P.S. Phosphate-containing glycolipids: a review on synthesis and bioactivity. Chemmedchem, 19, e202400315 (2024); DOI.
- Rottem, S. Unique choline-containing phosphoglycolipids in Mycoplasma fermentans. Chem. Phys. Lipids, 191, 61-67 (2015); DOI.
- Shimamoto, K., Fujikawa, K., Osawa, T., Mori, S., Nomura, K. and Nishiyama, K. Key contributions of a glycolipid to membrane protein integration. Proc. Japan Acad. Series B, Phys. Biol. Sci., 100, 387-413 (2024); DOI.
- Willis, L.M. and Whitfield, C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carb. Res., 378, 35-44 (2013); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: August 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
