Triacylglycerols: 2. Biosynthesis and Metabolism
In animals and plants, triacylglycerols are the primary storage lipids, which are relatively inert chemically and consist of three fatty acids linked to each of the positions of glycerol. All eukaryotic organisms and even a few prokaryotes are able to synthesise them, and in animals, many cell types and organs have this ability, but the liver, intestines and adipose tissue produce most with the last of these containing much of the body stores (see our web page on triacylglycerol structure and composition). Within all cell types, even those of the brain, triacylglycerols are stored as cytoplasmic 'lipid droplets' together with smaller amounts of other lipids, including oxylipins, enclosed by a monolayer of phospholipids and hydrophobic proteins such as the perilipins in adipose tissue or oleosins in seeds. Mycobacteria and yeasts have comparable lipid inclusions. These lipid droplets are now considered to be organelles with their own characteristic metabolic pathways and associated enzymes - no longer boring blobs of fat, and it is now recognized that adipose tissue especially is a central regulator of human physiology.
 |
| Figure 1. Structures of triacyl-sn-glycerols. |
The lipid serves as a store of fatty acids for energy, which can be released rapidly on demand, and as a reserve of fatty acids for structural purposes or as precursors for lipid mediators. Further, lipid droplets in cells serve as a protective agency to sequester any excess of bioactive and potentially harmful lipids such as free fatty acids, oxylipins, diacylglycerols, cholesterol (as cholesterol esters), retinol esters and coenzyme A esters.
While triacylglycerols are essential for normal physiology, an excessive accumulation in human adipose tissue and other organs results in obesity and other health problems, including insulin resistance, non-alcoholic fatty liver disease, cardiomyopathy and some cancers. Accordingly, there is considerable pharmaceutical interest in drugs that affect triacylglycerol biosynthesis and metabolism.
2.1. Biosynthesis of Triacylglycerols
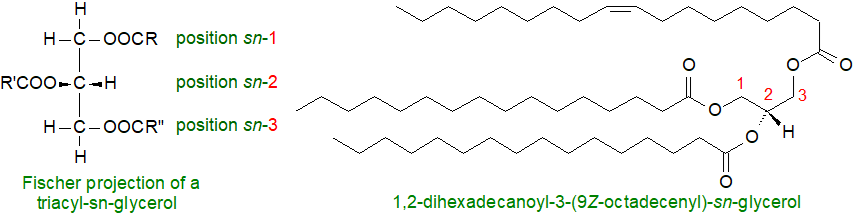
Three main pathways for triacylglycerol biosynthesis are known, the sn-glycerol-3-phosphate and dihydroxyacetone phosphate pathways, which predominate in liver and adipose tissue, and a monoacylglycerol pathway in the intestines. In maturing plant seeds and some animal tissues, a fourth route has been recognized in which a diacylglycerol transferase is the distinguishing enzyme.
Kennedy pathway: The main biosynthetic route to triacylglycerols is the sn-glycerol-3-phosphate or Kennedy pathway, first described by Professor Eugene Kennedy and colleagues in the 1950s, and this is the origin of more than 90% of liver triacylglycerols. In this pathway, the main source of the glycerol backbone was long believed to be sn-glycerol-3-phosphate from the catabolism of glucose (glycolysis), but it is now known that a significant proportion of the glycerol-3-phosphate originates in the cytoplasm by a process known as glyceroneogenesis via pyruvate, possibly the main source in adipose tissue, or to a lesser extent by the action of the enzyme glycerol kinase on free glycerol.
 |
| Figure 2. Kennedy pathway of triacylglycerol biosynthesis. |
Subsequent reactions occur primarily in or at the endoplasmic reticulum. First, the precursor sn-glycerol-3-phosphate is esterified by a fatty acid CoA ester in a reaction catalysed by glycerol-3-phosphate acyltransferases (GPAT3 and 4) at position sn-1 to form lysophosphatidic acid, and this is in turn acylated by an acylglycerophosphate acyltransferase (AGPAT or LPLAT) in position sn-2 to yield a key intermediate in the biosynthesis of all glycerolipids - phosphatidic acid, reactions described in greater detail in our web page on this lipid. Numerous isoforms of these enzymes are known; they are expressed with characteristic tissue and membrane distributions, and they are regulated in different ways.
Then, the phosphate group is removed by a family of enzymes - phosphatidic acid phosphohydrolases (PAPs or ‘phosphatidate phosphatases’ or ‘lipid phosphate phosphatases’ or 'lipins'), which produce sn-1,2-diacylglycerols as intermediates in the biosynthesis of triacylglycerols and of the phospholipids phosphatidylcholine and phosphatidylethanolamine (and of monogalactosyldiacylglycerols in plants). This is a significant branch-point in lipid biosynthesis as it may dictate the flow of lipids for storage or membrane biogenesis.
Three related cytoplasmic proteins, termed lipins, i.e., lipin-1, lipin-2 and lipin-3, are the main phosphatases that lead to triacylglycerol biosynthesis in animals. Unusually, these were characterized and named before the nature of their enzymatic activities was determined. Each of the lipins has a characteristic tissue distribution, but lipin-1 (PAP1) in three isoforms (designated 1α, 1β and 1γ) is the main PAP in adipose tissue and skeletal muscle in humans. Lipin-2 is the most abundant lipin in liver and is expressed substantially in the small intestine, macrophages and some regions of the brain; it is regulated dynamically by fasting and obesity (in mice). Lipin-3 overlaps with lipin-1 and lipin-2 and is found in the gastrointestinal tract and liver. Although they are cytosolic enzymes, lipins associate transiently with membranes to access their substrate, i.e., they are translocated to the endoplasmic reticulum in response to elevated levels of fatty acids within cells, although they do not have trans-membrane domains. Lipin-1 in the cytosol requires Mg2+ ions and is inhibited by N‑ethylmaleimide, whereas membrane-bound enzymes responsible for synthesising diacylglycerols as a phospholipid intermediate are independent of Mg2+ concentration and are not sensitive to the inhibitor.
 Perhaps surprisingly, lipin-1α has a dual role in that it operates in collaboration with known nuclear
receptors as a transcriptional coactivator to modulate lipid metabolism, while lipin 1β is associated with induction
of lipogenic genes such as those for the fatty acid synthase, stearoyl-CoA desaturase and DGAT.
By this means, they can have profound impacts upon signalling in a variety of cell types.
Abnormalities in lipin-1 expression are known to be factors in some human disease states that may lead to the metabolic syndrome
and inflammatory disorders.
Perhaps surprisingly, lipin-1α has a dual role in that it operates in collaboration with known nuclear
receptors as a transcriptional coactivator to modulate lipid metabolism, while lipin 1β is associated with induction
of lipogenic genes such as those for the fatty acid synthase, stearoyl-CoA desaturase and DGAT.
By this means, they can have profound impacts upon signalling in a variety of cell types.
Abnormalities in lipin-1 expression are known to be factors in some human disease states that may lead to the metabolic syndrome
and inflammatory disorders.
In the final step in this pathway, the 1,2-diacyl-sn-glycerol intermediate is acylated by diacylglycerol acyltransferases (DGAT), which can utilize a wide range of fatty acyl-CoA esters for triacyl-sn-glycerol production. There are two DGAT enzymes in animals, which are structurally and functionally distinct. DGAT1 is located mainly in the endoplasmic reticulum and is expressed in skeletal muscle, skin and intestine, with lower levels in liver and adipose tissue, and it is the only one present in the epithelial cells that synthesise milk fat in the mammary gland. It has a dual topology contributing to triacylglycerol synthesis on both sides of the membrane of the endoplasmic reticulum but esterifying only pre-formed fatty acids of exogenous origin to the cell in some tissues. As it can utilize a wider range of substrates, including monoacylglycerols, long-chain alcohols (for wax synthesis) and retinol, it helps to protect the endoplasmic reticulum from the lipotoxic effects of high-fat diets. Orthologues of this enzyme are present in most eukaryotes other than yeasts, and in plants, a soluble DGAT3 in has been identified in Arabidopsis.
DGAT2 is the relevant enzyme in hepatocytes and adipocytes (lipid droplets), although it is expressed much more widely in tissues. It is located in the endoplasmic reticulum, at the surface of lipid droplets and in mitochondria, and it esterifies fatty acids of both endogenous and exogenous origin. By means of a targeting domain, DGAT2 is able to tether between the endoplasmic reticulum and lipid droplet thereby channelling triacylglycerols from the site of synthesis to the nascent lipid droplet, where they accumulate and lead to the expansion of the latter (see below). Both enzymes are modulators of energy metabolism, and DGAT2 may control the homeostasis of triacylglycerols in vivo. In tumours, both enzymes can promote cancer progression.
As the glycerol-3-phosphate acyltransferase (GPAT) has the lowest activity of these enzymes, this step may be the rate-limiting one. On the other hand, DGATs are the dedicated triacylglycerol-forming enzymes, and they are seen as the best target for pharmaceutical intervention in obesity and attendant ailments with clinical studies of some DGAT1 inhibitors at the Phase 3 stage.
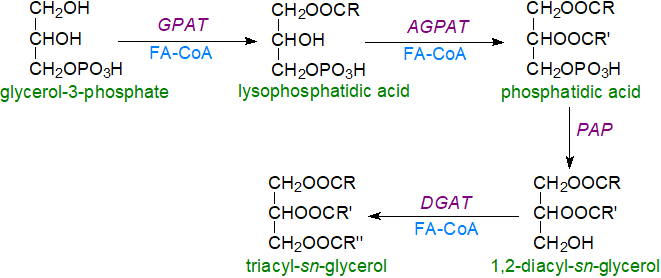
Dihydroxyacetone-phosphate pathway: In a second pathway for triacylglycerol biosynthesis, dihydroxyacetone-phosphate in peroxisomes or the endoplasmic reticulum can be acylated with fatty acid CoA esters by an acyltransferase to give 1-acyl dihydroxyacetone-phosphate, which is reduced by dihydroxyacetone-phosphate oxido-reductase to lysophosphatidic acid and can then enter the pathway above to triacylglycerols. The precursor dihydroxyacetone-phosphate has greater importance as part of the biosynthetic route to ether lipids, and neutral plasmalogens can be significant components of cytoplasmic droplets in many mammalian cell types but not in adipose tissue.
 |
| Figure 3. Biosynthesis of triacylglycerols via the dihydroxyacetone phosphate pathway. |
In prokaryotes, only the glycerol-3-phosphate pathway of triacylglycerol biosynthesis occurs, but in yeast both glycerol-3-phosphate and dihydroxyacetone-phosphate can be the primary precursors, and synthesis takes place in both cytoplasmic lipid droplets and the endoplasmic reticulum. In plants, the main pathway is via glycerol-3-phosphate, but these processes are discussed below in greater detail.
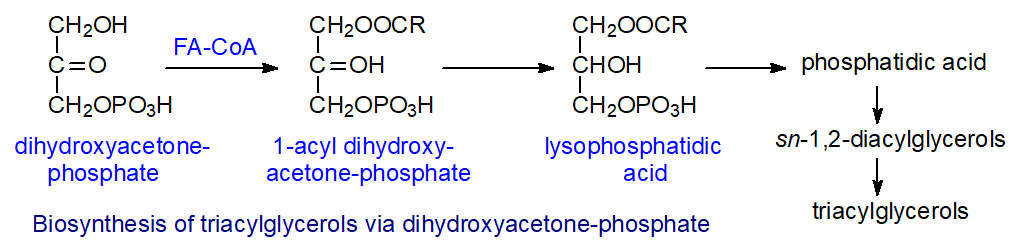
Monoacylglycerol pathway: In the enterocytes of intestines after a meal, up to 75% of the triacylglycerols are synthesised via a monoacylglycerol pathway. In this, 2-monoacyl-sn-glycerols and free fatty acids released from dietary triacylglycerols by the action of pancreatic lipase within the intestines (see below) are taken up by the enterocytes. There, the monoacylglycerols are first acylated by an acyl CoA:monoacylglycerol acyltransferase (MOGAT) and to some extent by DGAT1 with formation of sn-1,2-diacylglycerols as the first intermediate in the process, though some sn-2,3-diacylglycerols (~10%) are produced. In addition, 1‑monoacylglycerols can be synthesised by acylation of glycerol and then further acylated. There are three isoforms of the monoacylglycerol acyltransferase in humans of which MOGAT2 is the main enzyme in the intestines, although it is also present in liver where an appreciable proportion of the triacylglycerols result from the monoacylglycerol pathway. While MOGAT1 is the principal isoform in adipose tissue, the role of MOGAT3 is not clear, although it may operate in the intestines. Finally, acyl CoA:diacylglycerol acyltransferases (DGAT1 and DGAT2) react with the sn-1,2-diacylglycerols to yield triacylglycerols, but sn‑2,3‑diacylglycerols are esterified relatively slowly by these enzymes.
 |
| Figure 4. Biosynthesis of triacylglycerols via monoacylglycerols. |
Other pathways: In a fourth biosynthetic pathway, which is less well known, triacylglycerols are synthesised by a transacylation reaction between two racemic diacylglycerols that is independent of acyl-CoA. The reaction was first detected in the endoplasmic reticulum of intestinal microvillus cells and is catalysed by a diacylglycerol transacylase. Both diacylglycerol enantiomers participate in the reaction with equal facility to transfer a fatty acyl group with formation of triacylglycerols and a 2-monoacyl-sn-glycerol.
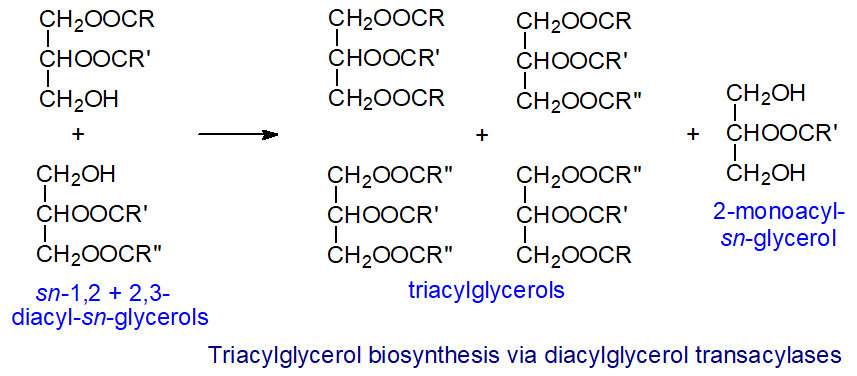 |
| Figure 5. Biosynthesis of triacylglycerols via diacylglycerol transacylases. |
A similar reaction has been observed in seed oils, where phospholipid diacylglycerol acyltransferases (PDATs) use endogenous phospholipids as fatty-acyl donors to generate triacylglycerols for storage in lipid droplets (CoA-independent pathway). This enzyme may part of the mechanism for remodelling triacylglycerol composition post synthesis and for regulation of the biogenesis of the endoplasmic reticulum by converting phospholipids into storage lipids. It is possible that analogous processes occur in the liver and adipose tissue, where extensive hydrolysis/re-esterification is known to occur. Phospholipase A2 group IVD (PLA2G4D) in humans and mice catalyses transacylase reactions using both phospholipids and acylglycerols as substrates, and in the presence of mono-and diacylglycerols, it generates diacylglycerols and triacylglycerols, respectively. Among other potential routes to the various intermediates, lysophosphatidic acid and phosphatidic acid can be synthesised in mitochondria, but they must then be transported to the endoplasmic reticulum before they enter the pathway for triacylglycerol production, while 1,2‑diacyl-sn-glycerols from the action of phospholipase C on phospholipids are utilized in the same way.
Transmembrane protein 68 (TMEM68) is a recently identified mammalian triacylglycerol synthase, with both MGAT and DGAT properties and high expression in the brain; it promotes triacylglycerol and lipid droplet formation independently of DGAT1/2 and regulates the composition of cellular glycerophospholipids. This enzyme is upregulated in breast cancer patients and higher levels are associated with poorer survival outcomes.
There is evidence for selectivity in the biosynthesis of different molecular species in a variety of tissues and organisms, a possible consequence of the varying biosynthetic pathways. In adipose tissue, fatty acids synthesised de novo are utilized in different ways from those from external sources in that they enter positions sn-1 and 2 predominantly, while a high proportion of the oleic acid synthesised in the tissue by desaturation of exogenous stearic acid is esterified to position sn-3. These phenomena can probably be explained by the different cellular locations of the DGAT and other enzymes. In a process akin to the Lands' cycle in phospholipids, dynamic remodelling of triacylglycerols occurs in adipose tissue and other organs in response to physiological changes, pharmacological interventions and pathological conditions.
In the glycerol-3-phosphate and other pathways, the starting material is of defined stereochemistry and each of the enzymes catalysing the various steps in the process is stereoselective and has preferences for certain fatty acids (as their CoA esters) and fatty acid combinations in the partially acylated intermediates. It should not be surprising, therefore, that natural triacylglycerols exist as enantiomers with each position of the sn-glycerol moiety esterified by different fatty acids, as discussed in Triacylglycerols - Part 1 with a discussion of the analytical methodology in Part 3.
2.2. Triacylglycerol Metabolism in the Intestines, Liver and Mammary Gland
Fat comprises up to 40% of the energy intake in the human diet in Western countries, and a high proportion of this is composed of triacylglycerols. The process of fat digestion is begun in the stomach by acid-stable gastric or lingual lipases, the extent of which is variable but in general is insignificant in quantitative terms in comparison to subsequent steps, although it may be required for efficient emulsification as an aid to digestion. Entry of triacylglycerol degradation products into the duodenum stimulates synthesis of the hormone cholecystokinin and causes the gall bladder to release bile acids, which are strong detergents and act to emulsify the hydrophobic triacylglycerols so increasing the available surface area. In turn, cholecystokinin stimulates the release of the hydrolytic enzyme pancreatic lipase and a co-lipase, and these together with bile salts and calcium ions act in a complex at the surface of the emulsified fat droplets to hydrolyse the triacylglycerols. The process is regiospecific and results in the release of the fatty acids from the 1 and 3 positions and formation of 2‑monoacyl-sn-glycerols, some of which can isomerize spontaneously to 1(3)-monoacyl-sn-glycerols for complete hydrolysis by the enzyme to glycerol and free fatty acids. Other lipases hydrolyse the phospholipids and other lipid esters in foods at the same time.
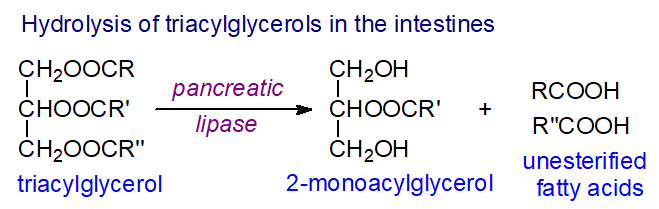 |
| Figure 6. Triacylglycerol hydrolysis in the intestines. |
This process is somewhat different in neonates and young infants, in whom pancreatic lipase is less important as it is effectively replaced by lipases in breast milk and by an acid gastric lipase (pH optimum 4 to 6). There is evidence that the regiospecific structure of dietary triacylglycerols affects the uptake of particular fatty acids and may influence the further lipid metabolism in humans, and for example, the presence of palmitic acid into position sn-2 of milk fat is of benefit to the human infant (as a source of energy for growth and development), although it increases the atherogenic potential for adults.
2‑Monoacylglycerols and 2-oleoylglycerol especially are signalling molecules in the intestines by activating a G‑protein coupled receptor GPR119, sometimes termed the ‘fat sensor’ to cause a reduction in food intake and body weight gain in rats and regulate glucose-stimulated insulin secretion; the free fatty acids released have a comparable effect, though by a very different mechanism via the receptor GPR40. Overall, it has become evident that triacylglycerol metabolism in the intestine has regulatory effects on the secretion of gut hormones and subsequently on systemic lipid metabolism and energy balance.
The free fatty acids and 2-monoacyl-sn-glycerols released are rapidly taken up by the intestinal cells from the distal duodenum to the jejunum by carrier molecules and by passive diffusion, and in the process, a fatty acid binding protein prevents a potentially toxic build-up of unesterified fatty acids and targets them for triacylglycerol biosynthesis. Within the cells, long-chain fatty acids are converted to the CoA esters and esterified into triacylglycerols by the monoacylglycerol pathway as described above, but in contrast, short and medium-chain fatty acids (C12 and below) are absorbed in the unesterified state and pass directly into the portal blood stream, where they are transported to the liver to be oxidized for energy production. Some of the dietary lipid is retained in cytoplasmic lipid droplets for later use, and some lipids/fatty acids may be absorbed from the circulation via the basolateral membrane for various purposes within the enterocytes.
Subsequently, the triacylglycerols are incorporated into lipoprotein complexes termed chylomicrons in the enterocytes by processes discussed in greater detail in our web page dealing with lipoproteins. In brief, chylomicrons consist of a core of triacylglycerols together with some cholesterol esters that is stabilized and rendered compatible with an aqueous environment by a surface film consisting of phospholipids, free cholesterol and one molecule of a truncated apoprotein B (48 kDa). These particles are secreted into the lymph and thence into the plasma for transport to the peripheral tissues for storage or structural purposes, and as they are chemically inert, triacylglycerols do not trigger side adventitious reactions on the way to their final destination. Adipose tissue in particular exports appreciable amounts of the enzyme lipoprotein lipase, which binds to the luminal membrane of endothelial cells facing into the blood, where it rapidly hydrolyses the passing triacylglycerols at the cell surface with release of free fatty acids, most of which are absorbed into the adjacent adipocytes and re-utilized for triacylglycerol synthesis within the cell.
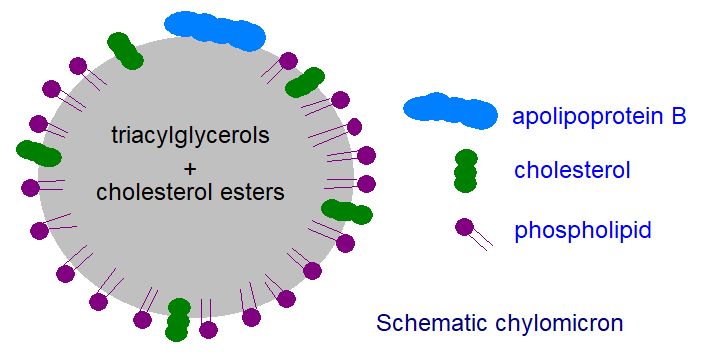 |
| Figure 7. A schematic chylomicron. |
The chylomicrons remnants eventually reach the liver, where the remaining lipids are hydrolysed at the external membranes by a hepatic lipase and absorbed. The fatty acids within the liver can be utilized for a variety of purposes, from oxidation to the synthesis of structural lipids, but a proportion is re-converted into triacylglycerols, and some of this is stored as lipid droplets within the cytoplasm of the cells (see next section). At the same time, a high proportion of the phosphatidylcholine from high-density lipoproteins taken up by the liver is eventually converted to triacylglycerols. In healthy liver, the levels of triacylglycerols are low (<5% of the total lipids), because the rates of acquisition of fatty acid from plasma and synthesis de novo within the liver are balanced by rates of oxidation and secretion into plasma. When these reactions are disturbed, excessive accumulation of storage triacylglycerols can occur and is often associated with fatty liver, insulin resistance and type 2 diabetes.
Most of the newly synthesised triacylglycerols are exported into the plasma as very-low-density lipoproteins (VLDL), consisting again of a triacylglycerol and cholesterol ester core, surrounded by phospholipids and free cholesterol, together with one molecule of full-length apoprotein B (100 kDa), apoprotein C and sometimes apoprotein E. These particles in turn are transported to the peripheral tissues, where they are hydrolysed and the free fatty acids absorbed before the remnants are eventually returned to the liver. Macrophages are also able to internalize and process triacylglycerol-rich lipoproteins with implications for foam cell production and the development of atherosclerosis.
In the mammary gland, triacylglycerols are synthesised in the endoplasmic reticulum and large lipid droplets are produced with a monolayer of phospholipids derived from this membrane. These are transported to the plasma membrane and bud off into the milk with their phospholipid envelope as milk fat globules and food for the newborn. The process is thus very different from that for the secretion of triacylglycerol-rich lipoproteins from other organs.
2.3. Triacylglycerol Metabolism in Adipocytes and Lipid Droplets
Adipose tissue and the adipocytes are characterized by accumulations of triacylglycerols, which act as the main energy store for animals while cushioning and insulating the body. Large fat depots occur around internal organs such as the liver and subcutaneously (see our web page on triacylglycerol composition), and each of these may react differently to metabolic constraints. For example in mice, adipose tissue in skin is highly reactive to diet, and with a high fat intake, much is delivered to dermal white adipose tissue and reduces heat transfer through the skin. In general, triacylglycerols stored when there is a surplus of nutrients are mobilized for energy production during starvation, but adipose tissue serves also as a reservoir of bioactive lipids, such as eicosanoids and lipid-soluble vitamins, and when required provides structural components, including fatty acids, cholesterol and retinol, for membrane synthesis and repair. They have sex-specific functions in relation to development, pregnancy, lactation and aging. By buffering against fatty acid accumulation that might exceed their capacity, non-adipose cells defend themselves against lipotoxicity while providing a rapid source of energy and essential metabolites by sensing and responding rapidly to changes in the systemic energy balance. Brown and beige fat have special properties and are discussed below, and bone marrow adipocytes (70% of the available space) are likewise distinctive.
Within most animal cells, including cell types in the brain, a proportion of the fatty acids taken up from the circulation is converted to triacylglycerols (>200 molecular species) as described above and incorporated into cytoplasmic lipid droplets (sometimes termed 'fat globules', 'oil bodies', 'lipid particles' or 'adiposomes'), which can range to up to 200 μm in diameter in adipocytes with other cell types containing smaller lipid droplets of the order of 50 nm in diameter. The triacylglycerols, packed together with cholesterol esters and other neutral lipids, are surrounded by a protective monolayer that includes phospholipids, cholesterol and hydrophobic proteins, the phospholipid components of which consists mainly of phosphatidylcholine and phosphatidylethanolamine derived from the cytosolic leaflet of the endoplasmic reticulum. Among the proteins, many are concerned directly with lipid metabolism, and these include acyltransferases, lipases, perilipins, caveolins and the Adipose Differentiation Related Protein (ADRP or adipophilin), which facilitate coordination and communication between different organelles and act as vital hubs of cellular metabolism. Similar lipid droplets occur within organelles such as the nucleus and mitochondria.
Based on profiling of the surface proteins and phospholipids, lipid droplets in cells are now considered to be complex organelles with a rich metabolism governed by a multitude of genes and are critical for many cellular and tissue processes. They supply fatty acids for various purposes, including membrane trafficking and recycling of both simple and complex lipids, while participating in immunometabolism, immune signalling and microbial killing. Potentially harmful lipids are in effect detoxified by sequestration in the core of the droplet. By secretion of bioactive molecules that engage in crosstalk with multiple organ systems, including the brain, adipocytes regulate whole-body homeostasis and enables adaptation to nutritional and environmental stresses. Dysfunction contributes to genetic and acquired diseases, which include hepatic steatosis, lipodystrophy, and cardiovascular, intestinal and infectious diseases.
Cytosolic lipid droplets and their associated metabolic processes are found in most eukaryotic cells, including those of the fruit fly Drosophila melanogaster, where many aspects of triacylglycerol processing and regulation parallel those in humans. They are also present in the plastids and other organelles of plants and in the cytoplasm of some prokaryotes (see below).
Lipid droplet assembly: This process takes place in sub-domains of the endoplasmic reticulum, where at least one isoform of each of the enzymes of triacylglycerol biosynthesis by the Kennedy pathway from acyl-CoA synthetases through to glycerol-3-phosphate acyltransferases (GPATs and LPLATs) is located probably in a protein assembly or 'interactome'. A dual-function regulator, calcineurin B homologous protein 1 (CHP1), stabilizes and activates GPATs to enable the coordinated recruitment of biosynthetic enzymes to lipid droplets, while a lipid transfer protein ATG2A transfers newly synthesised triacylglycerols, together with the intermediate diacylglycerols and phosphatidic acid, from the endoplasmic reticulum. As new triacylglycerols accumulate, they reach a critical level when a spontaneous condensation or nucleation by phase separation occurs, leading to an oil blister within the hydrophobic bilayer region, which attracts perilipins and other proteins that allow lipid droplets to grow further in patches of the membrane as lens-like swellings between the two membrane leaflets.
 |
| Figure 8. Seipin complex (Arlt, H. et al. DOI. Illustration by Matthew Conroy, Lipid Maps) |
A non-enzymatic protein seipin stabilizes the nascent droplets with minimal disruption to the membrane and enables them to mature. Seipin monomers assemble into a decameric cage-like structure and sit in the ER to provide a space that permits triacylglycerol molecules to interact with each other rather than with phospholipid acyl chains, a process that is probably aided by trans-membrane protein segments. This enables phase separation of the triacylglycerols, a lens-like structure and growth to a point where the seipin oligomer opens toward the cytoplasm so the lens can form a budding lipid droplet. In the nuclei of cells, seipin is utilized to stabilize lipid droplets, whose constituents interact with numerous proteins and regulate nuclear events, which differ from those in the cytoplasm.
As they grow, lipid droplets bud toward the cytosol, a process that is directed and aided by surface proteins such as perilipin, while the triacylglycerol core attracts and is largely surrounded by phospholipids from the outer leaflet of the endoplasmic reticulum. Growth continues through an extended endoplasmic reticulum/droplet junction or bridge until finally with the involvement of membrane curvature-inducing coat proteins, fat storage-inducing transmembrane ('FIT') proteins that bind diacylglycerols, lysophospholipids and phosphatidic acid (non-bilayer lipids), the droplets bud off into the cytoplasm with their surface monolayer of phospholipids and proteins, including the enzymes of triacylglycerol biosynthesis. This last step is probably aided by an asymmetric accumulation of phospholipids that decreases surface tension towards the cytosol. In adipocytes, lipid droplets can grow by fusion of smaller droplets, by a process in which CIDE proteins, CIDEA, CIDEB, and CIDEC are enriched at contact sites to create a channel for directional transfer of triacylglycerols from the smaller to the larger droplets. Similar processes occur in yeast, nematodes and plants.
 |
| Figure 9. Schematic representation of lipid droplet generation. Illustration by Henry Jacobowsky, LibretextsTM - Biology, with permission. |
Some of the surface proteins on lipid droplets can extend long helical hairpins of hydrophobic peptides deep into the lipid core, and prominent among these are the perilipins, which constitute a family of at least five phosphorylated proteins that bind to droplets in animals and share a common region, the so-called ‘PAT’ domain, named for the three original members of the family that include perilipin and ADRP. Proteins related evolutionarily to these are found in more primitive organisms, including insects, slime moulds and fungi, but not in the nematode Caenorhabditis elegans. In mammals, the perilipin PLIN1 (or 'perilipin A' or more accurately the splice variant 'PLIN1a') is a well-established regulator of lipolysis in adipocytes, maintaining a balance between lipid storage and utilization, and it may influence the production of the large lipid droplets in white adipose tissue. PLIN1 and PLIN2 serve in triacylglycerol metabolism in tissues other than adipocytes, and PLIN2 is the main perilipin in hepatocytes where it interacts with the surface monolayer lipids. PLIN3, which binds specifically to diacylglycerols, and to a lesser extent PLIN4, are ubiquitously expressed, and both take part in triacylglycerol biosynthesis and the formation of lipid droplets, while PLIN5 has many different functions but operates mainly in tissues that oxidize fatty acids such as the heart, muscle and brown adipose tissue. Other surface proteins of lipid droplets are enzymes intimately involved in triacylglycerol metabolism, although it has been suggested that cytoplasmic droplets may act as a storage organelle for hydrophobic proteins, which are needed elsewhere in the cell.
Subsequently, mitochondria, peroxisomes and other organelles may contribute or exchange lipids with lipid droplets at inter-organelle contact sites and cause changes in protein composition, but the endoplasmic reticulum is usually closest, and presumably this enables a dynamic response to any change in metabolic status sensed by this organelle. Within the liver, triacylglycerols are stored as lipid droplets in the cytoplasm adjacent to the endoplasmic reticulum where a triacylglycerol hydrolase can catalyse lipolysis to di- and monoacylglycerols that are more soluble in the membrane, which they are able to cross. They are then available for re-synthesis into triacylglycerols by luminally oriented acyltransferases before assembly into nascent lipoprotein complexes. The cytoskeleton is fundamental to this process by controlling the position and movement of lipid droplets and other organelles, while seipin is required to maintain junctions between cell membranes as well as the initial steps of their creation.
 Lipolysis: When fatty acids
are required by other tissues for energy or other purposes, they are released from the triacylglycerols by the sequential actions of three
cytosolic enzymes at neutral pH, i.e., adipose triacylglycerol lipase (ATGL), hormone-sensitive lipase (HSL) and monoacylglycerol lipase,
which cycle between the cytoplasmic surfaces of the endoplasmic reticulum and the surface layer of lipid droplets.
Simplistically, ATGL hydrolyses triacylglycerols to diacylglycerols, which are hydrolysed by HSL to monoacylglycerols
before these are hydrolysed by the monoacylglycerol lipase to complete the process.
Lipolysis proceeds in a highly ordered manner with stimulation through cell-surface receptors via neurotransmitters, hormones
and autocrine/paracrine factors that drive various intracellular signalling pathways that include kinases.
Lipolysis: When fatty acids
are required by other tissues for energy or other purposes, they are released from the triacylglycerols by the sequential actions of three
cytosolic enzymes at neutral pH, i.e., adipose triacylglycerol lipase (ATGL), hormone-sensitive lipase (HSL) and monoacylglycerol lipase,
which cycle between the cytoplasmic surfaces of the endoplasmic reticulum and the surface layer of lipid droplets.
Simplistically, ATGL hydrolyses triacylglycerols to diacylglycerols, which are hydrolysed by HSL to monoacylglycerols
before these are hydrolysed by the monoacylglycerol lipase to complete the process.
Lipolysis proceeds in a highly ordered manner with stimulation through cell-surface receptors via neurotransmitters, hormones
and autocrine/paracrine factors that drive various intracellular signalling pathways that include kinases.
Although many proteins interact with the three enzymes to modulate their activity, location and stability, perilipin (PLIN1) has been described as "the gatekeeper of the adipocyte lipid storehouse" that regulates lipolysis by acting as a barrier to the process in non-stimulated cells. On β‑adrenergic stimulation as during fasting, it is phosphorylated by the cAMP-protein kinase, which changes its shape and reduces its hydrophobicity, and by this means it triggers lipolysis. An isoform, perilipin A, is the main regulatory protein in white adipose tissue.
Adipose triacylglycerol lipase, which initiates the process, was discovered surprisingly recently and is related structurally to the plant acyl-hydrolases in that it has a patatin-like domain in the NH2-terminal region (patatin is a non-specific acyl-hydrolase in potato). Transport mechanisms guide ATGL from the endoplasmic reticulum membrane to lipid droplets, where it is located on the surface both in the basal and activated states. This lipase utilizes triacylglycerols containing long-chain fatty acids, preferentially cleaving ester bonds in the sn-1 or sn-2 position (but not sn-3), and it yields diacylglycerols and free fatty acids as the main products, but it hydrolyses diacylglycerols rather slowly and monoacylglycerols and cholesterol esters not at all. In addition, it is a transacylase and phospholipase, and it hydrolyses retinol esters in hepatic stellate cells. Adipose triacylglycerol lipase works together hormone-sensitive lipase, and they are rate limiting for the first step in triacylglycerol hydrolysis.
Regulation of these enzymes is a complex process, and for example, a lipid droplet protein designated Gene identification-58 (CGI-58 or ABHD5), is known to be required for hydrolysis of fatty acids from position sn-1. In the resting state, this protein binds to perilipin (PLIN1), but on β‑adrenergic stimulation, the latter is phosphorylated leading to dissociation and interaction of CGI-58 with phosphorylated ATGL to commence the first step in triacylglycerol hydrolysis. A second protein (G0S2) and insulin inhibit the enzyme. Mutations in adipose triacylglycerol lipase or CGI-58 are responsible for a syndrome in humans known as ‘neutral lipid storage disease’.
 |
| Figure 10. Sequential hydrolysis of triacyl-sn-glycerols in adipocytes and lipid droplets. |
Hormone-sensitive lipase in various isoforms is a structurally unique member of the large Ser-lipase/esterase family of enzymes in animals. It is regulated by the hormones insulin and noradrenaline by a mechanism that ultimately involves phosphorylation of the enzyme by cAMP-protein kinase (as with perilipin), thereby increasing its activity and causing it to translocate from the cytosol to the lipid droplet to initiate the second step in hydrolysis. It is regulated further by a variety of proteins that include PLINs and fatty acid binding proteins (FABP). Hormone-sensitive lipase has a broad substrate specificity compared to other neutral lipases, and as well as triacylglycerols, it will rapidly hydrolyse diacylglycerols, monoacylglycerols, retinol esters and cholesterol esters. With triacylglycerols, hormone-sensitive lipase preferentially hydrolyses ester bonds in the sn-1 and sn-3 positions, leaving free acids and 2‑monoacylglycerols as the main end products, although diacylglycerols are in fact hydrolysed ten times more rapidly than triacylglycerols.
The monoacylglycerol lipase hydrolyses monoacylglycerols only and not di- or triacylglycerols, and completes the process of lipolysis, releasing free glycerol and fatty acids. It is located in the cytoplasm, plasma membrane and lipid droplets. As it is the enzyme mainly responsible for hydrolysis of the endocannabinoid 2‑arachidonoylglycerol in malignant cancers, it is attracting pharmaceutical interest (as is the regulatory factor ABHD5). A further enzyme, α/β-hydrolase containing‑6 (ABHD6), is located on the inner leaflet of the plasma membrane and preferentially hydrolyses fatty acids at the sn-1 position over the sn‑2 position of monoacylglycerols, and it hydrolyses lysophospholipids and bis(monoacylglycerol)-phosphate; it has been associated with the development of insulin resistance and the progression of cancer. In the liver, further lipolytic enzymes, including carboxylesterases, operate against triacylglycerols in cytoplasmic lipid droplets.
Unesterified fatty acids released by the combined action of these three lipases can be exported into the plasma for transport to other tissues as albumin complexes, while the glycerol released is transported to the liver for metabolism by either glycolysis or gluconeogenesis. However, observations in vitro suggest that much of the fatty acids released are recycled and re-incorporated into triacylglycerols and thence into lipid droplets, often after desaturation or chain elongation (c.f., the Lands' cycle in phospholipids), a process sometimes referred to as the glyceride–fatty acid futile cycle, since this is energetically costly through consumption of ATP but with no net change in lipid concentration; all fatty acyl chains may exchange within 24 hours. Although uncertainties remain, the purpose may be to ensure a ready supply of accessible fatty acids for rapid generation of phospholipids or signalling molecules.
Eventually, the whole organelle can disappear, including the proteins, when they undergo lipophagy, a process of autophagy, i.e., the delivery of the organelles to lytic compartments for degradation. This can occur through direct lysosomal invagination or more often by a multistep process involving the formation of double-membrane vesicles termed 'autophagosomes' around lipid droplets with subsequent lysosomal fusion and degradation of the triacylglycerols by the lysosomal acid lipase. This process is important for the regulation of cellular lipid levels in various tissues and disease conditions, but especially during starvation as a rapid means of providing nutrients. It is relevant to tumorigenesis and cancer metastasis, and to neurodegenerative and neuroinflammatory diseases. While lipophagy is mechanistically distinct from lipolysis, there is cross talk between the two.
Control of fatty acid supply: Lipid droplets accumulate within many cell types (and organelles) other than adipocytes, including leukocytes, epithelial cells, hepatocytes and even astrocytes, often during infections, cancer and other inflammatory conditions. They are necessary for the cellular storage and release of hydrophobic vitamins, signalling precursors and other lipids that are not related to energy homeostasis, while reducing the dangers of lipotoxicity. On the other hand, excessive fatty acid accumulation is associated with lipotoxicity, endoplasmic reticulum stress and mitochondrial damage and dysfunction, so lipid storage in lipid droplets must be balanced by lipolysis for health. For example, during mitochondrial stress, release of linoleic acid from lipid droplets enables synthesis of the essential membrane constituent cardiolipin. This may be relevant to the storage of polyunsaturated fatty acids as lipid droplets act as antioxidant organelles that control lipid peroxidation, preserve organelles and prevent cell death, including by ferroptosis, with ferroptosis suppressor protein 1 (FSP1) as a critical regulator that prevents peroxidation by recycling coenzyme Q10 (CoQ10) in its lipophilic antioxidant state. They must exert precise control of the delivery of polyunsaturated fatty acids into metabolic and signalling pathways as unrestricted lipolysis could lead to the release of potentially harmful amounts.
 A variety of enzymes
are associated with lipid droplets, including protein kinases, which are involved in many different aspects of lipid
metabolism, such as cell signalling, membrane trafficking and control of the production of inflammatory mediators like the eicosanoids.
Lipolysis enables secretion of lipids termed lipokines (or 'adipokines') from adipocytes that signal in a hormone-like
fashion to other tissues, thereby modulating gene expression and physiology, including food intake, insulin sensitivity, insulin secretion
and related processes.
These molecules include the lipids palmitoleic acid (9-16:1) and
fatty acid esters of hydroxy fatty acids (FAHFA), though the circulating proteins adiponectin
and leptin have been studied more intensively.
Adiponectin is a powerful insulin sensitizer and suppressor of apoptosis and inflammation with anti-diabetic and anti-atherosclerotic properties,
often operating through its influence upon sphingolipid metabolism, while leptin exerts most of its effects on the brain to trigger behavioural,
metabolic and endocrine responses to control the body's fuel reserves.
A variety of enzymes
are associated with lipid droplets, including protein kinases, which are involved in many different aspects of lipid
metabolism, such as cell signalling, membrane trafficking and control of the production of inflammatory mediators like the eicosanoids.
Lipolysis enables secretion of lipids termed lipokines (or 'adipokines') from adipocytes that signal in a hormone-like
fashion to other tissues, thereby modulating gene expression and physiology, including food intake, insulin sensitivity, insulin secretion
and related processes.
These molecules include the lipids palmitoleic acid (9-16:1) and
fatty acid esters of hydroxy fatty acids (FAHFA), though the circulating proteins adiponectin
and leptin have been studied more intensively.
Adiponectin is a powerful insulin sensitizer and suppressor of apoptosis and inflammation with anti-diabetic and anti-atherosclerotic properties,
often operating through its influence upon sphingolipid metabolism, while leptin exerts most of its effects on the brain to trigger behavioural,
metabolic and endocrine responses to control the body's fuel reserves.
Indeed, there are now suggestions that lipid droplets in all cell types are essential for the response mechanisms to cellular stress, including autophagy, inflammation and immunity, and they act as hubs to integrate metabolic and inflammatory processes. Via their lipolytic machinery, they regulate the availability of fatty acids for production of oxylipins from polyunsaturated fatty acids and initiation of signalling pathways. For example, triacylglycerols in cytoplasmic lipid droplets of human mast cells, which are potent mediators of immune reactions and influence many inflammatory diseases, have a high content of arachidonic acid and this can be released by triacylglycerol lipases as a substrate for production of eicosanoids when the cells are stimulated appropriately. Metabolism in lipid droplets is required for the differentiation of monocytes and to sustain differentiated macrophages in relation to inflammation. Lipid droplets in the nervous system are seen mainly in glia, ependymal cells, neurons and microglia in vivo, and during ageing or stress due to redox imbalance or lipotoxicity, they can accumulate and are observed in some neurodegenerative diseases. The presence of lipid droplets has been detected in several types of cancer cells, such as hepatocellular, pancreatic and breast carcinomas, where they are a source of nutrients driving metastasis.
During apoptosis, triacylglycerols enriched in polyunsaturated fatty acids accumulate in lipid droplets, possibly as a protective mechanism against membrane damage caused by oxidative stress and any resulting hydroperoxides. Triacylglycerols in lipid droplets of the skin are the source of the linoleic acid that is required for synthesis of the O-acylceramides, which are components of the epidermal barrier. In osteoblasts, lipolysis in cytoplasmic lipid droplets provides fatty acids as a necessary source of energy for bone formation.
Endocrine functions: Not only does the adipocyte provide a store of energy but it is an endocrine organ that manages the flow of energy by means of the hormone leptin, which signals through the adiponectin receptors AdipoR1 and AdipoR2 and binds to the non-signalling interacting protein, T‑cadherin, all under control of the brain via the sympathetic nervous system. The result is stimulation of signalling cascades to communicate with other tissues though the secretion of cytokines and other mediators. The synthesis of leptin is tightly controlled by adipocytes mainly, although it is also produced by the stomach, placenta and mammary gland, and provides information on the state of fat stores to other tissues to regulate food intake and energy expenditure. Leptin was initially described as an anti-obesity hormone, but in fact, it serves as an adiposity signal that is vital in maintaining adipose tissue mass to ensure survival under conditions of negative energy balance and so protect against either a deficit or an excess of adiposity. Perilipin is required also for sensing purposes.
Insulin is the main hormone that affects metabolism and its receptor at the plasma membrane is located in caveolae. In adipose tissue, adiponectin is a potent insulin sensitizer and suppressor of cell death and inflammation, directly promoting anti-diabetic and anti-atherosclerotic outcomes, while release of proinflammatory cytokines can stimulate lipolysis and cause insulin resistance, in turn leading to tissue dysfunction and systemic disruption of metabolism. By such means, adipose tissue metabolism has profound effects on whole-body metabolism, and defects in these processes can have severe implications for such serious pathological conditions as diabetes, obesity, cardiovascular disease, fatty liver disease and cancer in humans. It is hoped that development of specific inhibitors for hormone-sensitive lipase will improve the treatment of such metabolic complications. As caveolae, which contain the proteins caveolins (and presumably sphingolipids) and are abundant in adipocytes, modulate the flux of fatty acids across the plasma membrane, and participate in signal transduction and membrane trafficking pathways, it is evident that they have a major role in this aspect of lipid metabolism.
Other functions: Vitamin E (tocopherols) and vitamin A as retinyl esters are stored in cytoplasmic lipid droplets, and the latter are present in appreciable concentrations in the stellate cells of liver. In endocrine cells of the gonads and adrenals, cholesterol esters stored in lipid droplets are a major source of cholesterol for the mitochondrial biosynthesis of various steroid hormones. In the nucleus of the cell, lipid droplets are generated at the inner nuclear membrane in a process similar to that in the endoplasmic reticulum, and as well as providing a reservoir of fatty acids for membrane remodelling, they can sequester transcription factors and chromatin components and generate the lipid ligands for certain nuclear receptors. A transient organelle termed the midbody in dividing cells in humans and rodents contains a unique triacylglycerol consisting of three fatty acids 16:1-12:0-18:1 (12:0 is rarely detected in human lipids), but its function is not known. Aside from lipid biochemistry, lipid droplets participate in protein degradation and glycosylation.
Some pathogenic viruses and bacteria such as Mycobacterium tuberculosis utilize lipid droplets as a source of nutrients with unfortunate consequences for the host, although in other infections, eukaryotic cells can rapidly accumulate lipid droplets with unique compositions, including many proteins with appreciable antibiotic properties, which are differentially regulated. In consequence, such lipid droplets and their enzyme systems have the potential to be innate immunity hubs and may be markers for disease states susceptible to pharmaceutical intervention.
Insects: In insects, the fat body is a multi-functional tissue and is the main metabolic organ, which integrates signals that control the immune system, moulting and metamorphosis and synthesises hormones that regulate innumerable aspects of metabolism. Lipids, carbohydrates and proteins are the substrates and products of many pathways for use in energy production or to act as reserves for mobilization at the appropriate stage of life (diapause, metamorphosis and flight). In relation to innate and acquired humoral immunity, the fat body produces bactericidal proteins and polypeptides such as lysozyme, and it the source of vitellogenin, the yolk protein needed for the development of oocytes. There is further discussion in our web page on lipoproteins.
2.4. Brown Adipose Tissue
Most adipose tissue depots ('white fat') serve primarily as storage and endocrine organs, which provide a reservoir of nutrients for release when the food supply is low, but a second specialized type of adipose tissue, brown fat, is multilocular, highly vascularized and rich in mitochondria and the iron-containing pigments that transport oxygen and give the tissue its colour and name. In humans, these depots are located in characteristic anatomical regions such as subcutaneous areas around the heart, kidney, pancreas and liver or the neck, where they can supply warm venous blood directly to the spinal cord and brain. They arise from progenitor cells that are closer to those of skeletal muscle than white adipocytes and can oxidize fat so rapidly that heat is generated ("non-shivering thermogenesis”), a feature that is important in young animals and those recovering from hibernation.
In brief, during cold exposure, release of noradrenaline and stimulation of β-adrenergic receptors in the nervous system initiate a catabolic program that commences with rapid breakdown of cellular triacylglycerol stores and release of unesterified fatty acids with transient activation of peroxisome proliferator-activated receptor gamma (PPARγ). These set in motion a signalling process that results in the efficient β-oxidation of fatty acids to generate heat. The key molecule is the uncoupling protein-1 (UCP1), which can be compared to a valve that uncouples electron transport in the respiratory chain from ATP production with a highly exothermic release of chemical energy, i.e., as heat rather than as ATP. Although many aspects of the mechanism are uncertain, it is clear that proton conductance by UCP1 is highly regulated and inducible. It is driven by free long-chain fatty acids and inhibited by purine nucleotides, i.e., fatty acids are not only the substrate for thermogenesis but act as self-regulating second messengers, and once initiated, the dense vasculature increases the delivery of fatty acids and glucose to the brown adipocytes and warms the blood passing through the tissue. Whether brown fat contributes to fever induction during infections is controversial.
The mitochondrial phospholipid cardiolipin, which is intimately involved in oxidative phosphorylation, is indispensable for stimulating and sustaining thermogenic fat, and in this tissue, B-cell factor 2 (EBF2), a transcription factor, regulates the integrity of the content and composition of this and other phospholipids in mitochondrial membranes. Peroxisomal synthesis of plasmenyl-phospholipids may regulate thermogenesis by acting upon mitochondrial metabolism. Further, monomethyl branched-chain fatty acids are rapidly synthesised and then catabolized in brown adipose tissue by ACOX2 in peroxisomes to support energy production and presumably heat generation, a futile cycle that is independent of UCP1. Sphingomyelin accumulates during differentiation of brown adipocytes and is a further factor in the regulation of non-shivering thermogenesis.
Acyl-CoA synthetases and thioesterases determine the availability of substrates for β-oxidation and consequently the thermogenic capacity. The constitutive G‑protein coupled receptor GPR3 maintains high cAMP levels and acts upon brown adipocytes to drive thermogenesis via intrinsic Gs‑coupling with oleic acid as a ligand (it was originally thought that no ligand was necessary). In brown adipose tissue of mice, cold stimulation induces the secretion of oleic acid to trigger Gs/cAMP/PKA signalling. The membrane-bound stearoyl-CoA (Δ9) desaturase (SCDI) is significantly increased in activity during long-term cold exposure and sustains thermogenesis by stimulating the release of norepinephrine, which acts through β1, β2, and β3 adrenergic receptors. Indeed, a wide array of circulating lipids contribute to thermogenic potential, including free fatty acids and triacylglycerols, and there is evidence that acylcarnitines produced in the liver from fatty acids released from white adipose tissue in response to cold exposure are transported in plasma to brown adipose tissue and can serve as a substrate for thermogenesis.
Synthesis of the lipokine (or 'batokine') octadecanoid 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) is induced by cold, and this stimulates brown adipose tissue by promoting the uptake of fatty acids, acting via G-protein-coupled receptors; it increases cardiac efficiency and cardiomyocyte respiration via enhanced calcium cycling. n‑3 Polyunsaturated fatty acids may promote adaptive thermogenesis via their 5- and 12‑lipoxygenase metabolites and endocrine factors (batokines) 5- and 12‑hydroxyeicosapentaenoic acids (5- and 12-HEPE), by improving glucose metabolism via increased uptake pf glucose into adipocytes and skeletal muscle. FAHFA may be relevant in this context, and there are suggestions that other dietary fatty acids can influence the metabolism of brown and beige fat, e.g., a deficiency in ELOVL3, responsible for the synthesis of very-long-chain monoenes, reduces the efficiency of thermogenesis.
 In hibernating
mammals, brown adipose tissue is metabolically essential, and even in laboratory animals such as mice,
it can consume about 50% of dietary lipids and glucose when the animals are exposed to cold.
In humans, the metabolism of brown adipose tissue is induced acutely by cold and is stimulated via the sympathetic nervous system,
and the relevance of this tissue to humans is now becoming apparent.
There are suggestions that brown adipose tissue can behave as an endocrine system to secrete batokines that may be favourable towards
cardiovascular risk, so research is underway to determine whether sustaining thermogenesis in brown fat by pharmaceutical means
could be beneficial towards this and other human disease states, including obesity and diabetes.
In hibernating
mammals, brown adipose tissue is metabolically essential, and even in laboratory animals such as mice,
it can consume about 50% of dietary lipids and glucose when the animals are exposed to cold.
In humans, the metabolism of brown adipose tissue is induced acutely by cold and is stimulated via the sympathetic nervous system,
and the relevance of this tissue to humans is now becoming apparent.
There are suggestions that brown adipose tissue can behave as an endocrine system to secrete batokines that may be favourable towards
cardiovascular risk, so research is underway to determine whether sustaining thermogenesis in brown fat by pharmaceutical means
could be beneficial towards this and other human disease states, including obesity and diabetes.
Beige adipocytes: This research has been stimulated by the observation that clusters of adipocytes with thermogenic capacity can be present in white adipose tissue and emerge in response to various physiological signals, including reactive oxygen species. They are termed 'beige or brite' adipocytes and arise from multipotent pre-adipocytes. While brown adipose tissue is a discrete organ in animals, beige adipose tissue is interspersed with white, and the two types have different developmental origins although they utilize the same machinery to release heat by oxidation of fatty acids under β-adrenergic stimulation. In adult humans, most of the deposits once thought to be classical brown adipose tissue do not contain the genetic markers for this tissue and are now recognized to be beige/brite fat, although brown adipose tissue per se is present in significant amounts in human new-borns and infants. In addition to the conventional UCP1-dependent thermogenesis, beige adipose tissue has three further thermogenic mechanisms that involve cycling of Ca2+, creatine substrate and triacylglycerols/fatty acids.
2.5. Other Functions of Triacylglycerol Depots
Subcutaneous depots act as a cushion around joints and serve as insulation against cold in many animals, as is obvious for marine mammals such as seals but perhaps less so for the pig, which is surrounded by a layer of fat. Those adipocytes embedded in the skin differ from the general subcutaneous depots and support the growth of hair follicles and regenerating skin, and they may have a defensive role both as a physical barrier and by responding metabolically to bacterial infection. Some insects that are tolerant of freezing synthesise triacylglycerols containing acetic acid, and these remain liquid at low temperatures; by interacting with water, they may play a part in cryoprotection.
In marine mammals and fish, the fat depots are less dense than water and so aid buoyancy with the result that less energy is expended in swimming. More surprisingly perhaps, triacylglycerols together with the structurally related glyceryl ether diesters and wax esters are the main components of the sonar lens used in echolocation by dolphins and toothed whales. In these, the triacylglycerols are unique in that they contain two molecules of 3‑methylbutyric (isovaleric) acid with one long-chain fatty acid. The relative concentrations of the various lipids in an organ in the head of the animals (termed the ‘melon’) are arranged anatomically in a three-dimensional topographical pattern to enable them to focus sound waves.
2.6. Triacylglycerol Metabolism in Plants and Yeasts
Fruit and seed oils are major agricultural products with appreciable economic and nutritional value. The mesocarp of fruits is a highly nutritious energy source that attracts animals that help to disperse the seeds, and in plants such as the oil palm and olive trees, much of the fruit flesh contains triacylglycerols. In seeds, triacylglycerols are the main storage lipid and can comprise as much as 60% of their weight. Fruit lipids are not intended for use by the plant per se and are stored in lipid droplets in large irregular structures that break down readily, but seed lipids are required for the development of the plant embryo and have been studied intensively. However, triacylglycerol biosynthesis and metabolism are required for many other purposes that include pollen viability and maintenance of lipid homeostasis in chloroplasts (see the note on plastoglobules below).
Seed oils: In seeds and other plant tissues, biosynthesis of fatty acids takes place in plastids, and they are stored as triacylglycerols in lipid droplets with a coherent surface layer of proteins and lipids in the embryo (e.g., Arabidopsis, soybean or sunflower) or endosperm (e.g., castor bean) tissues of seeds. The seed-specific transcription factor WRINKLED1 (WRI1) is considered to be a master regulator of seed oil accumulation, by upregulating many genes encoding enzymes involved in glycolysis and fatty acid biosynthesis, in conjunction with the upstream LAFL network. In addition to the common range of fatty acids synthesised in plastids, mainly palmitate and oleate, some plants produce novel fatty acids, including medium- and very-long-chain components and those with oxygenated and other structural features, but a means of diverting these to seeds for triacylglycerol production must exist to prevent disruption of the plant membranes.
Seed development occurs in three stages - rapid cell division with no accumulation of storage material, rapid deposition of triacylglycerols and other energy-rich metabolites, and finally desiccation. During the period of oil accumulation in seeds, the new acyl carrier protein (ACP) esters of fatty acids are first hydrolysed by two different classes of acyl-ACP thioesterases at the inner plastid envelope membrane, before the unesterified fatty acids are transported to the endoplasmic reticulum (ER), by a family of fatty acid export proteins (FAX) of which there are seven isoforms in Arabidopsis, two of which (FAX2 and FAX4) are highly expressed during the early stage of seed development. The unesterified fatty acids are shuttled across the plastid outer envelope, probably by vectorial acylation by long-chain acyl-CoA synthases, which catalyse CoA ester production.
In the ER, triacylglycerols and membrane lipids are synthesised by the Kennedy and other pathways described above. In yeast and plants, 1,2‑diacyl-sn-glycerol esterification is the only committed step in triacylglycerol production, and this occurs by mechanisms that can be either dependent or independent of acyl-CoA esters. The acyl-CoA-dependent route is catalysed by diacylglycerol:acyl-CoA acyltransferases (DGATs) with acyl-CoA and diacylglycerols as substrates, and two membrane-bound isoenzymes (DGAT1 and DGAT2) and a cytosolic isoenzyme (DGAT3) are known. DGAT1 is the main enzyme for triacylglycerol generation in developing seeds (together with the desaturase FAD2 for provision of linoleate), while DGAT2 is relevant in those plants with unusual fatty acid compositions. In Arabidopsis, DGAT3 has some specificity for polyunsaturated fatty acids in seed development. These enzymes differ greatly in structure, substrate preferences and expression patterns: DGAT1 is a member of the membrane-bound O-acyltransferase superfamily with nine or 10 trans-membrane domains in plants, while DGAT2 is one of the monoacylglycerol acyltransferase family with 2 or 3 putative transmembrane domains.
 |
| Figure 11. Triacylglycerol biosynthesis in the endoplasmic reticulum of plants via phosphatidylcholine. |
A substantial proportion of triacylglycerol biosynthesis in some plants (and algae and yeast) is synthesised by a flux through the membrane phospholipid phosphatidylcholine (or other phospholipids depending upon species), produced by what are sometimes termed inaccurately the 'eukaryotic and prokaryotic pathways' with differing positional distributions (see our web-page on galactosyldiacylglycerols), in which diacylglycerols are generated from phosphatidic acid by the action of a phosphatidate phosphatase as an intermediate. In the acyl-CoA-independent reaction, direct transfer of one fatty acid from phosphatidylcholine to diacylglycerol by the action of the phospholipid:1,2-diacyl-sn-glycerol-acyltransferase (PDAT) enzyme occurs with lysophosphatidylcholine as a by-product, which can be re-esterified for further reaction. It has been postulated that this pathway is a regulator of membrane lipid remodelling and organelle function that is important for maintaining cellular lipid homeostasis.
As phosphatidylcholine undergoes extensive remodelling, and its fatty acid components are converted by desaturation to linoleic and linolenic acids, the compositions and positional distributions within triacylglycerols generated in this way can be very different from those synthesised by the ‘classical’ pathways. In this manner, phosphatidylcholine is a carrier for the trafficking of acyl groups between organelles and membrane subdomains, and it has been suggested that an assembly of interacting enzymes may facilitate the transfer of polyunsaturated fatty acids from this phospholipid to triacylglycerols in seeds. Both sterol and sphingolipid biosynthesis are further factors necessary for efficient seed oil production. Surprisingly, substantial β-oxidation of fatty acids can take place in developing seeds simultaneously with synthesis de novo, but why this occurs and how it is regulated has still to be determined.
Some plants accumulate unique fatty acids in their seed triacylglycerols, but not in structural phospholipids, which often have commercial value in pharmaceuticals and oleochemicals. To make this possible, such plants have developed specialized metabolic pathways for remodelling of the triacylglycerols that utilize a unique lipase and two diacylglycerol acyltransferases with novel specificities (see our web page on hydroxy fatty acids).
As triacylglycerol synthesis continues, oil droplets accumulate between the leaflets of the endoplasmic reticulum and are surrounded by a monolayer of phospholipids, sterols and structural proteins, which in Arabidopsis include oleosins, a caleosin (which is also a peroxygenase), a steroleosin, a putative aquaporin and a glycosylphosphatidylinositol-anchored protein. Oleosins are the most abundant of these (~65%) and are small proteins (15-30 kDa) that contain cytosolic-facing N- and C-termini and a large hydrophobic domain necessary to target them to lipid droplets, where they affect the control of their size, stability and resistance to oxidation as a dense elastic interfacial layer. Eventually, lipid droplets "bud off” from the endoplasmic reticulum with their monolayer of phospholipids and proteins, and they are released into the cytosol by a mechanism that has still to be defined.
At the onset of germination, water is absorbed, and esterases/lipases are stimulated. The process of lipolysis begins at the surface of oil bodies, where the oleosins, which are the most abundant structural proteins, serve to assist the docking of lipases and to control the size and stability of lipid droplets in seeds. Several esterases/lipases have been cloned from various plants and possess a conserved catalytic triad of Ser, His and Asp or Glu, somewhat different from the animal lipases, as in patatin (an abundant lipolytic protein in potatoes), which are able to hydrolyse triacylglycerols but not phospho- or galactolipids. Of these, the most active is the 'sugar-dependent lipase 1 (SDP1)', which is a patatin-like lipase similar to the mammalian adipose triacylglycerol lipase discussed above and is located on the surface of the oil body. This hydrolyses triacylglycerols mainly to diacylglycerols but presumably works in conjunction with di- and monoacylglycerol lipases to generate free fatty acids and glycerol.
 The lipid droplets
in seeds exist in close proximity with glyoxysomes (broadly equivalent to peroxisomes).
These are the membrane-bound organelles that contain most of the enzymes required to oxidize fatty acids derived from the triacylglycerols
via acetyl-CoA to four-carbon compounds, such as succinate, which are then converted to soluble sugars to provide germinating seeds with energy
to fuel the growth of the seedlings and to produce shoots and leaves, while they supply structural elements before the seedlings develop
the capacity to photosynthesise.
How the products of lipolysis are transported to the glyoxysomes for further metabolism has still to be determined,
but a specific ‘ABC’ transporter is required to import fatty acids into the glyoxysomes in Arabidopsis.
The free acids are converted to their CoA esters by two long-chain acyl-CoA synthetases located
on the inner face of the peroxisome membrane before entry into the β‑oxidation pathway.
All of these processes are controlled by an intricate regulatory network, involving transcription factors
that crosstalk with signalling events from the seed maturation phase through to embryo development.
After about two days of the germination process, the glyoxysomes begin to break down,
but β-oxidation can continue in peroxisomes in leaf tissue.
The lipid droplets
in seeds exist in close proximity with glyoxysomes (broadly equivalent to peroxisomes).
These are the membrane-bound organelles that contain most of the enzymes required to oxidize fatty acids derived from the triacylglycerols
via acetyl-CoA to four-carbon compounds, such as succinate, which are then converted to soluble sugars to provide germinating seeds with energy
to fuel the growth of the seedlings and to produce shoots and leaves, while they supply structural elements before the seedlings develop
the capacity to photosynthesise.
How the products of lipolysis are transported to the glyoxysomes for further metabolism has still to be determined,
but a specific ‘ABC’ transporter is required to import fatty acids into the glyoxysomes in Arabidopsis.
The free acids are converted to their CoA esters by two long-chain acyl-CoA synthetases located
on the inner face of the peroxisome membrane before entry into the β‑oxidation pathway.
All of these processes are controlled by an intricate regulatory network, involving transcription factors
that crosstalk with signalling events from the seed maturation phase through to embryo development.
After about two days of the germination process, the glyoxysomes begin to break down,
but β-oxidation can continue in peroxisomes in leaf tissue.
Lipid droplets - plastoglobules: Triacylglycerol-rich lipid droplets (LD), sometimes termed 'plastoglobules', have been observed in most cell types, mainly in the cytosol but also in the nucleus, in vegetative tissues of plants as well as in seeds, and although their origin and function are poorly understood, they contain all the enzymes required for triacylglycerol metabolism together with phospholipases, lipoxygenases and other oxidative enzymes. Instead of oleosins, these lipid droplets in plants and algae contain a family of ubiquitously expressed 'LD-associated proteins' on the surface, together with a monolayer of membrane-derived phospholipids (mainly phosphatidylcholine), galactolipids such as sulfoquinovosyldiacylglycerol, and in some species betaine lipids. As in yeast and humans, seipins (three in Arabidopsis) are necessary for normal LD biogenesis, and here too, LD consisting of triacylglycerols, tocopherols, carotenoids and fatty acid phytyl esters are generated within the bilayer of the endoplasmic reticulum and pinch off into the cytoplasm. Lipid droplets have been observed to grow, shrink, move and make contact with other subcellular organelles within a cell to maintain lipid and energy homeostasis while supplying building blocks for membrane biogenesis and precursors for signalling oxylipins. For example, they can connect to peroxisomes to enable β‑oxidation of the fatty acids components for the ATP required for stomatal opening and no doubt many other purposes, and they connect to the plasma membrane via three novel proteins, although the purpose of this is not yet known.
In the thylakoid cisternae, plastoglobules are created in a similar manner by localized accumulation of triacylglycerols and other neutral lipids between the membrane leaflets and then pinch off into the stroma. They are not simply energy storage compartments but are involved in a wide range of dynamic metabolic pathways, which include biogenesis, membrane remodelling, regulation of energy homeostasis and stress responses, and during senescence, lipid droplets accumulate rapidly in leaves of A. thaliana. Antifungal compounds such as 2‑hydroxy-octadecatrienoic acid and other oxylipins are derived from α‑linolenic acid in these organelles, which can be considered to be intracellular factories for stable metabolites via unstable intermediates by concentrating the enzymes and hydrophobic substrates in an efficient manner. Further, plastoglobules are implicated in the biosynthesis and metabolism of vitamins E and K.
Abiotic stresses can induce remodelling of lipid membranes through lipase action with the formation of toxic lipid intermediates, and these can be sequestered by triacylglycerols in lipid droplets to inhibit membrane damage and potentially prevent cell death. During wounding of plant tissues, triacylglycerols containing mainly polyunsaturated fatty acid accumulate in lipid droplets, probably as a transient store for tissue regeneration. Aside from stress responses, lipid droplets serve in anther and pollen development, where triacylglycerols serve as a source of fatty acids for membrane biosynthesis and to recruit and transport proteins for both organ development and successful pollination.
Microalgae: Triacylglycerol biosynthesis in lipid droplets in microalgae is under intensive study because of their potential for nutraceutical and biodiesel production, and it seems that there is a similar metabolism as in higher plants but with a simpler genome encoding few redundant proteins. In the unicellular green model microalga Chlamydomonas reinhardtii, for example, lipid droplet proteins, lipases and enzymes of β‑oxidation have been characterized.
Yeasts: Lipid droplets in yeast are highly dynamic and diverse metabolic hubs that ensure stress resistance and cell survival by promoting membrane and organelle homeostasis. As most of the main biosynthetic and catabolic enzymes for triacylglycerol metabolism are conserved between yeasts and mammals, the former are proving to be useful models for the study of triacylglycerol production. In budding yeast, the lipid droplet assembly complex consists of seipin and four accessory factors, i.e. proteins designated Ldo45, Ldo16, Tgl4 and Pln1, which must be present in the correct stoichiometric proportions. The size and triacylglycerol content of lipid droplets in yeasts change appreciably in different stages of growth and development, and Saccharomyces cerevisiae contains a single phosphatidic acid phosphatase (Pah1), which is essential to this process. During vegetative growth, Pah1 in the cytosol is phosphorylated by multiple protein kinases, and this enables the synthesis of phospholipids rather than triacylglycerols. Some fatty acids derived from phospholipids are utilized for triacylglycerol biosynthesis at the inner nuclear membrane, and this is a requirement for nuclear integrity. As cells progress into stasis, the Pah1 is dephosphorylated and translocates to the endoplasmic reticulum, which ultimately leads to triacylglycerol synthesis for storage in lipid droplets.
2.7. Triacylglycerol Metabolism in Prokaryotes
Study of the biosynthesis of triacylglycerols in bacteria has been stimulated by an awareness that this lipid class is a factor in the pathogenesis of Mycobacterium tuberculosis and in the relationship with antibiotic biosynthesis by Streptomyces coelicolor. Triacylglycerols are an energy reserve for the long-term survival of M. tuberculosis during dormancy and the persistence phase of infection as well as a means by which unesterified fatty acids are detoxified; synthesis occurs by sequential acylation of glycerol-3-phosphate. Increasing numbers of bacteria from the genera Mycobacterium, Nocardia, Rhodococcus, Micromonospora, Dietzia and Gordonia are now known to produce triacylglycerols (sometimes wax esters), and these can be stored in lipid droplets ('intrabacterial lipid inclusions') in the organisms.
In most, the first three steps in triacylglycerol biosynthesis are catalysed by GPAT, LPAT and PAP enzymes comparable to those in other organisms, but it has become apparent that the DGAT can be a dual-function CoA-dependent acyltransferase, i.e., a wax ester synthase and diacylglycerol acyltransferase, which accepts a broad diversity of acyl-CoA substrates for esterification of diacylglycerols or long-chain fatty alcohols for the synthesis of triacylglycerols or wax esters, respectively, depending on which intermediates are present in the organisms. PAP regulates of the flux of phosphatidic acid towards triacylglycerol or glycerophospholipid synthesis. In mycobacteria, two pathways for biosynthesis of the precursor phosphatidic acid have been identified: one where acylation of sn-1 position of glycerol-3-phosphate precedes that of sn-2 and another in which acylations proceed in the reverse order, and these are catalysed by two unique acyltransferases designated PlsM and PlsB2.
Cyanobacteria, such as Synechocystis sp. contain multifunctional acyltransferases for synthesis of triacylglycerols, fatty acid phytyl esters and plastoquinol/plastoquinone esters. Bacteria that lack an enzyme of this kind are unable to make these non-polar lipids.
Other web pages on this site dealing with triacylglycerols are Triacylglycerols: Part 1 - their structure and compositions, and Triacylglycerols: Part 3 - regio- and stereospecific analysis procedures.
Recommended Reading
- Amari, C., Carletti, M., Yan, SQ., Michaud, M. and Salvaing, J. Lipid droplets degradation mechanisms from microalgae to mammals, a comparative overview. Biochimie, 227B, 19-34 (2024); DOI.
- Asis, A.C., Savoretti, F., Cabruja, M., Gramajo, H. and Gago, G. Characterization of key enzymes involved in triacylglycerol biosynthesis in mycobacteria. Sci. Rep., 11, 13257 (2021); DOI.
- Blot, C., Bertrand, M., Siponen, M. and Li-Beisson, Y. Lipid droplets on the move: remodeling, trafficking, and interaction with other organelles. J. Exp. Biol., in press (2025); DOI.
- Carman, G.M., Stukey, G.J., Jog, R. and Han, G.S. Insights into phosphatidic acid phosphatase and its potential role as a therapeutic target. Adv. Biol. Reg., 95, 101074 (2025); DOI.
- Chen, G.Q., Harwood, J.L., Lemieux, M.J., Stone, S.J. and Weselake, R.J. Acyl-CoA:diacylglycerol acyltransferase: Properties, physiological roles, metabolic engineering and intentional control. Prog. Lipid Res., 88, 101181 (2022); DOI.
- Cohen, P. (Editor) Brown and Beige Fat: From Molecules to Physiology. Biochim. Biophys Acta, Lipids, 1864, Issue 1, Pages 1-112 (2019) - special journal issue - several articles.
- Cook, J.R., Kohan, A.B. and Haeusler, R.A. An updated perspective on the dual-track model of enterocyte fat metabolism. J. Lipid Res., 63, 100278 (2022); DOI.
- Corvera, S. and others. Advances in adipose tissue biology. Endocrine Rev., in press (2025); DOI.
- Danielli, M., Perne, L., Jovicic, E.J. and Petan, T. Lipid droplets and polyunsaturated fatty acid trafficking: Balancing life and death. Front. Cell Developm. Biol., 11, 1104725 (2023); DOI.
- Farese, R.V. and Walther, T.C. Essential biology of lipid droplets. Annu. Rev. Biochem., 94, 447-477 (2025); DOI.
- Fujimoto, T. Nuclear lipid droplets – how are they different from their cytoplasmic siblings? J. Cell Sci., 135, jcs259253 (2022); DOI.
- Grabner, G.F., Xie, H., Schweiger, M. and Zechner, R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nature Metab., 3, 1445–1465 (2021); DOI.
- Guzha, A., Whitehead, P., Ischebeck, T. and Chapman, K.D. Lipid droplets: packing hydrophobic molecules within the aqueous cytoplasm. Annu. Rev. Plant Biol., 74, 195-223 (2023); DOI.
- Heier, C., Klishch, S., Stilbytska, O., Semaniuk, U. and Lushchak, O. The Drosophila model to interrogate triacylglycerol biology. Biochim. Biophys. Acta, Lipids, 1866, 1163-1184 (2021); DOI.
- Klemm, R.W. and Carvalho, P. Lipid droplets big and small: basic mechanisms that make them all. Annu. Rev. Cell Developm. Biol., 40, 143-168 (2024); DOI.
- Lee, J. and Ridgway, N.D. Substrate channeling in the glycerol-3-phosphate pathway regulates the synthesis, storage and secretion of glycerolipids. Biochim. Biophys. Acta, Lipids, 1865, 158438 (2020); DOI.
- Mathiowetz, A.J. and Olzmann, J.A. Lipid droplets and cellular lipid flux. Nature Cell Biol., 26, 331-345 (2024); DOI.
- Safi, R., Menéndez, P. and Pol, A. Lipid droplets provide metabolic flexibility for cancer progression. FEBS Letts, 598, 1301-1327 (2024); DOI.
- Safi, R., Sanchez-Alvarez, M., Bosch, M., Demangel, C., Parton, R.G. and Pol, A. Defensive-lipid droplets: Cellular organelles designed for antimicrobial immunity. Immun. Rev., 317, 113-136 (2023); DOI.
- Sah, S.K., Fan, J.L., Blanford, J., Shanklin, J. and Xu, C.C. Physiological functions of phospholipid:diacylglycerol acyltransferases. Plant Cell Physiol., 65, 863-871 (2024); DOI.
- Wei, W., Wang, L.F., Tao, J.J., Zhang, W.K., Chen, S.Y., Song, Q.X. and Zhang, J.S. The comprehensive regulatory network in seed oil biosynthesis. J. Integr. Plant Biol., 67, 649-668 (2025); DOI.
- Yang, S.Q., Liu, Y.K., Wu, X.X., Zhu, R.R., Sun, Y.L., Zou, S.Y., Zhang, D.J. and Yang, X.Q. Molecular regulation of thermogenic mechanisms in beige adipocytes. Int. J. Mol. Sci., 24, 6303 (2024); DOI.
- Zhang, S. and 11 others. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis., 13, 132 (2022); DOI.
- Zhao, Y.J., Cao, R., Li, J.C., Xu, Y.Y., Zhou, L.J. and Ye, Y.J. Lipid droplets in plants: turnover and stress responses. Front. Plant Sci., 16, 1625830 (2025); DOI.
- and in relation to the history of the topic -
- Coleman, R.A. The "discovery" of lipid droplets: A brief history of organelles hidden in plain sight. Biochim. Biophys. Acta, Lipids, 1865, 158762 (2020); DOI - see also DOI2.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: January 2026 | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
