Sphingolipids: Chemistry and Biochemistry
An Introduction
The sphingolipids comprise a wide range of complex lipids in which the defining component is a long-chain or sphingoid base (an amino-diol/triol), which in living tissues is usually linked to a fatty acid via an amide bond. J.L.W. Thudichum, a German chemist working in London, first coined the root term "sphingo-” in 1884 following his discovery of the first glycosphingolipids, because the enigmatic nature of the molecules reminded him of the riddle of the sphinx. Regretfully, the importance of his work was not recognized until 25 years after his death, and it was 1947 before the term "sphingolipide” was introduced by Herbert Carter and colleagues.
While they are much less enigmatic than they once were, sphingolipids are extremely versatile structural and signalling molecules that continue to fascinate as new knowledge is gained of their properties in healthy (and diseased) animal and plant tissues, and it is evident that many of the enzymes required for their biosynthesis and metabolism are highly conserved in most eukaryotes, although there are major differences in the nature and compositions of the sphingolipids in the various kingdoms of life. They are especially abundant in brain, which is second only to adipose tissue in its content of lipids. Although they are known to be synthesised by only a few bacterial genera, they are present in Sphingomonas, Sphingobacterium, and a few other species (often with distinctive compositions). Novel sphingolipid structures continue to be reported, and as an example at the last count, more than 200 of the complex glycosphingolipids classified as gangliosides, with variations in the complex carbohydrate component alone, had been characterized in vertebrates. Much research is concerned with animal lipids, but there have been fascinating new discoveries in plants.
The emphasis in publications has changed in recent years from complex sphingolipids as structural components of membranes, lipoproteins and skin to smaller molecules with more dynamic functions in cellular communication, and among many vital processes, sphingolipids have been directly or indirectly implicated in cell-cell interactions, tissue development, cell differentiation and migration, senescence and cell death, while aberrant metabolism is a factor in many diseases, including cardiovascular, metabolic and neurodegenerative disorders. A recent review argues that "based on this plethora of functions, most critical cell biological responses involve one or more bioactive sphingolipids at some juncture in the regulation of that specific response" (DOI). Indeed, I have suggested that "every individual lipid class has been found to have some unique role in tissues other than as a source of energy or as a simple construction unit of a cellular membrane" (here...).
1. The Basic Structures
Long-chain or sphingoid bases, of which sphingosine or (2S,3R,4E)-2-amino-4-octadecene-1,3-diol (illustrated) is typical, are the basic structural elements of sphingolipids. More than a hundred structural variants are known, and they can differ in chain-length and in the presence of various functional groups including double bonds of both the cis- and trans-configuration at different locations in the aliphatic chain. Likewise, ceramides, which contain sphingoid bases linked to fatty acids (see below) by amide bonds, vary appreciably in the compositions of both aliphatic components depending on their origins. As they tend to be relatively saturated molecules, they are much more resistant to oxidative stress than most glycerolipids. In addition to being precursors for structural lipids, long-chain bases and ceramides are intra- and inter-cellular signalling molecules, especially in animal cells, while another relatively simple sphingolipid, sphingosine-1-phosphate, is now implicated in countless aspects of animal metabolism. The concentrations of these bioactive lipids respond rapidly to the action of appropriate stimuli and then regulate downstream effectors and targets.
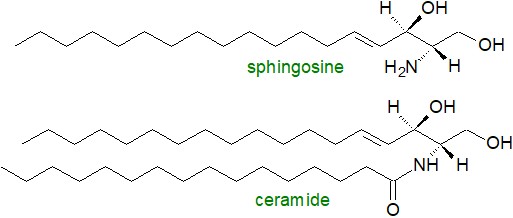 |
| Figure 1. The basic building blocks of sphingolipids. |
By addition of other polar moieties to the hydroxyl group in position 1 of the sphingoid base component, ceramides are the precursors of a multitude of complex sphingophospho- and sphingoglyco-lipids, which are required for innumerable purposes in membranes and tissues that are quite distinct from those of comparable glycerophospho- and glyceroglyco-lipids. In animals, sphingomyelin has structural similarities to phosphatidylcholine, but has very different physical and biological properties, while the complex oligoglycosylceramides and gangliosides (glycosphingolipids), of which glucosylceramide is the precursor, have no true parallels among the glyceroglycolipids. Plants contain different complex sphingophospholipids such as ceramide phosphorylinositol and phytoglycosphingolipids.
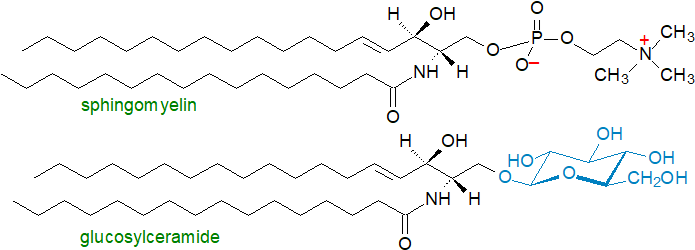 |
| Figure 2. Complex sphingolipids - a phosphosphingolipid and a glycosphingolipid. |
Complex sphingolipids are synthesised in the endoplasmic reticulum and Golgi, but they are transported to the plasma membrane of most mammalian cells where they support the membrane structure and also serve as adhesion sites for proteins from the extracellular tissue. The glycosphingolipids are essential for myelin formation in the brain, but sphingolipids are needed for crucial purposes in all cellular compartments, including the nucleus. The first five carbon atoms of the sphingoid base in sphingolipids have a highly specific stereochemistry and constitute a key feature that has been termed the ‘sphingoid motif’, which in comparison to other lipid species facilitates a relatively large number of molecular interactions (donor-acceptor) with other membrane lipids via hydrogen-bonding, charge-pairing, hydrophobic and van der Waals forces, and these can influence reactions with receptors and enzymes.
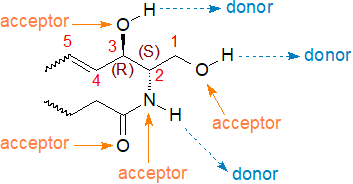 |
| Figure 3. The 'sphingoid motif'. |
In membranes, a distinctive property of sphingolipids is that they spontaneously form transient nanodomains termed 'rafts', usually in conjunction with cholesterol, where such proteins as enzymes and receptors congregate to carry out their signalling and other duties. Thus, in addition to their direct effects on metabolism, sphingolipids influence many aspects of biochemistry indirectly via their physical properties.
While it may be obvious that a well-balanced sphingolipid metabolism is necessary for health in animals, it has become evident that impaired sphingolipid metabolism and function are factors in the pathophysiology of many of the more common human diseases, which include diabetes, various cancers, microbial infections, Alzheimer's disease and other neurological syndromes, and diseases of the cardiovascular and respiratory systems. In humans, several genetic defects in sphingolipid metabolism or sphingolipidoses have been detected, mainly storage diseases associated with the lysosomal compartment where sphingolipids are catabolized. Sphingolipids and their metabolism are therefore likely to prove of ever-increasing interest to scientists.
There are appreciable differences in sphingolipid compositions and metabolism between animal and plant cells, both with respect to the aliphatic components and the polar head groups, although there are some similarities. While sphingomyelin is the most abundant sphingolipid in animals, it does not occur in plants and fungi. Although less is known of the role they play in plants, it has become apparent that complex sphingolipids are much more abundant in plant membranes than was once thought to be the case, and it is now recognized that they are major components of the plasma membrane and endomembrane system.
2. Some General Comments on Sphingolipid Metabolism
Animals: The biosynthesis and catabolism of sphingolipids involves many intermediate metabolites, all of which have biological activities of their own. In animals, the relationships between these metabolites have been rationalized in terms of a ‘sphingomyelin, sphingolipid or ceramide cycle’. Most of the reactions are reversible and the relevant enzymes are located in the endoplasmic reticulum, Golgi, plasma membrane and mitochondria. They are catabolized in the lysosomal compartment of cells.
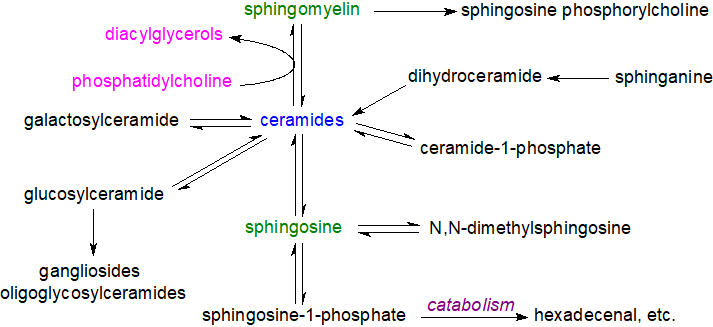 |
| Figure 4. The sphingomyelin/ceramide cycle in animals. |
The many different enzymes (and their isoforms) in these processes require numerous factors for efficient operation, including intracellular locations and mechanisms of activation. Each of the various compounds in these pathways has characteristic metabolic properties, and these are discussed in more detail on the web pages describing the individual sphingolipids. The simpler of these in structural terms, i.e., sphingosine, ceramides, sphingosine 1-phosphate and ceramide 1-phosphate, participate in signal transduction processes, sometimes in concert, but often in contradictory ways, especially in relation to inflammation and apoptosis. Thus, free sphingosine and other long-chain bases, which are the primary precursors of ceramides and thence of all the complex sphingolipids, serve as mediators of many cellular events, for example by inhibiting the enzyme protein kinase C. Ceramides participate in cellular signalling, in the regulation of apoptosis, cell differentiation, transformation and proliferation, and most stress conditions. In contrast, sphingosine-1-phosphate and ceramide-1‑phosphate promote cellular division (mitosis) as opposed to apoptosis, so that the balance between these lipids and ceramide and/or sphingosine levels in cells is critical and necessitates exquisite control in each cellular compartment.
The ‘structural’ sphingolipids, such as sphingomyelin, monoglycosylceramides, oligoglycosylceramides, gangliosides and sulfatides, all have unique and highly specific functions, some of which are due to their physical properties and location within raft nanodomains of membranes. Sphingolipids in the form of non-typical ceramides provide the permeability barrier in skin, where they are characterized by the presence of ultra-long fatty acyl components as well as fatty acyl groups linked to a hydroxyl group at the terminal end of the N‑linked fatty acids (thereby generating a three‑acyl-chain rather than a two‑chain molecule).
Plants: Metabolic pathways have been identified in plants that are comparable to those of the sphingomyelin cycle, and although they have not been studied as extensively as those in animals, sphingolipid metabolites such as sphingosine-1-phosphate (or analogues) have been linked to programmed cell death, signal transduction, membrane stability, host-pathogen interactions and stress responses. The unique range of complex sphingolipids in plant membranes, such as ceramide phosphorylinositol and the phytoglycosphingolipids, are now known to constitute a higher proportion of the total lipids than has hitherto been supposed, although their functions are only now being explored.
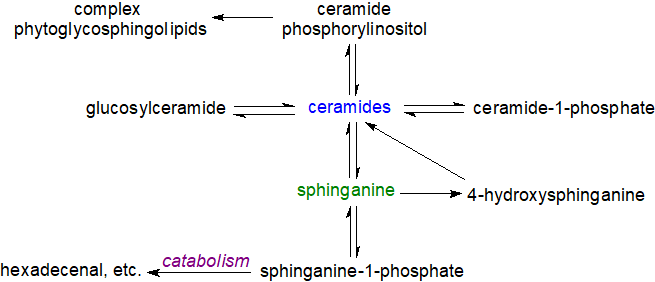 |
| Figure 5. Sphingolipid biosynthetic pathways in plants. |
Fungi: In fungi, there is a distinctive sphingolipid cycle that is comparable to that in plants in many ways, though with some significant differences, as for example, ceramide phosphoinositol mannosides are the main complex lipids. Saccharomyces cerevisiae has been an invaluable model because many of the enzymes of sphingoid base and ceramide biosynthesis were first identified in this species.
 |
| Figure 6. Complex ceramide phosphoinositol glycosides from plants and fungi. |
Bacteria: Sphingolipid biosynthesis has been confirmed in relatively few bacterial species, but those originating in the gut microbiome can have an impact upon the health of human hosts; other bacteria utilize host sphingolipids during infections to counter the immune defence systems as do many viruses. That said, a diverse and abundant range of sphingolipids has been identified in the lipidome of sediments from the Black Sea (20% of the total lipids) that are presumably synthesised by anaerobic bacteria in these deposits. Sulfono-analogues of long-chain bases and ceramides (capnoids) are produced by some bacteria, but for convenience, these are discussed with the sulfonolipids
3. Fatty acid Components of Sphingolipids
The fatty acids of sphingolipids are very different from those of glycerolipids, and they are linked mainly via amide bonds, which are chemically much more stable than O-ester bonds. In general, these consist of very-long-chain (up to C26) odd- and even-numbered saturated or monoenoic acyl groups and related 2R-hydroxy components, while even longer fatty acids (C28 to C36) occur in spermatozoa and the epidermis. The dienoic acid 15,18‑tetracosadienoate (24:2(n‑6)) derived from elongation of linoleic acid is found in the ceramides and other sphingolipids of several tissues, but at relatively low levels. Polyunsaturated fatty acids are only rarely present, although sphingomyelins of testes and spermatozoa are exceptions in that they contain such fatty acids, which are even longer in chain-length (up to 34 carbon atoms) and include 28:4(n‑6) and 30:5(n‑6). Skin ceramides also contain unusual very-long-chain fatty acids, while yeast sphingolipids contain mainly C26 fatty acids.
 |
| Figure 7. Some fatty acid components of sphingolipids. |
In plants and yeasts, a similar range of chain-lengths occur as in animals, but 2-hydroxy acids predominate sometimes accompanied by small amounts of 2,3‑dihydroxy acids; saturated fatty acids are most abundant, and the sphingolipids of rice (Oryza sativa) contain only saturated components, but monoenes are present in higher proportions in the Brassica family (including the model plant Arabidopsis thaliana) and a few other species. Some fungi contain monoenoic fatty acids with a trans-3 double bond and/or a hydroxyl group.
Very-long-chain saturated and monoenoic fatty acids for sphingolipid biosynthesis are produced from medium- or long-chain precursors by elongases (ELOVL) in the endoplasmic reticulum of cells in mammals, and there is increasing evidence that specific isoforms participate in the biosynthesis of certain ceramides. For example, ELOVL1 has been linked to the generation of ceramides with C24 fatty acids (saturated and unsaturated), while ELOVL4 is responsible for the ultra-long-chain fatty acids in skin. Yeasts possess three elongation enzymes: Elo1 (for medium to long-chain fatty acids), Elo2 (up to C22) and Elo3 (up to C26).
The hydroxyl group in hydroxy acids adds to the hydrogen-bonding capacity of the sphingolipids, and it helps to stabilize membrane structures and to strengthen the interactions with membrane proteins. In mammals, hydroxylation is accomplished by a fatty acid 2‑hydroxylase (FA2H), i.e., an NAD(P)H-dependent monooxygenase, which is an integral membrane protein of the endoplasmic reticulum and converts unesterified long-chain fatty acids to 2‑hydroxy acids, although further uncharacterized enzymes probably exist. The catalytic region consists of four conserved histidines that form a di-metal ion centre and adds the hydroxyl group stereospecifically to give only the R-enantiomer. Experimental evidence has been obtained that is consistent with 2‑hydroxylation occurring at the fatty acid level prior to incorporation into ceramides in the brain of mice and in the urinary bladder of mice and humans where the enzyme is expressed at high levels. In skin, 2‑hydroxy and non-hydroxy fatty acids as their CoA esters are used with equal facility for ceramide biosynthesis by ceramide synthases. As mutations in the fatty acid 2‑hydroxylase in humans and mice give rise to demyelination disorders, such as leukodystrophy, it is evident that there is an absolute requirement for sphingolipids containing 2‑hydroxy acids in membranes that cannot be substituted by non-hydroxy analogues. 2‑Hydroxy acids may be the source of some of the odd-chain fatty acids in brain by peroxisomal α-oxidation. In addition to their structural role, 2-hydroxy acids are reported to act as a chemical switch that influences glucose-stimulated insulin secretion.
 In plants, 2‑hydroxyl
groups are inserted into fatty acyl chains while they are linked to ceramide, as ceramide synthase does not accept hydroxy fatty acids in vitro
at least.
Two fatty acid 2‑hydroxylases (di-iron-oxo enzymes) have been found in Arabidopsis, one that utilizes very-long-chain fatty acids
(FAH1) and one for long chain fatty acids (FAH2).
In fungi, a hydroxyl group is inserted at C2 of the fatty acid in a dihydroceramide intermediate.
The double bond is introduced by a sphingolipid fatty acid desaturase in Arabidopsis, and this differs in structure from
that in the moss Physcomitrium patens.
In plants, 2‑hydroxyl
groups are inserted into fatty acyl chains while they are linked to ceramide, as ceramide synthase does not accept hydroxy fatty acids in vitro
at least.
Two fatty acid 2‑hydroxylases (di-iron-oxo enzymes) have been found in Arabidopsis, one that utilizes very-long-chain fatty acids
(FAH1) and one for long chain fatty acids (FAH2).
In fungi, a hydroxyl group is inserted at C2 of the fatty acid in a dihydroceramide intermediate.
The double bond is introduced by a sphingolipid fatty acid desaturase in Arabidopsis, and this differs in structure from
that in the moss Physcomitrium patens.
Although the fatty acid components are only occasionally considered in terms of the functions of sphingolipids, their influence is considerable in relation to their physical properties and location in membranes. Very-long-chain fatty acids may play a role in stabilizing highly curved membrane domains as is required during cell division, and the hydrophobic nature of the fatty acyl groups (together with the long-chain bases) enables the hydrogen bonding that is essential for the formation of raft nanodomains in membranes. As a rule, lipid bilayers containing sphingolipids with 2-hydroxy-fatty acyl or 4-hydroxy-sphingoid base moieties tend to generate condensed and more stable gel phases with higher melting temperatures than their non-hydroxylated equivalents, because they have a more extended and strengthened intermolecular hydrogen bonding network. Changes in fatty acid composition are seen in some disease states, and increased concentrations of fatty acids >C24 are a feature of adrenoleukodystrophy, an X-linked genetic disorder.
Removal of very-long-chain fatty acids from sphingolipids in mutants of Arabidopsis inhibits completely the development of seedlings, and in yeast mutants lacking sphingolipids, it has been demonstrated that synthetic glycerolipids must contain very-long-chain fatty acids (C26) to allow growth probably by stabilizing the proton-pumping enzyme H+-ATPase. Ceramides containing different fatty acids can be used in highly specific ways, and in fungi, C16 or C18 hydroxy acids are used exclusively for synthesis of glucosylceramide, while those containing very-long-chain C24 and C26 hydroxy acids are used only for synthesis of glycosyl inositol phosphorylceramide anchors for proteins. In plants, sphingolipids containing 2‑hydroxy acids are protective against oxidative and other biotic stresses.
4. Links between Glycerolipid and Sphingolipid Metabolism
Sphingolipid metabolism and glycerolipid metabolism have been widely treated as quite separate aspects of lipid science until relatively recently, partly for historical reasons and partly because the analysis of the two lipid groups requires different approaches and skills. However, there are many areas where the two overlap, not least because phosphatidylcholine is the biosynthetic precursor of sphingomyelin in animal cells, while in plants and fungi, phosphatidylinositol is the biosynthetic precursor of ceramide phosphorylinositol. In contrast, ethanolamine phosphate derived from the catabolism of sphingolipids via sphingosine 1-phosphate is recycled for the biosynthesis of phosphatidylethanolamine, and this is vital for survival in the protozoan parasite Trypanosoma brucei. Bis(monoacylglycero)phosphate in lysosomes is involved in the catabolism of glycosphingolipids. In studies in vitro, sphingosine 1‑phosphate has been shown to be an activator of the phospholipase C that hydrolyses the lipid mediator phosphatidylinositol 4,5‑bisphosphate with formation of diacylglycerols and inositol triphosphate with further bioactivity. In membranes, the location and functions of glycerophospholipids is influenced both positively and negatively by sphingolipid-rich domains or rafts.
There are several examples of phosphoinositides and other complex glycerolipids binding to enzymes of sphingolipid metabolism, either as part of a regulatory control upon their activities or to facilitate their location to various membranes. Thus, phosphatidylinositol 4-phosphate (PI(4)P) facilitates the conversion of ceramide at the Golgi to glucosylceramide by binding to the glucosylceramide transfer protein FAPP2 via its PH domain. Sphingosine kinase 2, one of the enzymes responsible for the biosynthesis of sphingosine 1-phosphate and the CERT protein involved in ceramide transport have binding sites for phosphatidylinositol monophosphates, while the ceramide kinase responsible for the biosynthesis of ceramide-1-phosphate must bind to phosphatidylinositol 4,5‑bisphosphate. Sphingomyelin production at the trans-Golgi network triggers a signalling pathway leading to dephosphorylation of PI(4)P, interrupting transport of both cholesterol and sphingomyelin. Phosphatidylserine, as well as the phosphoinositides, stimulates the neutral sphingomyelinase in brain. There are links to oxylipin metabolism, as ceramide 1‑phosphate (with phosphatidylinositol 4,5-bisphosphate) binds to the phospholipase A2 (cPLA2α) responsible for the hydrolysis of phosphatidylinositol and thence the release of arachidonic acid for the eicosanoid cascade.
The glyceroglycolipid seminolipid from male reproductive tissues and the sphingolipid sulfates have structural elements in common, and they use the same enzymes to introduce the carbohydrate and then the sulfate group to diacylglycerol and ceramide intermediates, respectively. In some types of glycosylphosphatidylinositol as protein anchors, the first mannose unit is decorated by the addition of a short oligosaccharide sequence starting with N‑acetylgalactosamine, before galactose is added by the action of a GM1 ganglioside synthase requiring the presence of lactosylceramide. Diacylglycerol acyltransferase 2 (DGAT2), a key enzyme in triacylglycerol biosynthesis, generates 1‑O‑acylceramides in skin and lipid droplets. Further, in the biosynthesis of cholesteryl glycosides in animals, a transfer of glucose occurs from glucosylceramide to cholesterol by means of cellular β‑glucocerebrosidases.
Suggested Reading
- AL Mughram, M.H., Kellogg, GE. and Wattenberg, B.W. Three kingdoms and one ceramide to rule them all. A comparison of the structural basis of ceramide-dependent regulation of sphingolipid biosynthesis in animals, plants, and fungi. Adv. Biol. Reg., 91, 101010 (2024); DOI.
- Backman, A.P.E. and Mattjus, P. Who moves the sphinx? An overview of intracellular sphingolipid transport. Biochim. Biophys. Acta, Lipids, 1866, 159021 (2021); DOI.
- Bai, X.Y., Ya, R., Tang, X.Y. and Cai, M.W. Role and interaction of bacterial sphingolipids in human health. Front. Microbiol, 14, 1289819 (2023); DOI.
- Breiden, B. and Sandhoff, K. Lysosomal glycosphingolipid storage diseases. Annu. Rev. Biochem., 88, 461-485 (2019); DOI.
- Carreira, A.C., Ventura, A.E., Varela, A.R. and Silva, L.C. Tackling the biophysical properties of sphingolipids to decipher their biological roles. Biol. Chem., 396, 597-609 (2015); DOI.
- Cassim, A.M., Grison, M., Ito, Y., Simon-Plas, F., Mongrand, S. and Boutte, Y. Sphingolipids in plants: a guidebook on their function in membrane architecture, cellular processes, and environmental or developmental responses. FEBS Letts, 594, 3719-3738 (2020); DOI.
- Chinnappa, K., Ballorin, F. and Francis, F. Fundamental neurochemistry review: sphingolipids and ceramides in brain development. J. Neurochem., 169, e70262 (2025); DOI.
- Dingjan, T. and Futerman, A.H. The role of the ‘sphingoid motif’ in shaping the molecular interactions of sphingolipids in biomembranes. Biochim. Biophys. Acta, Biomembranes, 1863, 183701 (2021); DOI - see also DOI2.
- Eckhardt, M. Fatty acid 2-hydroxylase and 2-hydroxylated sphingolipids: metabolism and function in health and diseases. Int. J. Mol. Sci., 24, 4908 (2023); DOI.
- Gomez-Larrauri, A., Larrea-Sebal, A., Martín, C. and Gomez-Muñoz, A. The critical roles of bioactive sphingolipids in inflammation. J. Biol. Chem., 301, 110475 (2025); DOI.
- Hannun, Y.A., Merrill, A.H. and Luberto, C. The bioactive sphingolipid playbook. A primer for the uninitiated as well as sphingolipidologists. J. Lipid Res., 66, 100813 (2025); DOI.
- Harrison, P.J., Dunn, T.M. and Campopiano, D.J. Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep., 35, 921-954 (2018); DOI.
- Jamjoum, R., Majumder, S., Issleny, B. and Stiban, J. Mysterious sphingolipids: metabolic interrelationships at the center of pathophysiology. Front. Physiol., 14, 1229108 (2024); DOI.
- Kuo, A. and Hla, T. Regulation of cellular and systemic sphingolipid homeostasis. Nature Rev. Mol. Cell Biol., 25, 802–821 (2024); DOI.
- Marquês, J.T., Marinho, H.S. and de Almeida, R.F.M. Sphingolipid hydroxylation in mammals, yeast and plants - an integrated view. Prog. Lipid Res., 71, 18-42 (2018); DOI.
- Merrill, A.H. Don't be surprised when these surprise you: some infrequently studied sphingoid bases, metabolites, and factors that should be kept in mind during sphingolipidomic studies. Int. J. Mol. Sci., 26, 650 (2025); DOI.
- Rodriguez-Cuenca, S., Pellegrinelli, V., Campbell, M., Oresic, M. and Vidal-Puig, A. Sphingolipids and glycerophospholipids - The "ying and yang" of lipotoxicity in metabolic diseases. Prog. Lipid Res., 66, 14-29 (2017); DOI.
- Tani, M. Biological importance of complex sphingolipids and their structural diversity in budding yeast Saccharomyces cerevisiae. Int. J. Mol. Sci., 25, 12422 (2024); DOI.
- Thomas, S., Samuel, S.V., Hoch, A., Syphurs, C. and Diray-Arce, J. The implication of sphingolipids in viral infections. Int. J. Mol. Sci., 24, 17303 (2023); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: January 2026. | ||
© The LipidWeb is open access and fair use is encouraged - but not text and data mining, AI training, and similar technologies (text scraping).
