Archaeal Lipids
The Archaea represent one of the primary kingdoms or domains of living organisms. They are single celled with a low deoxyribonucleic acid content, lacking a nuclear membrane, and include thermophiles, halophiles and acidophiles, collectively termed 'extremophiles', which some consider may resemble the dominant organisms in the primeval biosphere. While four main archaeal phyla were once recognized, the Euryarchaeota, Crenarchaeota, Thaumarchaeota and Korarchaeota, the taxonomy is changing as more DNA data sets become available (Archaea are not easy to culture), and the latest research suggests that there may be three superphyla. As the name suggests, extremophiles exist in extreme habitats, including hot springs and waters with high salt, alkali or acid conditions, but more recently it has become apparent that many such organisms exist in mild environments also. Several species are methanogenic, including those found in low temperature environments. There is a suggestion that Archaea may constitute up to 20% of the oceanic biomass, where they are significant contributors to global biogeochemical pathways including the methane and nitrogen cycles.
The lipids of Archaea are very different from those of Bacteria, although there are many parallels, and the differing structures and biosynthetic machinery constitute part of a continuing debate as to how and when the two groups diverged during evolution, sometimes termed the "lipid divide". As eukaryotes have features that may be of both archaeal and bacterial origin, it has been suggested that they could be descended from a chimeric cell derived from both ancestors. The membrane lipids of Archaea contain mainly isoprenoid moieties linked by ether bonds to glycerol with unique stereochemistry. These are impervious to many external environmental factors and enable the organisms to survive and thrive in extreme environments. Because these lipids are more resistant to degradation than many other biomolecules, they are often preserved in the geological record and are biomarkers, which contribute to knowledge of earth history and geological processes.
1. Basic Chemistry
The lipids of the Archaea are now known to contain many unique and characteristic polar lipids with the nature and relative amounts of the various lipid structures varying widely, depending on the specific organism and upon the environmental conditions. A unique characteristic is that they are based on 2,3-dialkyl-sn-glycerol backbones, i.e., the stereochemistry is the opposite of that found in the two other primary kingdoms, Bacteria (eubacteria) and Eukarya (eukaryotes) (see our web page on phosphatidic acid). The alkyl groups are isoprenoid in nature, and the simplest molecules of this type are derivatives of 2,3-diphytanyl-O-sn-glycerol (archaeol), i.e., with two C20 isoprenoid units attached to positions sn-2 and sn-3 of glycerol by ether linkages (sometimes designated C40 ether lipids). Forms with one sesterterpenyl (C25) and one phytanyl residue, often termed 'extended archaeol', are characteristic components of Haloarchaea, a clade of Archaea thriving in high saline environments, and further isoprenoid groups are found linked in this way in other Archaea, including macrocyclic diethers in which the ends of the two phytanyl chains are joined (e.g., cyclo-archaeol). In general, the alkyl chains are saturated, but forms with double bonds in various positions have been found in a few of the Archaea.
 |
| Figure 1. Basic structures of Archaeal ether lipids. |
In addition, a group of tetraethers with a strikingly different molecular architecture have been discovered as core lipids of the Archaea, especially in the thermophilic Crenarchaea. These molecules have one or two polar head groups, which need not be the same (see below), with the 2,3‑sn‑glycerol moieties linked by two C40 isoprenoid alkyl components (C80 ether lipids). The acronym GDGT is often used to denote such glycerol dialkyl glycerol tetraether lipids. Caldarchaeol (so-called because it is the predominant form in thermophilic species) has two C40 isoprenoid units linked from position 2 to position 3’ and from position 3 to position 2’ (anti-parallel chains), while in isocardarcheol (illustrated), they are linked from position 2 to position 2’ and from position 3 to position 3’ (parallel chains) of the two sn-glycerol moieties. While both forms can exist in the same organism, the anti-parallel forms tend to predominate.
Some of the Archaea contain lipids of this type with both methyl branches and one to four cyclopentane rings in each chain, or one to eight of the latter in total in different molecular forms, and it has been suggested that a nomenclature GDGT-x be employed, where x is the number of cyclopentane rings in the molecule. As the environmental temperatures increase and the pH decreases, the numbers of cyclopentane rings in lipids from thermoacidophilic species tend to increase. The stereochemistry at the ring structures adds to the complexity, and a further complication is that some from this phylum can have a single cyclohexane ring in the alkyl chains, while yet other lipids of this type have carbon-carbon links between the chains, forming an ‘H’-shape. Thaumarchaeota, and not Euryarchaeota, may be the main source of ring-containing forms in the marine environment, and this has taxonomic implications for studies of lipids in sediments. For many years, there was thought to be a relatively limited number of forms in living organisms, but improved methods of analysis are showing the great complexity that exists in nature. As an example, crenarchaeol from hyper-thermophilic organisms of the Thaumarchaeota is a 66‑membered macrocycle (82 carbons in total) with 22 stereo centres and four 1,3-trans-substituted cyclopentane moieties, one of which is connected by a single bond to a cyclohexane ring.

Yet other hydroxy-GDGT lipids have found with hydroxyl groups in position 3 of one of the alkyl chains, and structural analogues occur in marine sediments with only one glycerol moiety and with the alkyl groups terminating in hydroxyl groups, while unsaturated diether lipids are found in some Archaea that grow at very low temperatures (-20 to 10°C). Halophilic Archaea tend to contain mainly diether lipids with C20 or C25 isoprenoid chains, whereas the membranes of hyperthermophilic Archaea typically consist of a mixture of diphytanylglycerol diether and dibiphytanyl tetraether lipids, with or without cyclopentane rings. For example, the Halobacteriales only possess bilayer (mainly C20) membrane lipids, while the hyperthermophilic archaeon Aeropyrum pernix has C25 archaeols only. In contrast, the genera Pyrobaculum, Thermoplasma and Sulfolobus utilize caldarchaeols (C80), including those with cyclopentane ring structures.
As an alternative to the simple glycerol component, calditol a nine-carbon nonitol with a five-membered ring, i.e., 2‑hydroxymethyl-1,2,3,4,5-pentahydroxy-cyclopentane linked to a glycerol moiety at position sn-1, is present in a subset of thermoacidophilic Archaea of the Sulfolobales order within the Crenarchaeota phylum, such as Sulfolobus solfaricus. Positions sn-2 and 3 are attached to complex isoprene units to form a glycerol-dialkyl-calditol-tetraether. A radical S‑adenosylmethionine protein required for calditol synthesis has been identified in S. acidocaldarius, and as deletion of this renders the organism sensitive to low pH, calditol is essential to protect these archaeal cells from acidic stress. Similarly, tetraethers linked to butanetriol or pentanetriol instead of glycerol at one end of their core structures occur in the methanogenic archaeon Methanomassiliicoccus luminyensis, marine Bathyarchaeia and some anoxic sedimentary deposits.

While these core lipids are sometimes found in the free state in organisms, they are more often completed by a variety of polar head groups. These exist both as phospho- and glycolipids (and as a combination of both) and as sulfate esters of diglycosides. Most of the polar head groups of phospholipids are the same as those of organisms of the other primary kingdoms and include ethanolamine, L‑serine, glycerol, myo-inositol and even choline in phosphodiester-linkage, while the glycolipids comprise mainly glucosyl and gentiobiosyl (β‑D‑glucosyl-(1→6)-β-D-glucosyl) units linked to the core alkylglycerols. In the Halobacteriaceae, the basic diglycosyl residue is α‑D‑mannosyl(1-2)-α‑D-glucose, and in some Archaea there are some unique polar groups, such as di- and trimethylaminopentanetetrols, glucosaminyl-myo-inositol and glucosyl-myo-inositol. New structures are still being discovered.
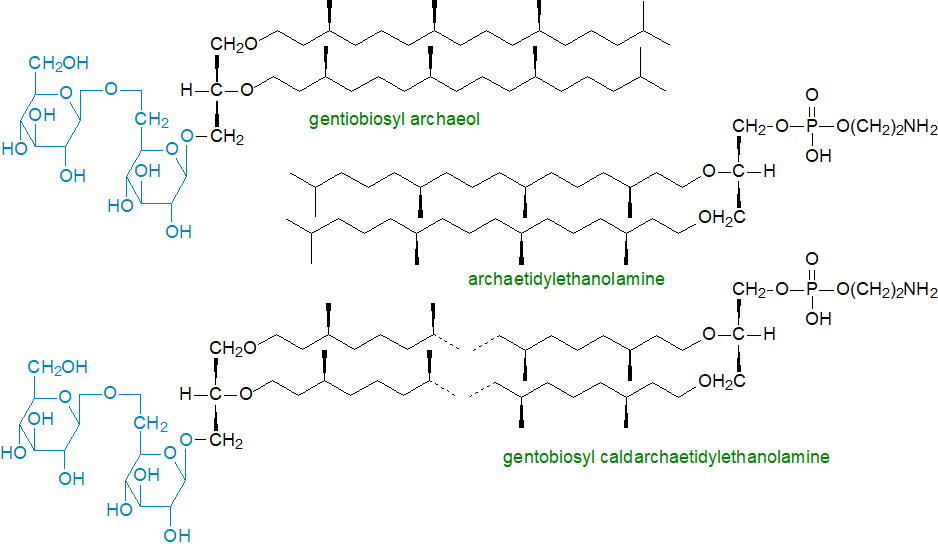 |
| Figure 2. Some examples of complex lipids from Archaea |
The simplest lipids of this type are based upon the archaeol backbone, so that archaetidic acid is the monophosphate ester of archeol and is the equivalent of phosphatidic acid from eukaryotes, while archaetidylethanolamine is analogous to phosphatidylethanolamine. Indeed, most of the conventional phospholipids have archaeal equivalents, including an analogue of cardiolipin with C20 and C25 isoprenoid chains. Gentiobiosyl archaeol could be considered to be analogue of the diglycosyldiacylglycerols found in higher plants. Other such lipids, which may lack eukaryotic or bacterial equivalents, include archaetidylglycerosulfate, archaetidylglycerophosphate methyl ester and triglycosyl archaeol lipids. While amino lipids and glycolipids containing pH-sensitive β-D-galactofuranosyl units are common in the methanogens, they are absent from thermophiles. Halobacterium salinarum and related species contain comparable lipids with sulfated tri-, di- and mono-glycosyl-diethers esterified to the phosphate group of phosphatidic acid, while Halobellus sp. contain similar molecular forms together with phosphatidylglycerosulfate.
Those lipids based on the caldarchaeol and other tetraether cores are much more complicated. Sometimes only one of the glycerol moieties is attached to a polar moiety, so caldarchaetidic acid is the monophosphate ester of caldarchaeol, but more often both glycerols are linked to polar moieties, and these are always different, e.g., glycosyl caldarchaetidylserine contains a glycosyl moiety at one end of the molecule and a serine phosphate at the other. Thus, the extensively studied Methanobacterium thermoautotrophicum contains four phospholipids (archaetidic acid, archaetidylethanolamine, archaetidylserine, archaetidyl-myo-inositol) and one glycolipid (gentiobiosyl archaeol) based on archaeol, together with four phospholipids (caldarchaetidic acid, caldarchaetidylethanolamine, caldarchaetidylserine, caldarchaetidyl-myo-inositol), one glycolipid (gentiobiosyl caldarchaeol) and three mixed glyco-phospholipids (gentiobiosyl caldarchaetidylethanolamine, gentiobiosyl caldarchaetidylserine, gentiobiosyl caldarchaetidyl-myo-inositol) based on caldarchaeol. The main polar lipid from S. acidocaldarius is characterized by a β-D-glucose bound to the nonitol group of calditol and a phosphate-bound myo-inositol at the glycerol end of the dibiphytanyl tetraether.
It is not always recognized that Archaea can contain appreciable amounts of lipids with conventional fatty acid components, and in the Haloarchaea, these are almost entirely saturated, mainly 18:0 (~80%) and 16:0 (11-20%). Most of these are not linked by ester bonds, so may be in unesterified form or as amide-linked components of amino-lipids, such as ornithine lipids, sphingolipids, or proteolipids, although esterified fatty acids amounted to 89% of the total phospholipid side chains in Methanothermus fervidus. It has been suggested that the requisite enzymes have been obtained from bacteria by horizontal gene transfer. Although sterols and hopanoids are not present, Archaea contain polyterpenes, i.e., carotenoids, polyprenols, quinones and apolar polyisoprenoids, which may have related functions in regulating the physical properties of membranes.
2. Archaeal Lipids in Membranes
Diether phospholipids resemble the more conventional diacyl phospholipids from eukaryotes in many aspects of their physical properties, and they have an ability to form bilayer membrane structures. Tetraether polar lipids, on the other hand, can span the membranes of the organisms to stiffen them at high growth temperatures and form in effect a membrane monolayer; physical chemical methods, such as freeze fracturing, are not able to separate two leaves. In contrast to non-archaeal membranes, the diether monopolar and tetraether bipolar lipids have a tendency to de-mix and cluster in separate domains that may not require the presence of stabilizing proteins. In aqueous solution, the bipolar ether lipids form remarkably stable liposomes of different sizes (uni- and multi-lamellar) and membrane packing densities. Vesicles prepared from archaeal lipids (‘archaeosomes’) are being developed for commercial use in intravenous drug delivery systems or as adjuvants for drugs and vaccines, where their high thermal and chemical stability, resistance to enzymatic degradation and immunomodulatory properties are assets.
The complex archaeal lipids are distributed asymmetrically in membranes, and a study of the distribution of lipids between the inner and outer leaflets of the membrane of Methanobacterium thermoautotrophicum has demonstrated that a high proportion of the gentiobiose units of both the di- and tetraether lipids are exposed on the outer aspect of the cells, where inter-glycosyl hydrogen bonding may assist in stabilizing the membrane structure. Similarly, much of the gentiobiose unit of gentiobiosyl caldarchaetidylethanolamine is on the outer surface with the phosphoethanolamine unit inside, although most of the archaetidylethanolamine (diether) is in the outer leaflet of the membrane bilayer. The phosphoserine and phosphoinositol residues of both diether and tetraether polar lipids are mainly oriented towards the cytoplasmic surface of the membrane as is expected in Eukaryotes.
 At the
growth temperature of any organism, membranes are in a liquid-crystalline state in which a high degree of lipid movement is possible to
enable membrane proteins to work properly and to maintain the barrier function, and most bacteria achieve this by changing the
chain-length and degree of unsaturation and/or branching of the fatty acid chains in response to environmental pressures.
In Archaea, the plasma membrane is in a liquid-crystalline state between a monolayer of dibiphytanyldiglycerol tetraether lipids and a bilayer
of diphytanylglycerol diether lipids, and the phytanyl chains of archaeal membranes maintain this phase over a wide range of growth temperatures.
In response to extreme temperature variations, the lipid compositions change with an increase in the proportion of tetraether
to diether lipids (GDGT/archaeol ratio) in membranes to improve their thermal stability, and for example in Thermococcus barophilus,
under its optimal growth conditions of 40 MPa and 85°C, caldarchaeol is the major ether form, but at higher pressures and lower
temperatures, archaeol predominates.
At the
growth temperature of any organism, membranes are in a liquid-crystalline state in which a high degree of lipid movement is possible to
enable membrane proteins to work properly and to maintain the barrier function, and most bacteria achieve this by changing the
chain-length and degree of unsaturation and/or branching of the fatty acid chains in response to environmental pressures.
In Archaea, the plasma membrane is in a liquid-crystalline state between a monolayer of dibiphytanyldiglycerol tetraether lipids and a bilayer
of diphytanylglycerol diether lipids, and the phytanyl chains of archaeal membranes maintain this phase over a wide range of growth temperatures.
In response to extreme temperature variations, the lipid compositions change with an increase in the proportion of tetraether
to diether lipids (GDGT/archaeol ratio) in membranes to improve their thermal stability, and for example in Thermococcus barophilus,
under its optimal growth conditions of 40 MPa and 85°C, caldarchaeol is the major ether form, but at higher pressures and lower
temperatures, archaeol predominates.
In general, ether lipids are much more stable to chemical attack via oxidation or acid/base treatment than acyl lipids, and there is increasing evidence that they have a major role in archaeal membranes in enabling the organisms to tolerate extremes of temperature, salt concentrations and pH. Halophiles can thrive at salinities greater than 20-25% and pressures of >50 MPa, and while the optimal growth temperature for many thermophiles is 80°C, some have survived a temperature as high as 120°C. Membranes containing tetraether lipids are also able to withstand high concentrations of metal ions, and some acidophiles can tolerate a pH of zero and pH gradients that approach 5 pH units. It has been demonstrated that the tetraether membrane monolayers have a limited permeability for protons even at the higher growth temperatures that have been observed, and Archaea adjust the composition of their membrane lipids to maintain their proton permeability within a narrow range so that a near neutral intracellular pH can be maintained for optimal activities of soluble and membrane-bound proteins. The addition of cyclic structures such as five-membered rings to the trans-membrane portion of the lipids is an adaptation to high temperatures, conferring enhanced membrane packing and reduced fluidity and permeability with strengthening of the hydrogen bonding, while the presence of a covalent bond between the alkyl chains in H‑shaped tetraethers may have the same effect to reinforce the strength of the membrane. and by reducing the distance between alkyl chains this produces denser membranes.
It has been proposed that magnesium ions can bridge the negative charges of adjacent anionic phospholipids to act in part as a surrogate for cardiolipin, a molecule that is known to control the curvature of membranes. However, there is a tight association between cardiolipin and cytochrome c oxidase in mitochondria and bacteria, and the same is true for the cardiolipin analogue in Archaea, suggesting that this is a truly universal lipid-protein interaction. Remarkably high concentrations of menaquinones are present in membranes of some extremophiles such as the Haloarchaea, where it has been suggested that in addition to their functions as electron and proton transporters, they act as ion permeability barriers and as powerful shields against oxidative stress.
3. Biosynthesis
The distinctive chirality of the glycerol molecule in Archaeal lipids is a consequence of the specificity of the enzyme that reduces dihydroxy acetone phosphate, i.e., the product is sn-glycerol 1-phosphate rather than sn‑glycerol 3-phosphate, as in bacteria and all eukaryotes.

In eukaryotes and most bacteria, there are a number of routes to the generation of sn-glycerol 3-phosphate, via D‑glyceraldehyde 3-phosphate as illustrated below (see our web page on ether lipids) and via glycolysis. Dihydroxyacetone phosphate (DHAP) is a critical intermediate, which in Archaea is converted to sn-glycerol 1-phosphate by an NADH-dependent reduction by G1P-dehydrogenase, which is completely different from the well-known G3P-dehydrogenase. Both use adenine nucleotides (NADH or NADPH), but G1P-dehydrogenase uses Zn2+ at its active site and transfers the pro-R hydrogen of NADH while G3P-dehydrogenase transfers the pro-S hydrogen. On the other hand, the G1P-dehydrogenase has now been detected in a few bacterial species (e.g., Bacillus subtilis) together with its product sn-glycerol 1‑phosphate, and vice versa, G3P‑dehydrogenase but not its product has been detected in Archaea; horizontal gene transfer may be involved.
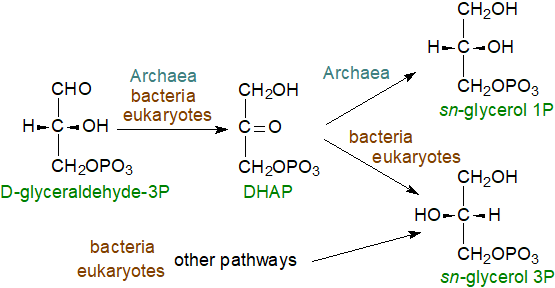 |
| Figure 3. Biosynthesis of sn-glycerol-1-phosphate. |
The isoprenoid chains in Archaea are synthesised by a mechanism that is similar if not identical to the classical mevalonic acid pathway (see our web page on cholesterol biosynthesis) at least in the first steps with production of the universal five-carbon subunits isopentenyl pyrophosphate and dimethylallyl pyrophosphate. Although the key enzyme HMG-CoA reductase from S. solfataricus showed more than 40% homology to eukaryal analogues, some of the later steps in isoprenoid biosynthesis are different from those in the classical mevalonic acid pathway, suggesting a divergence in archaeal metabolism from both bacteria and eukaryotes at a very early stage in their evolution from a putative common ancestor. There are also appreciable differences among the archaeal phyla (with some enzymes obtained by horizontal transfer from bacteria). The isoprenoid building blocks undergo sequential condensation reactions leading first to the formation of geranyl diphosphate (C10), then farnesyl (C15), geranylgeranyl (C20), farnesylgeranyl (C25) diphosphates and so forth, reactions catalysed by enzymes of a family of synthases common to all three domains of life.
Ether bonds are formed by coupling the terpenoid chains as geranylgeranyl units, first to position 3 of sn-glycerol 1-phosphate and then to position 2 to form sn‑2,3‑digeranylgeranylglycerol 1-phosphate by cytoplasmic prenyl transferases (geranylgeranylglyceryl diphosphate synthases) with all subsequent steps occurring through enzymes located on cell membranes.
 |
| Figure 4. Biosynthesis of complex archeol lipids. |
The membrane protein cytidine diphosphate (CDP)-archaeol synthase catalyses the transfer of the nucleotide to its archaeal lipid substrate, leading to the formation of a CDP-activated precursor (CDP-archaeol) to which polar head groups are attached. Molecular biology and gene studies have found several archaeal proteins with sequence similarities to members of the (CDP)-alcohol phosphatidyltransferase family that add the polar head groups, suggesting that the biosynthesis mechanisms for most archeol glycerol phospholipids resemble those for the bacterial analogues. As in the bacterial equivalent, archaetidylethanolamine is synthesised by decarboxylation of archaetidylserine, but that for the inositol lipids differs (see below). Archaeol glycolipids are synthesised by the transfer of glucose or gentiobiose from UDP-glucose or UDP-gentiobiose, respectively, to archaeol. The archaeal geranylgeranyl reductase can use the isoprenoid chains either in free form or bound to complex lipids, but hydrogenation of the double bond in position 2 is only possible when the isoprenoid chain is in bound form, so the fully saturated molecules may be formed at this final stage.
The formation of tetraether lipids has been one of the most intriguing problems in lipid biochemistry. Although it was presumed for many years that it must involve carbon-carbon bond formation between the two methyl termini of isoprenoid chains, i.e., at inert sp3-carbon centres, such a reaction is unprecedented in biochemistry, and alternative hypothetical mechanisms were proposed. However, the 'improbable' early suggestion is now known to be correct, as a radical S‑adenosylmethionine protein tetraether synthase was first isolated from S. acidocaldarius from the Thaumarchaeota, and when expressed in the methanogen Methanococcus maripaludis, which otherwise does not synthesise GDGTs but produces archaeol, it led to production of GDGT and structurally related lipids. The structure of this enzyme from Methanocaldococcus jannaschii and the mechanism of its action have now been elucidated.
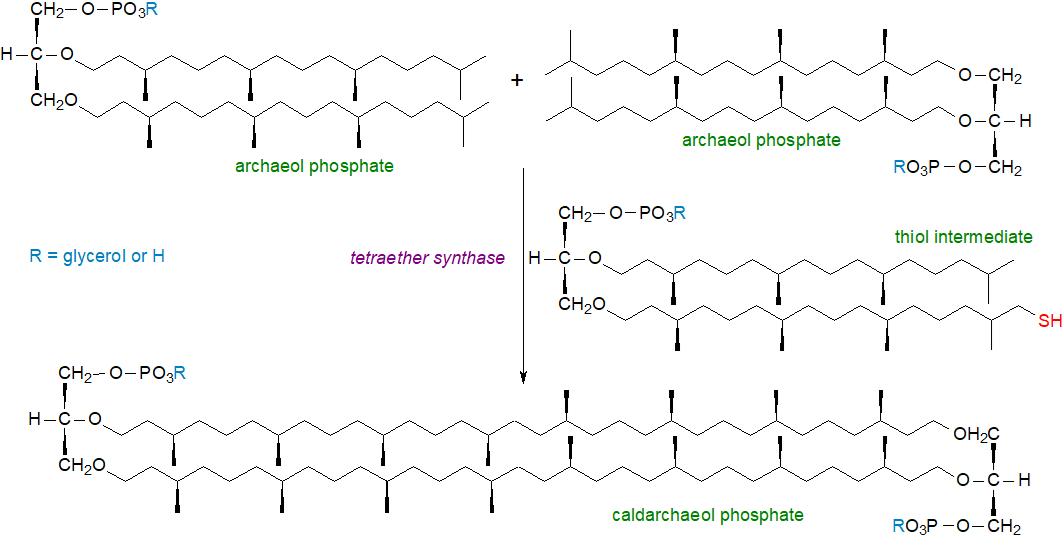 |
| Figure 5. Biosynthesis of glycerol dialkyl glycerol tetraether lipids. |
The tetraether lipid synthase from M. jannaschii is a radical S-adenosylmethionine (SAM) enzyme, which can cleave SAM reductively to yield methionine and a 5′‑deoxyadenosyl 5′-radical (5′-dA•). The latter is a potent oxidant that can initiate catalysis by abstracting a substrate hydrogen atom (H•), even from an unreactive carbon, and so is capable of biphytanyl chain formation of both macrocyclic archaeol and GDGT membrane lipids. The enzyme has four metallocofactors, three [Fe4S4] clusters and one mononuclear rubredoxin-like iron ion. Formation of the Csp3–Csp3 bond takes place on fully saturated archaeal lipid substrates (phosphate or glycerolphosphate) and proceeds via an intermediate bond between the substrate carbon and a sulfur of one of the [Fe4S4] clusters. This can interact with a further terminal carbon to form either cycloarchaeol or a tetraether lipid.
Similarly, a radical S‑adenosyl-L-methionine enzyme, Gms, has been identified that catalyses the formation of an additional Csp3–Csp3 linkage or bridge between adjacent methyl groups on the two isoprenoid chains of glycerol dialkyl glycerol (GDGT) tetraethers to produce the 'H'-shape (GDMT) contributing to increased membrane rigidity. A further enzyme, Gmm, can add further methyl groups in various positions adjacent to the bridge (Me-GDMT).
 |
| Figure 6. Biosynthesis of H-linked dialkyl glycerol tetraether lipids. |
Two enzymes have been identified that introduce cyclopentane rings into glycerol dibiphytanyl glycerol tetraethers in S. acidocaldarius. These ring synthases, designated GrsA and GrsB, are radical S-adenosylmethionine proteins, suggesting that the cyclization step may occur by a free radical mechanism, and that this occurs after formation of the ether bonds. GrsA introduces a cyclopentyl ring specifically at the C-7 position of the core tetraether lipid, while GrsB produces one at the C-3 position, and the reaction proceeds sequentially in this order. As the cyclization patterns are controlled by two separate enzymes, these are potentially influenced by different environmental factors. It has still to be determined whether the tetraether substrate must be unsaturated for cyclization and whether different phospholipid and hexose head groups influence ring formation, and no information is yet available on the biosynthesis of cyclohexane rings in this archaeal phylum.
Biosynthesis of the butanetriol and pentanetriol dibiphytanyl tetraethers in the methanogen M. luminyensis occurs by transfer(s) of the terminal methyl group of methionine, presumably from S-adenosylmethionine, to the glycerol analogues. Although the relevant enzymes have not been fully characterized, archaetidylethanolamine, archaetidylserine and archaetidylglycerol are probably synthesised by analogous routes to those for the corresponding phospholipids in bacteria. Enzymes that cleave the glycerol-ether bonds in the Archaea do not appear to have been identified, and they may not be important in terms of the overall metabolism. While some Archaeal cells seem to contain lipid droplets, proteomic studies have not detected any enzymes for triacylglycerol and wax ester biosynthesis, although the polyhydroxyalkanoate pathway was found.
Inositol lipids: The biosynthesis of the inositol lipids in Archaea and those few bacterial species that contain such lipids has proved of particular interest because the biosynthetic mechanism is very different from that in Eukaryotes, and this has evolutionary implications. Glucose-6-phosphate is converted to 1(L)‑myo‑inositol 1‑phosphate (synonymous with inositol 3-phosphate) by an inositol phosphate synthase, and this is reacted with CDP-archaeol to form archaetidylinositol 3-phosphate by an archaetidylinositol phosphate synthase. This differs from the mechanism in Eukaryotes in that it is the 1-hydroxyl group of inositol 3-phosphate that is transferred rather than the 1‑hydroxyl of free inositol. Finally, archaetidylinositol is produced via the action of a phosphatase.
 |
| Figure 7. Biosynthesis of archaetidylinositol. |
Cardiolipin: A phospholipase D motif–containing cardiolipin synthase has been characterized from the methanogen Methanospirillum hungatei that catalyses the condensation of two molecules of archaetidylglycerol to form archaetidylcardiolipin. The reaction is reversible, and the enzyme is promiscuous in that it can utilize a variety of substrates in vitro, including precursors with alkyl and acid moieties and of variable stereochemistry, to produce related lipids, many of which have not been found in nature.
 |
| Figure 8. Biosynthesis of archaetidylcardiolipin. |
Fatty acids: Much remains to be learned of fatty acid biosynthesis in Archaea, but acyl carrier protein is missing, and homologues of fatty acid synthase II detected in some may have been acquired from bacteria. Some of the enzymes for β-oxidation of fatty acids are present in Archaea, and it has been suggested that these might operate in reverse to synthesise fatty acids.
4. Archaeal Lipids and Evolution
Archaea and bacteria are widely considered to be distinct kingdoms of life that have diverged early in evolution from an organism designated the 'Last Universal Common Ancestor'. They are substantially different in their lipid compositions in that bacteria and eukaryotes have membrane lipids in which fatty acids are linked to sn‑glycerol-3-phosphate (G3P), while the main lipids in Archaea, as described above, have membranes in which the lipids have isoprenoid alkyl chains that are ether linked to sn‑glycerol-1-phosphate (G1P), i.e., of the opposite stereochemistry. However, bacteria from the Fibrobacteres-Chlorobi-Bacteroidetes superphylum, which are highly abundant in the deep anoxic waters of the Black Sea, encode a putative archaeal pathway for ether-bound isoprenoid membrane lipids in addition to the bacterial fatty acid membrane pathway, and the glycerolipids can possess either a G1P or G3P stereochemistry. Chiral chromatography of diradyl moieties of phospholipids suggests that this capacity may be more widespread among bacteria.
Of course, it is never possible to absolutely prove an event (the "lipid divide") that occurred in evolution billions of years ago, but the existence of 'mixed membranes' in natural environments and their stability over a long period in evolutionary history support the hypothesis of a common ancestor. Two recently discovered archaeal groups, i.e., marine Euryarchaeota group II/III and 'Lokiarchaeota', which may be descendants of the ancestor that lead to eukaryotes, lack the gene for synthesis of G1P and cannot synthesise archaeal membrane lipids, although they are able to produce 'chimeric' lipids with ether-bound isoprenoid chains or with one ester-bound fatty acid replacing an ether-bound isoprenoid. As Eukaryotes have features that seem to be of bacterial origin, these newly described organisms may reflect a transition stage between Archaea, Bacteria and Eukaryotes. Of course, only the evidence from lipid studies is discussed here, and much wider data sets are available to support such conclusions.
5. Geochemistry
Different Archaea can contain characteristic variants on the basic structures, and these are proving useful for taxonomic purposes and for studies of microbial ecology. Because of their saturated nature and the relatively stable ether bonds, residues of archaeal ether lipids can survive well in rocks and sediments, and they can serve as markers for the Archaea in general and even for particular organisms over geological time spans. Although slow degradation does occur via hydrolysis, oxidation and other reactions, the products are still recognizable as of Archaeal origin. Archaeal lipids are an important element of research in organic geochemistry as they are found in most environments, including soil, peat, marine and lacustrine water columns and sediments, hot springs and stalagmites. They have even been claimed to be the ‘most ubiquitous lipid on Earth’ (although there are other contenders).
6. Other Lipids with Apparently Similar Structures
Anaerobic soil bacteria of the genus Acidobacteria produce tetralkylglycerol ethers that superficially resemble those of the Archaea but are derived from 1,2‑di‑O‑alkyl‑sn-glycerol, and some contain membrane-spanning fatty acids such as iso-diabolic acid (13,16‑dimethyl-octacosanedioic acid).
The extremophile thermophilic bacterium, Limisphaera ngatamarikiensis produces a new and distinctive phospholipid class, termed 'serinophospholipids', which differ from glycero- and sphingophospholipids by having a serinol backbone, i.e. N,O-diacylserinophospho-N-methylethanolamine and N,O-diacylserinophosphoethanolamine, as major components (38% of the phospholipid mass). The fatty acids are mainly C16 and C18 saturated, some with methyl branches.
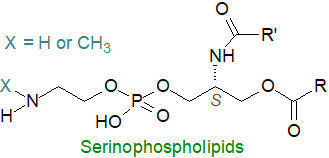
These lipids have an (S)-configured serinol core that is equivalent to the sn-glycerol-1-phosphate (G1P) arrangement characteristic of Archaea lipids and so very different from most bacteria. As they are not in fact Archaea, it is a matter of conjecture as to how these organisms fit in the evolutionary debate.
7. Analysis
Structural analysis of the archaeal lipids is technically daunting, and a lack of appropriate standards exacerbates the problem. The membranes are resistant to disruption for extraction of the lipids, but an improved method is now available that employs freeze-thaw cycles and the quaternary ammonium surfactant (hexadecyltrimethylammonium bromide) to give substantially improved lipid yields. Chemical degradative methods were first used for analysis, but modern mass spectrometric procedures have now come to the fore, especially with HPLC in combination with electrospray and atmospheric-pressure chemical ionization. Nuclear magnetic resonance (NMR) spectroscopy is then invaluable for determination of the stereochemistry of the various structural units. While it is simpler from a technical standpoint to analyse the core lipids after removal of the polar moieties, when both GC‑MS and HPLC-MS techniques can be used for analysis, technical improvements have made it easier (or less difficult!) to study the intact lipids by HPLC-MS methodology to determine the nature and proportions of the various head groups as well as of the alkyl moieties.
Suggested Reading
- Carbone, V., Schofield, L.R., Zhang, Y., Sang, C., Dey, D., Hannus, I.M., Martin, W.F., Sutherland-Smith, A.J. and Ronimus, R.S. Structure and evolution of the archaeal lipid synthesis enzyme sn-glycerol-1-phosphate dehydrogenase. J. Biol. Chem., 290, 21690-21704 (2015); DOI.
- De Kok, N.A.W. and Driessen, A.J.M. The catalytic and structural basis of archaeal glycerophospholipid biosynthesis. Extremophiles, 26, 29 (2022); DOI.
- Dibrova, D.V., Galperin, M.Y. and Mulkidjanian, A.Y. Phylogenomic reconstruction of archaeal fatty acid metabolism. Environm. Microbiol., 16, 907-918 (2014); DOI.
- Exterkate, M., de Kok, N.A.W., Andringa, R.L.H., Wolbert, N.H.W., Minnaard, A.J. and Driessen, A.J.M. A promiscuous archaeal cardiolipin synthase enables construction of diverse natural and unnatural phospholipids. J. Biol. Chem., 296, 100691 (2021); DOI.
- Kellermann, M.Y., Yoshinaga, M.Y., Valentine, R.C., Wormer, L. and Valentine, D.L. Important roles for membrane lipids in haloarchaeal bioenergetics. Biochim. Biophys. Acta, Biomembranes, 1858, 2940-2956 (2016); DOI.
- Law, K.P. and Zhang, C.L.L. Current progress and future trends in mass spectrometry-based archaeal lipidomics. Org. Geochem., 134, 45-61 (2019); DOI.
- Law, K.P., Li, X.X. and Zhang, C.L. Lipidomics in archaeal membrane adaptation to environmental stresses and growth conditions: A review of culture-based physiological studies. Sci. China, Earth Sci., 63, 790–807 (2020); DOI.
- Lloyd, C.T., Iwig, D.F., Wang, B., Cossu, M., Metcalf, W.W., Boal, A.K. and Booker, S.J. Discovery, structure and mechanism of a tetraether lipid synthase. Nature, 609, 197-203 (2022); DOI.
- Morii, H., Kiyonari, K., Ishino, Y. and Koga, Y. A novel biosynthetic pathway of archaetidyl-myo-inositol via archaetidyl-myo-inositol phosphate from CDP-archaeol and D-glucose 6-phosphate in methanoarchaeon Methanothermobacter thermautotrophicus cells. J. Biol. Chem., 284, 30766-30774 (2009); DOI.
- Řezanka, T., Kyselová, L. and Murphy, D.J. Archaeal lipids. Prog. Lipid Res., 91, 101237 (2023); DOI.
- Salvador-Castell, M., Tourte, M. and Oger, P.M. In search for the membrane regulators of Archaea. Int. J. Mol. Sci., 20, 4434 (2019); DOI.
- Schouten, S., Hopmans, E.C. and Damsté, J.S.S. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review. Org. Geochem., 54 19-61 (2013); DOI.
- Stonik, V.A., Makarieva, T.N., Shubina, L.K., Guzii, A.G. and Ivanchina, N.V. Structure diversity and properties of some bola-like natural products. Marine Drugs, 23, 3 (2025); DOI.
- Villanueva, L., von Meijenfeldt, F.A.B, Westbye, A.B., Yadav, S., Hopmans, E.C., Dutilh, B.E. and Damsté, J.S.S. Bridging the membrane lipid divide: bacteria of the FCB group superphylum have the potential to synthesize archaeal ether lipids. ISME J., 15, 168-182 (2021); DOI.
- Vyssotski, M., Lagutin, K., Mackenzie, A., Mitchell, K., Stewart, A.W., Scott, D., Stott, M.B. and Compton, B.J. Serinophospholipids: a third type of natural phospholipid discovered in a thermophilic bacterium. J. Nat. Prod., 88, 373-383 (2025); DOI.
- Zeng, Z., Chen, H., Yang, H., Chen, Y., Yang, W., Feng, X., Pei, H. and Welander, P.V. Identification of a protein responsible for the synthesis of archaeal membrane-spanning GDGT lipids. Nature Commun., 13, 1545 (2022); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: October 2025 | ||
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
