Mass Spectrometry of Dimethyloxazoline and Pyrrolidine Derivatives
Cyclic Fatty Acids
 This document
does not aim to be a complete account of mass spectrometry with
electron-impact ionization of fatty acids containing ring structures as 4,4‑dimethyloxazoline (DMOX)
and pyrrolidide derivatives, but rather is a personal account of our experience
of those encountered during our research activities and for which we have spectra available for illustrative purposes.
The web pages on pyrrolidides and DMOX
derivatives of monocarboxylic saturated fatty acids contain more introductory and mechanistic information,
together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.).
Spectra for methyl esters and
3‑pyridylcarbinol esters of cyclic fatty acids are described in separate documents.
These notes are intended as a practical guide rather than as a mechanistic account.
Where we are aware of prior illustrations of mass spectra in the literature, the appropriate papers are cited.
The occurrence and biological properties of cyclic fatty acids have been reviewed by
Sébédio and Grandgirard (1989), and there is further information on the chemistry, occurrence and biochemistry
of cyclic fatty acids on this website here...
This document
does not aim to be a complete account of mass spectrometry with
electron-impact ionization of fatty acids containing ring structures as 4,4‑dimethyloxazoline (DMOX)
and pyrrolidide derivatives, but rather is a personal account of our experience
of those encountered during our research activities and for which we have spectra available for illustrative purposes.
The web pages on pyrrolidides and DMOX
derivatives of monocarboxylic saturated fatty acids contain more introductory and mechanistic information,
together with links to pages dealing with additional practical methodology (sample concentration, derivative preparation, etc.).
Spectra for methyl esters and
3‑pyridylcarbinol esters of cyclic fatty acids are described in separate documents.
These notes are intended as a practical guide rather than as a mechanistic account.
Where we are aware of prior illustrations of mass spectra in the literature, the appropriate papers are cited.
The occurrence and biological properties of cyclic fatty acids have been reviewed by
Sébédio and Grandgirard (1989), and there is further information on the chemistry, occurrence and biochemistry
of cyclic fatty acids on this website here...
Despite having very different structures, dimethyloxazoline and pyrrolidide derivatives of a given fatty acid have identical molecular weights and fragment in similar ways under electron-impact ionization in the mass spectrometer, so it is convenient to discuss both together here. DMOX derivatives tend to give more abundant diagnostic ions and have better gas chromatography properties, but pyrrolidides are better when the functional group is in the terminal position.
Cyclopropyl Fatty Acids
Cyclopropyl fatty acids are common constituents of bacterial lipids and may accompany cyclopropene fatty acids as minor components of certain seed oils. DMOX derivatives are of limited value for the structural analysis of cyclopropyl fatty acids since they appear to undergo a rearrangement to form monoenes in a comparable manner as occurs with methyl esters (Zhang et al., 1987). This view of the mechanism is too simplistic but is serviceable. This is one of only a few examples where DMOX derivatives are less suitable, and 3‑pyridylcarbinol esters are undoubtedly best.
As an example, the mass spectrum of the DMOX derivative of 9,10-methylene-octadecanoate is illustrated -
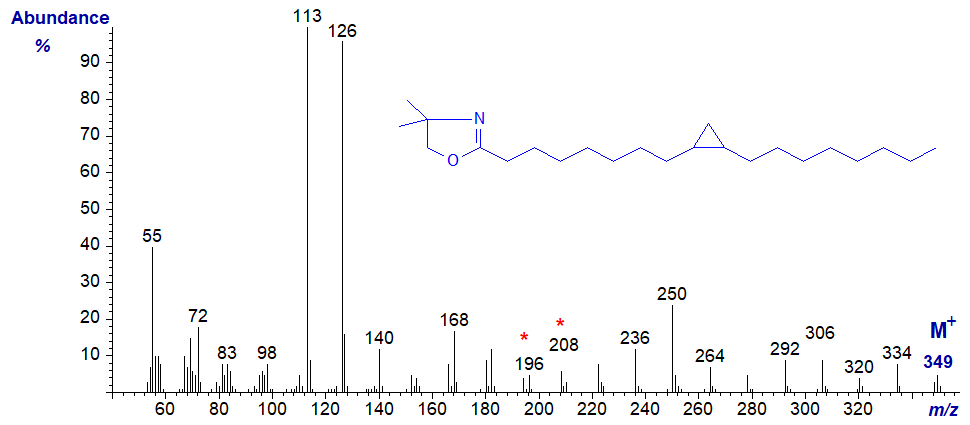
Although the structure can in theory be deduced from the fact the rearranged double bond in position 9 gives a characteristic gap of 12 amu between m/z = 196 and 208, this presupposes that the analyst is already aware that the fatty acid contains the ring structure - not a double bond (see the web page dealing with DMOX derivatives of monoenes). One clue might be the gas chromatographic (GC) retention time, which would be distinct from that of a monoene and would indicate that an unusual fatty acid was present.
The DMOX derivative of 11,12-methylene-octadecanoate (or lactobacillate) has the mass spectrum –
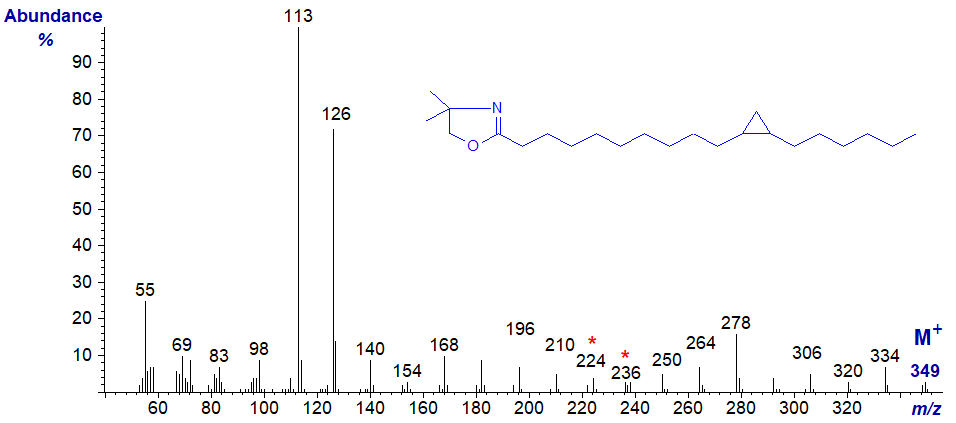
Again, the position of the ring can be deduced from the gap of 12 amu (between m/z = 224 and 236), assuming we recognize that it is a cyclopropyl fatty acid not a C19 monoene.
The mass spectra of pyrrolidide derivatives of cyclopropane fatty acids closely resemble those of the DMOX derivatives and like them give ambiguous spectra that are almost indistinguishable from those of monoenes one carbon longer in chain length, so the same caveats apply in interpreting the spectra. I am not aware of publication of the next two spectra elsewhere. In both derivatives, the ions at m/z = 113 and 126 are simply characteristic for the pyrrolidine or dimethyloxazoline rings.
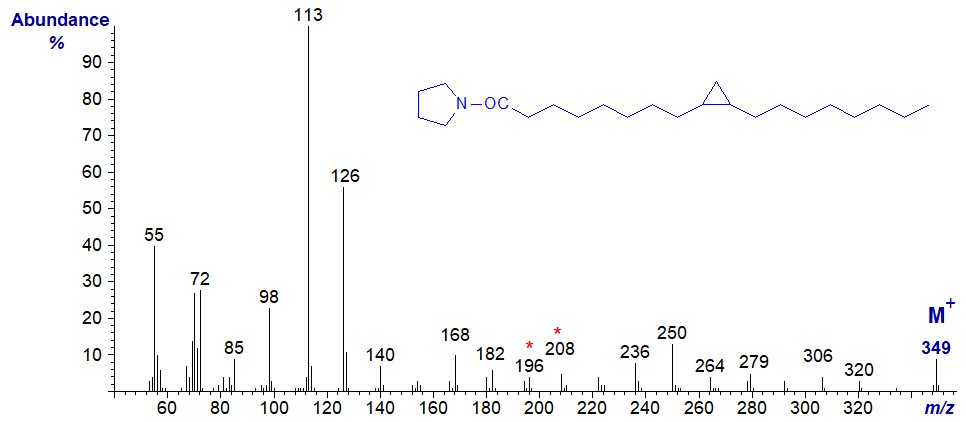
For example, the mass spectrum of the pyrrolidide of 9,10-methylene-octadecanoate is almost identical to that of 9-nonadecenoate, and the gap of 12 amu between m/z = 196 and 208 is that expected for a double bond in position 9 of the chain.
The mass spectrum of the pyrrolidide of 11,12-methylene-octadecanoate (or lactobacillate) is –
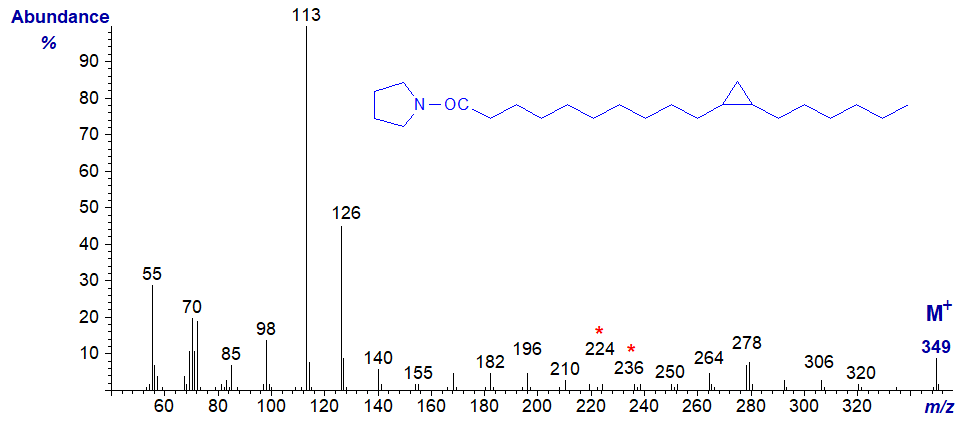
Although the position of the ring can in theory be deduced from this from first principles (from the gap of 12 amu between m/z = 224 and 236), in practice it may be better to regard it simply as a fingerprint. A recent publication in which more spectra were available for study has suggested that unequivocal identification may be possible by considering certain minor ions (Santalova and Denisenko, 2017), but whether this is true for DMOX derivatives has still to be determined. Again, GC retention times of pyrrolidide derivatives of monoenoic and cyclopropanoic fatty acids are different, and this can be useful information in deciding between possible structures.
Alternative mass spectrometric methods for locating cyclopropane rings in fatty acids involve ring opening by vigorous hydrogenation or by reaction with boron trifluoride-methanol (see our web page on methyl esters of cyclic fatty acids).
Cyclopropenyl Fatty Acids
It was long thought that GC and thus GC-MS of derivatives of cyclopropenyl fatty acid was impossible because of thermal degradation on the GC column, but modern capillary columns are relatively inert, and analysis by GC is straightforward, provided that appropriate derivatization methods are employed, i.e., that acidic conditions are avoided. The mass spectra of the DMOX derivatives of cyclopropenyl acids resemble those of a mono-acetylenic fatty acid and one carbon longer, presumably because rearrangement occurs in the mass spectrometer (Spitzer, 1991).
For example, the mass spectrum of the DMOX derivative of sterculic acid is illustrated next. The key diagnostic ions are for a gap of 10 amu between m/z = 196 and 206, the same as those that locate the triple bond in stearolic acid (see our web pages on mass spectra of DMOX derivatives of acetylenic fatty acids).
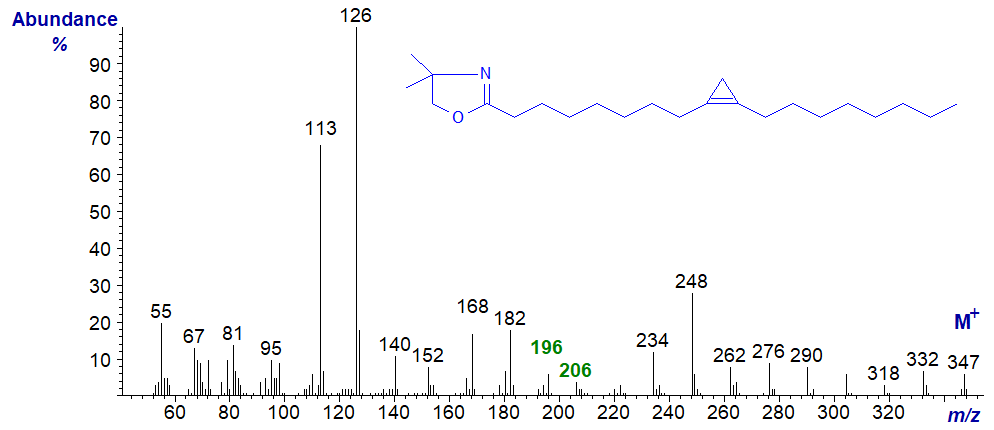
The spectrum of the pyrrolidide of sterculate is very similar to this (not published elsewhere), although the ions in the high mass range are less abundant, and it is illustrated below.
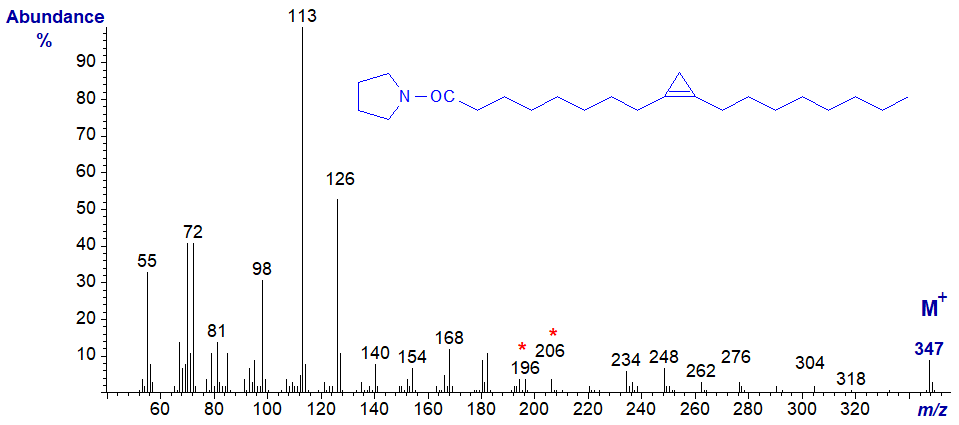
The key diagnostic ions are again for a gap of 10 amu between m/z = 196 and 206. The corresponding ions in the mass spectra of the malvalate derivatives of both types of derivative are all 14 amu lower, i.e., dmox and pyrrolidide.
Fatty Acids with Central Hexadienyl Rings
As far as I am aware fatty acids with central hexadienyl rings are not found in nature, but they can be formed as artefacts during derivatization of other natural fatty acids. For example, 7-(5-pentyl-cyclohexadienyl)-heptanoic acid was formed while preparing the DMOX derivative of crepenynic acid (octadec-9-en-12-ynoic acid) (Christie, 1998). The spectrum is -
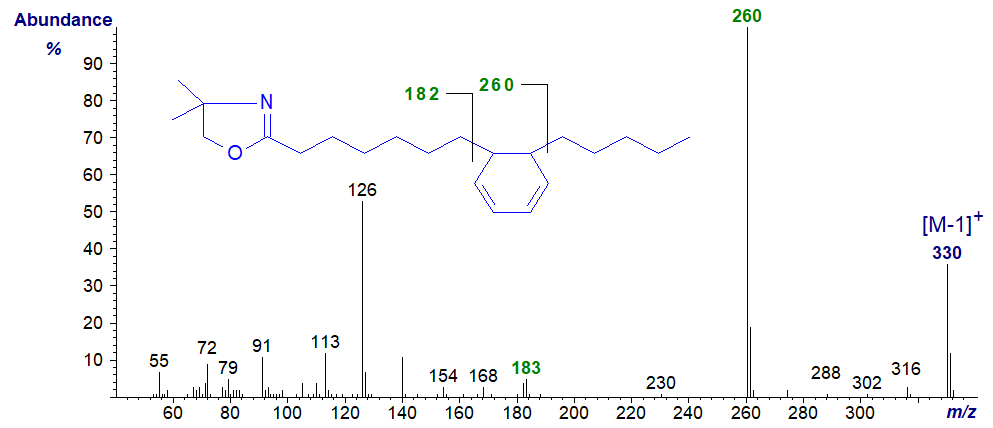
There is a highly characteristic gap of 77/78 amu between m/z = 182/183 and 260, which defines the position of the ring. We have the spectrum of the DMOX derivative of the isomeric 6-(6-hexyl-cyclohexadienyl)-hexanoate on file in the Archive section, which was produced as an artefact when derivatizing 6,8,12‑octadecatrienoic acid (Author, unpublished).
ω-Cyclopentyl and Cyclopentenyl Fatty Acids
Fatty acids with a terminal cyclopent-2-enyl moiety are found in high concentration in the seed oils of several species from the plant family Flacourtiaceae, though the corresponding cyclopentyl (saturated species) have been detected at low levels only. It is worth noting that although the nitrogen-containing derivatives permit location of most of the structural features with varying degrees of success, none fix the actual position of the double bond within the ring. For the latter purpose, chemical degradation, perhaps allied with mass spectrometry, is required (Christie et al., 1989).
The spectrum of the DMOX derivative of 11-cyclopentylundecanoate is -
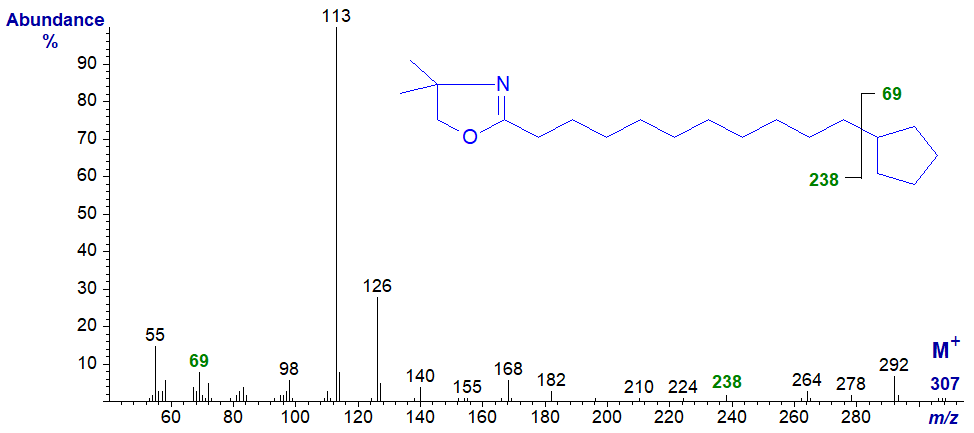
Here, one needs to use the eye of a true believer to see the gap of 69 amu, between the molecular ion and that at m/z = 238, for a terminal cyclopentane ring (Zhang et al., 1989). The problem is that the facile loss of methyl groups from the DMOX ring results in series of ions in the high mass range (m/z = 264, 278 and 292) that confound the picture (Hamilton and Christie, 2000).
There is much less confusion in identifying terminal structural features in the spectra of pyrrolidides, as can be seen from the spectrum of the pyrrolidide of 11‑cyclopentyl-undecanoate -
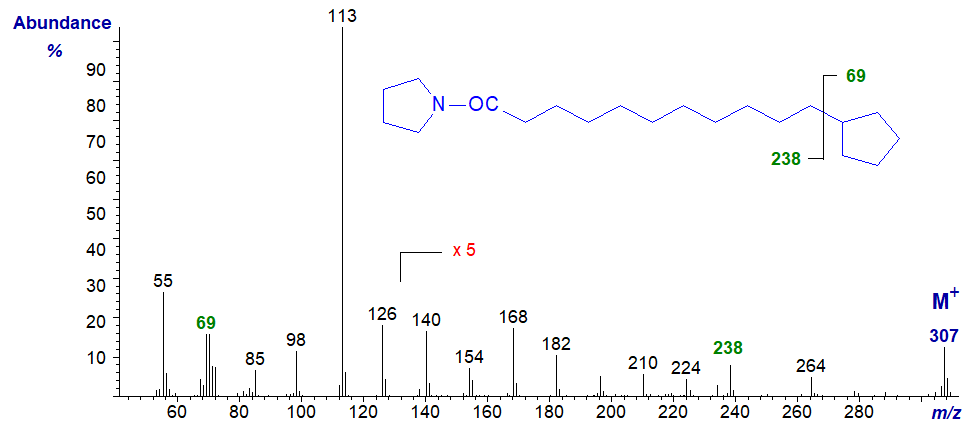
The gap of 69 amu between m/z = 239 and 307 clearly defines the ring, with only one anomalous ion at m/z = 264, which may represent the expulsion of a three-carbon fragment (C2 to C4) from the alkyl chain as has been studied from a mechanistic standpoint with methyl esters of saturated fatty acids.
In contrast, there is the spectrum of the DMOX derivative of hydnocarpate (11-cyclopentenylundecanoate) -
In fairness, the gap of 67 amu (m/z = 238 to 305) for loss of the terminal ring is a little more convincing, although interpretation is again confounded by ions that result from loss of methyl groups from the DMOX ring (cf., m/z = 290), a problem with all terminally substituted fatty acids. The ion representing the cyclopentene ring itself (m/z = 67) is certainly more abundant in this instance.
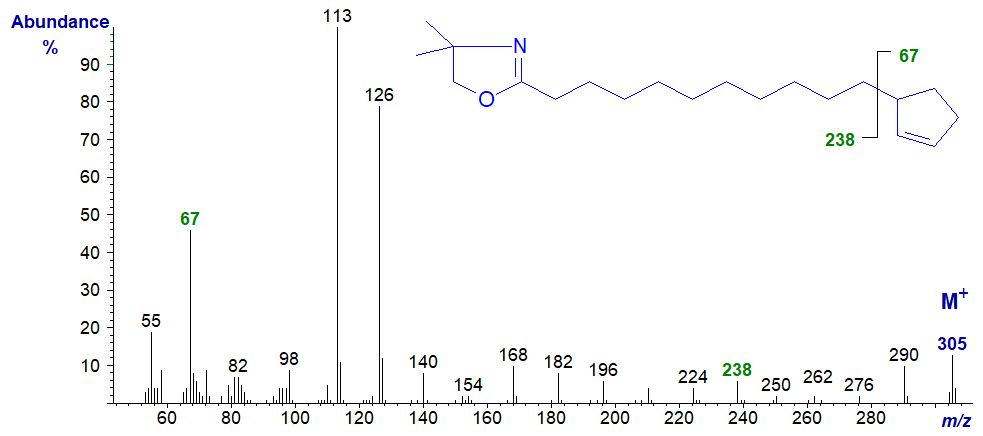
The pyrrolidide derivative of hydnocarpic acid has again a less confusing and much more useful spectrum -
In the high mass region, the gap of 67 amu between m/z = 238 and 305 for cleavage of the cyclopentenyl ring can be seen unambiguously. In all other respects, the spectrum is very similar to that of the corresponding DMOX derivative. We have the spectrum of the C18 analogue in the Archive pages. Neither of these spectra appears to have been published formally elsewhere.
Spectra of the DMOX derivatives of two of the natural cyclopentenyl fatty acids with double bonds in the chain acids follow, starting with that of 13‑cyclopent-2-enyltridec-4-enoate. Fingerprint ions at m/z = 152 and 166 locate the double bond in position 4 in the alkyl chain (see our web page on the relevant monoenes), while the gap of 67 amu between m/z = 264 and 331 defines the cyclopentene ring (as does the ion at m/z = 67 per se).
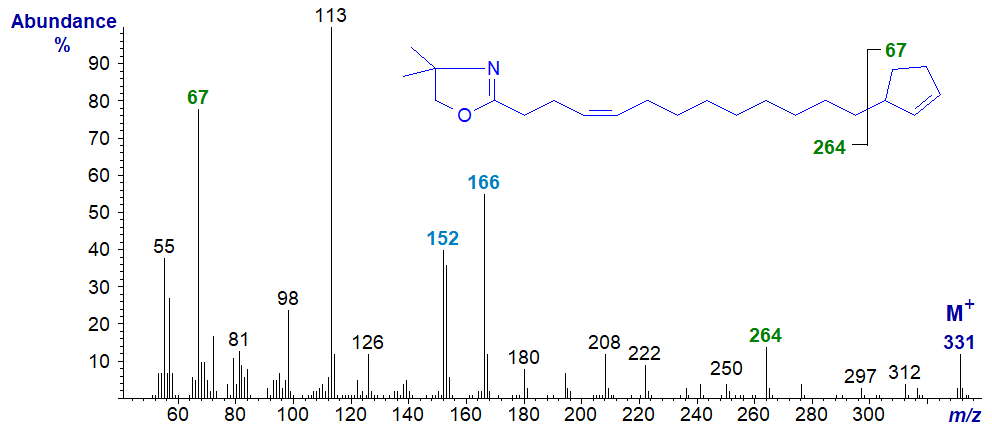
DMOX derivative of 15-cyclopent-2-enylpentadec-9-enoate or hormelate -
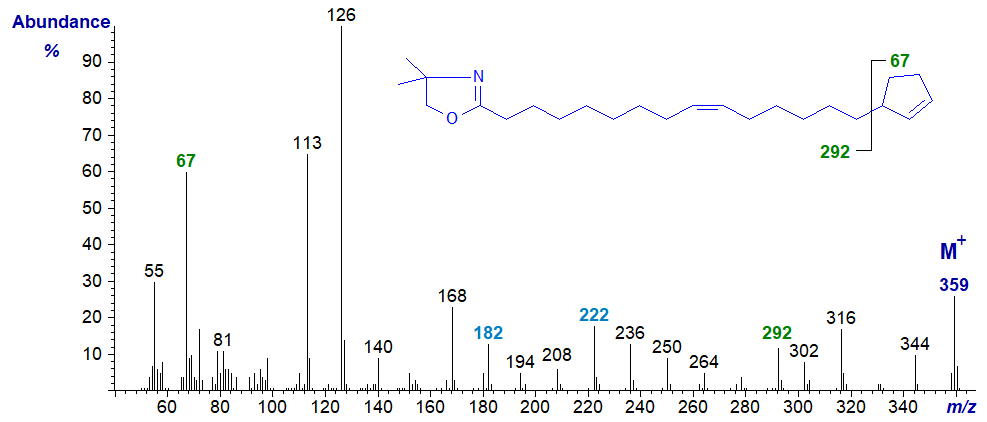
Definitive location of the cyclopentene ring is again problematic, though it should be recognized that each derivative gives a distinctive fingerprint at least, and the ion at m/z = 67 for the ion representing the terminal ring aids identification (Zhang et al., 1989). We do not see a clean gap between m/z = 292 and 359 as might be expected, again because methyl groups can be lost from the oxazoline ring to yield confounding ions (with other fragmentations). To define the location of the double bond in the chain, see the section of this web site on Mass spectrometry of DMOX derivatives of monoenes. With the last spectrum, it would be easy to conclude from an apparent gap of 12 amu between m/z = 182 and 194 that the double bond is in position 8 rather than 9 of the chain if no other information was available (the gap of 40 amu between m/z = 182 and 222 is a better guide). Such confusion does not occur with the 3-pyridylcarbinol ester or pyrrolidide. This does not mean that one derivative is better than another in all circumstances, and there are times when confirmatory evidence is desirable by using another derivative type.
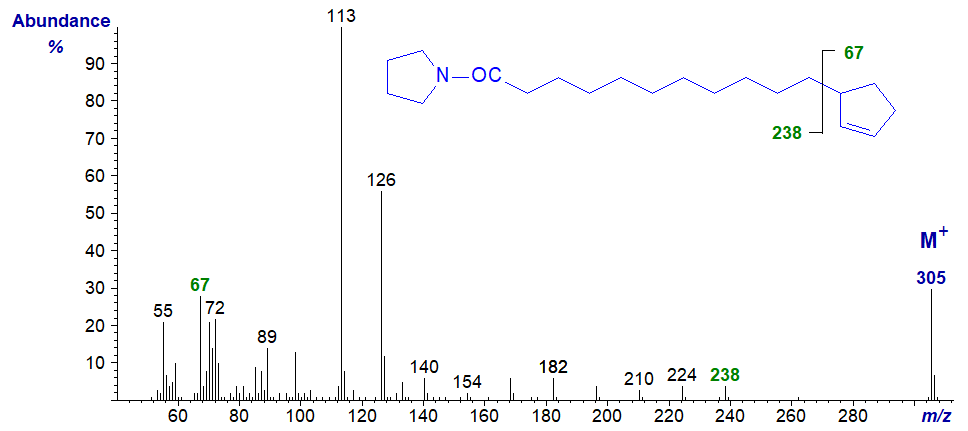
The pyrrolidide derivatives of terminal cyclopent-2-enyl fatty acids give mass spectra that resemble those of the DMOX derivatives, but the position of the ring is more clearly defined with the former. For example, in the spectrum of the pyrrolidide of 13‑cyclopent-2-enyltridec-4-enoate -
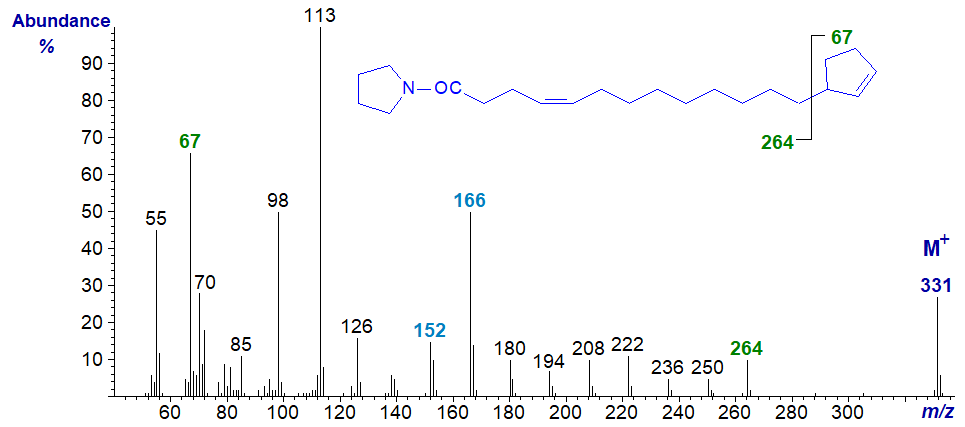
- the gap of 67 amu between m/z = 264 and 331 clearly locates the terminal ring structure, and there are no ions between them that confound the picture. The double bond is located from the fingerprint ions as in the analogous monoene (see our web pages on Pyrrolidine derivatives of monoenoic fatty acids).
The spectrum of the pyrrolidine derivative of 15-cyclopent-2-enylpentadec-9-enoate or hormelate -
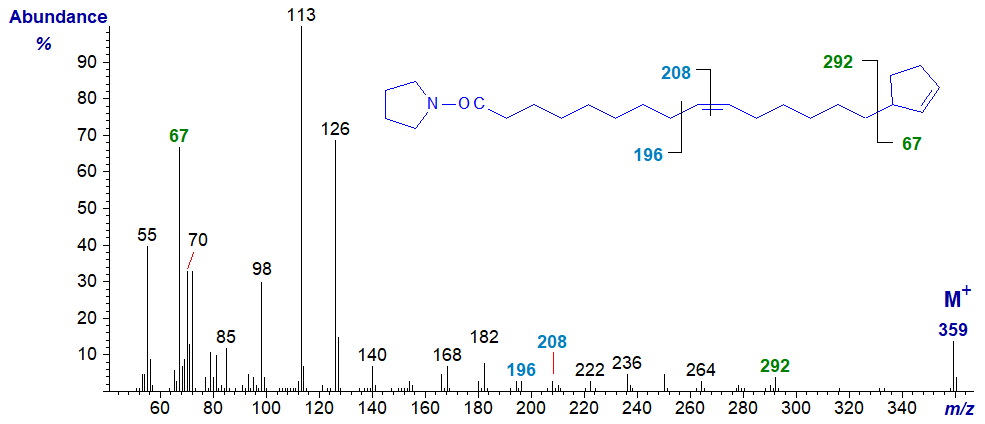
In this example, both the positions of the ring and of the double bond can be located easily as indicated on the spectrum.
Once more, in my opinion, 3-pyridylcarbinol esters are the best of the nitrogen-containing derivatives for structural characterization of ω‑cyclopentyl and ω-cyclopentenyl fatty acids.
ω-Phenyl Fatty Acids
Fatty acids with a terminal phenyl moiety are found in seed oils of the subfamily Aroideae of the Araceae and certain species of bacteria. Some of the available data have been published (Christie, 2003).
Mass spectrum of the DMOX derivative of 15-phenyl-pentadec-9-enoate –
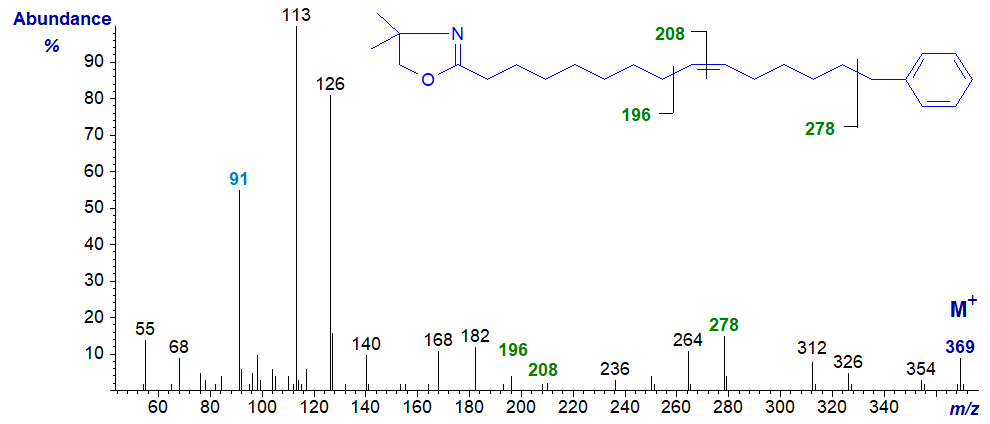
Mass spectra of pyrrolidides and DMOX derivatives exhibit a gap of 91 amu between the molecular ion and the next abundant ion in the high mass region (to m/z = 278), as illustrated above, and the tropylium ion at m/z = 91 is distinct (see the web page on methyl esters of tetraenes). Because of fragmentations involving the methyl group on the heterocyclic ring, the spectrum of the DMOX derivatives is confused in the high mass range.
Mass spectrum of the pyrrolidide of 15-phenyl-pentadec-9-enoate –
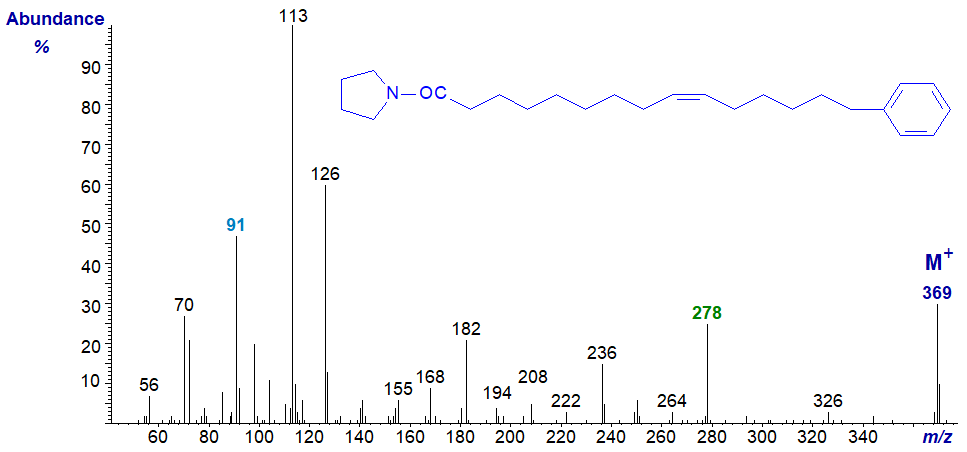
In this instance, the terminal phenyl group is very clear, but the position of the double bond could be confused as a gap of 12 amu between m/z = 182 and 194 suggests a double bond in position 8 rather than 9. The correct position could also be confirmed by preparing dimethyl disulfide derivatives (cf., the web page on this methodology). Again, this illustrates the value of using more than one derivative type for identification purposes.
Cyclic Fatty Acids Formed in Frying Oils
Fatty acids with internal five- and six-membered ring structures are formed when vegetable oils are heated to the high temperatures attained during frying or the physical refining process. The mechanism of cyclization is now believed to involve concerted thermal rearrangements with loss of one of the double bonds. As a host of basic structures can be formed from different fatty acid precursors, and cis-trans isomerization of double bonds and/or about the ring can occur, the range of cyclic products is very great. With my colleagues and collaborators, we have characterized most of these with the aid of both 3-pyridylcarbinol esters and dimethyloxazoline derivatives, and of course others have worked on the problem (reviewed by Christie and Dobson, 2000 and in the AOCS Lipid Library), but this is too specialized a topic for further discussion here.
There are mass spectra of many more cyclic fatty acids in our Archive section, i.e., of both DMOX and pyrrolidide derivatives, but without interpretation.
References
 Christie, W.W.
Mass spectrometry of fatty acids with methylene-interrupted ene-yne systems.
Chem. Phys. Lipids, 94, 35-41 (1998);
DOI.
Christie, W.W.
Mass spectrometry of fatty acids with methylene-interrupted ene-yne systems.
Chem. Phys. Lipids, 94, 35-41 (1998);
DOI.- Christie, W.W. 13-Phenyltridec-9-enoic and 15-phenylpentadec-9-enoic acids in Arum maculatum seed oil. Eur. J. Lipid Sci. Technol., 105, 779-780 (2003); DOI.
- Christie, W.W. and Dobson, G. Formation of cyclic fatty acids during the frying process. Eur. J. Lipid Sci. Technol., 102, 515-520 (2000); DOI.
- Christie, W.W., Brechany, E.Y. and Shukla, V.K.S. Analysis of seed oils containing cyclopentenyl fatty acids by combined chromatographic procedures. Lipids, 24, 116-120 (1989); DOI.
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Santalova, E.A. and Denisenko, V.A. Fatty acids from a glass sponge Aulosaccus sp. occurrence of new cyclopropane-containing and methyl-branched acids. Lipids, 52, 73-82 (2017); DOI.
- Sébédio, J.L. and Grandgirard, A. Cyclic fatty acids: natural sources, formation during heat treatment, synthesis and biological properties. Prog. Lipid Res., 28, 303-336 (1989); DOI.
- Spitzer, V. GC-MS (chemical ionization and electron impact modes) characterization of the methyl esters and oxazoline derivatives of cyclopropene fatty acids. J. Am. Oil Chem. Soc., 68, 963-969 (1991); DOI.
- Zhang, J.Y., Wang, H.Y., Yu, Q.T., Yu, X.J., Liu, B.N. and Huang, Z.H. The structures of cyclopentenyl fatty acids in the seed oils of Flacourtiaceae species by gas chromatography-mass spectrometry of their 4,4-dimethyloxazoline derivatives. J. Am. Oil Chem. Soc., 66, 242-246 (1989); DOI.
- Zhang, J.Y., Yu, Q.T. and Huang, Z.H. 2-Substituted 4,4-dimethyloxazolines as useful derivatives for the localisation of cyclopropane rings in long-chain fatty acids. J. Mass Spectrom. Soc. Japan, 35, 23-30 (1987); DOI.
I also recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
