Mass Spectrometry of Methyl Esters
Tetraenoic Fatty Acids
Methylene-Interrupted Tetraenes
Diagnostic Ions
As cautioned in the Introduction to these documents, the mass spectra of methyl esters obtained with electron-impact ionization afford limited information only concerning the structures of unsaturated fatty acids. There are a few key ions that help to identify the common polyenoic fatty acids with methylene-interrupted unsaturation, especially those of the biologically important (n-6) and (n-3) families, though these must be interpreted with caution. Thus, an ion at m/z = 150 is characteristic for fatty acids with an n-6 terminal moiety, while one at m/z = 108 defines an n-3 terminal group (omega ion) (Holman and Rahm, 1971; Brauner et al., 1982; Fellenberg et al., 1987). The presence of an alpha ion that contains the carboxyl group and the first two double bonds is less often recognized and tends to be small so easily missed, but it is equally important for characterization, as is discussed at greater length in the web page dealing with methyl esters of trienoic fatty acids, which contains a table of m/z values for all the expected structures.
In mass spectra of methylene-interrupted tetraenes, there is often an ion equivalent to the alpha ion + 27, and this is present also when there are three or more than four double bonds but tends to be lost amongst other minor ions. As examples, the bonds where simplistically these fragmentations can be considered to occur are depicted for 20:4(n‑3) and 20:4(n‑6) -
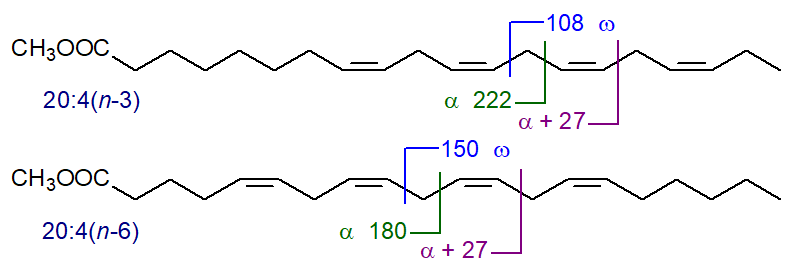 |
| Figure 1. Mass spectral fragmentations in relation to double bond positions in tetraenes. |
In comparison to the methylene-interrupted trienes, methyl esters of tetraenes, pentaenes and hexaenes tend to give molecular ions with very low abundances (if detectable at all), and the ion at [M−31/32]+ for loss of a methoxyl group is only rarely distinguishable. It can therefore be a difficult task to ascertain the molecular weight, so gas chromatographic retention data are especially important as additional aids to identification. This is rarely a problem with nitrogen-containing derivatives, such as the pyrrolidides, DMOX derivatives or 3-pyridylcarbinol esters. The McLafferty ion (m/z = 74) is always small in the mass spectra of methyl esters of polyunsaturated fatty acids, and in the lower molecular weight region, hydrocarbon ions of general formula [CnH2n‑5]+ tend to dominate the spectrum with the ion at m/z = 79 as the base ion.
 With fatty acids with multiple double bonds, a thermodynamically favoured rearrangement occurs
under electron impact ionization to produce a stable tropylium ion at m/z = 91,
and this becomes a progressively more important component of the mass spectrum with increasing numbers of double bonds.
Indeed, this dominates the spectrum of conjugated isomers such as methyl parinarate (see below).
With fatty acids with multiple double bonds, a thermodynamically favoured rearrangement occurs
under electron impact ionization to produce a stable tropylium ion at m/z = 91,
and this becomes a progressively more important component of the mass spectrum with increasing numbers of double bonds.
Indeed, this dominates the spectrum of conjugated isomers such as methyl parinarate (see below).
Some polyunsaturated fatty acids with double bond systems that are other than simply methylene-interrupted give 'fingerprint' spectra that are distinctive and can be used as a valuable guide to identification when authentic spectra are available for comparison, although the mechanisms of fragmentation may not be properly understood; a few in this category are discussed below. Some of the spectra depicted will have been published somewhere in the scientific literature (and hopefully readers will point out any citations that we have missed), but others are illustrated here for the first time.
Mass Spectra -
Of the C18 tetraenoic acids, 6,9,12,15-octadecatetraenoate (stearidonate or 18:4(n-3)) is probably the most common in plant (e.g., borage oil) and fish tissues at least, and the mass spectrum of its methyl ester is illustrated.
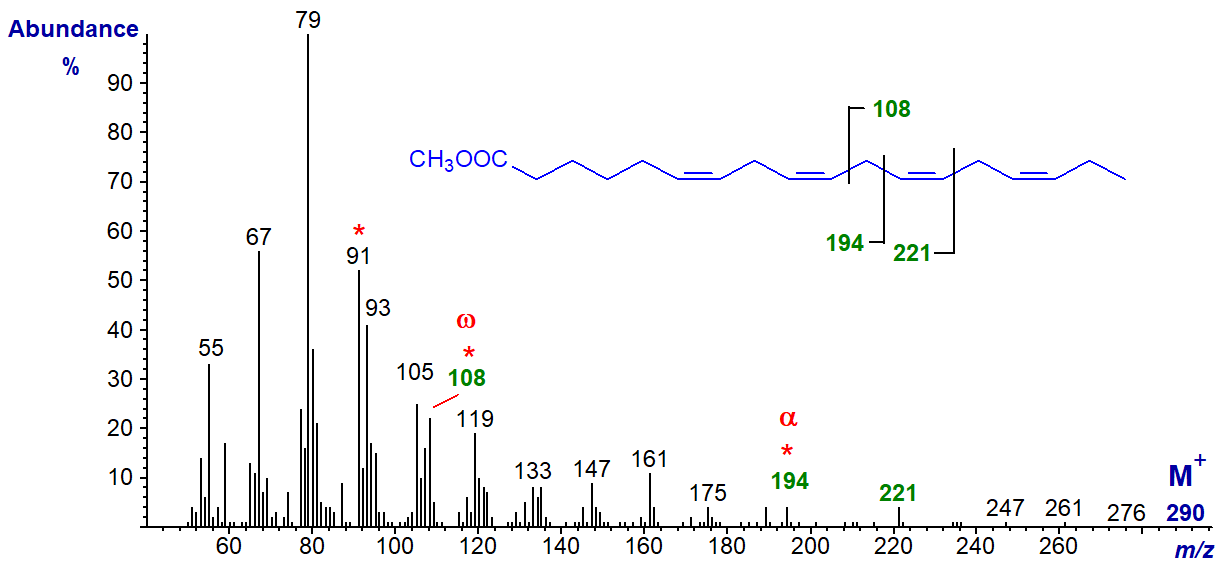
The molecular ion is barely distinguishable but the characteristic ion for the terminal n-3 structure, at m/z = 108, is apparent as is the alpha ion for Δ6,9 double bonds at m/z = 194 with the α+27 ion at m/z = 221.
In the spectrum of methyl 4,7,10,13-hexadecatetraenoate (16:4(n-3)), the ion at m/z = 108 is present as is the alpha ion at m/z = 166 (illustrated separately), while in the mass spectrum of methyl 5,8,11,14-octadecatetraenoate (18:4(n-4)) from fish oils, the alpha ion is at m/z = 180 and the omega ion at m/z = 122 (here..).
The spectrum of methyl 6,9,12,15-hexadecatetraenoate (16:4(n-1)) is different and can be interpreted to some extent in terms of the double bond positions. The α and α+27 ions are clearly seen at m/z = 194 and 221, respectively, although the expected ω ion (m/z = 80) is lost amongst the other hydrocarbon ions.
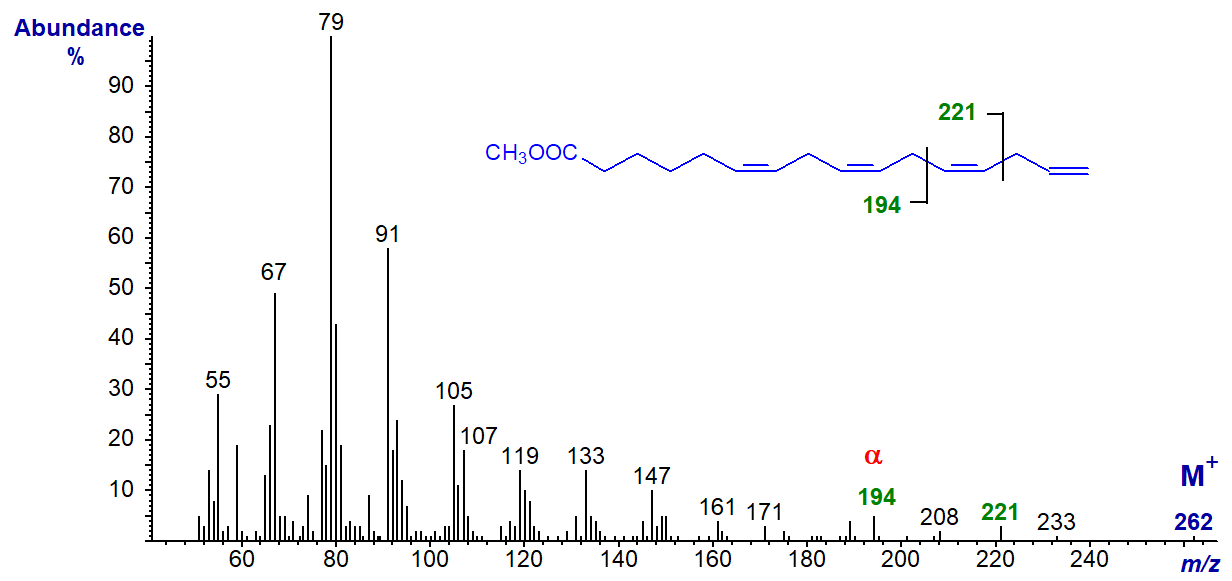
The expected α and α+27 ions are present in the spectra of 18:4(n-1) and 20:4(n-1), but not in those 16:3(n-1) and 18:3(n-1) (author, unpublished observation).
More important from a biological standpoint is the essential fatty acid, arachidonic acid (5,8,11,14-20:4 or 20:4(n‑6)), and the mass spectrum of methyl arachidonate follows -
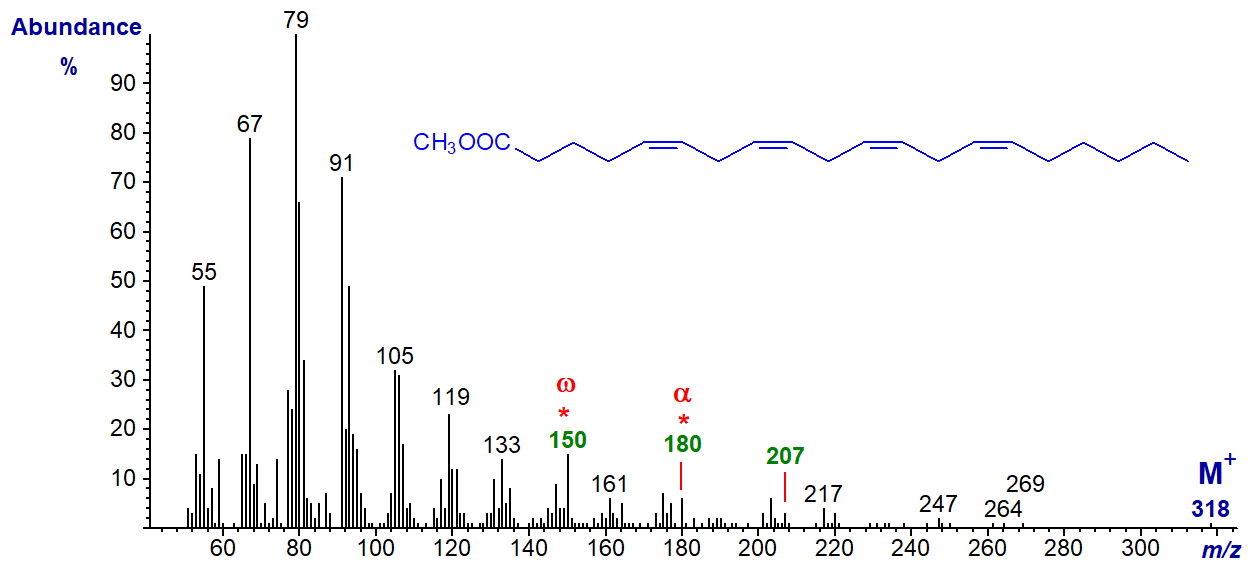
Once more the molecular ion at m/z = 318 is not very abundant, but the diagnostic ion for the n-6 moiety at m/z = 150 does stand out. The alpha ion for the Δ5,8 double bonds at m/z = 180 is small but distinctive, as is the α+27 ion at m/z = 207.
There are significant differences from the mass spectrum of methyl 8,11,14,17-eicosatetraenoate (20:4(n-3)) illustrated next -
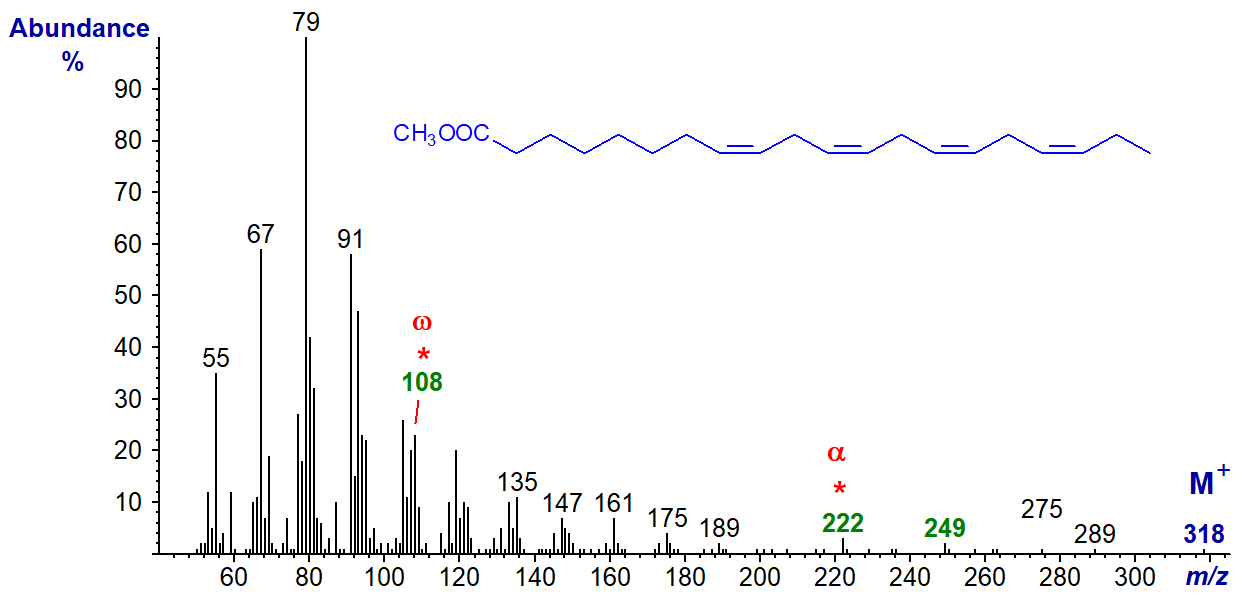
The 'diagnostic' omega ion for the n-3 family of fatty acids at m/z = 108 is indeed present, if somewhat hidden, while there is no ion at m/z = 150. Again, the alpha ion (m/z = 222) for Δ8,11 double bonds and that for α+27 are small but are clearly present.
Methyl 7,10,13,16-docosatetraenoate or 22:4(n-6) has the spectrum -
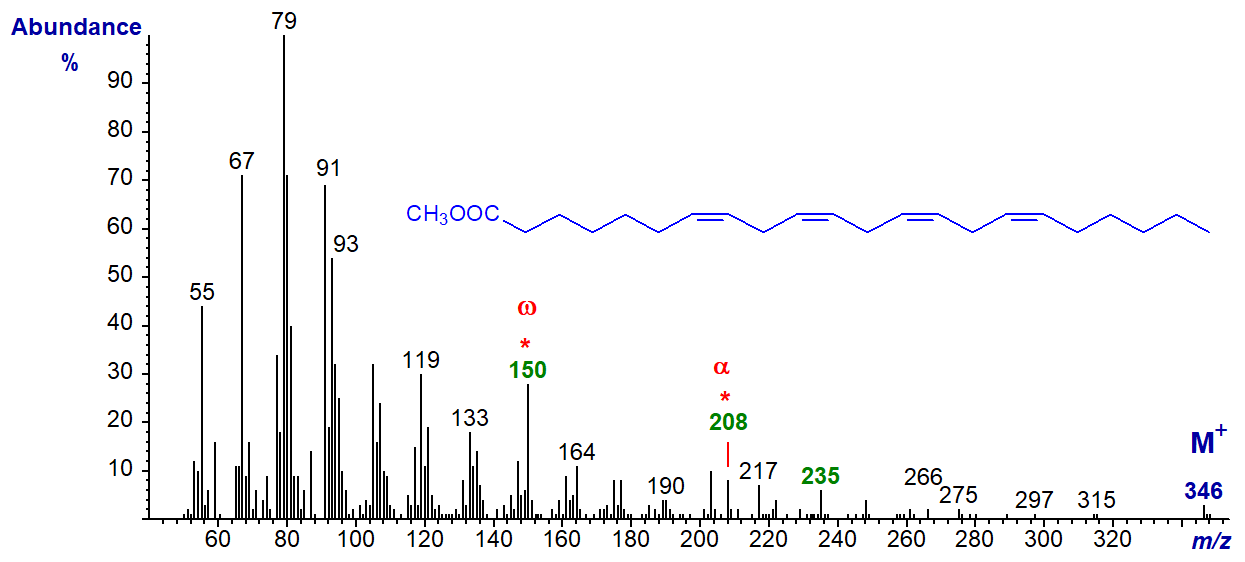
The characteristic ion at m/z = 150 for the n-6 family of fatty acids does again stand out, and this is present in the spectrum of 24:4(n-6) also. The alpha ion for the Δ7,10 double bonds at m/z = 208 is small but can be recognized, as is the α+27 ion (m/z = 235). The spectrum of 22:4(n-3) has the expected alpha and beta ions at m/z = 250 and 108, respectively.
Less Common Tetraenoic Fatty Acids
5,9,12,15-Octadecatetraenoic acid, with a bis-methylene-interrupted double bond system, is only rarely encountered in nature (in some conifer species mainly) and it its methyl ester has the spectrum -
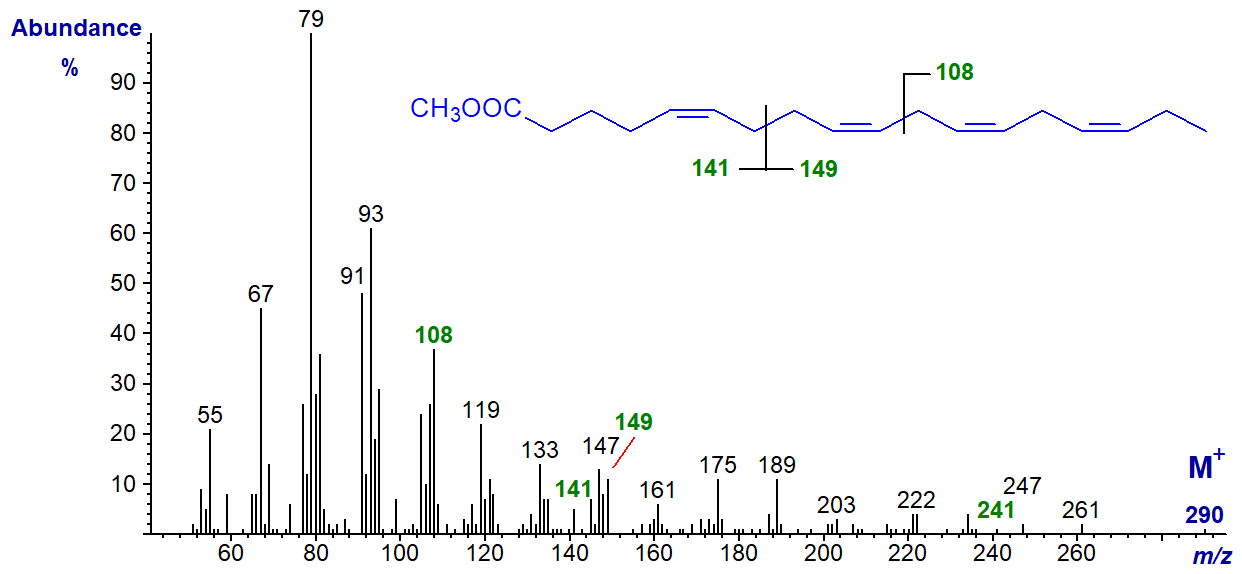
The omega ion at m/z = 108 for the (n-3) double bond system is present, as are ions representing cleavage at the centre of the bis-methylene-interrupted double bond system at m/z = 141 and 149. There is no obvious alpha ion, but the ion at m/z = 241 ([M-49]+), found in the spectra of methyl esters of other fatty acids with a Δ5,9 double bond system (see our web page on mass spectra of methyl esters of dienes), is present if in low abundance.
Methyl 3-trans,9-cis,12-cis,15-cis-octadecatetraenoate from the seed oil of Solidago vigaurea has four methylene groups between the first two double bonds. Its mass spectrum (not published elsewhere) is -
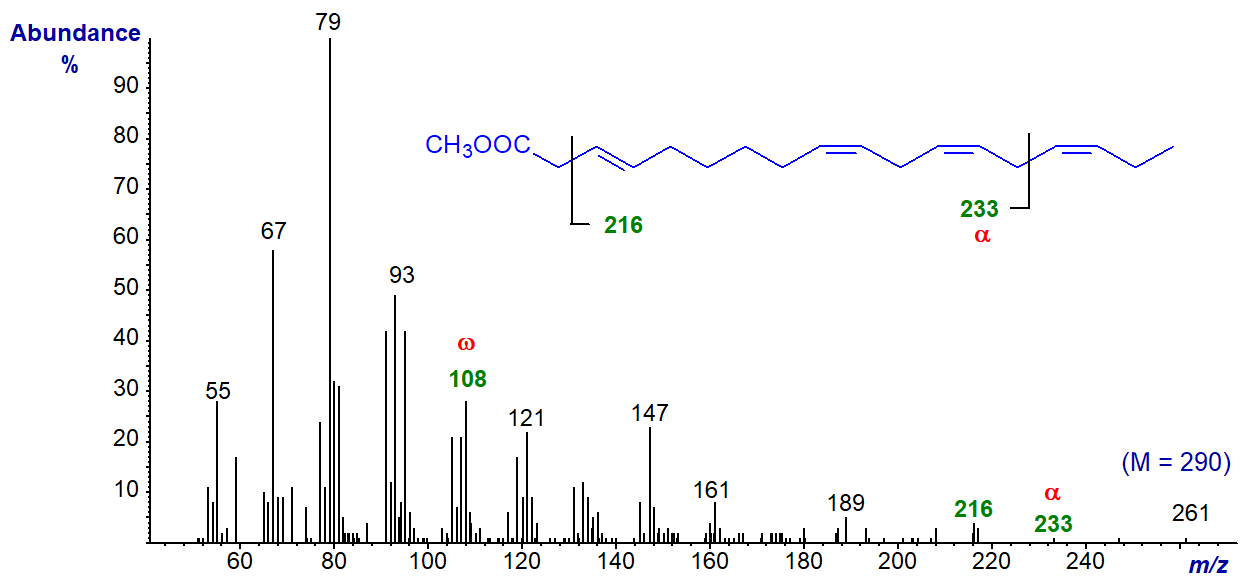
The molecular ion is not detected at normal magnification, but the omega ion for the n-3 moiety at m/z = 108 is prominent, and that at m/z = 233 may be the alpha ion (expected at m/z = 234). The fragment at m/z = 216 represents the loss of the McLafferty ion, confirming the identity of the analogous ion in the spectrum of methyl 3‑trans,9‑cis,12‑cis-octadecatrienoate (see our web page on mass spectra of methyl esters of trienes).
The mass spectrum of methyl 5,11,14,17-eicosatetraenoate, with four methylene groups between the first two double bonds (from a conifer species) -
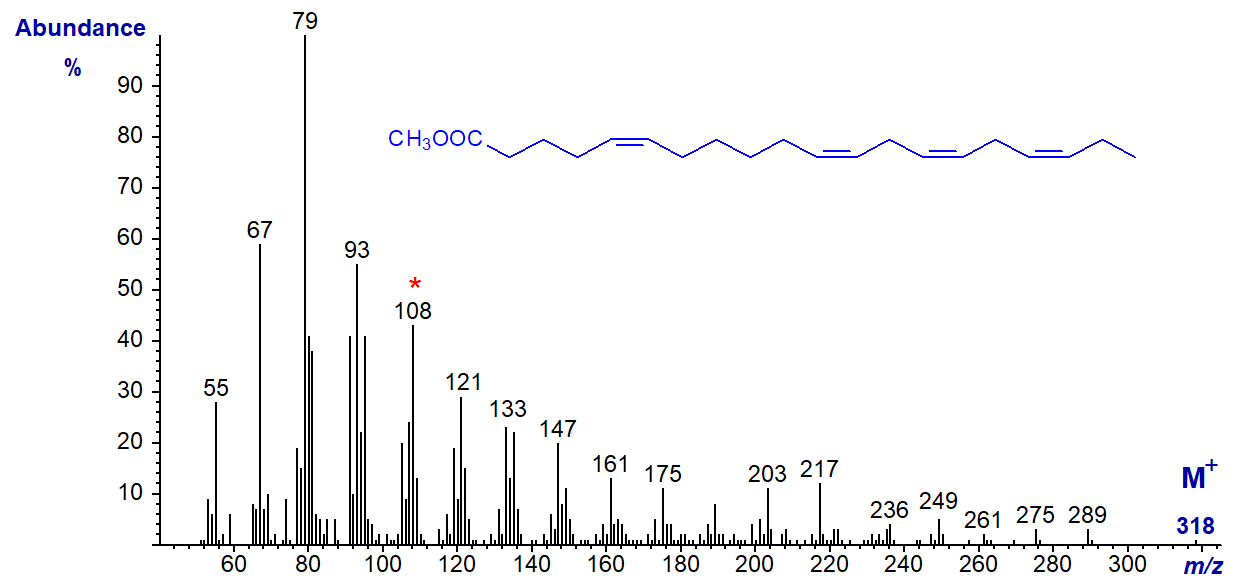
While the molecular ion is not very abundant once more, the diagnostic ion for the n-3 moiety at m/z = 108 is prominent, and there are other significant differences (though not necessarily interpretable in structural terms at present) from the spectrum of 20:4(n-3) illustrated earlier. The ion at m/z = 261 may be the alpha ion for the three methylene-interrupted double bonds.
The mass spectrum of the conjugated tetraene, methyl parinarate (9c,11t,13t,15c-18:4), from Impatiens noli-tangura seed oil is very different from the others. Like many here, it has probably not been published elsewhere.
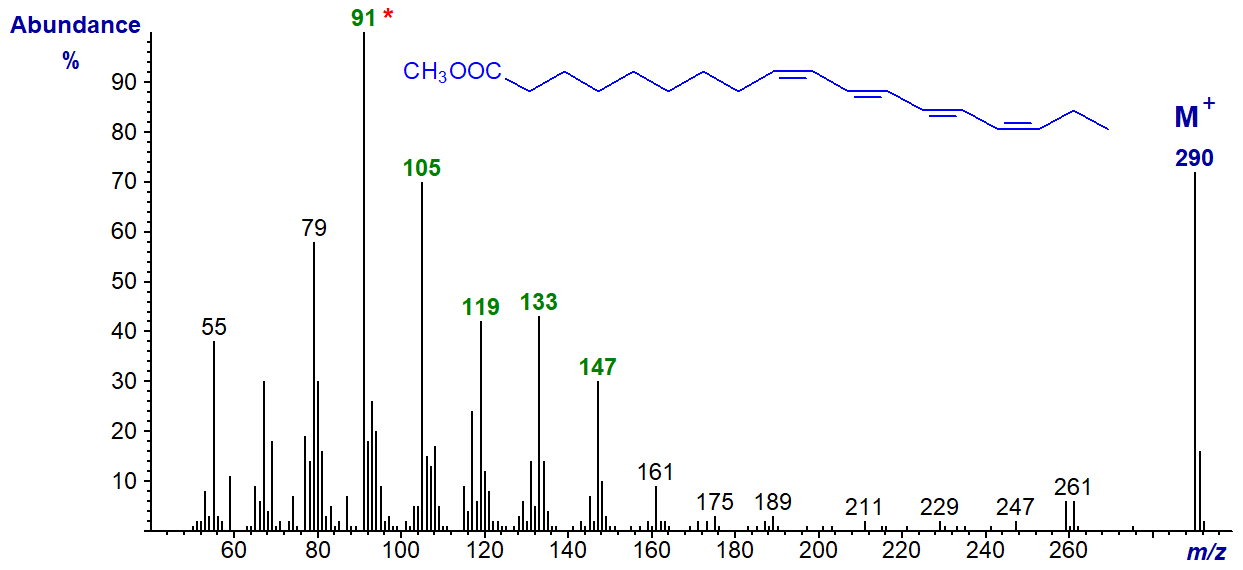
The molecular ion is especially abundant, and the base ion at m/z = 91 is a tropylium ion, which is characteristic for highly unsaturated molecules as described in the introductory paragraphs above. The homologous series of ions at m/z = 105, 119, 133, etc., are probably tropylium ions with alkyl substituents. While these ions are significant in the spectra of non-conjugated polyenes, they are much more abundant here. There are no ions that serve to locate double bonds.
We have spectra of a few more methyl esters of tetraenoic fatty acids on file, and they can be accessed (but without interpretation) from our Archive page.
References
- Brauner, A., Budzikiewicz, H. and Boland, W. Studies in chemical ionization mass spectrometry. 5. Localization of homoconjugated triene and tetraene units in aliphatic compounds. Org. Mass Spectrom., 17, 161-164 (1982); DOI.
- Fellenberg, A.J., Johnson, D.W., Poulos, A. and Sharp, P. Simple mass spectrometric differentiation of the n-3, n-6 and n-9 series of methylene interrupted polyenoic acids. Biomed. Environ. Mass Spectrom., 14, 127-130 (1987); DOI.
- Holman, R.T. and Rahm, J.J. Analysis and characterization of polyunsaturated fatty acids. Prog. Chem. Fats Other Lipids, 9, 15-90 (1971); DOI.
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - at Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
