Mass Spectrometry of DMOX Derivatives
Saturated Straight-Chain Fatty Acids
Introduction
 4,4-Dimethyloxazoline (DMOX) derivatives
give excellent mass spectra of fatty acids with electron-impact ionization that frequently permit unequivocal identification.
Any dubiety that may remain can be removed if the analyst has access to authentic spectra, and many
of those illustrated below and in the other documents in this website should prove useful for this purpose.
The gas chromatographic properties of DMOX derivatives are better than
those of 3‑pyridylcarbinol ('picolinyl') esters and pyrrolidides, and many analysts consider them the best single derivative
available for identification of fatty acids in mass spectrometric terms,
although this could be debated, especially when there are functional groups near the terminal end of the molecule.
In much of our research with novel samples, we have used more than one type of derivative as they provide useful complementary data.
One drawback of DMOX derivatives is that they are not very stable chemically; ring opening occurs rapidly on storage in the presence
of traces of moisture, although this problem can be ameliorated by storing them in the presence of desiccant.
There is as yet no simple clean-up step, as is available for other derivatives.
4,4-Dimethyloxazoline (DMOX) derivatives
give excellent mass spectra of fatty acids with electron-impact ionization that frequently permit unequivocal identification.
Any dubiety that may remain can be removed if the analyst has access to authentic spectra, and many
of those illustrated below and in the other documents in this website should prove useful for this purpose.
The gas chromatographic properties of DMOX derivatives are better than
those of 3‑pyridylcarbinol ('picolinyl') esters and pyrrolidides, and many analysts consider them the best single derivative
available for identification of fatty acids in mass spectrometric terms,
although this could be debated, especially when there are functional groups near the terminal end of the molecule.
In much of our research with novel samples, we have used more than one type of derivative as they provide useful complementary data.
One drawback of DMOX derivatives is that they are not very stable chemically; ring opening occurs rapidly on storage in the presence
of traces of moisture, although this problem can be ameliorated by storing them in the presence of desiccant.
There is as yet no simple clean-up step, as is available for other derivatives.
 Methods
for preparing DMOX derivatives are described elsewhere in this website
in a document on Preparation of derivatives.
They can be separated on a micro-preparative scale by reversed-phase HPLC, if a base-deactivated stationary phase is
employed (Christie, 1998 - and here...),
and this can be a useful means of enriching minor components for further analysis.
Methods
for preparing DMOX derivatives are described elsewhere in this website
in a document on Preparation of derivatives.
They can be separated on a micro-preparative scale by reversed-phase HPLC, if a base-deactivated stationary phase is
employed (Christie, 1998 - and here...),
and this can be a useful means of enriching minor components for further analysis.
DMOX derivatives are very similar in their fragmentation properties in mass spectrometry to pyrrolidides. Indeed, despite the differences in structure, the two have identical molecular weights, and the McLafferty ions from each have the same m/z value. The same is true for most ions of diagnostic value, although the relative abundances can be very different, and DMOX derivatives usually have ions of greater relative intensity in the high mass range. While pyrrolidides appear to have fallen out of fashion, they are more stable chemically and have advantages when functional groups are near the terminal carbon atom. DMOX derivatives are better with unsaturated fatty acids.
There is a comprehensive list of publications on DMOX derivatives in the Bibliography Section of these pages. A review of the topic, including mechanistic aspects, has been published by Spitzer (1997), although there are alternative explanations for the origins of some key ions (Hamilton and Christie (2000)). References are listed here when we are aware of prior formal publication of spectra in the scientific literature (please inform us of any we have missed), but many of the following spectra will not have been published elsewhere.
The Spectra
DMOX derivatives are not at their best with saturated fatty acids as the ions of higher mass tend to be of low intensity relative to the base ion, and troublesome rearrangement ions occur in greater abundance than with unsaturated fatty acid derivatives. However, they do at least give a usable molecular ion. This together with GC retention data is usually sufficient for definitive identification purposes, as the main source of confusion is likely to be with iso/anteiso-methyl branched analogues which elute separately. The mass spectrum of the DMOX derivative of palmitic acid (16:0) is illustrated first (Zhang et al., 1988).
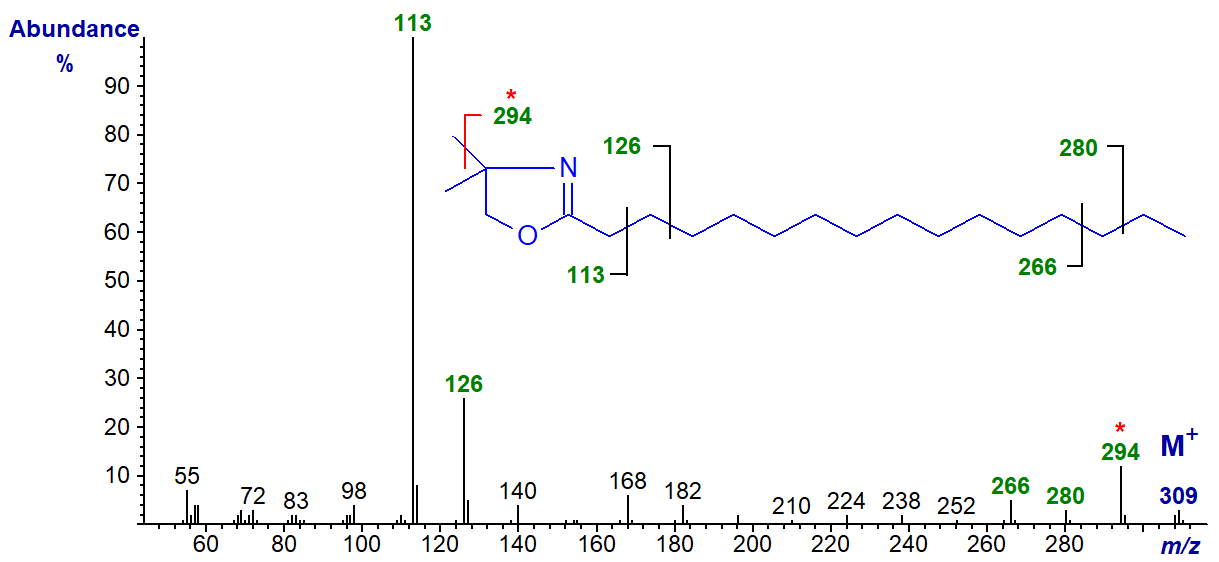
The McLafferty ion at m/z = 113 is the expected base ion (see the web pages on methyl esters of saturated fatty acids for a more general mechanistic discussion of this ion), and this is always an abundant ion in DMOX spectra except when there is some other functional moiety on carbon 2. It is usually accompanied by a prominent ion at m/z = 126. In general, the best approach to interpretation of the mass spectra of DMOX derivatives is to start with the molecular ion and work backwards. The molecular ion (m/z = 309) is small but recognizable in this instance, and then there is a gap of 15 amu to m/z = 294, followed sequentially by ions 14 amu apart (m/z = 280, 266, 252 and so forth), which can be considered simplistically as cleavage at successive methylene groups.
Studies with derivatives of isotopically labelled fatty acids have demonstrated that the ion representing [M‑15]+, at m/z = 294 in this instance, in the spectra of DMOX derivatives is derived mainly from loss of a methyl group from the ring and not from the terminal methyl group on the fatty acyl chain (Hamilton and Christie, 2000). For example, the spectrum of the DMOX derivative of 2H35-stearic acid (18:0) -
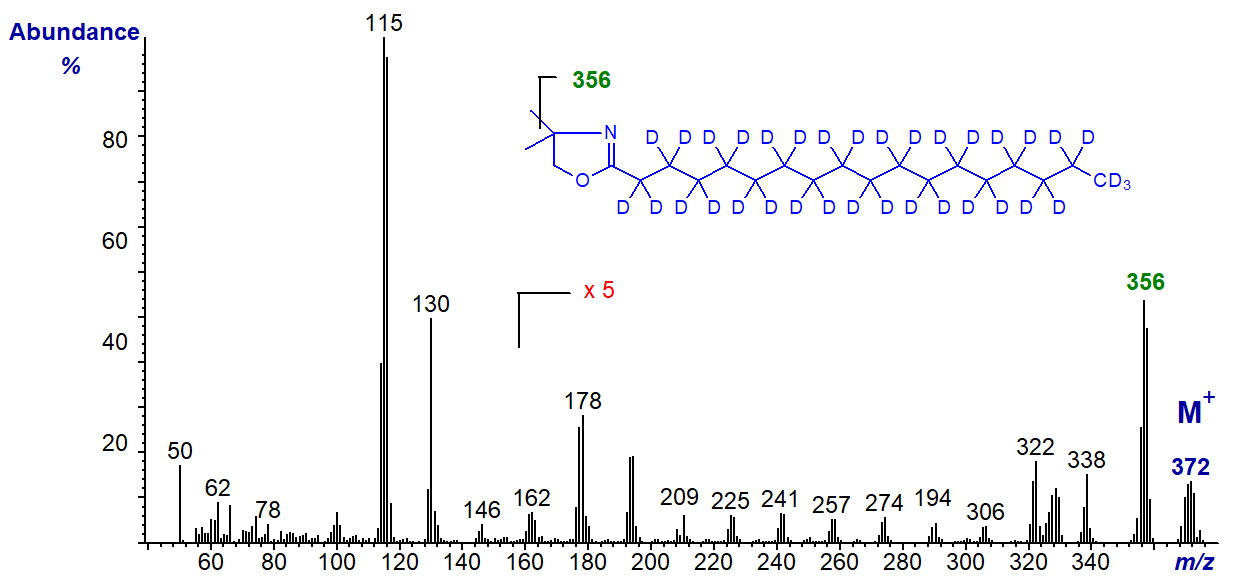
There is no ion representing loss of [M‑18]+ as might be expected for a terminal trideutero-carbon, but a substantial ion for [M‑15]+ at m/z = 356, which can only arise from the loss of a methyl group from the ring. This mechanistic feature is important in that it confounds the interpretation of the spectra of most fatty acids with terminal functional groups. Ions representing loss of 2 and 3 carbon units (carbons 2 to 4) in the high mass range can also cause problems of interpretation. The spectrum of the DMOX derivative of 2,2‑1H,2H33‑octadecanoate illustrated here.., produced from the latter by over-vigorous derivatization conditions, may be instructive (Hamilton and Christie, 2000).
This web document is intended as a practical guide to the interpretation of mass spectra as opposed to a mechanistic study, but some of these features can be seen in the spectrum of the DMOX derivative of 4,4-2H-palmitate -
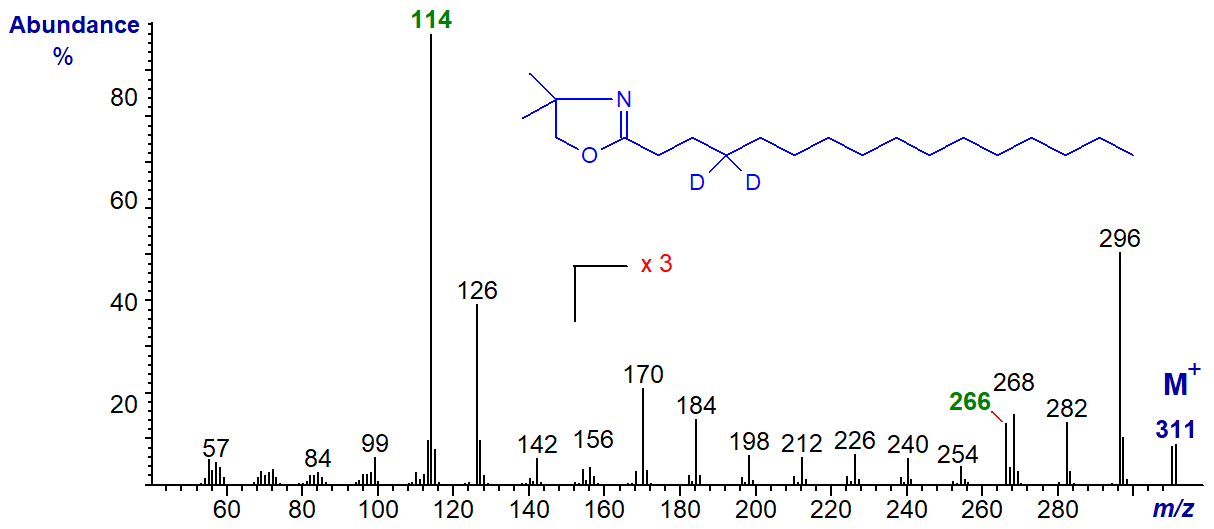
I have highlighted two significant ions; the McLafferty ion is now at m/z = 114 as its formation requires an interaction with the hydrogens/deuteriums on carbon 4, while that at m/z = 266 (M‑45]+) represents the expulsion of a fragment consisting of carbons 2 to 4 (formed with methyl esters). I will leave readers to do the work of any further interpretation, or to consult Hamilton and Christie (2000) or the mass spectra on file in the Archive section of these web pages for further derivatives labelled with stable isotopes. The origin of the ion at m/z = 126 may be of interest to those with mechanistic inclinations.
The mass spectra of the DMOX derivatives of all other saturated straight-chain fatty acids are then easy to understand and a few with a range of different chain lengths are now illustrated without further interpretation.
The mass spectrum of the DMOX derivative of hexanoate (6:0) -
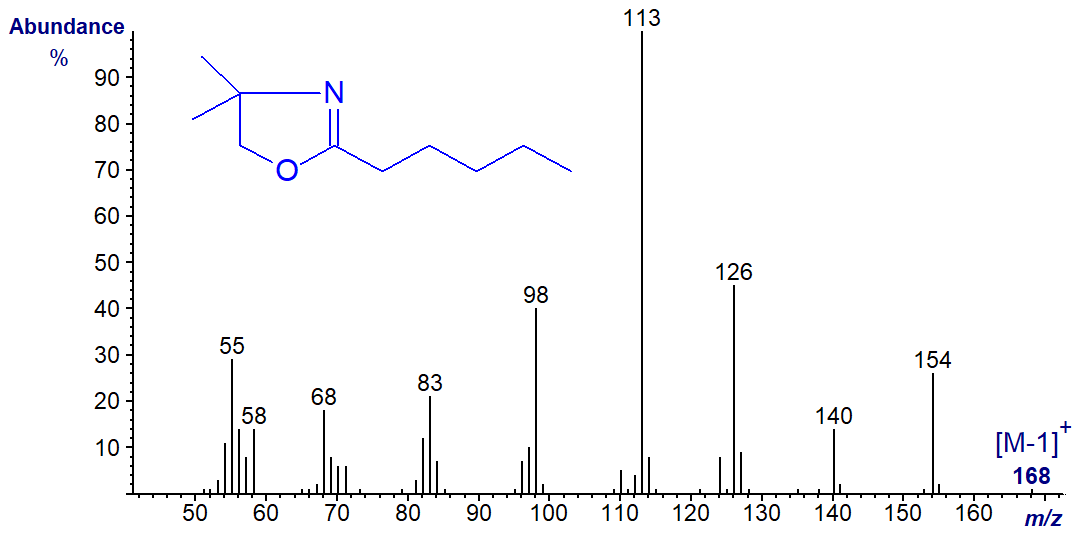
The mass spectrum of the DMOX derivative of dodecanoate (12:0) -
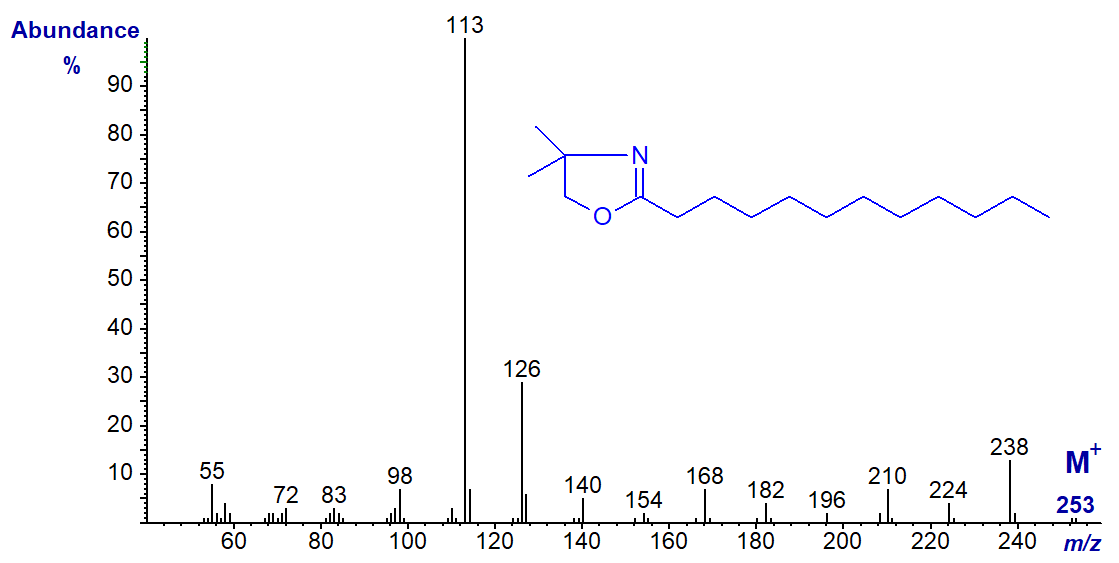
The mass spectrum of the DMOX derivative of docosanoate (22:0) -
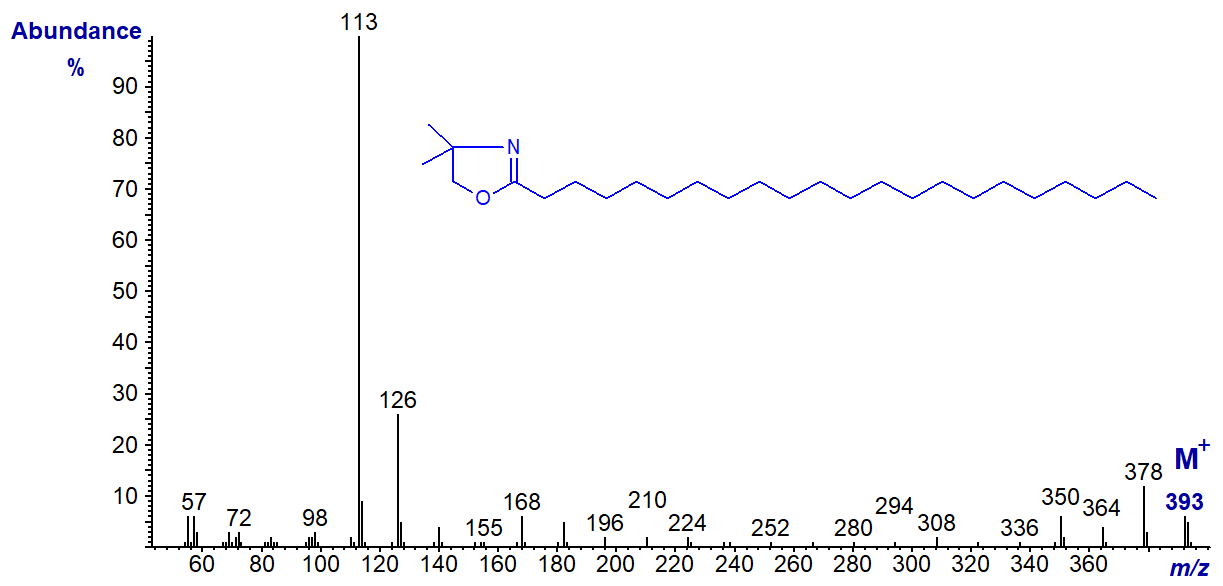
We have mass spectra on file for the DMOX derivatives of many more saturated fatty acids (6:0 to 30:0), which are illustrated without interpretation in the Archive section of these web pages.
References
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids, 33, 343-353 (1998); DOI.
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Spitzer, V. Structure analysis of fatty acids by gas chromatography - low resolution electron impact mass spectrometry of their 4,4-dimethyloxazoline derivatives - a review. Prog. Lipid Res., 35, 387-408 (1997); DOI.
- Zhang, J.Y., Yu, Q.T., Liu, B.N. and Huang, Z.H. Chemical modification in mass spectrometry IV. 2-Alkenyl-4,4-dimethyloxazolines as derivatives for double bond location of long-chain olefinic acids. Biomed. Environ. Mass Spectrom., 15, 33-44 (1988); DOI.
| © Author: William W. Christie |  |
|
| Updated: November 8th, 2023 | Contact/credits/disclaimer | |
