Mass Spectrometry of DMOX Derivatives
Monoenoic Fatty Acids
Straight-Chain C18 Monoenoic Fatty Acids
The mass spectra of DMOX derivatives of monoenoic fatty acids obtained under electron-impact ionization tend to be distinctive and permit facile location of the double bond, especially when it is located centrally. When the double bond is near either end of the molecule, interpretation of spectra can be more difficult, a task greatly simplified by publication of details of the mass spectra of all the positional isomers of octadecenoic acid (2- to 17-18:1) (Christie et al., 2000); these were synthesised by Gunstone and Ismail (1967). Here, the spectra of all these isomers are illustrated. To begin, the spectrum of the most common natural monoene, the DMOX derivative of octadec-9-enoate (oleate), is illustrated -
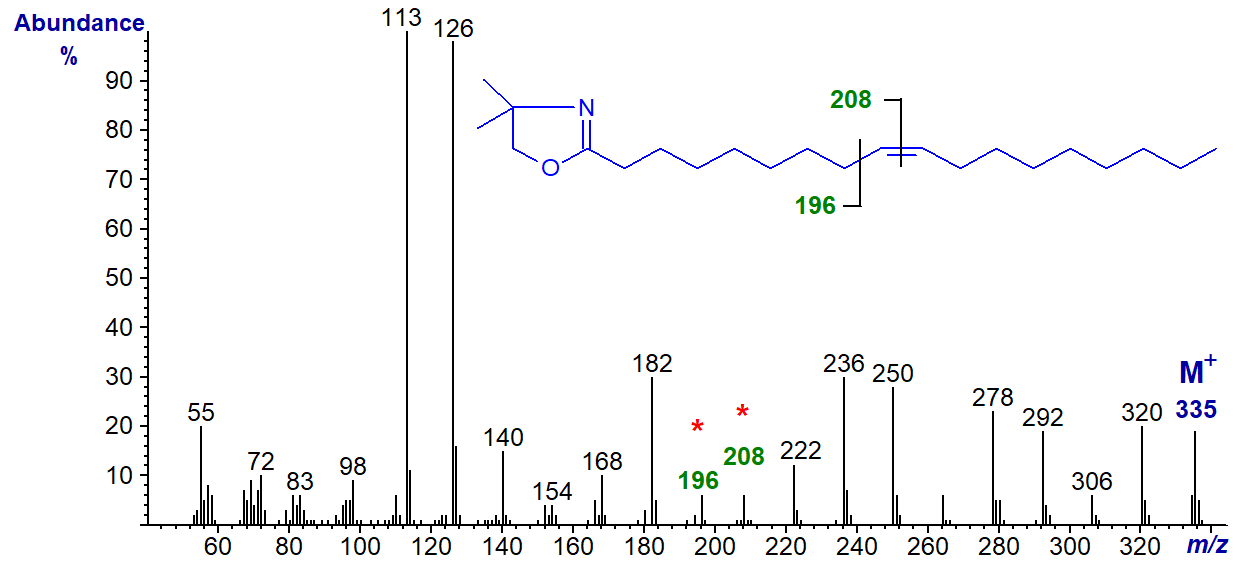
DMOX derivatives of unsaturated fatty acids tend to give more abundant ions in the higher mass range in comparison to saturated. Zhang et al. (1988) formulated an empirical rule:
"If a mass separation of 12 instead of the regular 14 amu is observed between two neighbouring even-mass homologous fragments containing n-1 and n carbon atoms of the original acid moiety, a double bond exists between carbons n and n+1 in the chain".
In this instance, the gap of 12 amu between m/z = 196 and 208 locates the double bond in position 9. It should be noted that this rule was first formulated for pyrrolidides, which have very similar mass spectra in spite of the structural differences (see the web page dealing with pyrrolidides of monoenoic fatty acids).
As more information has become available, it has become apparent that the above rule only applies from about position 6 onwards, but it is only really clear for the 8-18:1 to 15-18:1 isomers. Nonetheless, when the double bond is closer to the carboxyl group (or rather to the heterocyclic ring), each isomer gives distinctive fragmentation patterns or fingerprints so compounds can be identified by comparison with authentic spectra. Note: These fingerprints are invaluable for the location of the first double bond in polyunsaturated derivatives.
The two prominent ions at m/z = 236 and 250 are formed in rearrangements that give stable conjugated double bond systems, and these and the analogous ions for other isomers can be useful diagnostic indicators when fatty acids are not fully resolved by gas chromatography or when the background noise level is high.
DMOX derivative of 2-octadecenoate (2-18:1) -
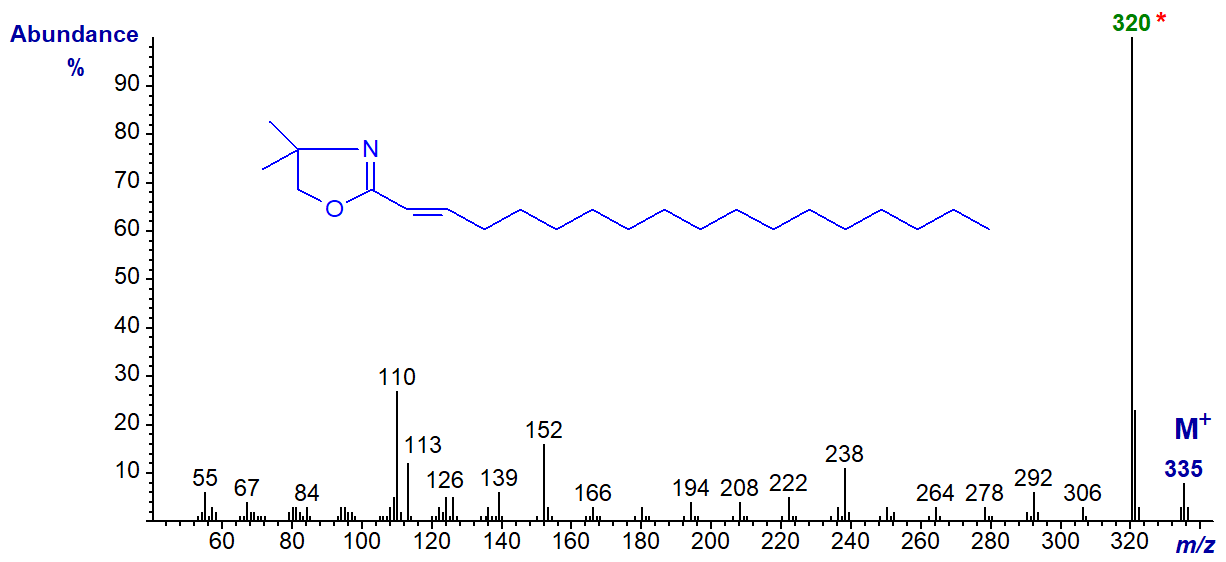
Note that the [M-15]+ ion (m/z = 320) is the base ion, while the usual ions at m/z = 113 and 126 are relatively inconspicuous. This spectrum differs appreciably from that published by Lamberto and Ackman (1995), and it now seems certain that they misidentified the spectrum of the DMOX derivative of 2-octadecenoate as the 3-isomer and vice versa. The error is understandable, as we now know that isomerization can occur very easily during the derivatization step if an inappropriate method is used (see - Christie et al. (2000) and Hamilton and Christie, 2000 or our web page on derivatization methods - for a full explanation including a mechanistic discussion). Similarly, a reported spectrum for the DMOX derivative of 3-decenoate (Luthria and Sprecher, 1993) is probably that of 2-decenoate having isomerized during derivatization.
DMOX derivative of 3-octadecenoate (3-18:1). The ions at m/z = 152 (the base ion) and 166 are the noteworthy components of a distinctive fingerprint. The mechanism for their formation is discussed in the paper by this author and colleagues cited above. It is my impression from limited practical experience that cis-3 double bonds are easily isomerized to position 2 during derivatization, but that this is less of a problem with trans-3 double bonds.
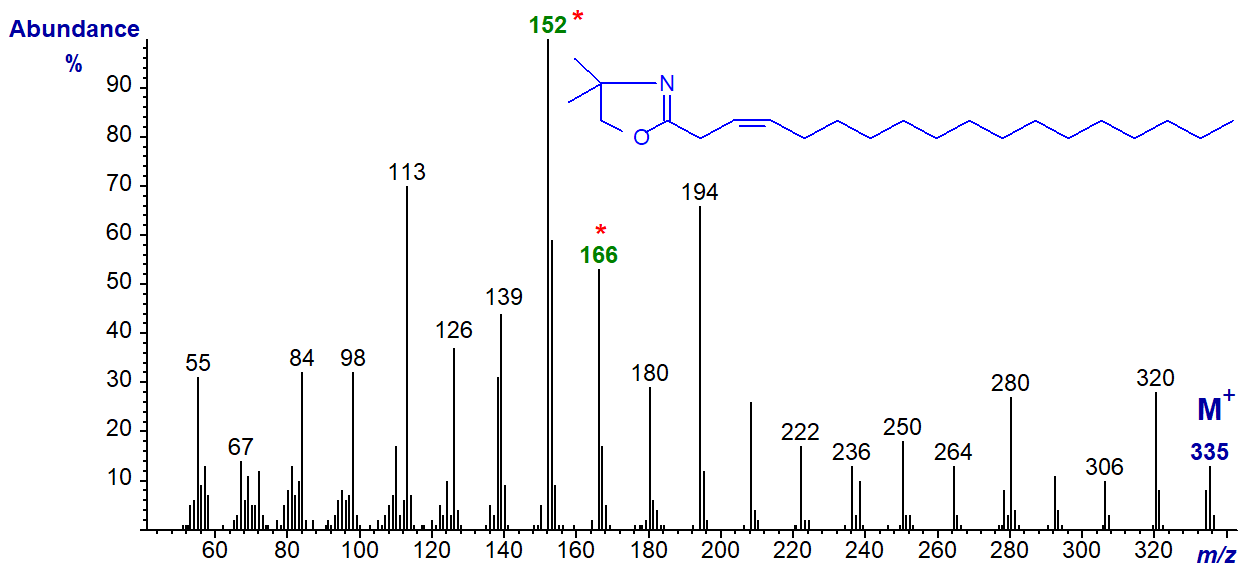
DMOX derivative of 4-octadecenoate (4-18:1) -
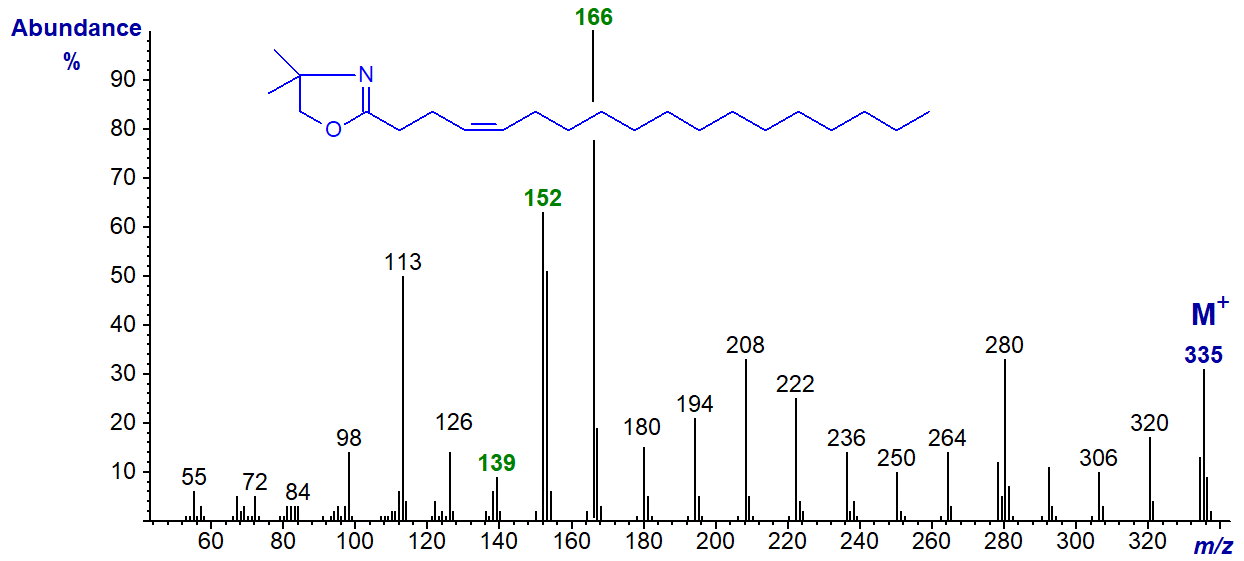
This spectrum is very similar to the previous with the abundant ions at m/z = 152 and 166 (the base ion) standing out, though with a reversed order of abundance. The only other distinctive feature is that the ions at m/z = 139, 180 and 194 are less abundant than with the 3‑isomer (see the archived spectrum of the DMOX derivative of 4-16:1 ).
DMOX derivative of 5-octadecenoate (5-18:1) -
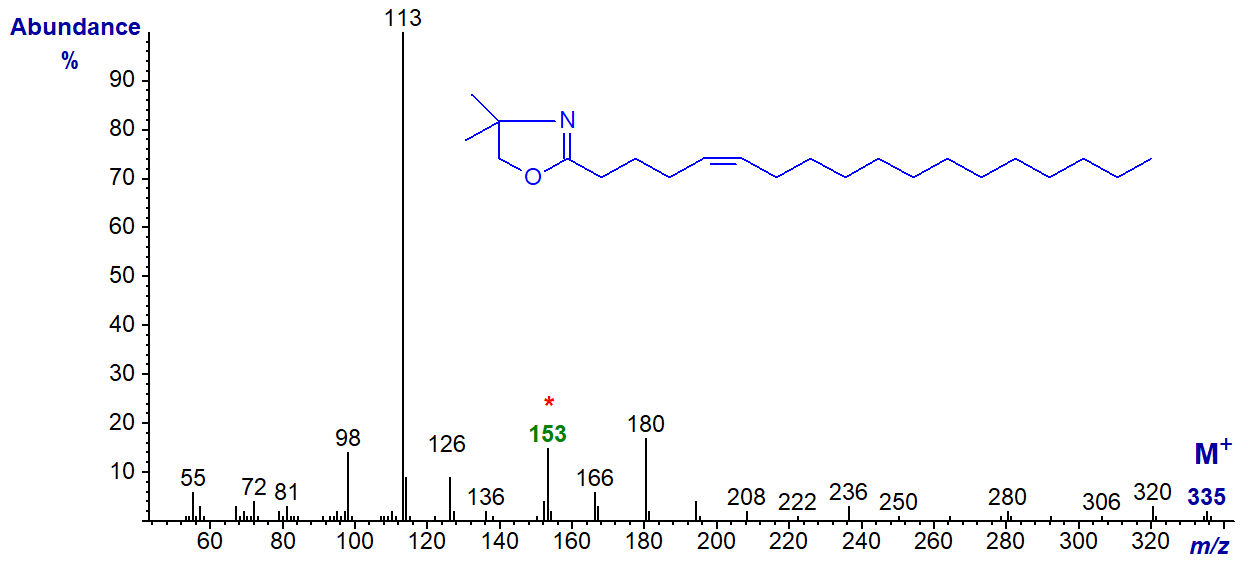
Here the base ion is the McLafferty ion at m/z = 113, but the odd-numbered ion at m/z = 153 is a useful diagnostic guide. With this and DMOX derivatives of other fatty acids with a double bond in position 5, the ions in the higher mass range always appear to be of low abundance in relation to the base ion at m/z = 113 (cf., the spectrum of 5-16:1 in the Archive)
DMOX derivative of 6-octadecenoate (6-18:1) (Zhang et al., 1988) -
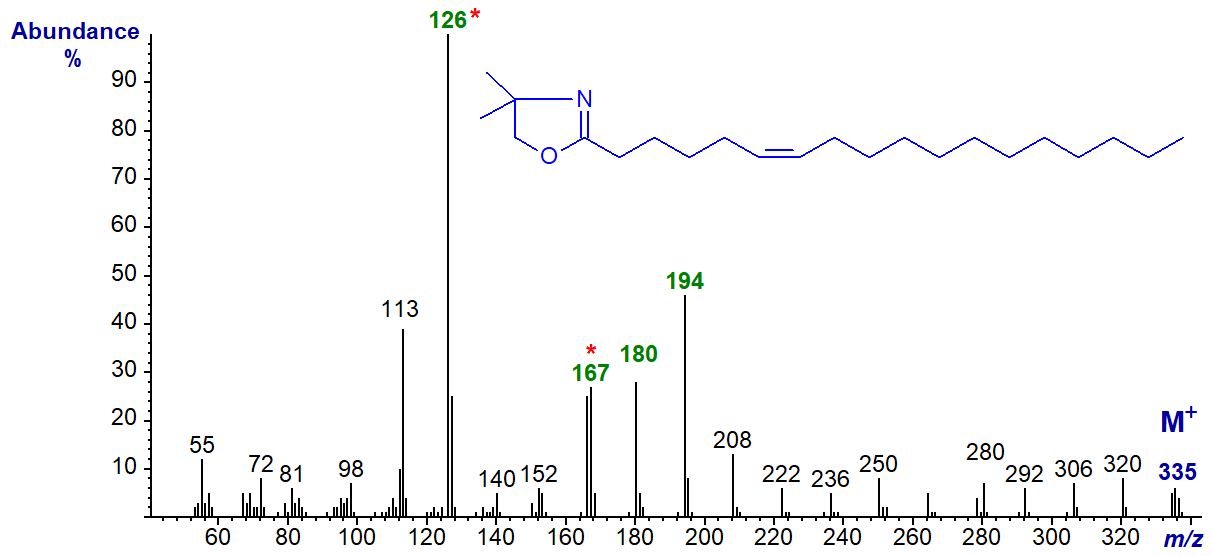
Here the base ion is at m/z = 126 (as opposed to 113 when the double bond is in position 5). This appears to be a constant feature for double bonds in positions 6 to 8, so can be of diagnostic value. In this instance, the odd-numbered ion at m/z = 167 (or the triplet at 167, 180 and 194) is a further guide to location of the double bond in position 6. See the spectrum of the DMOX derivative of 6-16:1 in the Archive web pages.
DMOX derivative of 7-octadecenoate (7-18:1)-
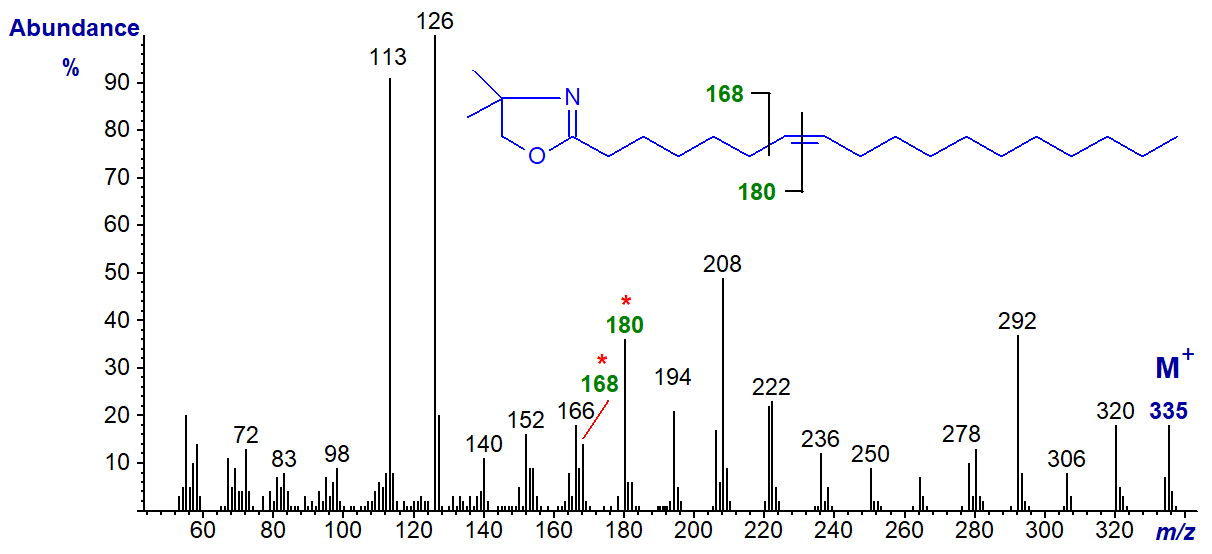
It requires some imagination to see the gap of 12 amu for the double bond in position 7 between m/z = 168 and 182, but the fingerprint spectrum is distinctive.
The diagnostic gap of 12 amu is as expected in the spectra of the following isomers until the double bonds are near the terminal end of the molecule. These are illustrated without further comment, although the diagnostic ions are highlighted. DMOX derivative of 8‑octadecenoate (8-18:1) -
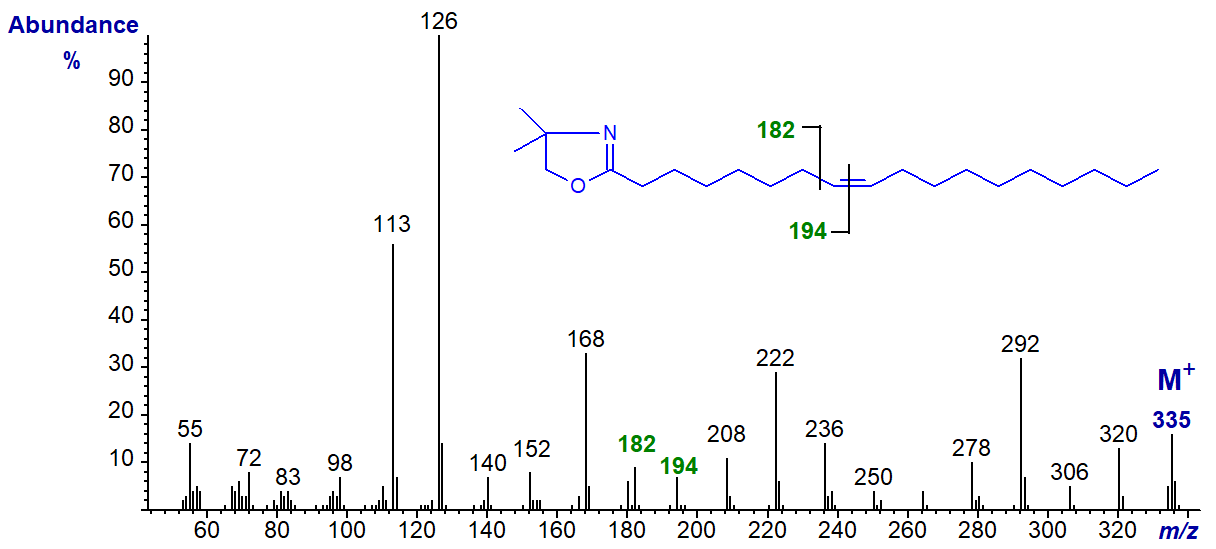
DMOX of 9-octadecenoate (9-18:1) - see top of this web page.
DMOX derivative of 10-octadecenoate (10-18:1) -
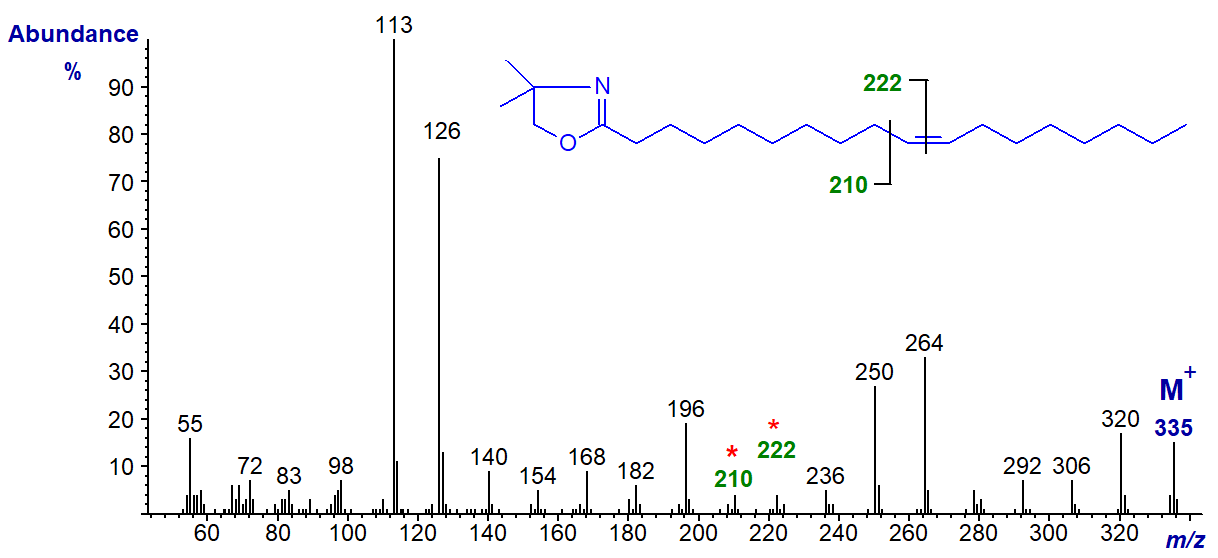
DMOX derivative of 11-octadecenoate (11-18:1) (see Zhang et al. (1988)) -
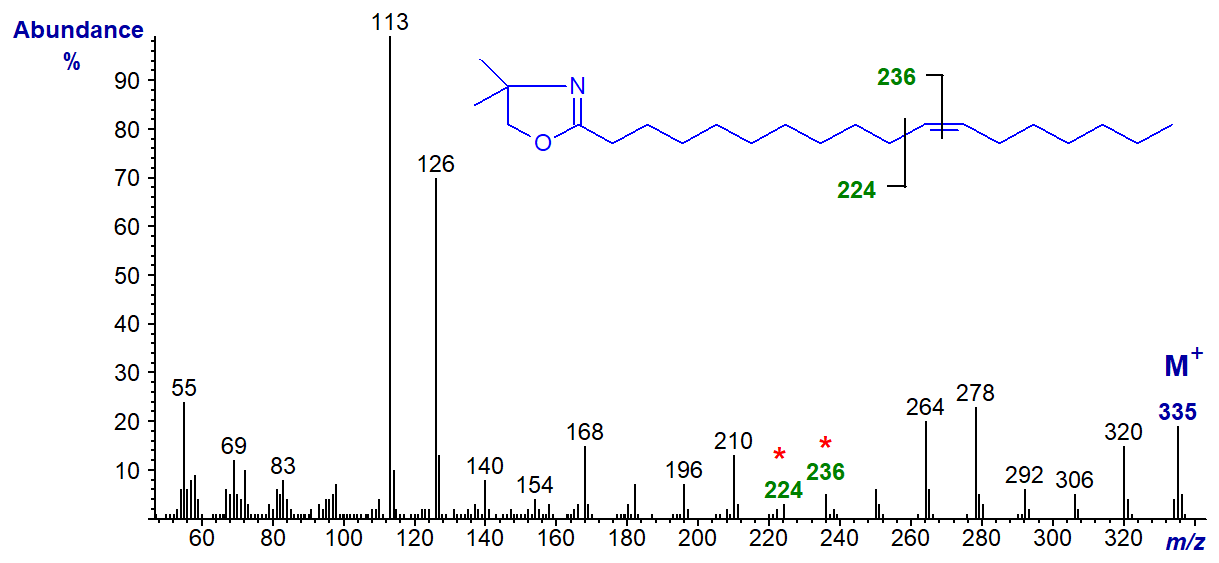
DMOX derivative of 12-octadecenoate (12-18:1) -
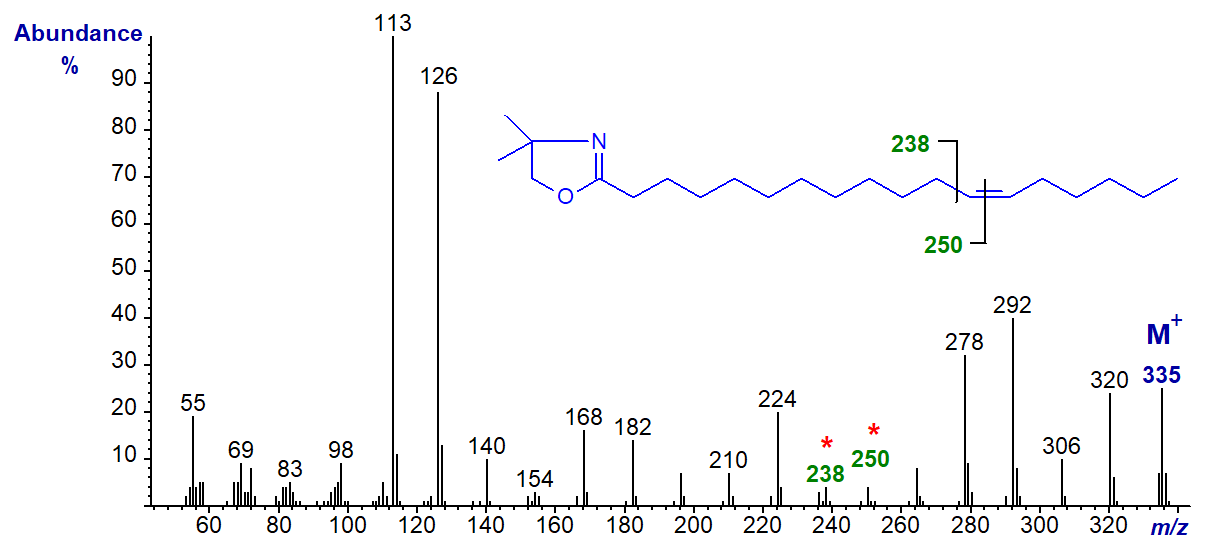
DMOX derivative of 13-octadecenoate (13-18:1) (see Zhang et al. (1988)) -
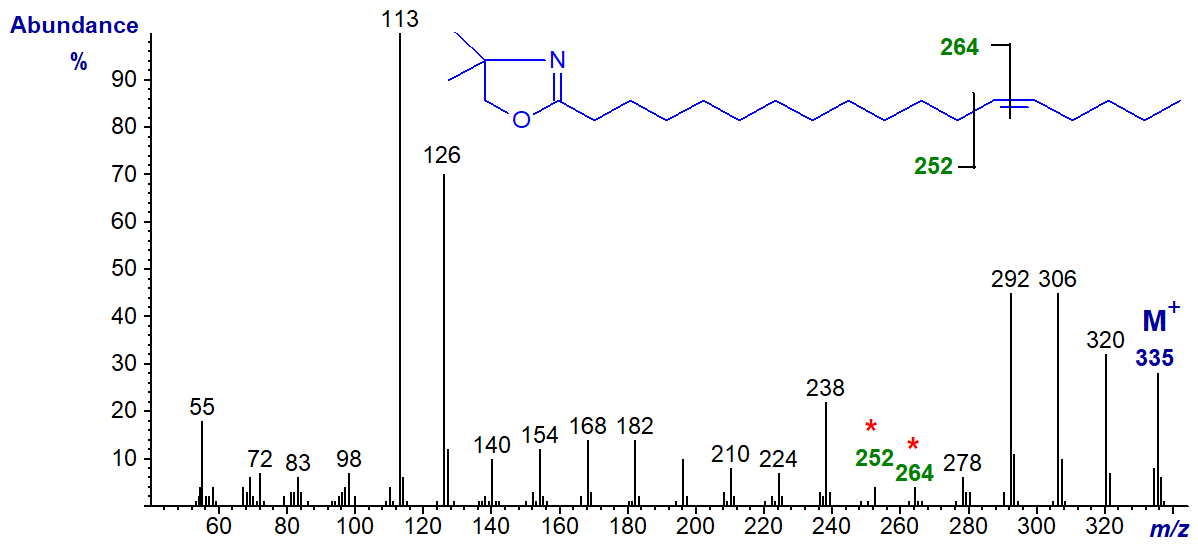
DMOX derivative of 14-octadecenoate (14-18:1) -
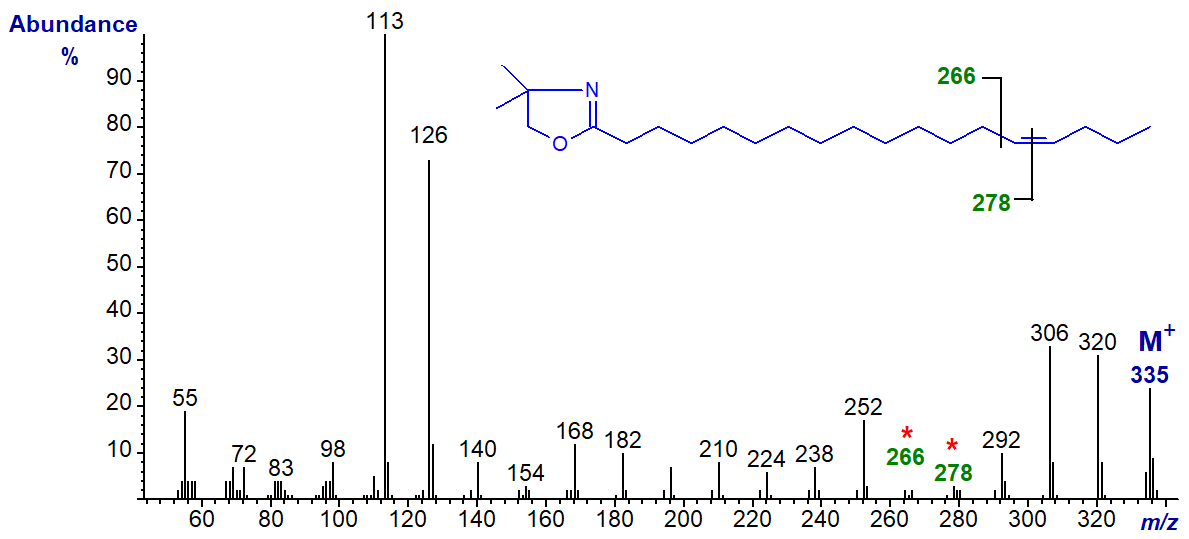
Note that although the ions for cleavage of the double bond (gap of 12 amu) are becoming less distinct, the two prominent ions at m/z = 306 and 320 in this example, formed in rearrangements that give stable conjugated double bond systems, remain useful diagnostic indicators.
DMOX derivative of 15-octadecenoate (15-18:1) -
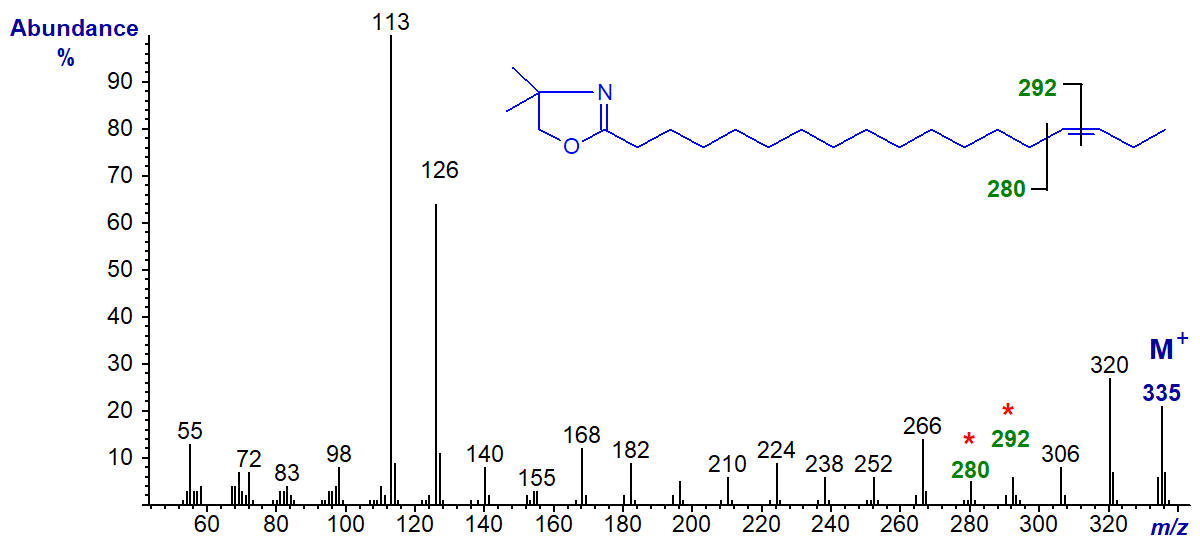
DMOX derivative of 16-octadecenoate (16-18:1) -
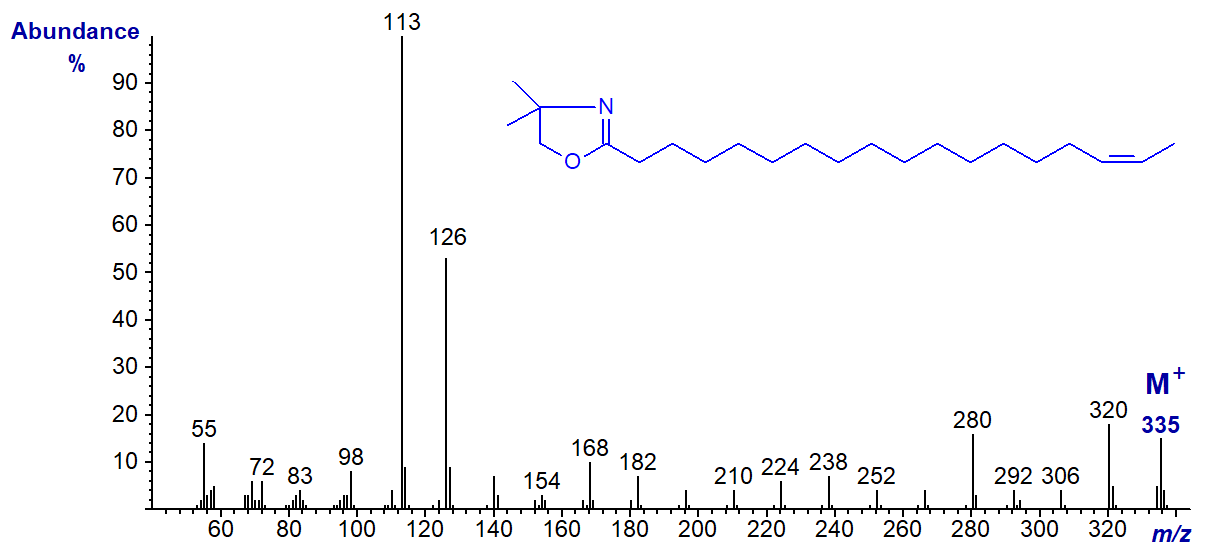
In this instance, although the rule to locate the gap of 12 amu may still operate, it is disguised by other fragmentations, and it would be easy to interpret this spectrum incorrectly as that of the 15-isomer.
DMOX derivative of 17-octadecenoate (17-18:1) -
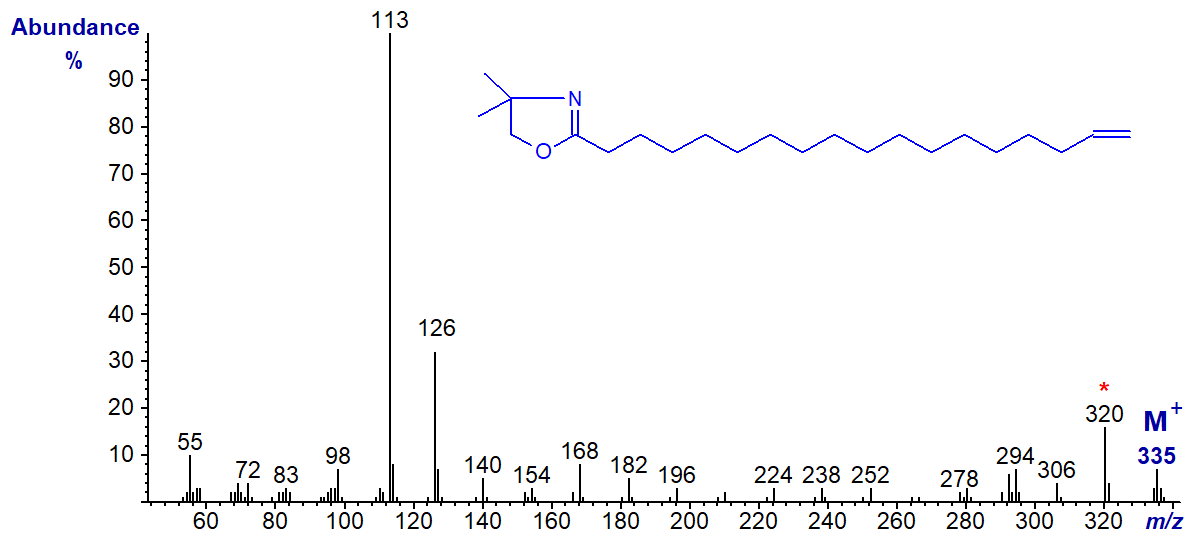
The [M-15]+ ion (m/z = 320), representing loss of a methyl group from the heterocyclic ring (Hamilton and Christie, 2000), is the most abundant ion in the higher mass range adding confusion to the ions that might locate the double bond. However, both this and the previous isomer give distinctive fingerprints for comparison with spectra of appropriate standards.
Straight-chain Monoenoic Fatty Acids of Other Chain-Lengths
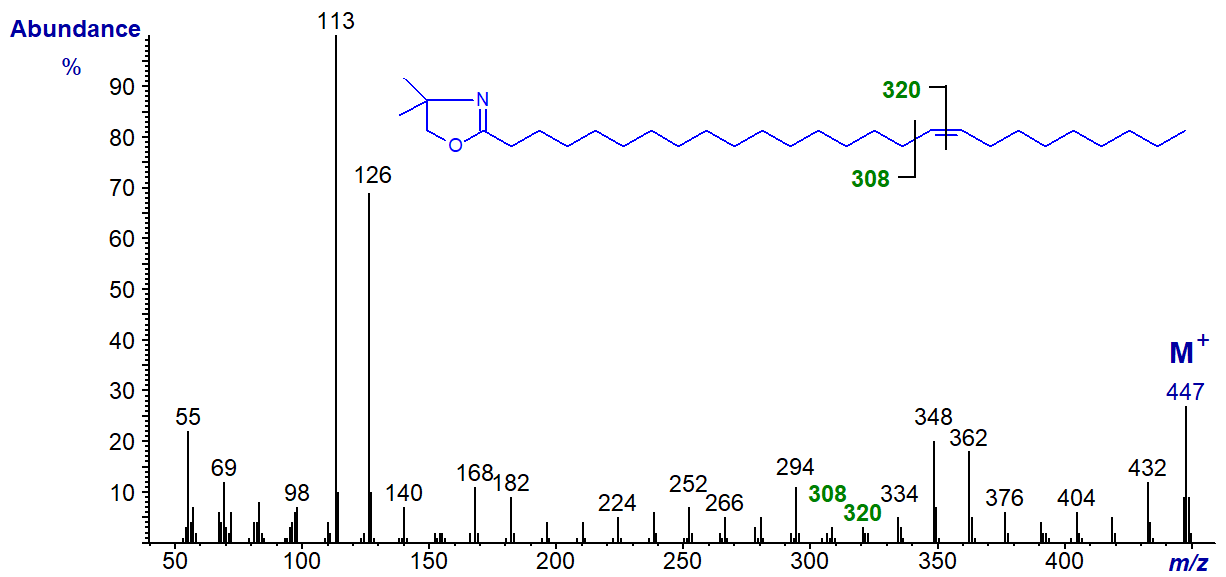
The same features are seen in the spectra of all monoenes regardless of the chain-length, but the spectrum of the DMOX derivative of 9‑decenoate (9-10:1) would be expected to be similar to that of an isomer with a terminal double bond, while that of 9‑docenoate (9‑12:1) has the expected fragmentations for a double bond in a more central position 9 (cf., the spectrum of the oleate derivative). The spectrum of the DMOX derivative of 17-hexacosenoate (17-26:1) does not resemble that of 17-18:1 above in which the double bond is terminal but has the expected gap of 12 amu between m/z = 308 and 320 for a double bond in a central position. These three spectra are illustrated below without further comment.
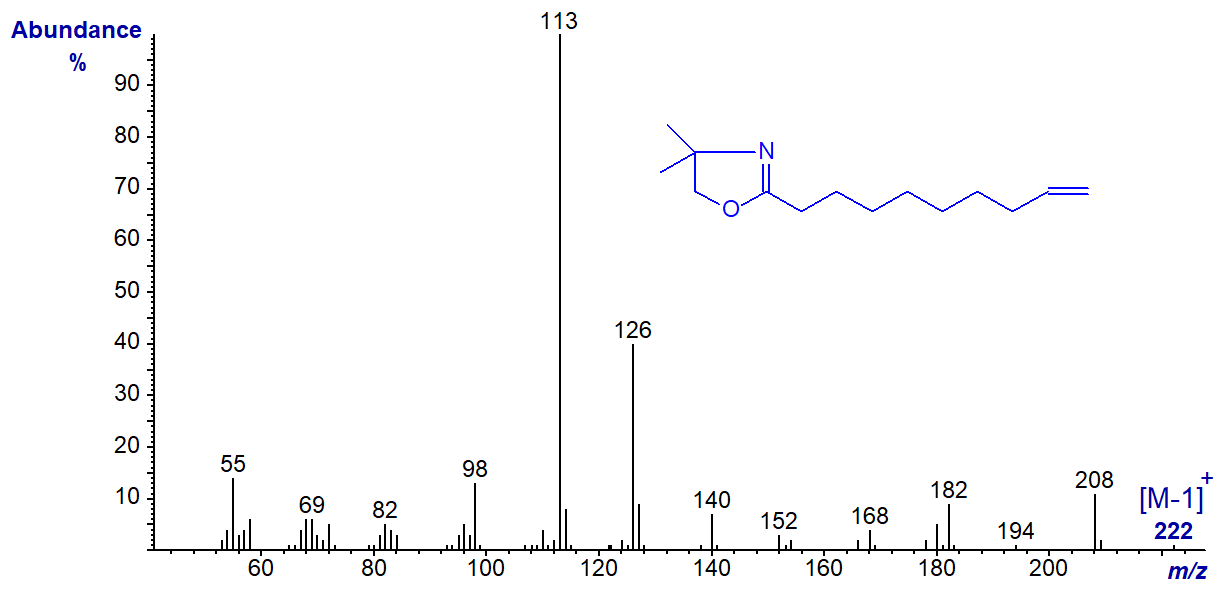
DMOX derivative of 9-decenoate (9-10:1) -
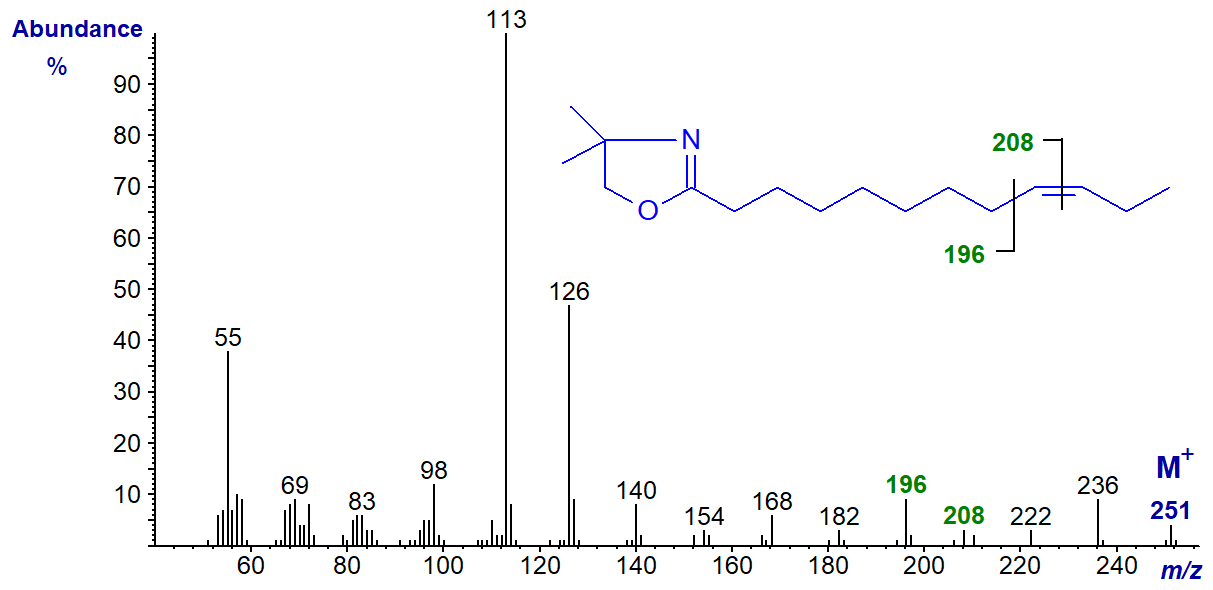
DMOX derivative of 9-dodecenoate (9-12:1) -
DMOX derivative of 17-hexacosenoate (17-26:1) -
We have unpublished mass spectra of the DMOX derivatives of many more monoenoic fatty acids with a range of chain-lengths, and they are available in the Archive section of these web pages, but without interpretation.
Branched-Chain Monoenoic Fatty Acids
We have mass spectra of the DMOX derivatives of two methyl-branched monoenoic fatty acids, starting with that of 15-methyl-hexadec-9-enoate found in a sponge -
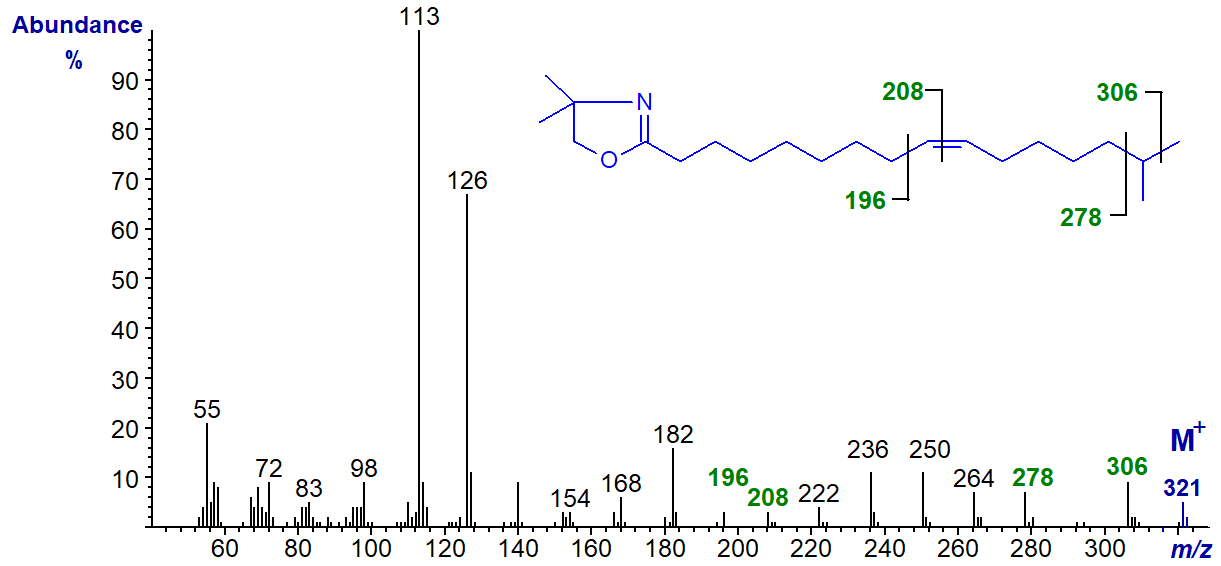
The double bond position is determined by the interval of 12 amu between m/z = 196 and 208, while the branch point is revealed by the gap of 28 amu between m/z = 278 and 306 for the loss of carbon 15 and its methyl group. It is noteworthy that an iso-methyl group is not easily distinguished with saturated branched-chain fatty acids in this way with DMOX derivatives.
The DMOX derivative of 7-methyl-hexadec-6-enoate -
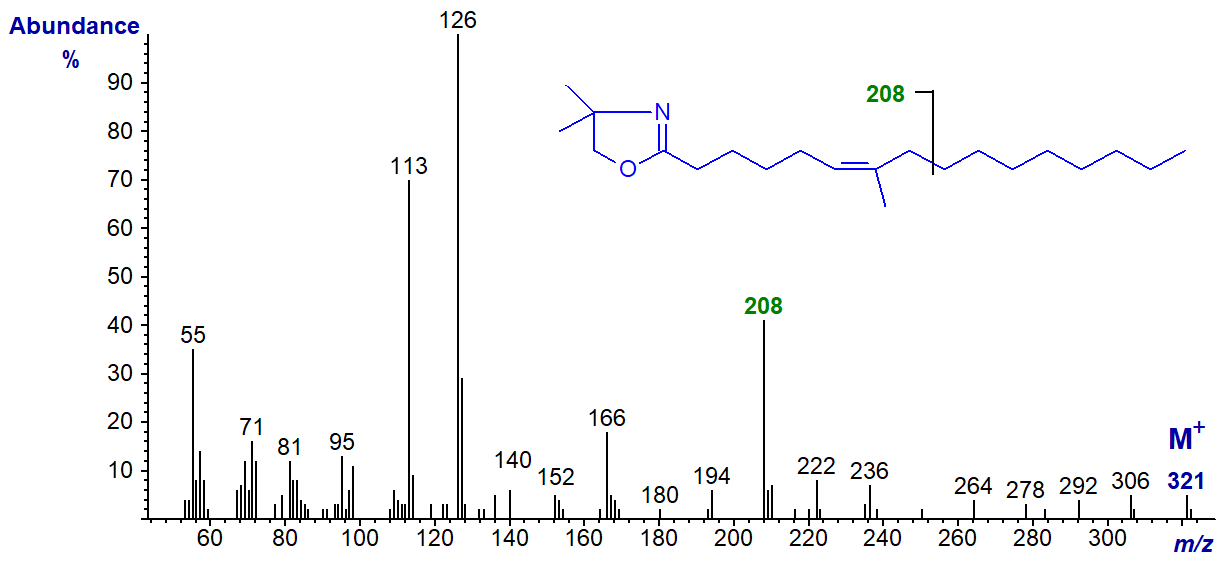
From its mass spectrum alone, we would not have been confident of the identification of this fatty acid from fish and other marine organisms from tropical seas, if we had not had additional information from another source. Presumably the ion at m/z = 208 is from cleavage between carbons 9 and 10 of the chain, beta to the double bond and methyl branch, as illustrated. The presence of the methyl group on carbon 7 distorts the fingerprint we expect for a double bond in position 6, although the most abundant ion is at m/z = 126 as with 6-18:1 above.
References
- Christie, W.W., Robertson, G.W., McRoberts, W.C. and Hamilton, J.T.G. Mass spectrometry of the 4,4-dimethyloxazoline derivatives of isomeric octadecenoates (monoenes). Eur. J. Lipid Sci. Technol., 102, 23-29 (2000); DOI.
- Gunstone, F.D. and Ismail, I.A. Fatty acids. Part 13. The synthesis of all the cis n-octadecenoic acids. Chem. Phys. Lipids, 1, 209-224 (1967); DOI.
- Hamilton, J.T.G. and Christie, W.W. Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4,4-dimethyloxazoline derivatives of fatty acids. Chem. Phys. Lipids, 105, 93-104 (2000); DOI.
- Lamberto, M. and Ackman, R.G. Positional isomerization of trans-3-hexadecenoic acid employing 2-amino-2-methylpropanol as a derivatizing agent for double bond location by gas-chromatography/mass spectrometry. Anal. Biochem., 230, 224-228 (1995); DOI.
- Luthria, D.L. and Sprecher, H. 2-Alkenyl-4,4-dimethyloxazolines as derivatives for the structural elucidation of isomeric unsaturated fatty acids. Lipids, 28, 561-564 (1993); DOI.
- Zhang, J.Y., Yu, Q.T., Liu, B.N. and Huang, Z.H. Chemical modification in mass spectrometry IV. 2-Alkenyl-4,4-dimethyloxazolines as derivatives for double bond location of long-chain olefinic acids. Biomed. Environ. Mass Spectrom., 15, 33-44 (1988); DOI.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
