Mass Spectrometry of Methyl Esters
Derivatization of Double Bonds in Fatty Acids
for Structural Analysis
Sometimes it is necessary or helpful to derivatize methyl esters of fatty acids further by reaction at the double bonds to locate these and other structural features by GC-MS. The useful techniques of hydrogenation and deuteration are described below, together with the preparation and mass spectral properties of dimethyl disulfide (DMDS) and 4-methyl-triazoline-3,5-dione (MTAD) adducts. Information on preparation of methyl esters of fatty acids for mass spectrometry with suitable protocols is available on its own web page. The methods that follow are valuable complementary techniques and not merely alternatives to the use of the nitrogen-containing derivatives (3‑pyridylcarbinol esters, pyrrolidides, DMOX), preparation of which is described here...
Hydrogenation
Catalytic hydrogenation is a simple procedure that provides invaluable structural information regarding fatty acid identity when combined with GC or GC-MS analysis. It is best carried out with methyl esters, but then there may sometimes be advantages in subsequent conversion to 3-pyridylcarbinol ester or DMOX derivatives for mass spectrometric characterization, e.g., to locate branch-points or ring structures.
Some needlessly complex procedures involving high pressures and temperatures are sometimes described in the literature, but the following method is both practical and convenient [1].
| Laboratory protocol: The unsaturated ester (1-2 mg) in a test-tube is dissolved in methanol (1 mL) and Adams' catalyst (platinum oxide; 1 mg) is added. The tube is connected via a two-way tap both to a reservoir of hydrogen (e.g., in a balloon or football bladder) at or just above atmospheric pressure and to a vacuum pump. The tube is alternatively evacuated and flushed with hydrogen several times to remove any air, after which it is shaken vigorously while an atmosphere of hydrogen at a slight positive pressure is maintained for 2 hr. Then, the hydrogen supply is disconnected, the tube is flushed with nitrogen and the solution is filtered to remove the catalyst. The solvent is evaporated in a stream of nitrogen or under reduced pressure, and the required saturated ester is taken up in hexane or diethyl ether for GC analysis. |
At its simplest, hydrogenation is used merely to determine the chain length of components. By eliminating all unsaturated centres in fatty acid methyl esters from most samples of natural origin, a simple set of peaks is obtained for the saturated even-numbered homologous series on GC analysis, and these can be compared with authentic standards. Any anomalous peaks may be an indication of novel structures, and in samples of animal origin, small amounts of odd-chain fatty acids may be detected in the GC trace, together with methyl-branched fatty acids, usually iso- closely followed by anteiso-isomers, which are best identified as the 3‑pyridylcarbinol ester or pyrrolidide derivatives.
Deuteration
While hydrogenation eliminates unsaturation and aids identification and location of other functional groups, selective deuteration will assist both in locating double and triple bonds and characterizing other moieties in the aliphatic chain. Indeed, deuteration has been used since the early days of mass spectrometry of lipids as a means of locating double bonds and for unravelling fragmentation mechanisms, but the value of the procedure for structure determinations is limited with methyl ester derivatives as the wide range of rearrangement ions formed during mass spectrometry leads to some scrambling of the deuterium atoms in the alkyl chain. However, by using nitrogen-containing derivatives, which give clean radical-induced fragmentations with minimal rearrangement, most such problems have been eliminated. Again, the reaction is best carried out with methyl esters prior to conversion to 3-pyridylcarbinol ester or DMOX derivatives for mass spectrometry. Methodology of the kind described above has enabled us to identify many different fatty acids of marine, plant and animal origins.
 |
| Figure 1. Deuteration with Wilkinson's catalyst - monoene. |
Deuteration with deuterium gas and Wilkinson's catalyst (tris(triphenylphosphine)-rhodium(I) chloride) is employed for the purpose. Gaseous deuterium is available commercially in small cylinders, or it can be generated in situ by reaction of deuterium chloride with sodium borodeuteride. It is essential to have a good excess of deuterium so that the reaction goes rapidly to completion, otherwise some isomerization of the double bonds and scrambling of the hydrogen atoms is possible (author, unpublished). The following method is based on that of Dickens et al. [2].
| Laboratory protocol: Methyl esters of unsaturated fatty acids are subjected to deuteration with deuterium gas and Wilkinson's catalyst. The fatty ester (up to 2 mg) and Wilkinson's catalyst (5 mg) in dioxane (1 ml) are degassed with helium in a tube fitted with a septum. The vessel is purged with five volumes of deuterium with constant stirring and then is left with an atmosphere of deuterium at 60°C for 2hr. The solvent is removed in a stream of nitrogen and the required ester is obtained by adsorption chromatography on a small column of Florisil™ (0.5 g), eluted with hexane-acetone (96:4, v/v). |
The technique was first used in conjunction with pyrrolidide derivatives and has been used with DMOX derivatives, but 3‑pyridylcarbinol esters appear best for the purpose as they give cleaner radical-induced fragmentations with fewer rearrangement ions and more abundant ions of high molecular weight with saturated fatty acids. On mass spectral analysis, clear diagnostic ion fragments are obtained that permit the determination of the positions of the original double bonds in the alkyl chain.
To illustrate this, an unusual fatty acid with two double bonds and a triple bond in conjugation in the seed oil of Tanacetum corymbosum was identified as octadeca-8,10-dien-12-ynoic acid by deuteration of the methyl ester derivative prior to conversion to the 3‑pyridylcarbinol ester for analysis by GC-MS [3]. The spectrum is -
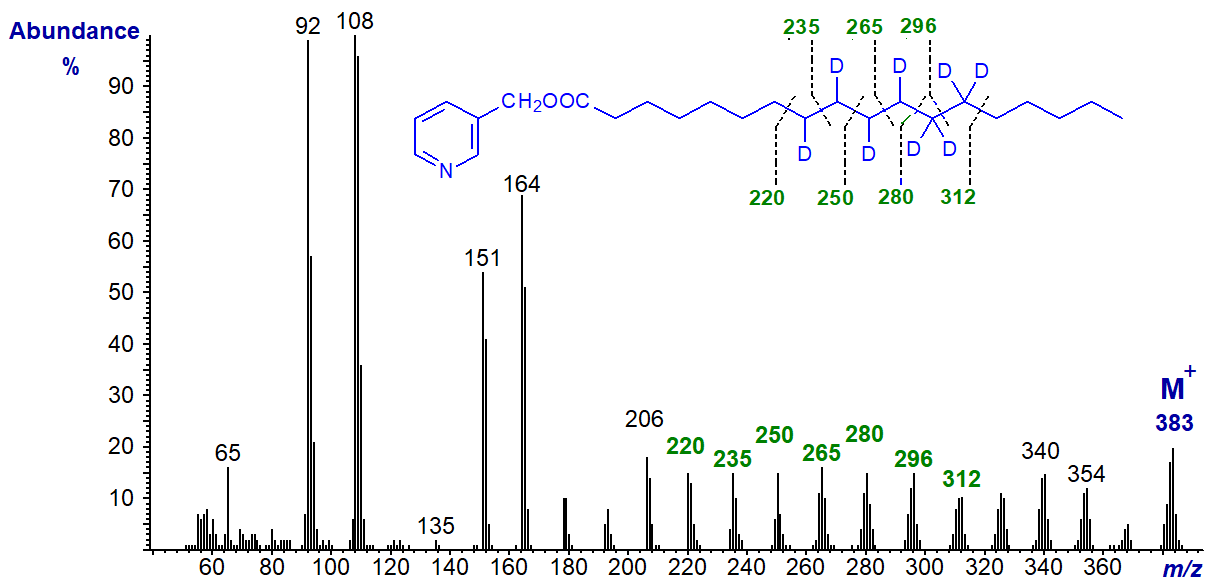
Mass spectra of 3-pyridylcarbinol esters are described elsewhere in these web pages, and it must suffice for the moment to point out that one deuterium atom is added to each carbon of the double bonds and two to each carbon of the triple bonds; these are easily identified from the mass spectrum. Thus, there are gaps of 15 amu instead of 14 amu for carbon atoms with one deuterium atom (m/z = 220 to 235 to 250 to 265 to 280), then of 16 amu for carbons with two deuterium atoms (m/z = 280 to 296 to 312). A further example of the methodology was the proof of structure of 12‑oxo‑octadec-9-enoic acid from milk fat [4] and discussed on this website here....
Dimethyl Disulfide Adducts
Monoenoic fatty acids: To get round the problem of locating double bonds, it is possible to prepare specific derivatives of unsaturated fatty acids that ‘fix’ the double bond. The most useful of these for monoenes are the dimethyl disulfide adducts, as they have excellent mass spectrometric properties and are prepared in a simple one-pot reaction [5]. A number of interesting publications describing its use have appeared, and these have been detailed in a substantial review [6] (a comprehensive list of relevant references is available here...). Indeed, there have been more than 100 publications using aspects of this method for fatty acids alone, and many more for pheromones, hydrocarbons, terpenes and others. The adduct adds substantially to the molecular weight of the original ester, and it tends to elute at a temperature about 40°C higher than the latter from a GC column containing a non-polar silicone phase.
 |
| Figure 3. The reaction of dimethyl disulfide with a monoenoic fatty acid derivative. |
Adduct formation has been shown to be entirely stereospecific, presumably by trans addition, so that threo- and erythro-derivatives are formed from cis- and trans-isomers, respectively. Although the different geometrical isomers have indistinguishable mass spectra, they are eluted separately from GC columns with that derived from the cis-isomer eluting first. Where appropriate standards are available, the spectrum together with the retention time can therefore be used to determine the geometry of double bonds, and there is a potential to use this as a method for determining total trans monoenoic fatty acids in samples. The most widely used preparation procedure is outlined below (although a more rapid method has been described [7]).
| Laboratory protocol: The monoenes (1 mg) are dissolved in dimethyl disulfide (0.2 mL), and a solution (0.05 mL) of iodine in diethyl ether (60 mg/mL) is added. The mixture is stirred for 24 hours, then hexane (5 mL) is added, and the mixture is washed with dilute sodium thiosulfate solution, dried over anhydrous sodium sulfate, and the solvent evaporated. The product is taken up in fresh hexane for injection directly onto the GC column. |
The mass spectrum of the dimethyl disulfide adduct of methyl oleate is illustrated first -
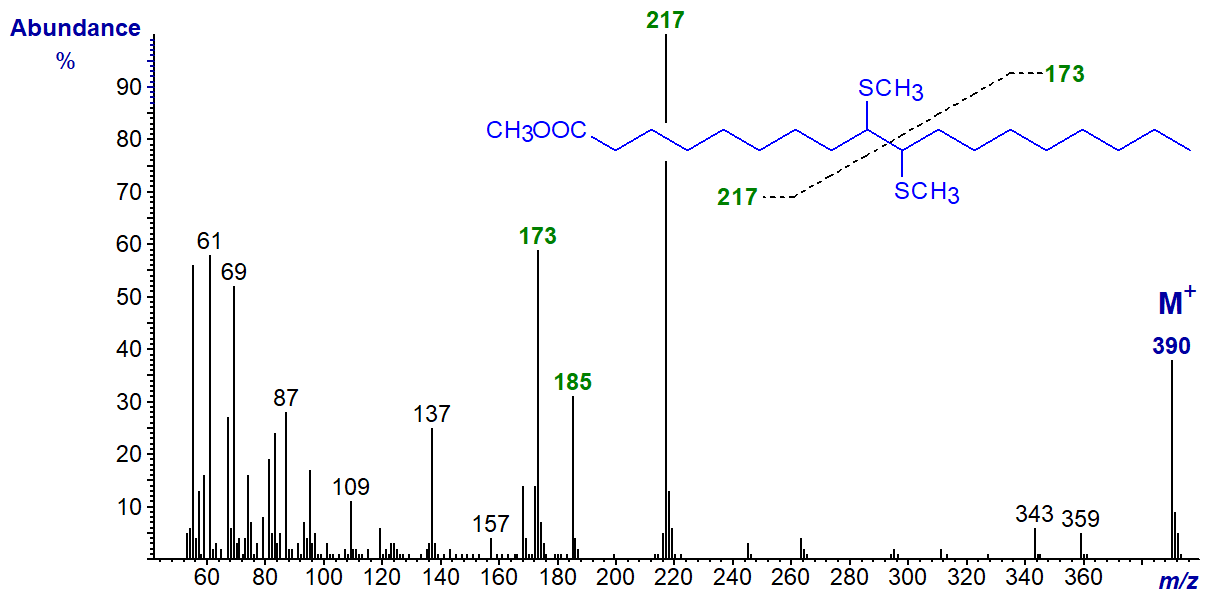
Cleavage occurs between the carbons that originally constituted the double bond to yield two substantial fragment ions, i.e., that containing the terminal methyl part of the molecule at m/z = 173 and that with the carboxyl group at m/z = 217. A further prominent ion at m/z = 185 corresponds to the latter fragment with the loss of the elements of methanol from the carboxyl group.
The mass spectrum of the dimethyl disulfide adduct of methyl 11-octadecenoate (cis-vaccenate) follows -
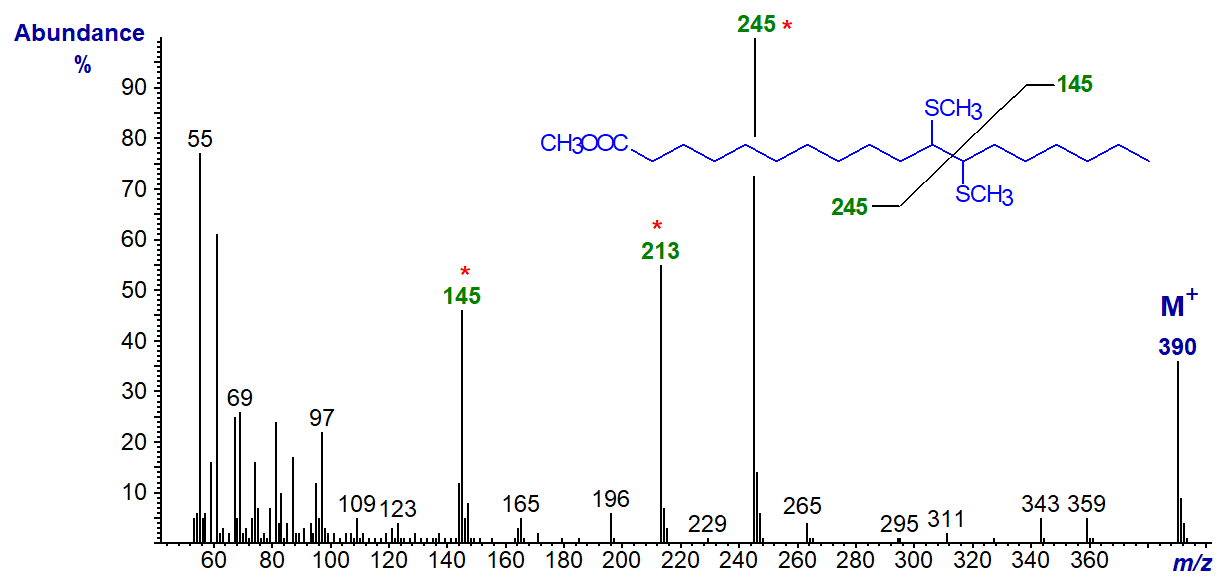
The spectrum differs from the previous one in that the diagnostic ions are shifted by 28 amu as expected.
Positional isomers as DMDS adducts can be resolved better than the unchanged esters on GC columns, and this property has been used to separate and quantify petroselinic, oleic and vaccenic acids in seed oils [8]. While simpler alternatives may be available for this specific purpose, it may be worth keeping it in mind for confirmation or for other difficult analyses. It has been used successfully for hydroxy, methoxy, branched-chain and benzene ring-containing fatty acids with one double bond in the aliphatic chain, and for ether lipids.
Dienoic fatty acids present more of a problem than monoenes for the technique. The considerable increase in molecular weight means that rather high temperatures are required for GC analysis, and when the two double bonds are in proximity, complications can arise in the reaction with dimethyl disulfide. There is no problem when double bonds are separated by more than four carbon atoms, although this is a relatively rare occurrence in nature, and 9,15-octadecadienoic acid (with four methylene groups between the double bonds) from mango pulp was characterized simply as the bis-DMDS derivative [9]. Similar types of dienoic fatty acids have been characterized from sponges in the same way mainly in the laboratory of Néstor M. Carballeira.
When the double bonds are closer together, a variety of products is possible. Most natural dienoic fatty acids have methylene-interrupted double bonds, and when dimethyl disulfide was reacted under mild conditions (30 minutes reaction, 35°C) with methyl linoleate, only one double bond reacted [10]. An equimolar mixture of methyl 9,10‑bis(methylthio)octadec-12-enoate and methyl 12,13-bis(methylthio)octadec-9-enoate was formed, and again distinctive mass spectra were obtained that permitted location of the double bonds. With cis,trans-isomers of linoleate, only the cis-double bond reacted with the reagent, so limiting the value of the technique in such circumstances [11]. When higher temperatures (up to 60°C) and longer reaction times (40 hours) were employed, a second mole of dimethyl disulfide was added, and cyclization occurred giving heterocyclic compounds with thietane, tetrahydrothiophene and tetrahydrothiopyran structures (4-, 5- and 6-membered rings), as illustrated [12,13] -
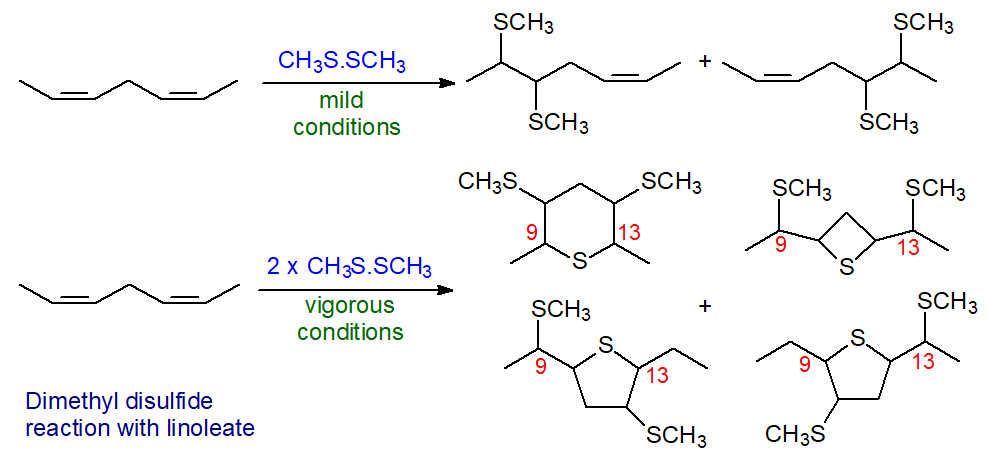 |
| Figure 6. Part structures of reaction of DMDS with linoleate. |
These heterocyclic compounds can give characteristic and diagnostic spectra, so that the technique continues to have some practical value. In one report, thetane formation only was observed, but in more systematic studies four distinct products were obtained in proportions that varied according to the reaction conditions. Di‑cis and di-trans forms of linoleate gave products with distinct stereochemistry and different chromatographic properties, suggesting again that the technique might have value for determining the geometry of double bonds in such fatty acids [13].
Long-chain fatty acids with 5,9-diene systems are common constituents of marine sponges. Reaction of these with dimethyl disulfide under appropriate conditions gives a 5-membered cyclic thioether substituted with two alkyl chains, each containing a methylthio group on the carbons immediately adjacent to the ring as illustrated [14].
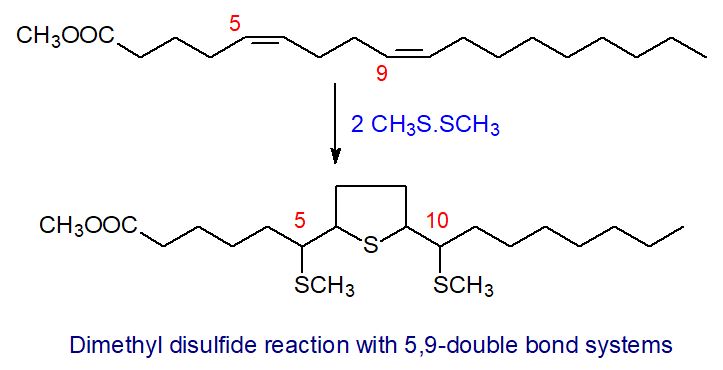 |
| Figure 7. Reaction of 5,9-18:2 with DMDS. |
These compounds give characteristic mass spectra that permit location of the double bonds, and many different demospongic acids of this type have been identified in this way, including some containing bromine atoms and methyl branches as well as the 5,9-double bond system.
Polyunsaturated fatty acids. Another approach is to generate monoenes from polyunsaturated fatty acids before this reaction is used for identification purposes. Thus, heneicosapentaenoic acid (21:5(n-3)) from eel lipids was first isolated by silver ion TLC, before being subjected to partial hydrogenation with hydrazine to a mixture of monoenes; these were then converted to DMDS derivatives for structural analysis by GC-MS [15]. Further unusual trienoic fatty acids have been identified by this means from such natural sources as mites and slime moulds. A modification to the reaction protocol that enables higher yields of monoene products with minimal by-product formation directly from polyunsaturated fatty acids has been described with some practical examples [16]. The procedure has been adapted for LC-MS [17].
In addition to the difficulties with dienes and polyenes, DMDS derivatives were not suitable for determining the position of the double bond in a cyclopentene ring in my experience, although confirmation of the positions of double bonds in the aliphatic chain of natural fatty acids with this structural feature was obtained.
Diels-Alder (MTAD) Adducts for Conjugated Double Bonds
A useful derivative specific for determination of double bond positions in conjugated dienes is to form a Diels-Alder adduct, i.e., a six-membered ring structure, with the methyl ester of the fatty acid by reaction with the reagent, 4-methyl-1,2,4-triazoline-3,5-dione (MTAD). Such derivatives have excellent mass spectrometric properties, enabling determination of the positions of the original double bonds in such samples as commercial conjugated linoleic acid (CLA) and the metabolites formed from this in animal tissues. The nature of the reaction with geometrical and positional isomers has been investigated in some detail [18]. Reaction occurs almost instantaneously at room temperature and must be stopped immediately by adding 1,3-hexadiene to mop up excess reagent [19].
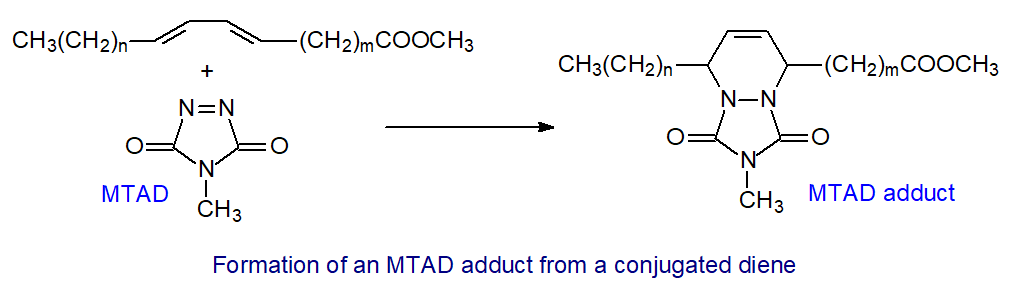 |
| Figure 8. Reaction of 4-methyl-1,2,4-triazoline-3,5-dione (MTAD) with a conjugated diene. |
| Laboratory protocol: The CLA methyl ester (220 μg; 1.15mM) and MTAD (425 μg; 5.8 mM) in dichloromethane (650 μL) are mixed in a test-tube at 0°C by agitating for less than 10 seconds. The reaction is stopped immediately by addition of 1,3-hexadiene (12 mM), followed by agitation for a few seconds. Excess reagents are removed in a stream of nitrogen at 30°C, and the sample is re-dissolved in dichloromethane for analysis by GC-MS. |
The mass spectrum of the MTAD adduct of methyl 9-cis,11-trans-octadecadienoate is illustrated below -
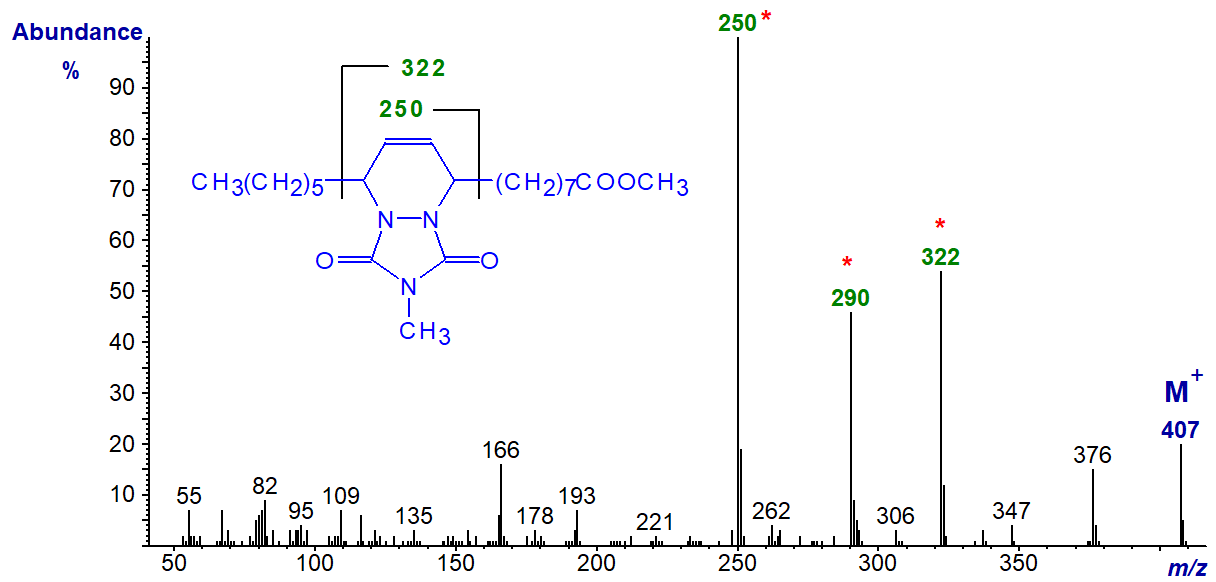
Cleavage occurs on either side of the six-membered ring, enabling simple location of the carbons that originally constituted the conjugated double bond system, i.e., at m/z = 250 and 322. Confirmatory evidence comes from the ion representing loss of methanol from the ion containing the carboxyl moiety, which is at m/z = 290 in this instance. Indeed, these ions can be used with selective ion monitoring to quantify positional isomers in the presence of mixtures.
For comparison, the spectrum of the MTAD adduct of the 10,12-18:2 isomer follows -
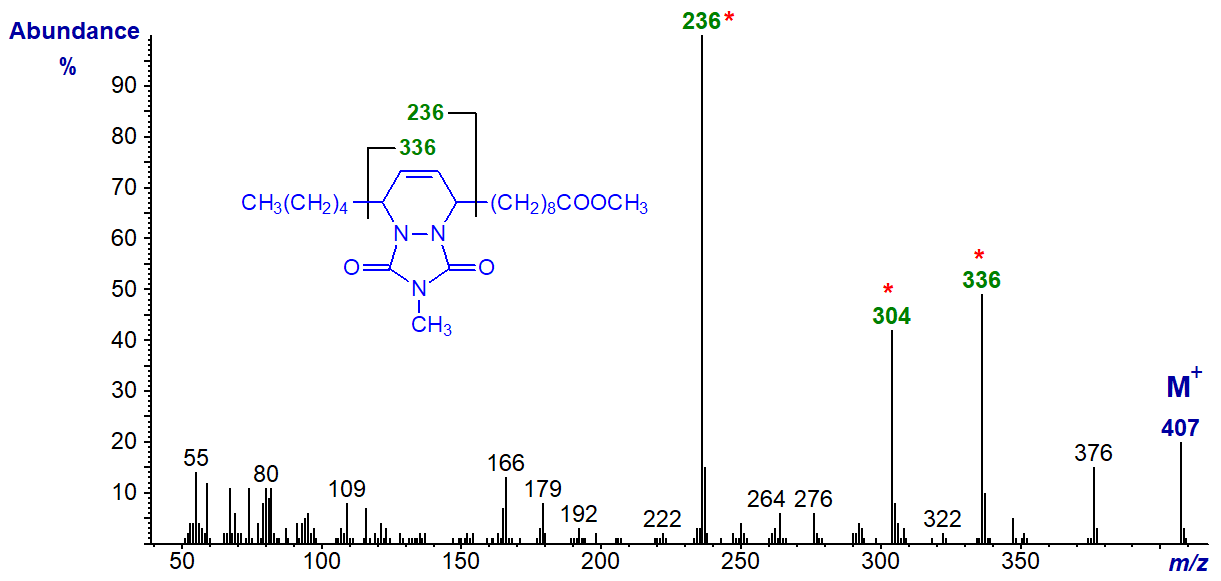
The carbons that were part of the original conjugated double bond system can be located by the ions at m/z = 336 and 236, with that at 304 representing loss of methanol from the carboxyl-containing ion. We have spectra of more MTAD adducts of conjugated dienes on file, and they can be accessed (but without interpretation) from our Archive page.
When the reagent is reacted with a conjugated triene, such as the methyl ester of punicic (9,11,13-octadecatrienoic) acid, two possible products are formed.
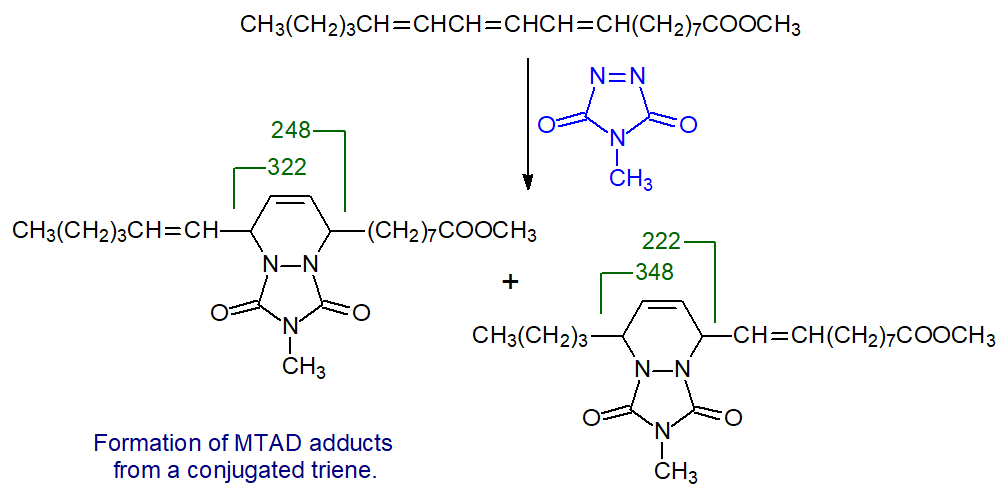 |
| Figure 11. Reaction of MTAD with the methyl ester of punicic acid. |
Both adducts are separated by GC and give diagnostic spectra, as illustrated first with the 9,11-double bonds reacting (upper) and then with the 11,13-double bonds reacting (lower) -
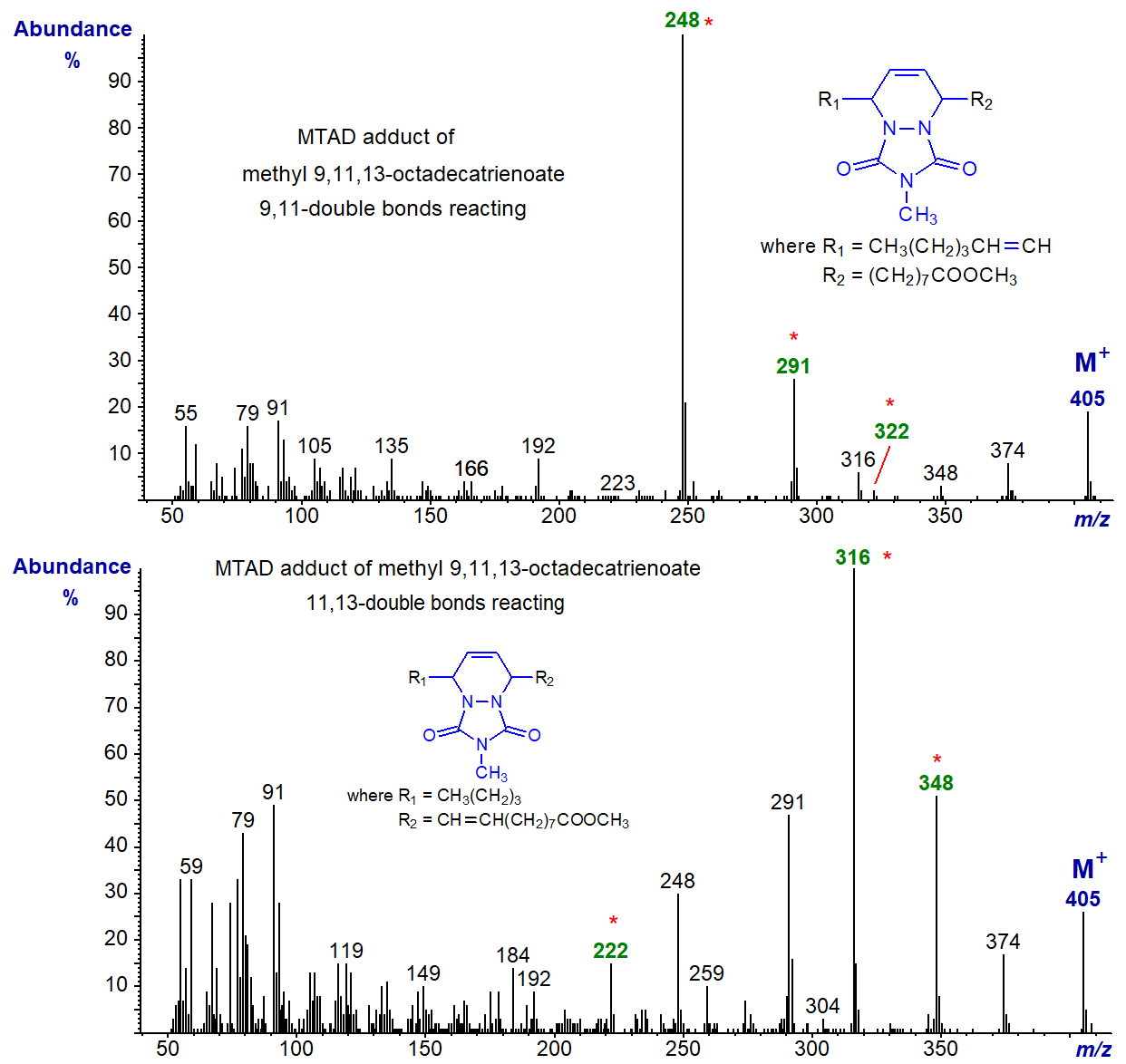
The key diagnostic ions are expected to be at m/z = 248 and 322, but with the latter ion further fragmentation has occurred with loss of a methoxyl group so that the ion at m/z = 291 helps to define the structure.
With the second spectrum, interpretation is less straight forward, and the original article by Dobson [19] should be consulted. The important diagnostic ions at m/z = 222 and 348 are present, as is the latter less the elements of methanol (m/z = 316).
The method can be used, but with care, to locate conjugated dienes in the presence of non-conjugated double bonds, as was demonstrated for 5,8,11,13-eicosatetraenoate (mass spectrum of the MTAD adduct) [20]. If possible, the reagent is best used with fractions enriched in conjugated fatty acids, as a small amount of reaction with methylene-interrupted double bonds, moving them into conjugation, can sometimes occur (author, unpublished observation).
References
 Christie, W.W.
and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis
(4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see
Science Direct.
Christie, W.W.
and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis
(4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see
Science Direct.- Dickens, B.F., Ramesha, C.S. and Thompson, G.A. Quantification of phospholipid molecular species by coupled gas chromatography-mass spectrometry of deuterated samples. Anal. Biochem., 127, 37-48 (1982); DOI.
- Tsevegsuren, N., Christie, W.W. and Lösel, D. Tanacetum (Chrysanthemum) corymbosum seed oil: a rich source of a novel conjugated acetylenic acid. Lipids, 33, 723-727 (1998); DOI.
- Brechany, E.Y. and Christie, W.W. Identification of the unsaturated oxo fatty acids in cheese. J. Dairy Res., 61, 111-115 (1994); DOI.
- Francis, G.W. Alkylthiolation for the determination of double-bond position in unsaturated fatty acid esters. Chem. Phys. Lipids, 29, 369-374 (1981); DOI.
- Christie, W.W. Structural analysis of fatty acids. In: Advances in Lipid Methodology - Four, pp. 119-169 (edited by W.W. Christie, Oily Press, Dundee) (1997).
- Shibahara, A., Yamamoto, K., Kinoshita, A. and Anderson, B.L. An improved method for preparing dimethyl disulfide adducts for GC/MS analysis. J. Am. Oil Chem. Soc., 85, 93-94 (2008); DOI.
- Thies, W. Determination of the petroselinic acid content in seeds of Coriandrum setivum by GLC. Fat Sci. Technol., 95, 20-23 (1993); DOI.
- Shibahara, A., Yamamoto, K., Shinkai, K., Nakayama, T. and Kajimoto, G. cis-9,cis-15-Octadecadienoic acid: a novel fatty acid found in higher plants. Biochim. Biophys. Acta, 1170, 245-252 (1993); DOI.
- Yamamoto, K., Shibahara, A., Nakayama, T. and Kajimoto, G. Determination of double bond positions in methylene-interrupted dienoic fatty acids by GC-MS as their dimethyl disulfide adducts. Chem. Phys. Lipids, 60, 39-50 (1991); DOI.
- Shibamoto, S., Murata, T. and Yamamoto, K. Determination of double bond positions and geometry of methyl linoleate isomers with dimethyl disulfide adducts by GC/MS. Lipids, 51, 1077-1081 (2016); DOI.
- Carballeira, N.M., Shalabi, F. and Cruz, C. Thietane, tetrahydrothiophene and tetrahydrothiopyran formation in reaction of methylene-interrupted dienoates with dimethyl disulfide. Tet. Letts., 35, 5575-5578 (1994); DOI.
- Carballeira, N.M. and Cruz, C. Dimethyl disulfide derivatization of ethyl (9Z,12Z)-9,12-octadecadienoate and ethyl (9E,12E)-9,12-octadecadienoate. Chem. Phys. Lipids, 84, 81-85 (1996); DOI.
- Carballeira, N.M. and Shalabi, F. Novel brominated phospholipid fatty acids from the Caribbean sponge Petrosia sp. J. Nat. Prod. - Lloydia, 56, 739-746 (1993); DOI.
- Yamamoto, K., Shibahara, A., Nakayama, T. and Kajimoto, G. Double-bond localization in heneicosapentaenoic acid by a gas chromatography/mass spectrometry (GC/MS) method. Lipids, 26, 948-950 (1991); DOI.
- Liao, S.A. and Huang, Y.S. Preferential formation of mono-dimethyl disulfide adducts for determining double bond positions of poly-unsaturated fatty acids. J. Am. Oil Chem. Soc., 99, 279-288 (2022); DOI - see also DOI2
- Olfert, M., Knappe, C., Sievers-Engler, A., Masberg, B. and Lämmerhofer, M. Determination of double bond positions in unsaturated fatty acids by pre-column derivatization with dimethyl and dipyridyl disulfide followed by LC-SWATH-MS analysis. Anal. Bioanal. Chem., 417, 2753–2766 (2025); DOI.
- Reaney, M.J.T., Liu, Y.D. and Taylor, W.G. Gas chromatographic analysis of Diels-Alder adducts of geometrical and positional isomers of conjugated linoleic acid. J. Am. Oil Chem. Soc., 78, 1083-1086 (2001); DOI.
- Dobson, G. Identification of conjugated fatty acids by gas chromatography mass spectrometry of 4-methyl-1,2,4-triazoline-3,5-dione adducts. J. Am. Oil Chem. Soc., 75, 137-142 (1998); DOI.
- Sébédio, J.L., Juanéda, P., Dobson, G., Ramilison, I., Martin, J.C., Chardigny, J.M. and Christie, W.W. Metabolites of conjugated isomers of linoleic acid (CLA) in the rat. Biochim. Biophys. Acta, 1345, 5-10 (1997); DOI.
| © Author: William W. Christie |  |
|
| Updated: June 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
