Mass Spectrometry of Methyl Esters
Normal Saturated Fatty Acids
 Methyl esters are by far the most used derivatives for fatty acid analysis in general, and a great deal of information
is available on their chemical, physical, chromatographic and spectroscopic properties, but as cautioned in the
'Introduction' to these documents, mass spectrometry with electron-impact ionization
of methyl esters affords limited information only concerning the structures of fatty acids, especially double bond positions.
Some limited information on double bond positions in polyenoic fatty acids may be ascertainable, but only rarely for monoenes or dienes.
The molecular ion is usually distinct, and this is an important piece of information for identification purposes.
If gas chromatographic retention data are added to this, it is often possible to be 90% certain of the identity of a fatty acid.
In addition, the positions of structural features such as branch points and oxygenated groups can usually be deduced.
Such intricacies are left for later, and in this document, mass spectra of methyl esters of linear saturated fatty acids only are described.
Methods of preparing methyl esters are described on another web page.
Methyl esters are by far the most used derivatives for fatty acid analysis in general, and a great deal of information
is available on their chemical, physical, chromatographic and spectroscopic properties, but as cautioned in the
'Introduction' to these documents, mass spectrometry with electron-impact ionization
of methyl esters affords limited information only concerning the structures of fatty acids, especially double bond positions.
Some limited information on double bond positions in polyenoic fatty acids may be ascertainable, but only rarely for monoenes or dienes.
The molecular ion is usually distinct, and this is an important piece of information for identification purposes.
If gas chromatographic retention data are added to this, it is often possible to be 90% certain of the identity of a fatty acid.
In addition, the positions of structural features such as branch points and oxygenated groups can usually be deduced.
Such intricacies are left for later, and in this document, mass spectra of methyl esters of linear saturated fatty acids only are described.
Methods of preparing methyl esters are described on another web page.
The Spectra
Methyl esters of straight-chain fatty acids have characteristic spectra, and some representative examples are illustrated below with brief details of interpretation only. Those of the more common fatty acids were published in the early days of mass spectrometry (Ryhage, R. and Stenhagen, E. Mass spectrometric studies. I. Saturated normal long-chain methyl esters. Arkiv Kemi, 13, 523-542 (1959)).
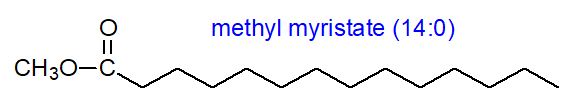
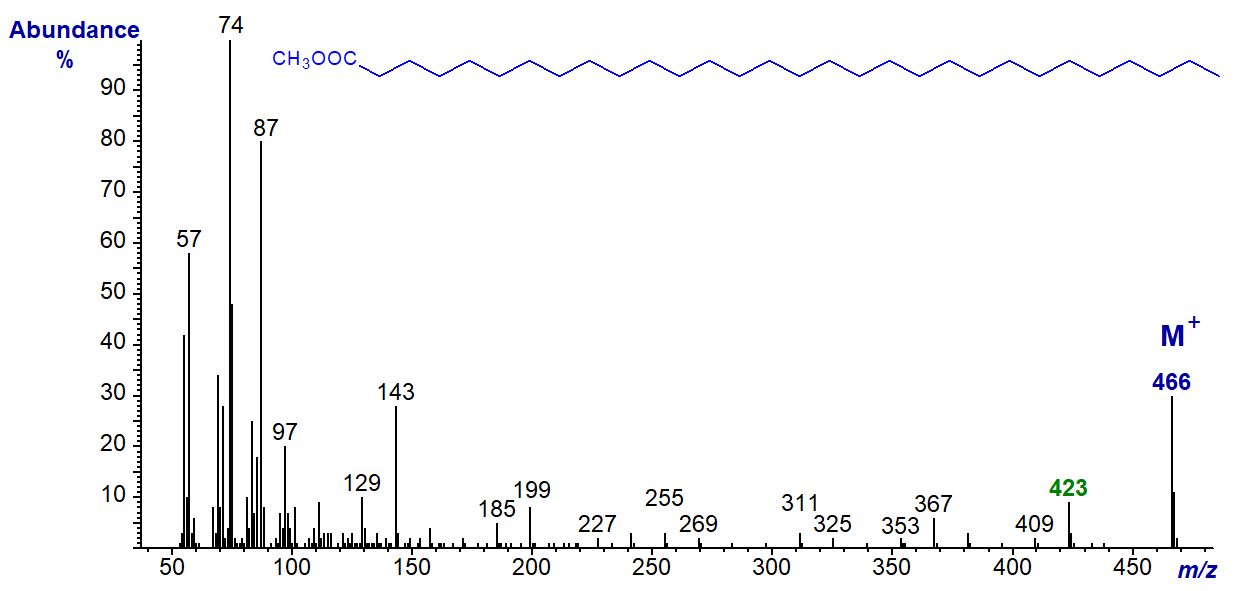
The spectra are relatively simple as the main ions tend to occur singly (14 amu apart) and not in clusters as with unsaturated esters. The mass spectrum of methyl palmitate (16:0) is -
The molecular ion at m/z = 270 is clearly seen, as is an ion at 239 ([M-31]+) representing loss of a methoxyl group, and confirming that it is indeed a methyl ester. An ion at m/z = 227 ([M‑43]+) represents loss of a C3 unit (carbons 2 to 4) via a complex rearrangement, while that m/z = 241 ([M‑29]+; carbons 2 and 3) is formed similarly and is also diagnostic. The ion at m/z = 74 has a special significance as it is the McLafferty rearrangement ion (see below), although the cleavage point is shown here simplistically for illustrative purposes. It affords further confirmation that the spectrum is that of a methyl ester. The long homologous series of related ions uniformly 14 amu apart at m/z = 87, 101, 115, 129, 143, 157, 199 and so forth of general formula [CH3OCO(CH2)n]+ is evidence that there are unlikely to be other functional groups in the chain. The origins of many of these ions are discussed below.
Although mass spectra of fatty acids with iso- and anteiso-methyl branches are easily confused with those of linear analogues (see the web page on methyl esters of branched-chain fatty acids), GC relative retention times should eliminate any doubts. In the mass spectra of methyl esters of unsaturated fatty acids in contrast, hydrocarbon ions predominate, and the McLafferty ion becomes much less distinct.
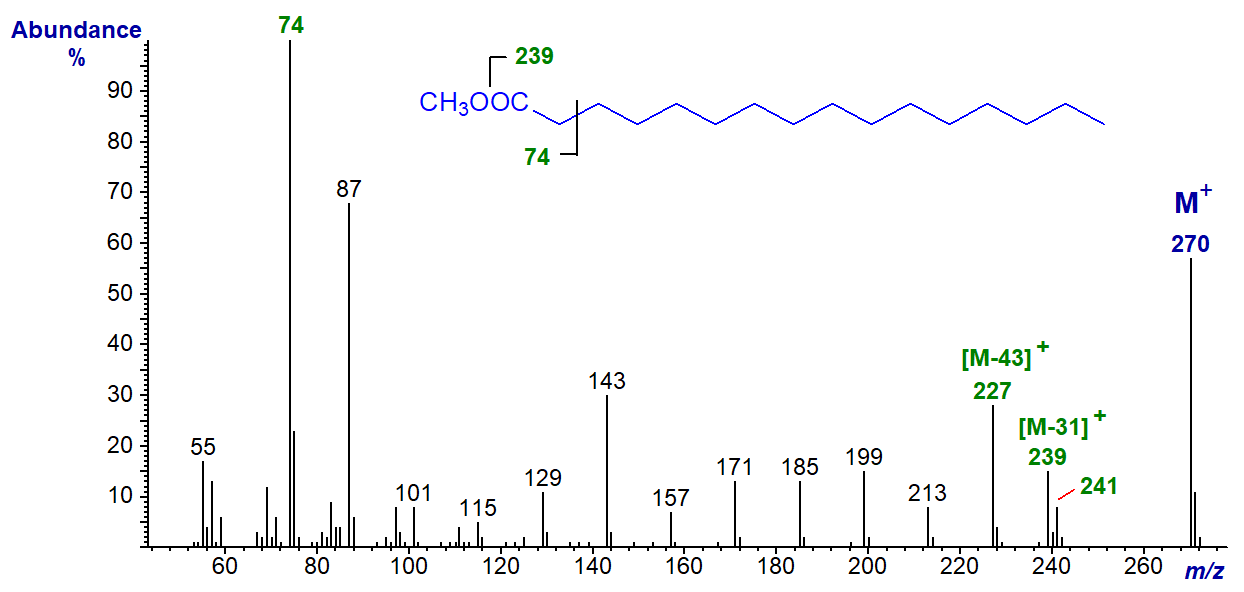
The mass spectrum of methyl docosanoate (22:0) is shown next -
In essence, it shows all the same features as that of methyl palmitate except that the molecular ion and other ions in the high mass range, e.g., that for loss of the methyl group, are shifted upwards by 84 amu.
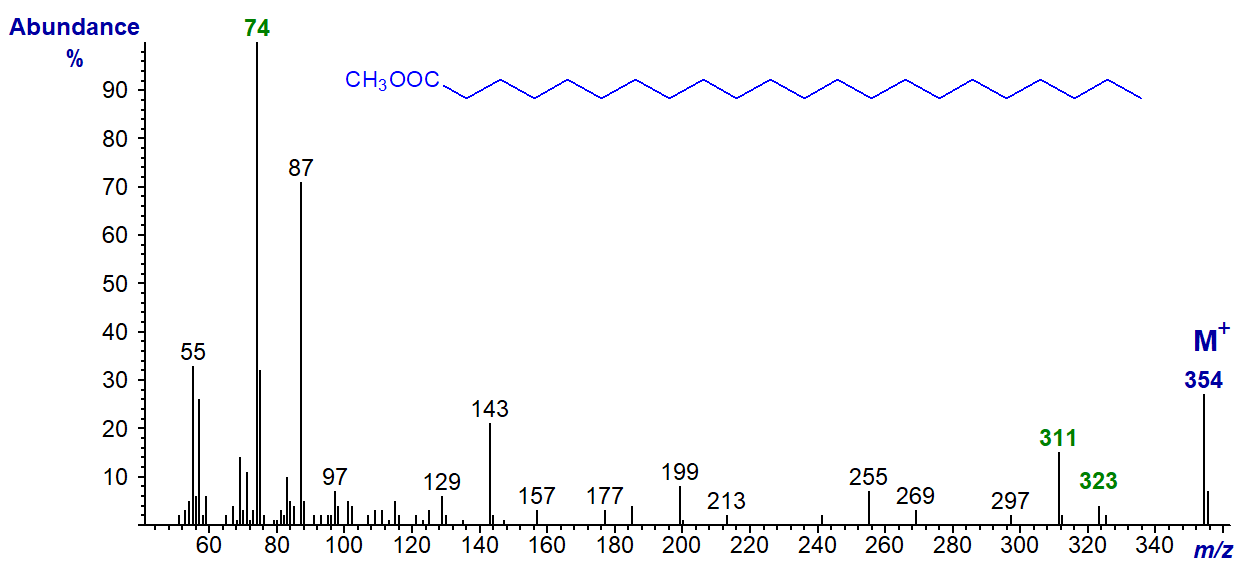
With spectra of methyl esters of shorter-chain fatty acids, the molecular ion tends to become progressively smaller, and the only significant ion in the high mass range may be that for the [M‑31]+ ion, in this instance at m/z = 127, which must then be used to determine the molecular weight. Otherwise, comparable features are seen in the mass spectra of methyl esters of other saturated fatty acids, and the spectrum of the short-chain methyl octanoate (8:0) is -
- and then the very-long-chain methyl tricosanoate (30:0) -
Mechanistic Aspects
Although these pages are not intended to be a treatise on mechanistic aspects of mass spectrometry, the McLafferty rearrangement ion is central to the identification of most ester derivatives of fatty acids, so some digression in this direction seems desirable here. In fact, the McLafferty rearrangement is one of the most widely occurring and thence most studied processes in mass spectrometry. The resulting ion is always of diagnostic importance.
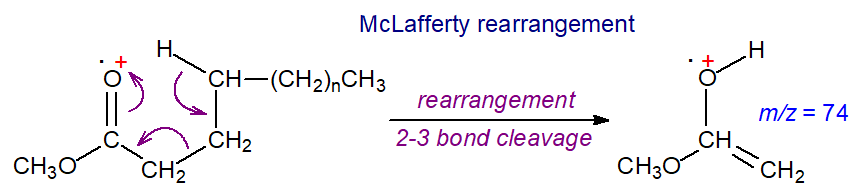 |
| Figure 6. The McLafferty rearrangement of a methyl ester. |
A site-specific rearrangement is involved in which a hydrogen atom from position 4 of the aliphatic chain migrates to the carbo-methoxy group as illustrated, presumably through a six-membered transition state, which is sterically favoured. One result is elimination of the elements of a neutral olefin. If one of the hydrogen atoms on carbon 4 is substituted, then the McLafferty ion will be appreciably lower in intensity than expected. This may explain why it is less evident in the mass spectra of derivatives of unsaturated fatty acids with increasing numbers of double bonds, which can readily migrate to position 4 under electron bombardment. If both hydrogens on carbon 4 are substituted (other than with deuterium), the McLafferty ion cannot form (see the spectra of the deuterated fatty acids below and of 4-thia fatty acids on the appropriate web page).
If there is a substituent on position 2, the m/z value of the McLafferty ion will be increased according to the size of the substituent. When the nature of the alcohol moiety varies, so will the size of the McLafferty ion: to m/z = 88 for ethyl esters, m/z = 102 for propyl esters and m/z = 113 for dimethyloxazoline and pyrrolidine derivatives. However, it is now recognized that the ion at m/z = 151 in spectra of 3-pyridylcarbinol ('picolinyl') esters is not formed in a McLafferty rearrangement as was first reported.
In addition to the McLafferty ion, there is a series of related ions of general formula [(CH2)nCOOCH3]+ of which that at m/z = 87 is most abundant, followed by ions at m/z = 101, 115, 129, 143 and so forth; these are formed by losses of neutral aliphatic radicals from the terminal part of the molecule.
The ion at [M-43]+ at m/z = 227 is believed to be formed via a rearrangement of the chain and one hydrogen atom, followed by expulsion of a propyl radical (carbons 2 to 4), again via a six-membered transition state (I have seen this erroneously ascribed to an iso-methyl branch in a recent publication in the journal Lipids). An ion at [M-29]+ is presumed to arise in an analogous manner following an initial cleavage between carbons 3 and 4. These ions can be useful diagnostically when carbons 2 to 4 in a fatty acid chain are substituted, for example by a methyl branch.
Confirmatory evidence for these mechanisms can be seen in the mass spectra of methyl palmitate (16:0) deuterated in specific positions, which can be compared with that of the normal ester above. In the first instance, the spectrum of methyl 2,2-dideutero-hexadecanoate is illustrated.
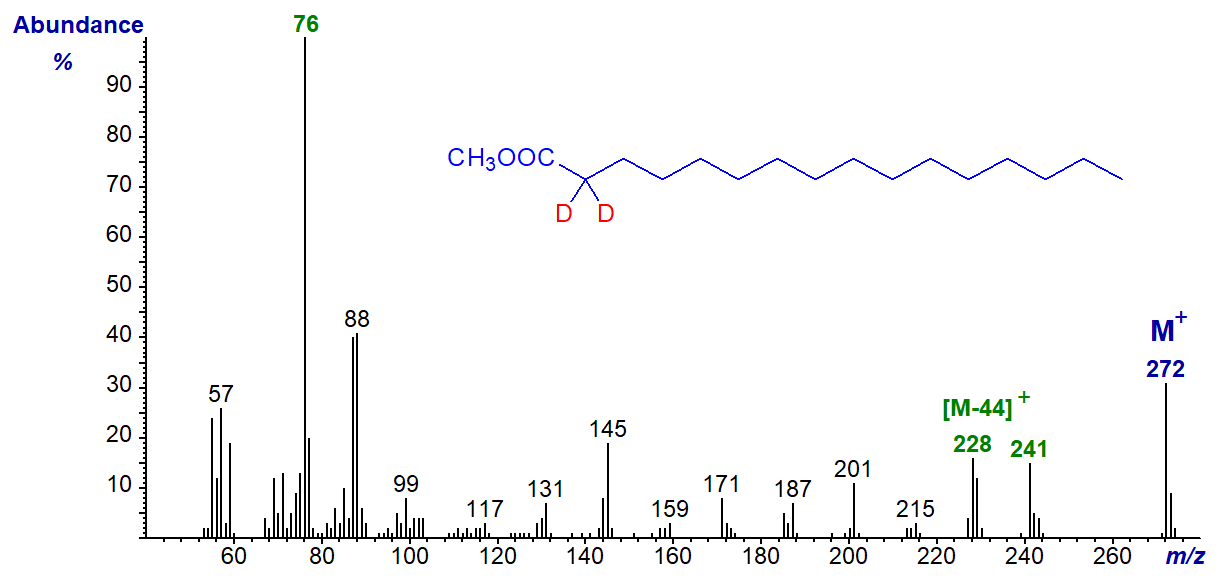
Both the molecular ion and the McLafferty rearrangement ion have increased by 2 units, the latter to m/z = 76. As would be anticipated, the ion representing the loss of a methoxyl group is still at [M-31]+ (m/z = 241). On the other hand, the ion representing loss of carbons 2 to 4 is now equivalent to the loss of 44 amu (at m/z = 228), as is explained by the accepted mechanism for the formation of this ion. In this and the next spectrum, the ions resulting from the loss of carbons 2 and 3 are now equivalent to [M‑31]+ and coincide with that for the loss of the methoxyl group (m/z = 241) in a low-resolution spectrum.
Next the spectrum of methyl 3,3-dideutero-hexadecanoate -
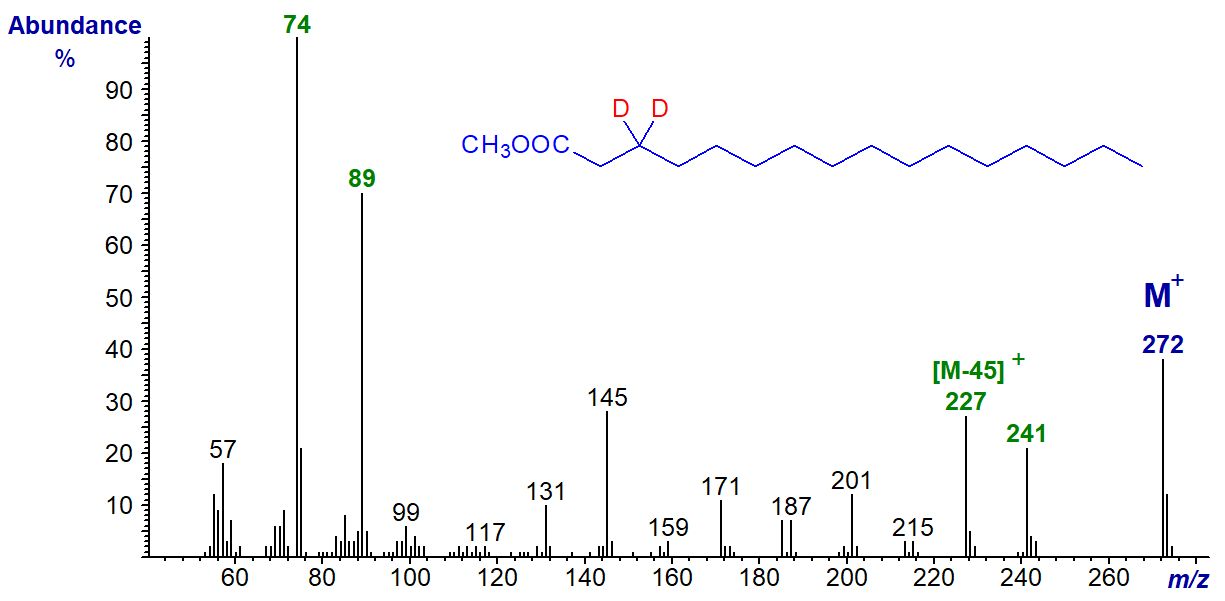
Now the McLafferty ion is at m/z = 74 again, but the ion for [CH3OCO(CH2)2]+, formerly at m/z = 87 has now increased to 89. The ion representing loss of carbons 2 to 4 is now at [M-45]+ (m/z = 227).
And now, the spectrum of methyl 4,4-dideutero-hexadecanoate -
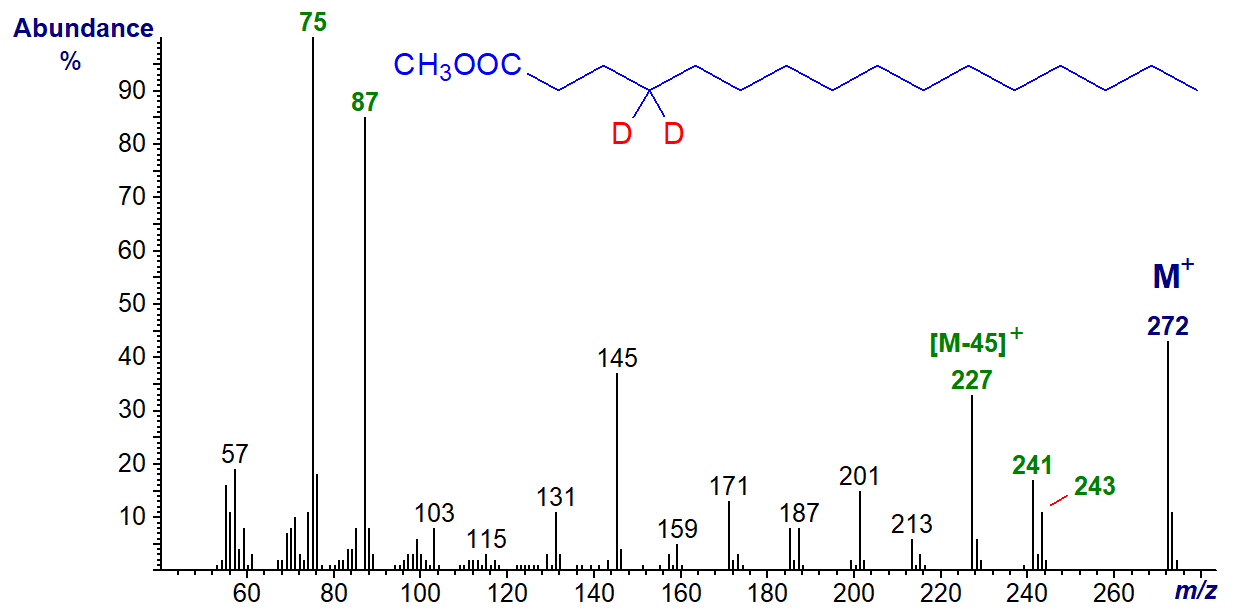
The McLafferty ion is now at m/z = 75 as one of the deuterium atoms from carbon 4 has been abstracted during the rearrangement. The ion representing loss of carbons 2 to 4 is again at [M-45]+ (m/z = 227), while that for loss of carbons 2 and 3 has reappeared at [M-29]+ (m/z = 243).
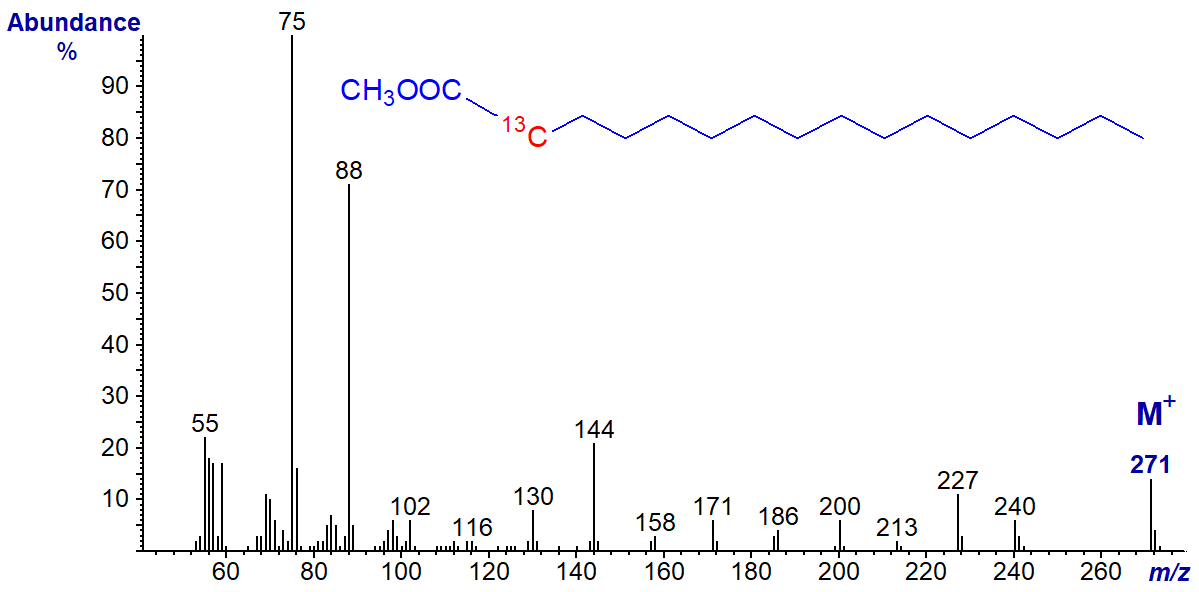
Finally, the spectrum of methyl 2-13C-hexadecanoate is illustrated, but I leave the task of comparison and interpretation to readers -
Spectra of many more methyl esters of saturated fatty acids (4:0 to 30:0), including some labelled with the stable isotopes, can be accessed from our Archive pages (without interpretation).
I also recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - from Science Direct.
| © Author: William W. Christie |  |
|
| Updated: May 2025 | Contact/credits/disclaimer | |
© The LipidWeb is open access and fair use is encouraged but not text and data mining, AI training, and similar technologies.
