Mass Spectrometry of Methyl Esters
Cyclic Fatty Acids
This document does not aim to be a complete account of mass spectrometry with electron-impact ionization of naturally occurring fatty acids containing ring structures as the methyl esters, but rather is a personal account of our experience of those encountered during our research activities and for which we have spectra available for illustration purposes. Spectra for 3-pyridylcarbinol ('picolinyl') esters and DMOX derivatives with pyrrolidides are described in separate documents. Where we are aware of prior illustrations of mass spectra in the literature, the appropriate papers are cited. These notes are intended as a practical guide rather than as a mechanistic account, although some important general mechanistic elements are described in our web page on methyl esters of saturated fatty acids. Methods of preparing methyl esters are described on another web page. The occurrence and biological properties of cyclic fatty acids have been reviewed by Sébédio and Grandgirard (1989), and there is further information on the chemistry, occurrence and biochemistry of cyclic fatty acids on this website here...
Cyclopropyl Fatty Acids
Cyclopropyl fatty acids are common constituents of bacterial lipids and may accompany cyclopropenyl fatty acids as minor components of certain seed oils. Unfortunately, mass spectra of methyl esters of cyclopropyl fatty acids are not very informative as the ring appears to rearrange under electron bombardment in the mass spectrometer to give a double bond. Spectra are thus indistinguishable from those of monoenoic fatty acids with an alkyl chain one carbon longer (Christie and Holman, 1966). The mass spectrum of methyl 9,10-methylene-hexadecanoate is illustrated as an example -
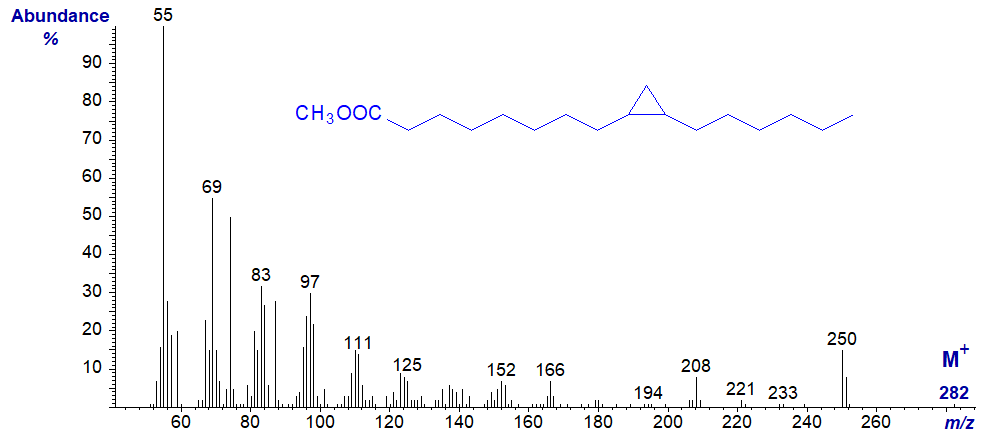
The spectrum is in essence identical to that of methyl 8-heptadecenoate. Although there have been suggestions in the literature that subtle differences exist between the mass spectra of cyclopropanes and monoenes that might serve for diagnosis, these are not very convincing to this author. They may work with a limited range of pure model compounds but are less likely to be of practical value in the analysis of complex mixtures, especially when instrumental variations are taken into account. On the other hand, the GC retention times of methyl esters of monoenoic and cyclopropanoic fatty acids are very different, and this is useful information in deciding between possible structures.
3-Pyridylcarbinol esters are the best derivative for locating the position of a cyclopropane ring. If this is not available, vigorous hydrogenation causes ring opening with formation of methyl branches, which can be located by mass spectrometry (McCloskey and Law, 1967). Alternatively, a robust reaction with boron trifluoride-methanol reagent causes ring opening with formation of methoxy derivatives, which can be identified in a similar manner (Minnikin, 1972). Dimethyloxazoline and pyrrolidide derivatives give less informative spectra than 3-pyridylcarbinol esters.
Cyclopropenyl Fatty Acids
It was long thought that gas chromatography (GC) and thus GC-MS of derivatives of cyclopropenyl fatty acids was impossible because of thermal degradation on the GC column, but modern capillary columns are relatively inert, and analysis by GC should be straightforward, provided that appropriate derivatization methods are employed, i.e., acidic methylation conditions must be avoided as they can cause alterations to the ring structure.
The mass spectra of methyl ester derivatives of cyclopropenoid fatty acids tend to resemble those of dienoic fatty acids, so methyl sterculate (9,10‑methylene-octadec-9-enoate) has a spectrum that differs in minor ways only from that of methyl nonadecadienoate, and there are no obvious ions that serve to locate the ring (Pawlowski et al., 1974).
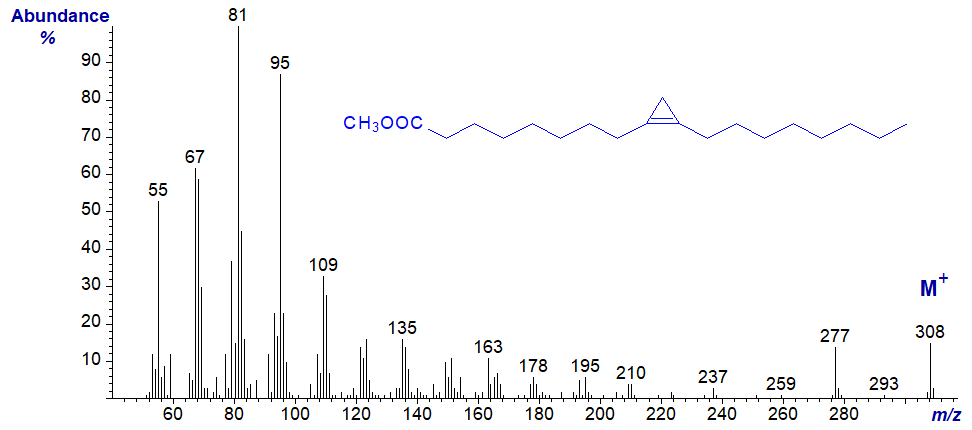
Chemical ionization spectra (rather than electron-impact ionization) have features that indicate the presence of a cyclopropene ring but not its location (Spitzer, 1991). Again, 3-pyridylcarbinol derivatives are the best alternative derivative for locating the position of a cyclopropene ring, though derivatization by addition of methane thiol across the double bond is another possibility (see Hooper and Law, 1968). Dimethyloxazoline and pyrrolidide derivatives are less suitable. Note that silver ion chromatography causes ring opening of cyclopropenyl fatty acids, and some have used this property for characterization purposes.
ω-Cyclopentenyl and Cyclopentyl Fatty Acids
Fatty acids with a terminal cyclopent-2-enyl moiety are found in high concentration in the seed oils of several species from the plant family Flacourtiaceae, though the corresponding cyclopentyl (saturated species) have been detected at low levels only. It is worth noting that although the nitrogen-containing derivatives permit location of most of the structural features with varying degrees of success (see the separate documents for 3‑pyridylcarbinol esters and DMOX-pyrrolidides), none fix the actual position of the double bond within the ring. For the latter purpose, chemical degradation, perhaps allied with mass spectrometry, is required (Christie et al., 1989).
While the mass spectra of the methyl ester derivatives have limited value only for characterization purposes, the ring structure itself can be detected and confirmed (Christie et al., 1969). Thus, the mass spectrum of methyl hydnocarpate (11-cyclopentenylundecanoate) is -
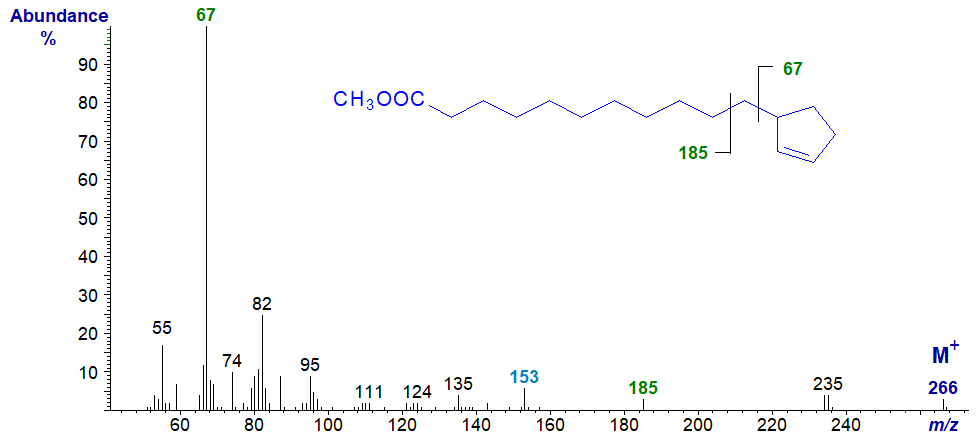
The base ion at m/z = 67 is presumed to be the ionized cyclopentene ring per se, but no corresponding fragment at m/z = 199 is detectable. Instead, an ion at m/z = 185 represents the remainder of the molecule with cleavage beta to the ring (together with an ion at m/z = 153 for loss of a methoxyl group from this ion). In the high mass range, the molecular ion is small but distinct, and there is an ion representing loss of a methoxyl group from this (at m/z = 235).
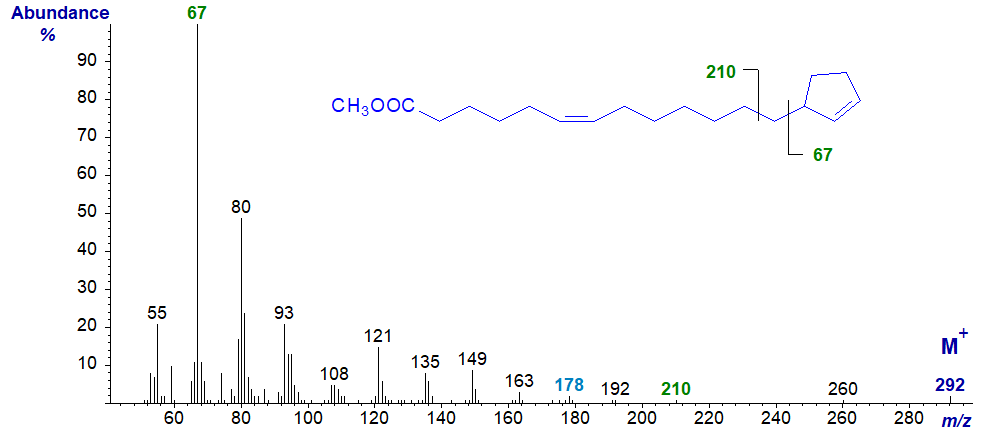
In the mass spectrum of methyl gorlate (13-cyclopent-2-enyltridec-6-enoate), the base ion is again at m/z = 67, but the ion representing cleavage beta to the ring (at m/z = 210) together with the associated ion at m/z = 173 are barely distinguishable in this instance. There is no feature that serves to locate the position of the double bond in the alkyl chain, but this can be accomplished by preparing the dimethyl disulfide derivative prior to mass spectrometry. The ions in the low mass range differ somewhat from those in the previous spectrum.
The mass spectrum of methyl 11-cyclopentylundecanoate, i.e., with a saturated ring, is rather different –
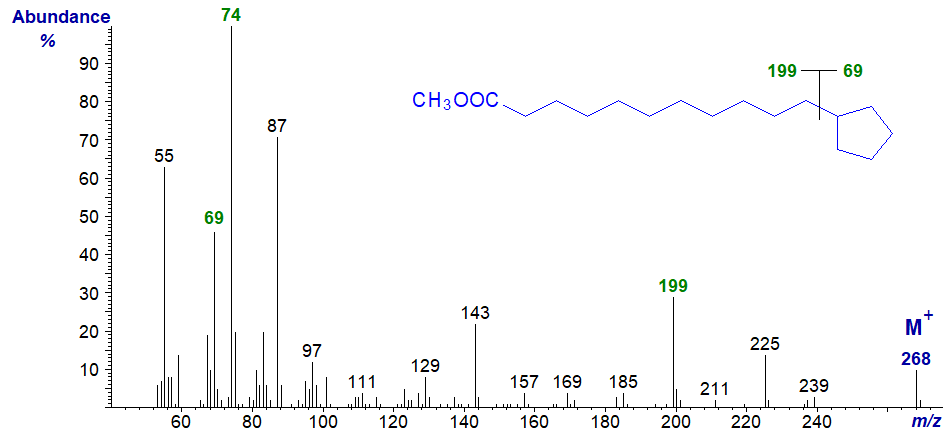
An ion representing the ring fragment (m/z = 69) does stand out, as does an ion for the loss of the ring at m/z = 199 (i.e., cleavage alpha to the ring in this instance). Otherwise, the spectrum resembles that of a normal saturated ester (apart from the fact that the molecular ion is two units amu lower because a ring is present), and the McLafferty ion at m/z = 74 is now the base ion.
ω-Cyclohexyl Fatty Acids
ω-Cyclohexylundecanoic acid is a minor component of cow's milk fat, although it probably originates in rumen bacteria, and this was the source here for the mass spectrum of methyl 11-cyclohexylundecanoate (first published by Schogt and Begemann, 1965) -
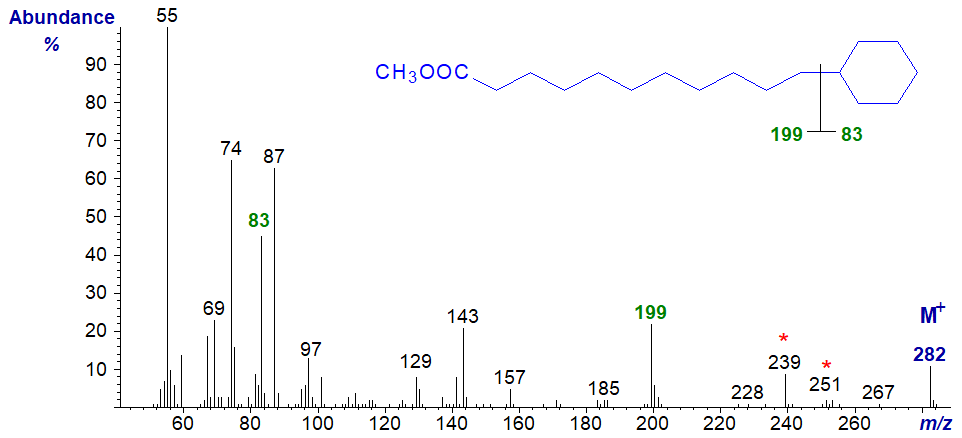
The ion at m/z = 199 defines the position of the cyclohexane ring as illustrated, via fragmentation adjacent to the ring (as with the cyclopentyl fatty acids above), while the other fragment ion at m/z = 83 is also prominent. The ion at m/z = 239 ([M‑43]+) is common in saturated esters and represents a complex rearrangement involving expulsion of carbons C2 to C4, while that at m/z = 251 is produced by the loss of the methoxyl group. Spectra of further homologues have been published by Schröder and Vetter (2013). While I would expect useful spectra from 3-pyridylcarbinol and pyrrolidide derivatives, I am not aware of any that have been published.
ω-Phenyl Fatty Acids
Fatty acids with a terminal phenyl moiety are found in seed oils of the subfamily Aroideae of the Araceae and certain species of bacteria. Some of the available data have been published (Christie, 2003). The mass spectrum of methyl 13-phenyltridecanoate, which is usually the main component, is -
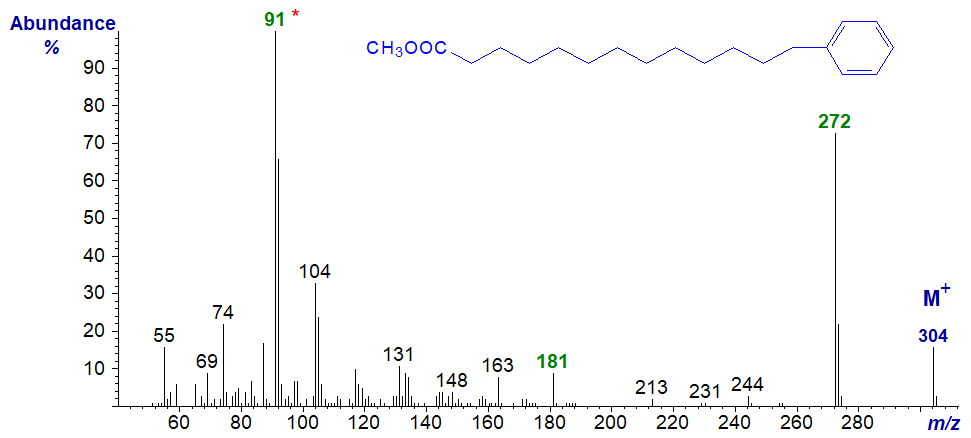
 There
is a distinct molecular ion at m/z = 304, and an abundant ion at m/z = 272 reflects the loss of the elements of methanol.
The base ion at m/z = 91 is presumably a tropylium ion, which is typical for spectra of aromatic compounds (or of
polyunsaturated fatty acids), while that at m/z = 181 is presumably formed by loss of a tropylium from
the ion representing [M‑32]+.
There
is a distinct molecular ion at m/z = 304, and an abundant ion at m/z = 272 reflects the loss of the elements of methanol.
The base ion at m/z = 91 is presumably a tropylium ion, which is typical for spectra of aromatic compounds (or of
polyunsaturated fatty acids), while that at m/z = 181 is presumably formed by loss of a tropylium from
the ion representing [M‑32]+.
The mass spectrum of methyl 13-phenyl-tridec-9-enoate differs somewhat from this in that the homologous series containing the tropylium ion is especially prominent, i.e., at m/z = 91, 104 (now the base ion), 117 and 131. The base ion at m/z = 104 does not reflect the proximity of the ring to the double bond, as the same feature is seen in the spectrum of methyl 15-phenylpentadec-9-enoate.
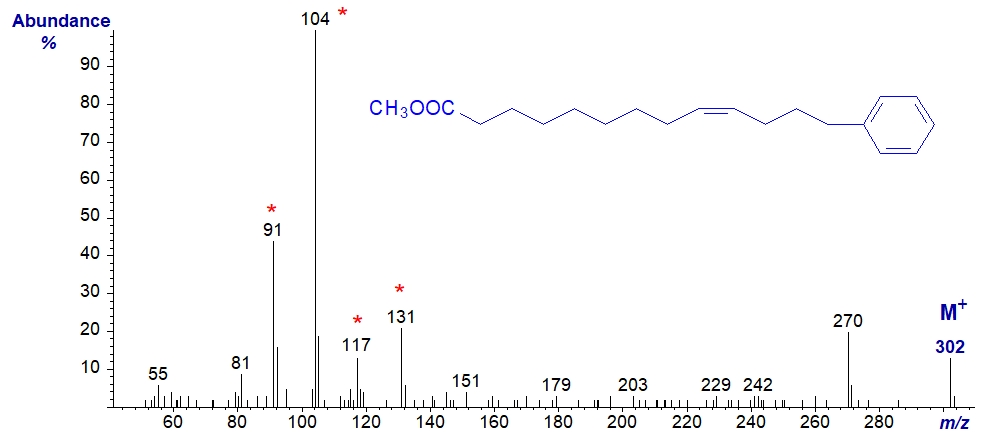
Therefore, as might be anticipated there are no ions that locate the position of the double bond in the alkyl chain, although this has been accomplished by preparing the dimethyl disulfide adducts (Meija and Soukup, 2004). Spectra of further homologues with saturated alkyl chains have recently been published (Schröder, M. et al., 2014). While 3-pyridylcarbinol and pyrrolidide derivatives have been employed successfully, DMOX derivatives tend to be less useful for ω-substituted derivatives.
There are mass spectra of methyl esters of many more cyclic fatty acids for reference or comparison purposes in our Archive section, and these include spectra of methyl esters of phenolic acids such as coumaric, caffeic, ferulic and sinapic acids, which are common constituents of plant surface waxes and cutins (all without interpretation).
References
 Christie, W.W.
13-Phenyltridec-9-enoic and 15-phenylpentadec-9-enoic acids in
Arum maculatum seed oil. Eur. J. Lipid Sci. Technol.,
105, 779-780 (2003); DOI.
Christie, W.W.
13-Phenyltridec-9-enoic and 15-phenylpentadec-9-enoic acids in
Arum maculatum seed oil. Eur. J. Lipid Sci. Technol.,
105, 779-780 (2003); DOI.- Christie, W.W. and Holman, R.T. Mass spectrometry of lipids. 1. Cyclopropane fatty acids. Lipids, 1, 176-182 (1966); DOI.
- Christie, W.W., Brechany, E.Y. and Shukla, V.K.S. Analysis of seed oils containing cyclopentenyl fatty acids by combined chromatographic procedures. Lipids, 24, 116-120 (1989); DOI.
- Christie, W.W., Rebello, D. and Holman, R.T. Mass spectrometry of derivatives of cyclopentenyl fatty acids. Lipids, 4, 229-231 (1969); DOI.
- Hooper, N.K. and Law, J.H. Mass spectrometry of derivatives of cyclopropene fatty acids. J. Lipid Res., 9, 270-275 (1968); DOI).
- McCloskey, J.A. and Law, J.H. Ring location in cyclopropane fatty acid esters by a mass spectrometry method. Lipids, 2, 225-230 (1967); DOI.
- Meija, J. and Soukup, V.G. Phenyl-terminated fatty acids in seeds of various aroids. Phytochemistry, 65, 2229-2237 (2004); DOI.
- Minnikin, D.E. Ring location in cyclopropane fatty acid esters by boron trifluoride-catalyzed methoxylation followed by mass spectrometry. Lipids, 7, 398-403 (1972); DOI.
- Pawlowski, N.E., Eisele, T.A., Lee, D.J., Nixon, J.E. and Sinnhuber, R.O. Mass spectra of methyl sterculate and malvalate and 1,2-dialkylcyclopropenes. Chem. Phys. Lipids, 13, 164-172 (1974); DOI.
- Schogt, J.C.M. and Begemann, P.H. Isolation of 11-cyclohexylundecanoic acid from butter. J. Lipid Res., 6, 466-470 (1965); DOI.
- Schröder, M. and Vetter, W. Detection of 430 fatty acid methyl esters from a transesterified butter sample. J. Am. Oil Chem. Soc., 90, 771-790 (2013); DOI.
- Schröder, M., Abdurahman, H., Ruoff, T., Lehnert, K. and Vetter, W. Identification of aromatic fatty acids in butter fat. J. Am. Oil Chem. Soc., 91, 1695-1702 (2014); DOI.
- Sébédio, J.L. and Grandgirard, A. Cyclic fatty acids: natural sources, formation during heat treatment, synthesis and biological properties. Prog. Lipid Res., 28, 303-336 (1989); DOI.
- Spitzer, V. GC-MS (chemical ionization and electron impact modes) characterization of the methyl esters and oxazoline derivatives of cyclopropene fatty acids. J. Am. Oil Chem. Soc., 68, 963-969 (1991); DOI.
I can recommend - Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - from Science Direct.
| © Author: William W. Christie |  |
|
| Updated: November 15th, 2023 | Contact/credits/disclaimer | |
